Abstract
OX2 (CD200) is a type-1 membrane glycoprotein that contains two immunoglobulin superfamily domains and which is expressed on a variety of lymphoid and non-lymphoid cells in the rat. The recent characterization of a receptor for OX2 (OX2R) on myeloid cells, and the phenotype of an OX2-deficient mouse, suggests that OX2 may regulate myeloid cell activity in anatomically diverse locations. Here we report the tissue distribution of the human homologue of the rat OX2 glycoprotein using a new monoclonal antibody (mAb), OX104, raised against recombinant human OX2. Human OX2 was expressed at the cell surface of thymocytes, B cells, T cells, neurons, kidney glomeruli, tonsil follicles, the syncytiotrophoblast and endothelial cells. This broad, but not ubiquitous, distribution pattern is very similar to that observed in rats, suggesting that OX2 may regulate myeloid cell activity in a variety of tissues in humans.
Introduction
Analysis of the leucocyte-cell surface has been facilitated by the ‘shotgun’ production of monoclonal antibodies (mAbs) that recognize extracellular structures.1 Obviously, molecules that were restricted to leucocytes were good candidates for leucocyte-specific functions, but several proteins had rather broad distributions, making it difficult to predict their biological roles. These proteins were not expressed on all cell types and hence were unlikely to be involved in general housekeeping functions. These proteins included Thy-1, L1, NCAM and OX2 glycoproteins, which were present both on leucocytes and brain cells, and also on some other cell types.2
The OX2 cell-surface glycoprotein was defined by a mAb raised against glycoproteins prepared from rat thymocytes.3 The major sites of OX2 expression in rat are thymocytes, activated T cells, B cells, follicular dendritic cells, neurons, vascular endothelium, kidney glomeruli, the granulosa of degenerating corpora lutea, trophoblasts and some smooth muscle.4–8 Data in the mouse show that OX2 is expressed on thymocytes, some T cells and brain tissueA1 (G. J. Wright, M. H. Brown & A. N. Barclay, unpublished). The OX2 protein, like many other leucocyte-surface proteins, contains two immunoglobulin superfamily (IgSF) domains, suggesting that it functions through interactions with other cell-surface proteins.2 The cytoplasmic region of the OX2 protein is short (19 amino acids) and contains no known signalling motifs.9 The broad tissue distribution of OX2 and apparent lack of signalling capability that might result from interactions of the extracellular domains, made deduction of function difficult.
OX2 has recently been shown to interact with another protein (known as the OX2 receptor, or OX2R), which also contains two IgSF domains but, in contrast to OX2 itself, the OX2R is only expressed by cells of the myeloid lineage and has a large cytoplasmic region that contains tyrosines, which are known sites of phosphorylation, including an NXPY PTB-binding motif.10 The distribution and molecular nature of the OX2/OX2R proteins suggested that they might be involved in the tissue-specific regulation of myeloid functions. Indeed, the phenotype of an OX2-deficient mouse showed defects in myeloid cellular biology, which included elevated numbers of macrophages within tissues that normally express OX2, and the brain microglia appeared to be more numerous and in a more activated state.A1 This phenotype indicated that the role of OX2 was to control myeloid cellular activity in a restrictive manner via interaction with the OX2R.10,A1
If OX2 serves a similar role in human as that implicated in rodents, then one would expect the unusual distribution of the OX2 protein to be conserved across these species. Prior to this study, Northern blot analysis had shown the presence of human OX2 mRNA in two B-cell lymphomas (MAJA and WI-L2) and in normal brain,11 and an antibody had been reported to recognize OX2 on dendritic cells.12 Therefore, the distribution of the human OX2 glycoprotein is critical in revealing the tissues that have the potential ability to regulate myeloid function through this pathway. We report the production of recombinant human OX2 protein, its use in raising a mAb (OX104) that recognizes native human OX2, and the distribution of OX2 protein in lymphoid and non-lymphoid organs.
