Abstract
It is well established in animal models that production of the cytokine tumour necrosis factor-α (TNF-α) is essential to the proper expression of acquired specific resistance following infection with Mycobacterium tuberculosis. This gives rise to an apparent state of chronic disease which over the next 100–200 days is characterized by slowly worsening pathological changes in the lung. To determine whether continued TNF-α production was harmful during this phase mice were treated with a TNF-α inhibitor, pentoxifylline. It was observed that although this therapy did not alter the numbers of bacteria recovered from the lungs of the infected mice, tissue damage within the lung was accelerated. These data thus demonstrate that production of TNF-α, already known to be important during the early expression of resistance to tuberculosis, remains important and beneficial during the chronic stage of the disease.
Introduction
It is well established that the cytokine tumour necrosis factor-α (TNF-α) plays an important role in the expression of protective immunity to mycobacterial infection.1 Addition of exogenous TNF-α in vivo leads to an increased ability of mice to control mycobacterial infection2 whereas blocking the cytokine by specific antibodies prevents adequate development of granulomas in mice infected with bacillus Calmette–Guèrin.3 More recently, it has been shown using TNF-α gene-disrupted,4,5 or TNF-α receptor-disrupted,6 mice that these animals have significantly reduced survival times after infection with Mycobacterium tuberculosis, thus demonstrating the necessity for TNF-α production during the early stages of this infection. The pathology of such mice4,6 showed impairment of granuloma formation, with a distinct absence of epithelioid cells,6 and evidence of necrosis.
Tuberculosis often presents as a chronic disease of the lungs. Thus while the use of the above technical approaches clearly demonstrates a beneficial role for TNF-α in early protection, such models do not provide any information regarding the role of TNF-α during the chronic stage of the disease when the bacterial load is static or slowly rising. It is quite possible that increased, or continued, expression of TNF-α would be associated with local necrosis and blood vessel destruction. We hypothesized, in keeping with the ‘doubled-edged sword’ concept,1 that although TNF-α was initially helpful, its continued production by activated macrophages within the granuloma might eventually be harmful and would contribute to the slowly increasing necrosis and breakdown of the granuloma that we have documented elsewhere.7
To test this hypothesis, mice in a state of chronic disease after aerosol infection were treated with pentoxifylline, a methyl xanthine derivative, which inhibits the synthesis of TNF-α,8–10 then examined for changes in lung pathology. Contrary to our hypothesis, such mice showed evidence of accelerated damaging lung pathology. These data thus indicate that production of TNF-α continues to be helpful and beneficial during the chronic tuberculosis disease state.
Materials and methods
Mice
Specific pathogen-free female 6–8-week-old C57BL/6 were purchased from Jackson Laboratories (Bar Harbor, ME). Infected mice were kept in BL-3 biohazard facilities throughout the experiments and maintained with sterile water, bedding and mouse chow. The specific pathogen-free nature of the mouse colonies was demonstrated by testing sentinel animals. These were shown to be negative for 12 known mouse pathogens.
Bacterial infections
Mycobacterium tuberculosis Erdman was originally obtained from the Trudeau Mycobacteria Collection, (Saranac Lake, NY). Bacteria were grown in Proskauer–Beck liquid medium containing 0·05% Tween-80 to mid log phase and frozen in aliquots at −70° until needed. Mice were infected via the aerosol route with a low dose of bacteria. Briefly, the nebulizer compartment of an airborne infection apparatus (Middlebrook, Terre Haute, IN) was filled with a suspension of bacteria resulting in the uptake of 50–100 viable bacteria per lung during a 30-min exposure. The numbers of viable bacteria in the lung and spleen were determined at various time-points by plating serial dilutions of whole organ homogenates onto Middlebrook 7H11 agar (Life Technologies, Gaithersburg, MD) and counting bacterial colonies after 20 days of incubation at 37°. The data are expressed as the log10 value of the mean number of bacteria recovered per organ (n = 4 animals).
Pentoxifylline treatment
Pentoxifylline (1-(5-oxohexyl)-3,7-dimethylxanthine) (Sigma, St Louis, MO) was prepared as a 40-µg/ml stock solution in saline. Mice were given 200 µl by intraperitoneal injection once weekly beginning 120 days post-aerosol and continuing throughout the course of the study.
