Abstract
While the T-cell receptor (TCR) repertoire of the newborn is highly diverse, a gradual alteration in diversity of the expressed TCR repertoire, in particular the oligoclonality of CD8+ T cells, occurs with increasing age. The timing of the initiation of this process is unknown. These changes are associated with an accumulation of T-cell expansions, thought to be in response to chronic antigen stimulation, frequently by persistent viruses such as Epstein–Barr virus (EBV) and cytomegalovirus (CMV). Using reverse transcription–polymerase chain reaction heteroduplex analysis we have characterized the TCR expression of CD4 and CD8 cells from healthy young children and adults in order to delineate the age range at which these oligoclonal populations appear. We demonstrate that considerable oligoclonality can occur, even in healthy young children, and also that these expanded clonotypes persist. These are shown by heteroduplex to be exclusively within the CD28− subpopulation. The presence of such oligoclonal expansions correlates closely with the percentage of CD8+ cells that have the CD28− phenotype. However, we also show that control of chronic infection with EBV or CMV may coexist with a highly diverse, polyclonal TCR repertoire well into adulthood. These studies suggest that many factors affect the overall regulation of clone size in response to chronic antigens during the development of the immune system.
Introduction
The potential diversity of the human T-cell receptor (TCR) repertoire has been estimated to be as great as 1015 different TCR.1 While the TCR repertoire of the newborn is highly diverse,2 direct measurements of TCR transcripts suggest that the actual number of αβTCR expressed receptors in adults is closer to 107, and that the complexity of the expressed repertoire varies between functionally distinct subpopulations of T cells.3 The presence of clonal T-cell expansions following antigenic exposure, in particular within the CD8+ population, is associated with viral infections including human immunodeficiency virus (HIV), cytomegalovirus (CMV) and Epstein–Barr virus (EBV).4–6 CD8+ T-cell expansions, which have been demonstrated in both healthy young adults7 and in the elderly,8 can be very large, are long lived and frequently have the CD28− CD57+ phenotype.9 They are frequently oligoclonal, arising from a very limited number of unique TCR clonotypes.9 Clonal T-cell expansions are not detectable in cord or neonatal blood.10,11
The precise definition of ‘memory’ T cells remains controversial and there is now good evidence for functionally distinct subsets of memory T cells which can be distinguished phenotypically.12 It has become clear that CD45 is not a reliable marker of memory, in particular for CD8+ T cells since primed CD45RO+ CD8 T cells may revert to a CD45RA phenotype.13,14 Cells which express a CD28− CD57+ phenotype appear to be ‘terminally differentiated’, have undergone multiple divisions and contain a proportion of antigen-experienced effector cells.15–17 Recently the CD27 molecule has also been shown to be down-regulated on effector memory cells, and this CD27– population closely overlaps the CD28− phenotype.18
The use of tetrameric major histocompatibility complex (MHC)–peptide complexes has demonstrated large T-cell expansions in response to acute viral infections in both humans and mice,19,20 and some of the clonotypes from the acute response persist in chronic infection.21 The properties which allow some clones to become long-lived memory or effector cells, while others do not persist, are unknown.22,23
We have previously demonstrated large clonal CD8+ T-cell expansions with the CD28− CD57+ phenotype in the blood of a child with autoimmune chronic arthritis, which were not present in the inflamed joint.24 Others have reported clonal T-cell expansions in association with other autoimmune diseases, such as the CD4+ CD28− expansions seen in adult rheumatoid arthritis,25 as well as an overall perturbation of the repertoire size in these patients.26 However, there is very little known about the TCR repertoire of healthy young children. In view of the changes in T-cell populations and repertoire with age, it may be difficult to interpret TCR data from children with autoimmune conditions if no data from normal age-matched controls are available. Therefore, we wished to determine the normal pattern of clonal T-cell expansions in healthy children, in particular the timing and frequency of the development of these expansions and their functional significance. In this study we have characterized the normal TCR repertoire of healthy children and compared them to young adults, using flow cytometry and the highly sensitive heteroduplex method.27 We have defined clonal expansions as cells whose TCR sequences are detectable by heteroduplex (HD; which has a sensitivity of 1 cell in approximately 10 000)28 above the DNA smear of multiple TCR sequences formed by a polyclonal population.