Materials and methods
Construction, expression and purification of a human OX2CD4d3+4 soluble fusion protein
The two IgSF domains that comprise the extracellular region of the human homologue of the OX2 glycoprotein were amplified by the polymerase chain reaction (PCR) using the oligonucleotides GTCTAGACACACCATGGGCAGTCCGGTGATCAGGATGCCCTTC (sense) and ATGGATGTCGACCCTTTGTTGACGGTTTG (antisense), which were designed using the known genomic sequence11 and human spleen cDNA (kindly provided by Dr A. McKnight, King’s College Hospital, London, UK) as a template. The first four amino acids of the leader sequence, which were not identified in the genomic sequence, were replaced by the rat sequence: MGSP. The products were blunt-end ligated using the pBluescript cloning kit (Stratagene, La Jolla, CA) and subcloned into appropriately digested rCD4d3+4 pBluescript.13 Clones containing inserts were sequenced and one was found to contain a single amino acid substitution that was attributed to a PCR error by comparison to the genomic sequence.11 This clone was used as a template for a further PCR using the oligonucleotides CCCGGGGGATTCTAGACACACCATGGGCAGTCCG (OX2 sense) and CCATCTCAACTCTCCCTGC (CD4d3+4 antisense), which allowed cloning in-frame with CD4d3+4 in pEF-BOS using XbaI and SalI restriction endonuclease sites.13 The mutant OX2CD4d3+4 was corrected using the mutagenesis kit from Bio-Rad (Hemel Hempstead, UK). The wild-type OX2CD4d3+4 construct was tested in a transient mammalian expression system and was expressed at high levels (≈ 10 µg/ml). This construct was then subcloned into the expression vector pEE14, a CHO.K1 cell line was transfected using the CaPO4 method and a stably secreting line was established.13 OX2CD4d3+4 was purified from spent tissue culture medium by immunoaffinity chromatography using the OX68 mAb that recognizes the CD4d3+4 protein tag.13
Bead-binding assay
Bead-binding assays were performed essentially as described previously,14,15 with the exception that biotinylated, fluorescein isothiocyanate (FITC)-loaded plastic beads (Spherotech Inc., Libertyville, IL) were used instead of Covaspheres™.
Generation of OX104 mAb
Six-week-old male BALB/c mice were immunized, subcutaneously, with 10–20 µg of purified OX2CD4d3+4 in complete Freund's adjuvant (once) and incomplete Freund's adjuvant (twice). A mouse generating good immune responses to the immunogen was boosted and 4 days later the spleen was removed and hydridomas generated, using standard procedures, by fusion with the NS-1 cell line. Hybridoma supernatants were initially screened by enzyme-linked immunosorbent assay (ELISA) using the immunogen OX2CD4d3+4 as a target and then with soluble rat CD4 to eliminate hydridomas secreting mAbs reactive to the rat CD4 portion of the chimaeric protein. The remaining hybridoma supernatants were screened for the ability to stain the MAJA human B-cell lymphoma that had been identified as a potential source of OX2 by Northern blot.11 One hybridoma was recloned three times, isotyped as a mouse immunoglobulin G1 (IgG1) and named OX104.
mAbs
All cells were freshly stained at 4° in the presence of 10 mm azide, according to standard procedures, on a fluorescence-activated cell sorter (FACScan; Becton-Dickinson, Palo Alto, CA). OX104 mAb was detected using a phycoerythrin (PE)-conjugated donkey anti-mouse secondary antibody (Chemicon, Harrow, UK). Double and triple staining was performed, respectively, using biotinylated anti-human CD3 (UCHT1 mouse IgG1) and CD19 (HIB19 mouse IgG1) (both from BD Pharmingen, San Diego, CA), and anti-human CD4 (RPA-T4 FITC-conjugated mouse IgG1), CD8 (3B5 Cy5-conjugated mouse IgG2a) and CD25 (M-A251 FITC-conjugated mouse IgG1) (all Serotec, Kidlington, UK). OX1 (anti-rat CD45), OX45 (anti-rat CD48) and OX102 (anti-rat OX2 receptor) are all mouse IgG1 mAbs.