Histological analysis
The lower right lung lobe was collected from each mouse and inflated with, and stored in, 10% formal saline. Tissues were prepared routinely and sectioned for light microscopy with lobe orientation designed to allow for the maximum surface area of each lobe to be seen. Tissues were stained with haematoxylin and eosin to determine granuloma formation and were examined by a qualified veterinary pathologist with no previous knowledge of the sample groups. Figures shown are representative of the experimental groups.
Reverse transcription–polymerase chain reaction (RT-PCR)
A portion of lung tissue was suspended in Ultraspec (Cinna/Biotecx, Friendswood, TX), homogenized, and frozen rapidly for storage at −70°. Total cellular RNA was extracted and reverse transcribed using murine Moloney leukaemia virus reverse transcriptase (Life Technologies, Grand Island, NY). PCR was performed with specific primers for TNF-α, interferon-γ (IFN-γ), macrophage chemoattractant protein (MCP-1), macrophage inflammatory protein-1α and 1β (MIP-1α, MIP-1β). The PCR product was Southern blotted and probed with specific, labelled oligonucleotides, and the blots were developed using the enhanced chemiluminescence kit (ECL; Amersham, Arlington Heights, IL.). The hypoxanthine phosphoribosyltransferase (HPRT) house-keeping gene was also amplified for each sample and used to confirm that equivalent amounts of readable RNA were present in all the samples.
Results
M. tuberculosis infection is unaltered in mice receiving pentoxifylline
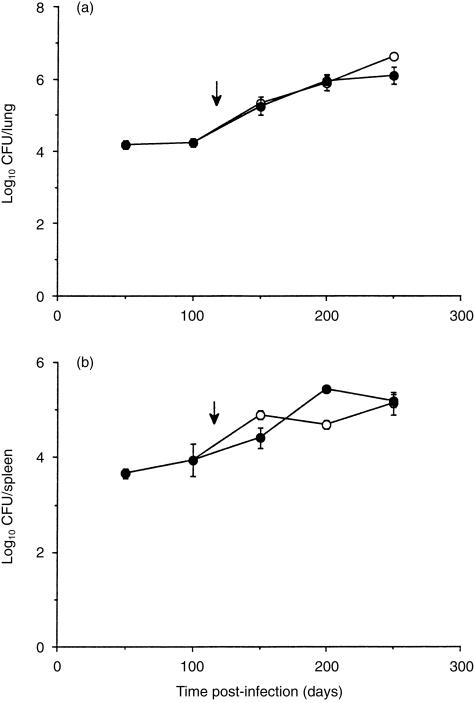
Mice were infected via the respiratory route with 102 M. tuberculosis Erdman. After 120 days mice were given pentoxifylline by intraperitoneal injection, once weekly, until the completion of the experiment. The bacterial load was similar between the control mice and the mice receiving pentoxifylline in both the lung (Fig. 1a) and spleen (Fig. 1b).
Figure 1.
Progression of M. tuberculosis infection in mice treated with pentoxifylline. Mice were infected by aerosol exposure with 102 M. tuberculosis and the course of the infection was followed over time in the lung (a) and spleen (b). Mice receiving pentoxifylline (•) were compared to a control group which had not received the drug (○). Data are expressed as the mean value from four individual mice, ± SEM. Arrows depict initiation of pentoxifylline treatment.
TNF-α mRNA is reduced in mice receiving pentoxifylline
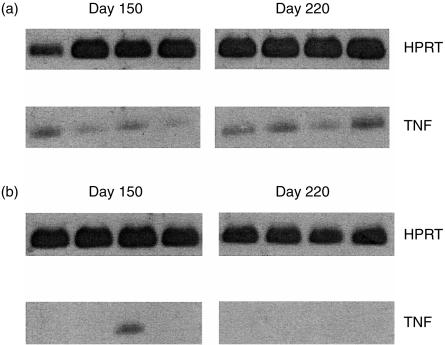
To confirm that TNF-α production was in fact reduced in mice receiving pentoxifylline we collected lung tissue throughout the course of the experiment, isolated the RNA, and probed for the presence of TNF-α by RT-PCR. Message for TNF-α was detectable in the lungs of wild-type mice (Fig. 2a), whereas TNF-α mRNA was not detectable in the lungs of the mice treated with TNF-α inhibitor at two individual time-points throughout the experiment (Fig. 2b). TNF-α message was absent from 11 out of the 12 mice over the three time-points analysed. These data therefore demonstrate that pentoxifylline treatment did indeed result in the inhibition of TNF-α synthesis.