Materials and methods
Samples
Ethical approval for the study was obtained from Great Ormond Street Ethics Committee. Venous blood samples were taken from 10 healthy children undergoing minor surgery, with parental consent, and from 10 healthy laboratory volunteers. Peripheral blood mononuclear cells (PBMC) were separated by Ficoll density centrifugation. Cells were separated into CD4+ and CD8+ or CD28+ and CD28− populations using magnetic beads (Milteyni Biotec, Surrey, UK), giving fractions which were routinely > 96% pure. Serological testing for evidence of infection with EBV and CMV was performed by the Virology Lab, UCLH.
Flow cytometry
For flow cytometry, PBMC were washed in phosphate-buffered saline (PBS)/1% fetal calf serum (FCS)/0·1% sodium azide before staining by standard methods. For TCR beta chain variable (TCRBV) analysis, antibodies to CD3, CD4, CD8, CD45RO, CD28, CD57 and a panel of 15 anti-TCRBV monoclonal antibodies (mAbs; as described24) were used. Between 20 000 and 30 000 events were collected per condition on a FACStar cytometer (Becton Dickinson, CA) and data were analysed using CellQuest software (Becton Dickinson). A live CD3+ gate was set in order to exclude CD4+ macrophages or CD8α+ non-T cells (such as natural killer cells). Phycoerythrin (PE) -conjugated MHC/peptide tetramer staining reagent, human leucocyte antigen (HLA)-B8/RAKFKQLL (immunodominant peptide derived from the EBV lytic EBV protein BZLF119), was generously supplied by Dr M. Callan, Oxford UK. For analysis of tetramer-positive cells, PBMC were stained using HLA-B8/RAKFKQLL tetramer in conjunction with CD3-fluorescein isothiocyanate (FITC) and CD8-QR antibodies, and 1 000 000 events were collected.
Heteroduplex analysis
Reverse transcription–polymerase chain reaction (RT-PCR)-HD analysis was performed as described.24 Briefly, total RNA was prepared using RNAzol (Biogenesis, Poole, UK) and 5 µg was used for first-strand cDNA synthesis with oligo-dT. Twenty-six TCR PCR reactions using TCRBV primers and an internal TCRBC primer as described27 were performed for each sample, in parallel with 26 HD carrier PCR reactions using an external TCRBC primer. HD reactions were performed by mixing carrier and sample PCR products, heating to 95° for 5 min and then annealing at 50° for 1 hr. HD products were analysed on a 12% non-denaturing polyacrylamide/0·5% TBE gel (Tris/Borate/EDTA), blotted onto Hybond N+ membrane and probed using a carrier-specific TCRBC probe.24