Human tissue
Human blood was obtained the day after collection from the National Blood Centre, John Radcliffe Hospital, Oxford. Blood was diluted with an equal volume of RPMI-1640 (Gibco BRL, Paisley, UK) before 25 ml was layered over 15 ml of Ficoll–Hypaque (Pharmacia Biotech, Little Chalfont, Buckingham, UK) and erythrocytes/granulocytes removed by centrifugation. The leucocyte interface was removed and the remaining erythrocytes were lysed using an ammonium chloride solution and then washed until the supernatant was clear (platelet free). T-cell blasts were generated by culturing peripheral blood mononuclear cells (PBMCs), at a concentration of 1·5 × 106 cells/ml, for 3 days in RPMI-1640/10% fetal calf serum (FCS) in the presence of 5 µg/ml of concanavalin A (Pharmacia Biotech). Human thymi were obtained from the John Radcliffe Hospital and used on the day of thymectomy.
Immunohistochemistry
Immunohistochemistry was performed on acetone-fixed cryostat sections of human tissue using a standard indirect peroxidase technique and then counterstained with haematoxylin.16 Sections were viewed on an Olympus BX 40 microscope (Olympus Optical Company, London, UK) and images stored electronically using a JVC KY F-30 digital camera (JVC Professional, London, UK).
Results
A human OX2CD4d3+4-binding reagent binds the rat OX2R
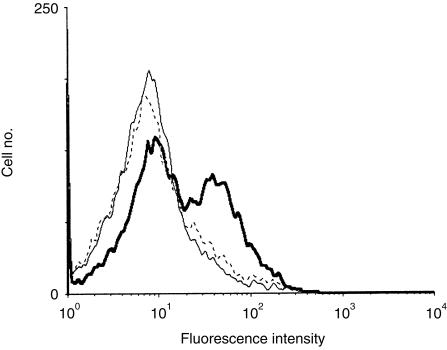
The two IgSF domains that comprise the extracellular region of the human OX2 molecule were expressed as a soluble chimaera with domains three and four of rat CD4 (CD4d3+4). Soluble OX2CD4d3+4 protein, when presented as a multivalent OX2-binding reagent around fluorescent beads, bound rat resident peritoneal cells (RPCs) that primarily consist of macrophages (Fig. 1). This binding could be prevented by preincubating the RPCs with the OX102 mAb that recognizes the rat OX2R and is able to block the OX2–OX2R interaction.10 This demonstrated that human OX2 was able to bind the rat OX2 receptor and that the chimaeric protein was correctly folded. This OX2CD4d3+4 protein was purified and used to immunize mice for mAb production. The hybridoma supernatants were screened on both recombinant human OX2 and the MAJA cell line (data not shown), and one mAb, OX104, was cloned and analysed in detail.
Figure 1.
Recombinant human OX2CD4d3+4 binds rat OX2 receptor (OX2R). Flow cytometry analysis shows that human OX2-coated beads bound rat resident peritoneal cells (RPCs) coated in OX45 (anti-rat CD48) monoclonal antibody (mAb) (thick line), but that binding was blocked back to a negative control (CD4d3+4-coated beads, thin line) by OX102 (anti-rat OX2R) mAb (broken line).