Figure 2.
Expression of TNF-α message in the lungs of M. tuberculosis-infected mice. Lung lobes were collected from individual mice at several time-points throughout the study. Total RNA was isolated, transcribed, and probed using primers specific for HPRT and TNF. Messenger RNA from the lungs of four individual wild-type mice (a), or four individual mice which had received pentoxifylline (b), are shown at either 150 or 220 days postinfection.
Chemokine mRNA was unaltered in mice receiving pentoxifylline
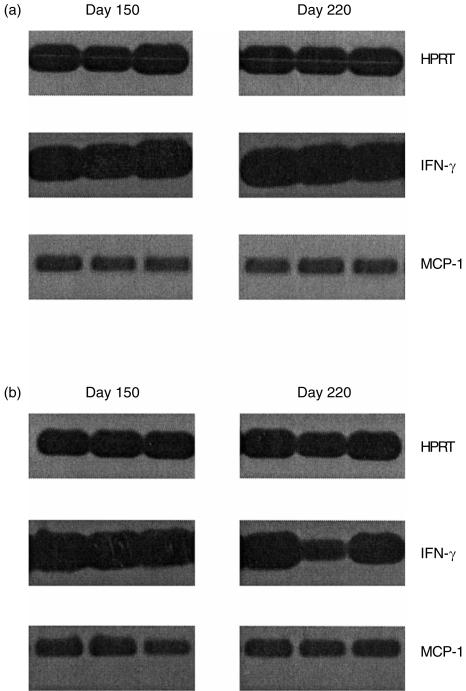
Pentoxifylline treatment, in addition to blocking TNF-α production, may also influence the cellular influx into the infected lung. In this regard, mRNA isolated from infected lung tissue was probed for the presence of several macrophage and lymphocyte chemoattractants. Figure 3 shows that there were no differences between the wild-type group and the group of mice receiving pentoxifylline for the expression of MCP-1 within the lung tissue. Similar results were obtained with MIP-1α and MIP-1β (data not shown). In addition, the expression of IFN-γ was comparable between the wild-type mice and those treated with pentoxifylline.
Figure 3.
Expression of MCP-1 and IFN-γ message in the lungs of M. tuberculosis-infected mice. Lung lobes were collected from individual mice at several time-points throughout the study. Total RNA was isolated, transcribed, and probed using primers specific for HPRT, MCP-1 and IFN-γ. Messenger RNA from the lungs of three individual wild-type mice (a), or three individual mice which had received pentoxifylline (b), are shown at either 150 or 220 days postinfection.
Pentoxifylline treatment accelerates degenerative pathology in the lung
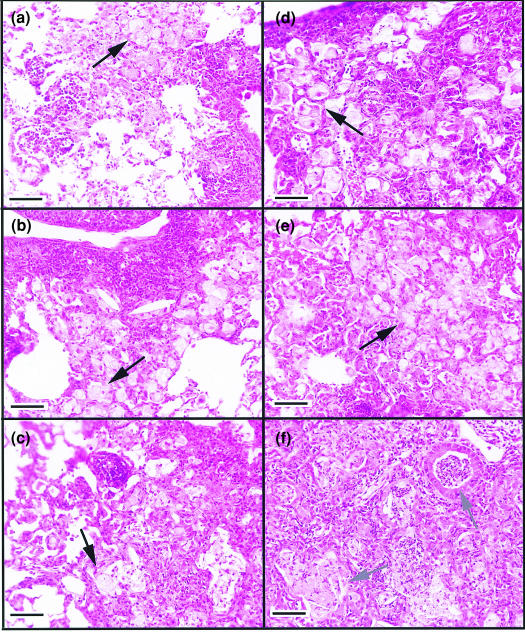
The changes within the lungs are summarized in Table 1 and representative lung lesions are depicted in Fig. 4. The lung tissues from control mice consisted of typical multifocal lesions containing mixtures of both lymphocytes and macrophages for the first 100 days (Fig. 4a) after infection. Lymphocytic foci can be seen associated with macrophage populations. As the experiment progressed through 200 (Fig. 4b) and 250 days (Fig. 4c) post-aerosol, a gradual increase in foci of degenerating (foamy) macrophages and occasional cholesterol deposition was apparent, however, the lymphocyte foci were still evident within the lesions. In contrast, lung sections from mice receiving pentoxifylline throughout the latter part of the infection had evidence of altered cellular composition in comparison to the control group. For example, on days 150 (Fig. 4d) and 200 (Fig. 4e), the lesions in this group contained substantially fewer lymphocytes, instead consisting predominantly of large foamy macrophages with lymphocytes interspersed within the lesions. In addition, the appearance of these lesions suggested a more rapid progression towards tissue degeneration, depicted by much larger areas of macrophage breakdown and deposition of cholesterol as represented in Fig. 4(f) 250 days after M. tuberculosis infection.