Statistics
Data were analysed using the spss software (version 7·5).
Results
Flow cytometric analysis underestimates the T-cell oligoclonality present in healthy young children
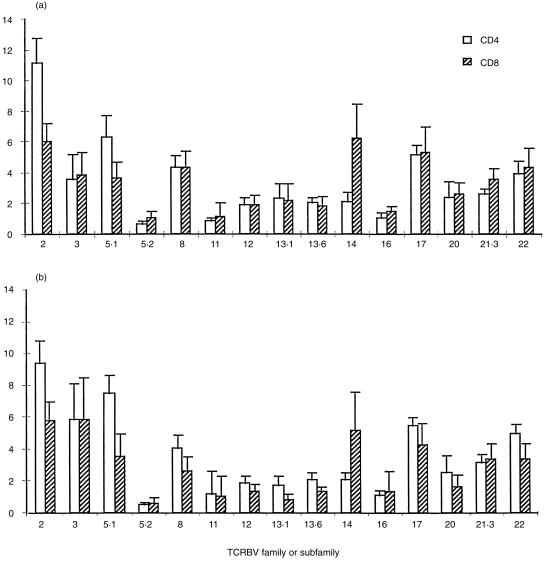
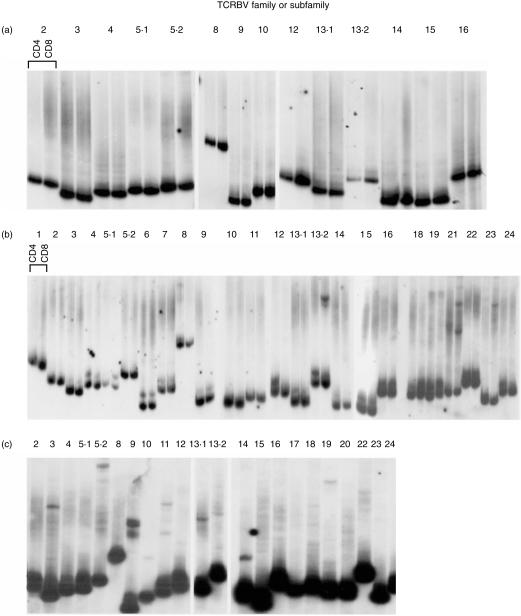
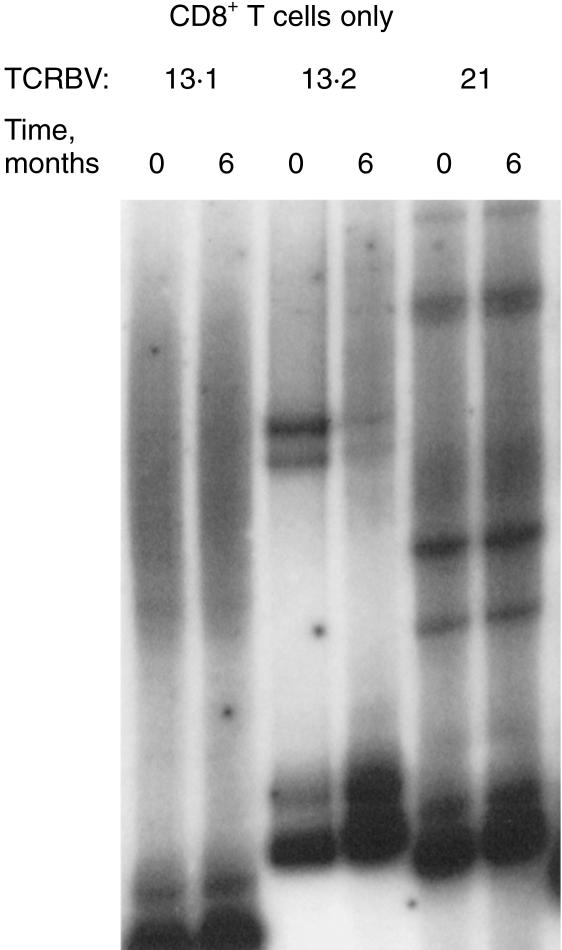
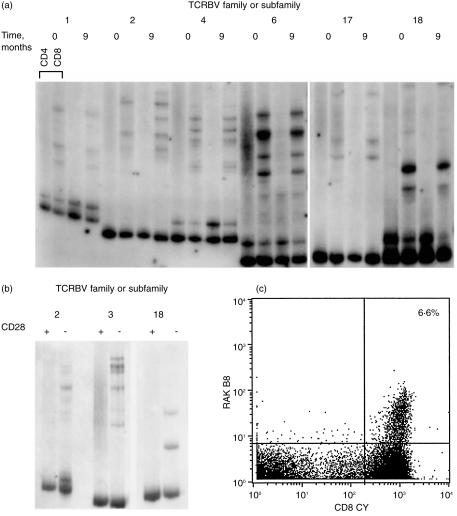
Samples were obtained from 10 children (aged from 1 year 7 months to 14 years 9 months) and 10 adults (aged between 21 and 41 years). All were healthy and well at the time of sampling with no history of chronic infection, allergy, or autoimmunity. A panel of 15 mAb to TCRBV families or subfamilies were used in triple staining with anti-CD3 and CD4 or CD8 mAb. The CD4 : CD8 ratios were not different between the two groups (Table 1). Expression of TCRBV in the children, as measured by the panel of anti-TCR mAbs, did not differ significantly from that in the adults in this study (Fig. 1). Several TCRBV families showed a skew towards either the CD4 or the CD8 population in children, as previously described in adults,29–31 indicating that these subset differences are established early in the development of the T-cell repertoire. HD analysis was then performed on separated CD4+ and CD8+ cells from seven samples from children. Of these, only one (N1, a child aged 5 years) was entirely polyclonal in both CD4 and CD8 subsets, indicated in this assay by a DNA smear (multiple HD molecules, each at low frequency) running above carrier DNA (Fig. 2a). Five of the seven children had HD bands in only a small number of TCRBV families, indicating the presence of clonal expansions within these families. A representative HD analysis is shown in Fig. 2(b) (child N9, aged 7 years 10 months). One child (N7, aged 7 years 6 months, Fig. 2c) showed a highly oligoclonal TCR repertoire, with clonal bands detectable in 50% of the TCRBV families. A second sample obtained from child N9 showed identical clonotypic bands, indicating that the CD8+ expansions were persistent over at least 6 months (Fig. 3). None of the 10 children had expanded TCR expression by FACS analysis, as defined as greater than mean ± 2SD of the normal adult data set, illustrating that flow cytometry can greatly underestimate the presence of expanded T-cell clones.
Table 1.