Human OX2 is expressed in lymphoid tissue
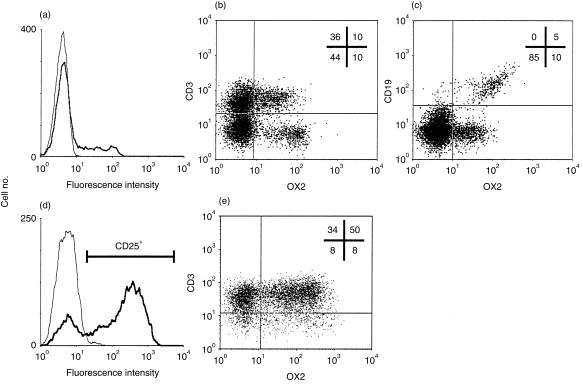
The distribution of the human OX2 antigen was analysed using the OX104 mAb, as summarized in Table 1. The OX104 mAb stained ≈ 20% of PBMCs (Fig. 2a). This OX2+ population could be separated into both CD19+ and CD3+ subpopulations using multicolour cytofluorimetry (Fig. 2b, 2c). All CD19+ cells were OX2+, and 20% of CD3+ cells expressed OX2 at the cell surface. T blasts were generated by culturing PBMCs in concanavalin A for 3 days and were shown to have an activated phenotype, as demonstrated by CD25 expression (Fig. 2d). 60% of CD25+ CD3+ cells expressed elevated levels of OX2, showing that OX2 is up-regulated on a population of activated T cells (Fig. 2e).
Table 1.
Localization of human OX2 protein expression, as detected by the OX104 monoclonal antibody (mAb)
| Tissue | Details |
|---|---|
| Lymphoid | All CD19+ B cells |
| Subset (≈ 20%) CD3+ T cells | |
| Up-regulated on most (60%) CD3+ CD25+ T-cell blasts | |
| Follicular dendritic cells | |
| Thymocytes (elevated expression on single positives) | |
| Neural | Cerebellum, peripheral nerve bundles |
| Other tissues | Vascular endothelium, smooth muscle, kidney glomeruli, syncytiotrophoblast |
Figure 2.
OX2 is expressed on a subset of T cells and all B cells. (a) Flow cytometry shows that OX104 monoclonal antibody (mAb) (thick line) stained ≈ 20% of total peripheral blood mononuclear cells (PBMCs) when compared with an isotype-matched negative control, OX1 (thin line). Approximately 20% of CD3+ (b) and all CD19+ (c) cells were OX2+. (d) Three-day concanavalin A-stimulated PBMCs were gated according to the activation marker, CD25 (thick line). (e) Approximately 60% of the CD25+ CD3+ cells were OX2 positive. The number of events in each quadrant is expressed as a percentage of the total gated.
Human OX2 expression increases upon thymocyte lineage commitment
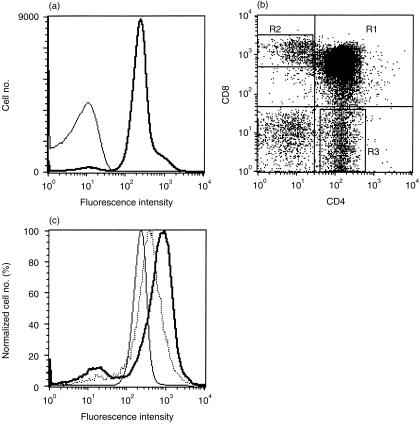
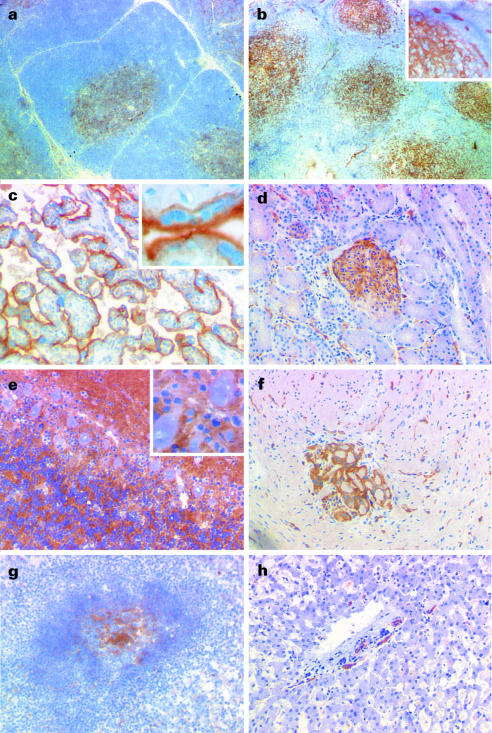
Almost all human thymocytes were densely stained with the OX104 mAb (Fig. 3a). Triple colour staining using CD4 and CD8 mAbs showed that the mean level of OX2 expression was higher on single-positive (SP) lineage-committed thymocytes (Fig. 3b, 3c). The CD4 SP thymocytes had a higher mean level of OX2 expression than the CD8 SP population although there was a distinct, but small, percentage of CD4 SP cells that were OX2 negative. This correlated with the immunohistochemistry staining of the thymus that showed a more intense staining of the thymic medulla when compared with the cortical regions (Fig. 4a).