Table 1.
Cellular changes within the lung of mice infected with M. tuberculosis
| Time postinfection | Wild-type mice | Pentoxifylline-treated mice |
|---|---|---|
| Day 50 | Occasional areas of macrophages associated with dense lymphocyte infiltrations. | No treatment group |
| Day 100 | Expanded lesions consisting of lymphocytes associated with foamy macrophages. | No treatment group |
| Day 150 | Foamy macrophages and tissue degeneration. Fewer lymphocytes. | Fewer lymphocytes than wild-type. Much faster progression towards cellular degeneration. More necrosis. |
| Day 200 | Foamy macrophages and some cellular breakdown. Lymphocytic response still present. | Continuing tissue breakdown. Much more necrosis than wild-type. Occasional lymphocyte foci. |
| Day 250 | Cellular degeneration. Necrosis, cholesterol deposits. Lymphocytes still present within the lesions. | Distinct absence of lymphocytes. Much cellular degeneration, necrosis, and cholesterol deposition. |
Lung tissue was collected from wild-type mice, or mice receiving pentoxifylline, and sectioned and stained with haematoxylin and eosin. Sections were examined by a veterinary pathologist without prior knowledge of sample grouping. Tissue was subjectively graded for both quality and quantity of cellular accumulations from four individual mice/group at each experimental time-point.
Figure 4.
Cellular changes within the lung of M. tuberculosis-infected mice. Representative tissue from control mice, or mice receiving pentoxifylline, are depicted. Lesions in wild-type mice consisted of both lymphocyte aggregates associated with foamy macrophages after 150 days of M. tuberculosis infection (a). As the infection progressed, the lesions became more degenerative with aggregates of foamy macrophages associated with lymphocyte foci (b, 200 days). After 250 days of M. tuberculosis, the lesions again presented with lymphocyte foci associated with foamy macrophages (c). The lung lesions of mice receiving pentoxifylline had fewer lymphocyte foci throughout the duration of the experiment, although lymphocytes were scattered within the rafts of foamy macrophages (d, 150 days). As the infection progressed, there was evidence of markedly more macrophage degeneration (e, 200 days) which was associated with regions of necrotic cellular debris associated with cholesterol deposition (f, 250 days). Magnification ×200, size bar = 100 µm. Black arrows depict foamy macrophages; grey arrows show necrotic cellular debris and cholesterol deposition.
Discussion
This study shows that administration of a drug that prevents the production of the cytokine TNF-α during the chronic phase of M. tuberculosis infection does not significantly alter the bacterial load recovered from these animals. However, there was a clear acceleration in the disease progression in terms of the degree of macrophage degeneration, tissue necrosis and cholesterol deposition.7
Accordingly, we hypothesize that TNF-α plays an important role during the course of the chronic stage of the disease. It does not appear to be a direct anti-microbial role, because the bacterial load was not affected, even though this does gradually increase as the pathology worsens.7 An alternative possibility therefore would be in terms of maintenance of the continued integrity of the granuloma. We suspect that TNF-α is a key molecule in the regulation of this event, mediated directly or indirectly by the production of mononuclear cell-directed chemoattractant chemokines.1 It is therefore possible that blocking the production of TNF-α subsequently alters the mechanisms that control the size and cellular composition of the granuloma, promoting a continued macrophage influx but failing to recruit lymphocytes, thus resulting in the degenerative type of lesions observed here. Analysis of macrophage and lymphocyte chemoattractants however, showed no differences in the ability of mice receiving pentoxifylline to synthesize several chemokines, thus suggesting that TNF-α itself, or perhaps other chemokines, may be influencing granuloma formation. What we cannot definitively conclude from these data is the hypothesis that the production of TNF-α was itself responsible for the gradual progression of the disease and development of necrosis we have documented previously.7 In this regard, TNF-α can act as an inflammatory mediator, up-regulating the expression of adhesion molecules on the endothelium and allowing the trafficking of lymphocytes into the infected tissues.11,12 However, IFN-γ production was evident in both experimental groups therefore suggesting that despite the absence of lymphocytic foci within the lungs of pentoxifylline-treated mice, the capacity to produce IFN-γ was not compromised. TNF-α message was noticeably absent from the lung, thus demonstrating that pentoxifylline treatment did indeed inhibit the synthesis of TNF-α, however, pentoxifylline has also been shown to inhibit the activation and adhesion molecules of human lymphocytes in vitro.13 If this observation can be extrapolated to an in vivo system then the absence of activated cells within the lung might also account for the differences in pathological changes within the lung that we observed.