Summary data of the age and T-cell subsets of donors analysed
| Children (n = 10) | Adults (n = 10) | |
|---|---|---|
| Age, years* | 7·1 (± 2·6) | 30·9 (± 6·4) |
| CD3+ of PBMC† | 73·0 (± 10·7) | 70·4 (± 9·8) |
| CD4+ of CD3+ | 57·2 (± 7·4) | 55·3 (± 11·9) |
| CD8+ of CD3+ | 30·9 (± 10·1) | 37·3 (± 10·5) |
| CD28− of CD3+ | 7·7 (± 5·1) | 15·1 (± 11·8) |
| CD28− of CD8 CD3+ | 14·6 (± 11·3) | 24·5 (± 14·4) |
| CD45RO+ of CD3+ | 18·8 (± 6·8) | 32·9 (± 12·7) |
| Mean percentage TCRBV families with HD bands | 15·0 ± 6·0 | 33·1 ± 12·1‡ |
*Mean values±SD;
cell phenotypes are mean (± SD) percentages;
these figures are mean±1 SD for n = 7 children, and n = 6 adults, analysed by HD.
Figure 1.
Expression of TCRBV proteins on peripheral blood CD4+ and CD8+ T cells of (a) children (n = 10) and (b) adults (n = 10). Three-colour flow cytometry was performed using mAb to CD3, CD4, or CD8 and a panel of TCRBV antibodies. After gating on live CD3+ cells, the per cent positive for each TCRBV in CD4+ or CD8+ cells were calculated. Bars represent mean values and error bars represent the SD.
Figure 2.
Expressed TCR repertoire in young children shows a wide variation in degree of oligoclonality. Heteroduplex (HD) analysis of TCR expression by bead-separated CD4 and CD8 T cells (a and b) or unseparated PBMC (c). RT-PCR using 26 TCRBV primers and HD reactions were performed, and analysed on a 12% polyacrylamide gel under non-denaturing conditions. Gels were blotted and probed with a TCRBC probe. Dark lower bands represent carrier DNA. (a) Polyclonal pattern, child N1, aged 5 years; (b) oligoclonal bands in TCRBV13.2, 21 and 23, child N9, aged 7 years 10 months; and (c) oligoclonal pattern in many TCRBV families, child N7, aged 7 years 6 months.
Figure 3.
Expanded clonotypes in children persist. RT-PCR-HD reactions for TCRBV 13.1 (polyclonal smear), 13.2 and 21 (persisting oligoclonal bands) performed on purified CD8+ T cells from child N9, taken 6 months apart as shown.
The wide spectrum of CD8+ oligoclonality correlates with the size of the CD28− population even in young subjects
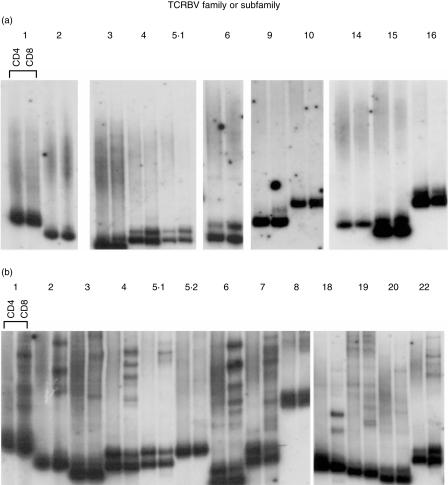
We compared the data from the children studied with those from healthy adult donors. Again we observed a wide variability in the degree of oligoclonality and, as in the children, the majority of clonal bands were within the CD8 population. The majority of adults had some BV families showing oligoclonality, but a minority (two out of six), retained a highly polyclonal repertoire well into their fourth decade. The TCR HD results of two representative adult donors are shown in Fig. 4 (donors A7, aged 32 years and A11, aged 29 years). The expanded clones were shown to be long lived by analysis of CD8+ separated cells from samples taken from donor A11 9 months apart (Fig. 5a). HD analysis after separation into CD28+ and CD28− T cells from donor A11, showed that the identical clonotypic patterns seen in CD8+ cells were also within the CD28− population (Fig. 5b).
Figure 4.
RT-PCR-HD analysis on separated CD4 and CD8 T cells. (a) Highly diverse repertoire of A7 (aged 32 years) and (b) oligoclonal TCR repertoire of A11 (aged 29 years).
Figure 5.