Figure 3.
OX2 is expressed on thymocytes and at increased levels on single positive, lineage-committed thymocytes. (a) Flow cytometry shows that almost all thymocytes were stained with the OX104 monoclonal antibody (mAb) (thick line) above an isotype-matched negative control, OX1 (thin line). (b) Thymocytes were gated, according to CD4 and CD8 expression, into: (R1) CD4+ CD8+ double-positives (DPs); (R2) CD8+ single-positives (SPs); and (R3) CD4+ SPs. The level of OX2 expression was plotted as a function of the normalized cell number from each region in (b) with R3 CD4+ SPs (bold line), R2 CD8+ SPs (dotted line) and R1 CD4+ CD8+ DPs (thin line) (c).
Figure 4.
Immunohistochemistry of frozen human tissue stained with OX104 monoclonal antibody (mAb). (a) Thymus. The medullary regions were strongly stained, with diffuse and less prominent staining within the cortex. (b) Tonsil. The follicles in the tonsil were highly stained, consistent with the staining of follicular dendritic cells and/or activated T cells. In addition, endothelial-like structures in the extrafollicular regions were positive. The inset, at a higher magnification, demonstrates both the diffuse nature of the staining in the follicle (bottom left) and endothelial-like staining (top). (c) Placenta. Strong staining of syncytiotrophoblast membranes, which is shown more clearly in the inset. (d) Kidney. The glomeruli of the kidney were strongly stained. (e) Cerebellum. The molecular layer was strongly stained with patches of staining in the granular layer. The Purkinjé cells and glial cells appear unstained (inset). (f) Gut. Scattered blood vessels were stained and one prominent nerve bundle. (g) Spleen. The majority of staining was in the follicular region with occasional positive cells in both the white and red pulps. (h) Liver. Largely negative but with scattered staining that was mainly confined to the vascular endothelium.
Human OX2 is expressed on lymphoid, neural and other tissues
Immunohistochemistry showed that OX2 expression in the secondary lymphoid organs (tonsil Fig. 4b and spleen Fig. 4g) was primarily restricted to the follicles. The diffuse nature of the staining pattern was consistent with the positive cell being the follicular dendritic cell (Fig. 4b, inset). The B-cell areas were not stained, suggesting that the level of OX2 on B cells was below the sensitivity of the immunohistochemistry technique used.
The vascular endothelium (particularly the high endothelial venules) was highly stained in tonsil (Fig. 4b, inset). Staining of the vasculature was selective and seemed primarily restricted to the smaller vessels. The membranes of the syncytiotrophoblast were highly positive (Fig. 4c), and the glomeruli of the kidney were brightly stained and expression appeared to be localized to the endothelium (Fig. 4d).
OX2 was highly expressed on both central and peripheral nerve tissue (Fig. 4e, 4f). The staining in the cerebellum was almost confluent in the molecular layer with a more patchy staining in the granular layer. The large Purkinjé cells appeared to be negative (Fig. 4e, inset) but it is not possible to determine whether their membranes were labelled. The staining pattern was very similar to that reported in rats, where most neurons and endothelium were clearly positive.4 Peripheral nerve bundles were highly stained and were observed in the gut (Fig. 4f) and liver (data not shown). Endothelial structures were stained in several tissues, including liver tissue (Fig. 4h).