Contrary to findings by other investigators using gene-disrupted mouse models,4,6 we did not see elevated bacterial numbers in the organs of mice receiving the TNF-α inhibitor, thus indicating that the anti-microbial role of TNF-α is limited to the initial phase of immunity to the infection. TNF is expressed rapidly by macrophages after infection with mycobacteria,14,15 and in conjunction with IFN-γ can stimulate the production of the anti-microbial molecule nitric oxide.16,17 Thus the absence of TNF-α during the initiation of infection would allow the mycobacteria to grow uncontrollably and as demonstrated elsewhere4–6 prove fatal.
Studies of human tuberculosis show that TNF-α messenger RNA is elevated within the pleural fluid of tuberculoid pleurisy patients, a form of disease associated with a rigorous immune response to the infection.18 In addition, increased TNF-α mRNA in lymph nodes of tuberculosis patients has been demonstrated to be associated with a lack of necrosis within the granuloma,19 an observation which supports the data presented here. Clearly, in humans, the continued production of TNF-α in response to chronic M. tuberculosis infection also appears to be beneficial. However, patients with severe tuberculosis have increased TNF-α production during the initial 7 days of anti-tuberculosis drug therapy, perhaps in response to the abundance of lipoglycans and other antigens from the dead mycobacteria.20 This was, however, associated with some degree of clinical deterioration thus demonstrating that when TNF-α production is excessive this can also be deleterious to the host.
Acknowledgments
This work was supported by NIH grants AG-06946 and AI-44072.
References
- 1.Orme IM, Cooper AM. Cytokine/chemokine cascades in immunity to tuberculosis. Immunol Today. 1999;20:307–12. doi: 10.1016/s0167-5699(98)01438-8. [DOI] [PubMed] [Google Scholar]
- 2.Champsi J, Young LS, Bermudez LE. Production of TNF-alpha, IL-6 and TGF-beta, and expression of receptors for TNF-alpha and IL-6, during murine Mycobacterium avium infection. Immunology. 1995;84:549–54. [PMC free article] [PubMed] [Google Scholar]
- 3.Kindler V, Sappino A-P, Grau GE, Piguet P-F, Vassalli P. The inducing role of tumor necrosis factor in the development of bactericidal granulomas during BCG infection. Cell. 1989;56:731–40. doi: 10.1016/0092-8674(89)90676-4. [DOI] [PubMed] [Google Scholar]
- 4.Bean AG, Roach DR, Briscoe H, France MP, Korner H, Sedgwick JD, Britton WJ. Structural deficiencies in granuloma formation in TNF gene-targeted mice underlie the heightened susceptibility to aerosol Mycobacterium tuberculosis infection, which is not compensated for by lymphotoxin. J Immunol. 1999;162:3504–3511. [PubMed] [Google Scholar]
- 5.Kaneko H, Yamada H, Mizuno S, Udagawa T, Kazumi Y, Sekikawa K, Sugawara I. Role of tumor necrosis factor-alpha in Mycobacterium-induced granuloma formation in tumor necrosis factor-alpha-deficient mice. Lab Invest. 1999;79:379–86. [PubMed] [Google Scholar]
- 6.Flynn JL, Goldstein MM, Chan J, et al. Tumor necrosis factor-alpha is required in the protective immune response against Mycobacterium tuberculosis in mice. Immunity. 1995;2:561–72. doi: 10.1016/1074-7613(95)90001-2. [DOI] [PubMed] [Google Scholar]
- 7.Rhoades ER, Frank AA, Orme IM. Progression of chronic pulmonary tuberculosis in mice aerogenically infected with virulent Mycobacterium tuberculosis. Tubercle Lung Disease. 1997;78:57–66. doi: 10.1016/s0962-8479(97)90016-2. [DOI] [PubMed] [Google Scholar]