Discussion
The distribution of the human OX2 cell-surface glycoprotein is very similar to the unusual expression patterns reported in rat. The well-conserved pattern of OX2 protein expression across these species on a broad range of evolutionary diverse cells and tissues suggests that the distribution of OX2 is central to its function and unlikely to be a result of aberrant expression. This wide, but not ubiquitous, tissue distribution argues against OX2 playing a role in general ‘housekeeping’ functions and it is more probable that it acts to control myeloid cell activity in tissues through its interaction with its recently described receptor.10
In the steady state, macrophages are involved in a wide range of tissue maintenance functions17–21 but can also play a role in the initiation and perpetuation of inflammatory processes that can cause local tissue damage and subsequent loss of function.22 Macrophages isolated from different tissues exhibit a broad variety of different morphologies and functional phenotypes,23,24 despite originating from an apparently equivalent pool of circulating monocytes in the blood. It is widely thought that the local tissue microenvironment plays a large role in determining the function of both resident and recruited macrophages. Only relatively few cell-surface interactions have been shown to influence macrophage biology, in contrast to the widely reported roles of soluble factors.10
OX2 is highly expressed on both follicular dendritic cells and the kidney glomeruli and, interestingly, both of these tissues associate with a potent macrophage-activating stimulus: immune complexes. Follicular dendritic cells harbour immune complexes on their cell surface (and this antigen retention is the basis of the humoral memory response),25 and the kidney glomeruli are known to trap immune complexes involved in the disease glomerulonephritis. Therefore, inappropriate macrophage activation in both the glomerulus and at the follicular dendritic cell surface is an immunological problem that might be controlled by the presence of OX2.
Mechanisms to explain maternal tolerance to a semiallogeneic fetus have primarily focused on the nature of the trophoblast, the specialized cell type that forms the boundary between maternal and fetal tissues. The cell surface of the trophoblast lacks the classical human leucocyte antigen (HLA) class I and class II molecules,26 and expresses apoptosis-inducing molecules, such as Fas ligand27 and TRAIL.28 In addition, the non-classical HLA-G molecule that is expressed on the extravillous trophectoderm has recently been shown to interact with ILT-4, an inhibitory receptor expressed by cells of the myelomonocytic lineage, which suggests that negative regulation of myeloid cells is important in maintaining immune privilege.29 The high levels of OX2 expression on the syncytiotrophoblast implicates the OX2–OX2R interaction in the regulation of myeloid cells during the maintenance of maternal tolerance.
The finding that human OX2 has a tissue distribution virtually identical to that of rat is compatible with a function of OX2 that involves the marking of a range of cell types and tissues that have a common need to regulate myeloid cellular biology. The failure to see dramatic effects in knockout mice in certain OX2+ tissues (such as the thymus and placenta)A1 would imply that this is one of many control mechanisms that locally control macrophage activity through cell–cell contact, in both healthy and inflamed tissue.
Acknowledgments
We thank Ruth Goddard for her help in creating the OX2CD4d3+4 stable line. This work was supported by the Medical Research Council.
Note Added In Proof
After this paper had been put into production, a submitted article was published. The reference A1 has been used to cite this article throughout the text.
A1 Hoek RM, Ruuls SR, Murphy CA et al. Down-regulation of the macrophage lineage through interaction with OX2(CD200). Science 2000; 290: 1768.
References
- 1.Williams AF, Galfre G, Milstein C. Analysis of cell surfaces by xenogeneic myeloma-hybrid antibodies: differentiation antigens of rat lymphocytes. Cell. 1977;12:663. doi: 10.1016/0092-8674(77)90266-5. [DOI] [PubMed] [Google Scholar]
- 2.Barclay AN, Brown MH, Law SKA, McKnight AJ, Tomlinson MG, van der Merwe PA. Leucocyte Antigens Factsbook. 2. London: Academic Press; 1997. [Google Scholar]
- 3.McMaster WR, Williams AF. Identification of Ia glycoproteins in rat thymus and purification from rat spleen. Eur J Immunol. 1979;9:426. doi: 10.1002/eji.1830090603. [DOI] [PubMed] [Google Scholar]
- 4.Webb M, Barclay AN. Localisation of the MRC OX-2 glycoprotein on the surfaces of neurones. J Neurochem. 1984;43:1061. doi: 10.1111/j.1471-4159.1984.tb12844.x. [DOI] [PubMed] [Google Scholar]
- 5.Barclay AN. Different reticular elements in rat lymphoid tissue identified by localization of Ia, Thy-1 and MRC OX 2 antigens. Immunology. 1981;44:727. [PMC free article] [PubMed] [Google Scholar]
- 6.Barclay AN, Ward HA. Purification and chemical characterisation of membrane glycoproteins from rat thymocytes and brain, recognised by monoclonal antibody MRC OX 2. Eur J Biochem. 1982;129:447. doi: 10.1111/j.1432-1033.1982.tb07070.x. [DOI] [PubMed] [Google Scholar]
- 7.Bukovsky A, Presl J, Zidovsky J, Mancal P. The localization of Thy-1.1, MRC OX 2 and Ia antigens in the rat ovary and fallopian tube. Immunology. 1983;48:587. [PMC free article] [PubMed] [Google Scholar]
- 8.Bukovsky A, Presl J, Zidovsky J. Association of some cell surface antigens of lymphoid cells and cell surface differentiation antigens with early rat pregnancy. Immunology. 1984;52:631. [PMC free article] [PubMed] [Google Scholar]
- 9.Clark MJ, Gagnon J, Williams AF, Barclay AN. MRC OX-2 antigen: a lymphoid/neuronal membrane glycoprotein with a structure like a single immunoglobulin light chain. EMBO J. 1985;4:113. doi: 10.1002/j.1460-2075.1985.tb02324.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wright GJ, Puklavec MJ, Willis AC, Hoek RM, Sedgwick JD, Brown MH, Barclay AN. The lymphoid/neuronal cell surface OX2 glycoprotein recognises a novel receptor on macrophages implicated in the control of their function. Immunity. 2000;13:233. doi: 10.1016/s1074-7613(00)00023-6. [DOI] [PubMed] [Google Scholar]
- 11.McCaughan GW, Clark MJ, Barclay AN. Characterization of the human homolog of the rat MRC OX-2 membrane glycoprotein. Immunogenetics. 1987;25:329. doi: 10.1007/BF00404426. [DOI] [PubMed] [Google Scholar]
- 12.Ragheb R, Abrahams S, Beecroft R, Hu J, Ni J, Ramakrishna V, Yu G, Gorczynski RM. Preparation and functional properties of monoclonal antibodies to human, mouse and rat OX-2. Immunol Lett. 1999;68:311. doi: 10.1016/s0165-2478(99)00060-7. 10.1016/s0165-2478(99)00060-7. [DOI] [PubMed] [Google Scholar]
- 13.Brown MH, Barclay AN. Expression of immunoglobulin and scavenger receptor superfamily domains as chimeric proteins with domains 3 and 4 of CD4 for ligand analysis. Protein Eng. 1994;7:515. doi: 10.1093/protein/7.4.515. [DOI] [PubMed] [Google Scholar]
- 14.Brown MH, Preston S, Barclay AN. A sensitive assay for detecting low-affinity interactions at the cell surface reveals no additional ligands for the adhesion pair rat CD2 and CD48. Eur J Immunol. 1995;25:3222. doi: 10.1002/eji.1830251204. [DOI] [PubMed] [Google Scholar]