- 8.Prada J, Prager C, Neifer S, Bienzle U, Kremsner PG. Production of Interleukin-6 by human and murine mononuclear leukocytes stimulated with Plasmodium antigens is enhanced by Pentoxifylline, and tumor necrosis factor secretion is reduced. Infect Immun. 1993;61:2737–40. doi: 10.1128/iai.61.6.2737-2740.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Strieter RM, Remick DG, Ward PA, Spengler Rn, III, Larrick JPL, Kunkel SL. Cellular and molecular regulation of tumor necrosis factor-alpha production by pentoxifylline. Biochem Biophys Res Comms. 1988;155:1230–6. doi: 10.1016/s0006-291x(88)81271-3. [DOI] [PubMed] [Google Scholar]
- 10.Ward A, Clissold SP. Pentoxifylline: A Review of its Pharmacodynamics and Pharmacokinetic Properties, and Its Therapeutic Efficacy. Auckland: ADIS Press Limited; 1987. [DOI] [PubMed] [Google Scholar]
- 11.Furth Rv, Zwet TLv, Buisman AM, Dissel JTv. Anti-tumor necrosis factor antibodies inhibit the influx of granulocytes and monocytes into an inflammatory exudate and enhance the growth of Listeria monocytogenes in various organs. J Infectious Diseases. 1994;170:234–7. doi: 10.1093/infdis/170.1.234. [DOI] [PubMed] [Google Scholar]
- 12.Pober JS. Effects of tumour necrosis factor and related cytokines on vascular endothelial cells. Ciba Foundation Symposium. 1987;131:170–84. doi: 10.1002/9780470513521.ch12. [DOI] [PubMed] [Google Scholar]
- 13.Gonzalez-Amaro R, Portales-Perez D, Baranda L, Redondo JM, Martinez-Martinez S, Yanez-Mo M, Garcia-Vicuna R, Cabanas C, Sanchez-Madrid F. Pentoxifylline Inhibits Adhesion and Activation of Human T Lymphocytes. J Immunol. 1998;161:65–72. [PubMed] [Google Scholar]
- 14.Silver RF, Li Q, Boom WH, Ellner JJ. Lymphocyte-dependent inhibition of growth of virulent Mycobacterium tuberculosis H37Rv within human monocytes: requirement for CD4+ T cells in purified protein derivative-positive, but not in purified protein derivative-negative subjects. J Immunol. 1998;160:2408–17. [PubMed] [Google Scholar]
- 15.Rhoades ER, Cooper AM, Orme IM. Chemokine response in mice infected with Mycobacterium tuberculosis. Infect Immun. 1995;63:3871–7. doi: 10.1128/iai.63.10.3871-3877.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chan J, Xing Y, Magliozzo RS, Bloom BR. Killing of virulent Mycobacterium tuberculosis by reactive nitrogen intermediates produced by activated murine macrophages. J Exp Med. 1992;175:1111–22. doi: 10.1084/jem.175.4.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Flesch IEA, Hess JH, Oswald IP, Kaufmann SHE. Growth inhibition of Mycobacterium bovis by IFN-γ stimulated macrophages: regulation by endogenous tumor necrosis factor-α and by IL-10. Int Immunol. 1994;6:693–700. doi: 10.1093/intimm/6.5.693. [DOI] [PubMed] [Google Scholar]
- 18.Barnes PF, Fong S-J, Brennan PJ, Twomey PE, Mazumder A, Modlin RL. Local production of tumor necrosis factor and IFN-γ in tuberculosis pleuritis. J Immunol. 1990;145:149–54. [PubMed] [Google Scholar]
- 19.Bergeron A, Bonay M, Kambouchner M, Lecossier D, Riquet M, Soler P, Hance A, Tazi A. Cytokine patterns in tuberculous and sarcoid granulomas: correlations with histopathologic features of the granulomatous response. J Immunol. 1997;159:3034–43. [PubMed] [Google Scholar]
- 20.Bekker LG, Maartens G, Steyn L, Kaplan G. Selective increase in plasma tumor necrosis factor-alpha and concomitant clinical deterioration after initiating therapy in patients with severe tuberculosis. J Infect Dis. 1998;178:580–4. doi: 10.1086/517479. [DOI] [PubMed] [Google Scholar]