- 15.Preston S, Wright GJ, Starr K, Barclay AN, Brown MH. The leukocyte/neuron cell surface antigen OX2 binds to a ligand on macrophages. Eur J Immunol. 1997;27:1911. doi: 10.1002/eji.1830270814. [DOI] [PubMed] [Google Scholar]
- 16.Bastin JM, Jones M, O'Callaghan CA, Schimanski L, Mason DY, Townsend AR. Kupffer cell staining by an HFE-specific monoclonal antibody: implications for hereditary haemochromatosis. Br J Haematol. 1998;103:931. doi: 10.1046/j.1365-2141.1998.01102.x. [DOI] [PubMed] [Google Scholar]
- 17.Hopkinson-Woolley J, Hughes D, Gordon S, Martin P. Macrophage recruitment during limb development and wound healing in the embryonic and foetal mouse. J Cell Sci. 1994;107:1159. doi: 10.1242/jcs.107.5.1159. [DOI] [PubMed] [Google Scholar]
- 18.Schaffer CJ, Nanney LB. Cell biology of wound healing. Int Rev Cytol. 1996;169:151. doi: 10.1016/s0074-7696(08)61986-5. [DOI] [PubMed] [Google Scholar]
- 19.Sunderkotter C, Steinbrink K, Goebeler M, Bhardwaj R, Sorg C. Macrophages and angiogenesis. J Leukoc Biol. 1994;55:410. doi: 10.1002/jlb.55.3.410. [DOI] [PubMed] [Google Scholar]
- 20.Unanue ER. Antigen-presenting function of the macrophage. Annu Rev Immunol. 1984;2:395. doi: 10.1146/annurev.iy.02.040184.002143. [DOI] [PubMed] [Google Scholar]
- 21.Platt N, da Silva RP, Gordon S. Class A scavenger receptors and the phagocytosis of apoptotic cells. Immunol Lett. 1999;65:15. doi: 10.1016/s0165-2478(98)00118-7. 10.1016/s0165-2478(98)00118-7. [DOI] [PubMed] [Google Scholar]
- 22.Erwig LP, Rees AJ. Macrophage activation and programming and its role for macrophage function in glomerular inflammation. Kidney Blood Press Res. 1999;22:21. doi: 10.1159/000025905. [DOI] [PubMed] [Google Scholar]
- 23.Naito M, Umeda S, Yamamoto T, et al. Development, differentiation, and phenotypic heterogeneity of murine tissue macrophages. J Leukoc Biol. 1996;59:133. doi: 10.1002/jlb.59.2.133. [DOI] [PubMed] [Google Scholar]
- 24.Gordon S. The macrophage. Bioessays. 1995;17:977. doi: 10.1002/bies.950171111. [DOI] [PubMed] [Google Scholar]
- 25.Klaus GG, Humphrey JH, Kunkl A, Dongworth DW. The follicular dendritic cell: its role in antigen presentation in the generation of immunological memory. Immunol Rev. 1980;53:3. doi: 10.1111/j.1600-065x.1980.tb01038.x. [DOI] [PubMed] [Google Scholar]
- 26.Parham P. Immunology: keeping mother at bay. Curr Biol. 1996;6:638. doi: 10.1016/s0960-9822(09)00436-9. [DOI] [PubMed] [Google Scholar]
- 27.Runic R, Lockwood CJ, Ma Y, Dipasquale B, Guller S. Expression of Fas ligand by human cytotrophoblasts: implications in placentation and fetal survival. J Clin Endocrinol Metab. 1996;81:3119. doi: 10.1210/jcem.81.8.8768884. [DOI] [PubMed] [Google Scholar]
- 28.Phillips TA, Ni J, Pan G, Ruben SM, Wei YF, Pace JL, Hunt JS. TRAIL (Apo-2L) and TRAIL receptors in human placentas: implications for immune privilege. J Immunol. 1999;162:6053. [PubMed] [Google Scholar]
- 29.Allan DS, Colonna M, Lanier LL, Churakova TD, Abrams JS, Ellis SA, McMichael AJ, Braud VM. Tetrameric complexes of human histocompatibility leukocyte antigen (HLA)-G bind to peripheral blood myelomonocytic cells. J Exp Med. 1999;189:1149. doi: 10.1084/jem.189.7.1149. [DOI] [PMC free article] [PubMed] [Google Scholar]