Oligoclonal populations are CD28− and correlate with the high number of T cells which bind specific MHC/peptide tetramers. (a) Persistence of CD8+ expansions in highly oligoclonal repertoire (donor A11). RT-PCR-HD reactions for TCRBV 1, 2, 4, 6, 17, 18, on CD4 and CD8 T cells separated from PBMC taken 9 months apart. (b) Clonotypic HD bands segregate with the CD28− fraction. RT-PCR-HD reactions for TCRBV 2, 3 18 on CD28+ and CD28− T cells separated from PBMC of donor A11. (c) PBMC from donor A11 were stained with PE-conjugated HLA-B8/RAKFKQLL tetrameric complex, combined with antibodies to CD3 and CD8. A dot plot shows data gated on CD3+ cells, with tetramer staining on the y-axis (RAKB8). The number of CD8+ T cells staining with the tetramer reagent is shown in the upper right quadrant.
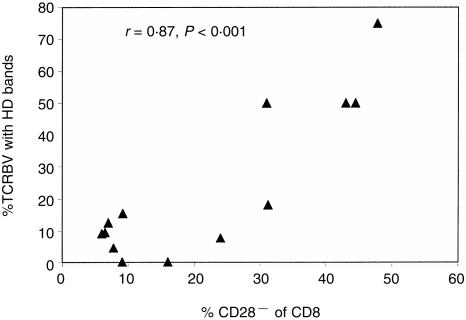
Using all 13 sets of HD data (seven children, six adults), we calculated an estimate of clonality within each CD8+ TCR repertoire by expressing the number of TCRBV families showing HD CD8+ clonal bands on a standard exposure autoradiograph as a percentage of the total of TCRBV families analysed. The mean (± 1 SD) values for each group are shown in Table 1. We also analysed all samples (10 children, 10 adults) for the expression of CD45RO, CD28 and for adult samples, CD57, on defined T-cell subsets. As expected, both the percentage of CD45RO+ T cells, and the appearance of the CD8+ CD28− and CD8+ CD57+ populations increased with age (Table 1). However, these two parameters were highly variable. In both adults and children, the presence of clonal bands on HD in the CD8+ population was strongly correlated with the size of the CD28− population (r = 0·87, P < 0·001) but only weakly correlated with age (r = 0·52, P = 0·08) (Fig. 6). No association between HLA type and clonality of TCR repertoire was seen (data not shown).
Figure 6.
Association between oligoclonality in CD8+ T cells, as estimated by the per cent of the whole CD8+ TCR repertoire showing HD clonal bands, with the CD28− phenotype of the CD8+ population, for 13 individuals (seven children, six adults).
Together these data confirm that the development of oligoclonal expansions within the CD8+ population, which closely mirrors the presence of CD8+ CD28− cells, can occur early in childhood, or alternatively not at all until well into adult life. We were interested to delineate factors which might be responsible for these differences.
Chronic infection with EBV or CMV may coexist with a highly polyclonal TCR repertoire
Oligoclonal CD8+, CD28−, or CD57+ populations have been associated with several chronic viral infections, including the gammaherpes viruses EBV and CMV.5,16,32 We therefore asked the question whether a highly diverse TCR repertoire would correlate with no prior exposure to such viruses. We performed serological testing for EBV and CMV on all 10 adult donors. Unfortunately, serum samples were not available from the child donors. The serological status of adult donors represented in HD figures is shown in Table 2. In all donors with positive serology, virus-specific immunoglobulin G (IgG) was positive while IgM was not detected, indicating previous infection. The majority of such individuals are likely to carry these DNA viruses as persistent infections. Surprisingly, both viral infections were found to coexist in some donors with a highly polyclonal repertoire. Thus both donor A7 and A14 had highly polyclonal TCR repertoires by HD and low numbers of CD28− cells in the CD8+ population (Fig. 4a and Table 2), yet had evidence of previous EBV or CMV infection. However, all donors with a high number of CD28− cells within the CD8+ population, paralleled by clonal bands on HD analysis, had evidence of infection by one or other virus. The only adult donor in our cohort who was negative for antibodies to EBV and CMV (A19) had no HD bands detectable and had a polyclonal repertoire producing a smear on HD (data not shown). For HLA-B8-positive donors, an MHC/peptide staining tetramer reagent was used to stain for CD8+ T cells specific for the EBV peptide RAKFKQLL (peptide derived from the EBV lytic protein BZLF119) in the context of HLA-B8. The donor with a strikingly restricted diversity of TCR expression (A11) had 6·6% of all CD8+ cells specific for this viral peptide alone (Fig. 5c). Of the HLA-B8/RAKFKQLL tetramer+ CD8+ T cells, 65% were also CD28−, while 48% of the CD8+ cells as a whole were CD28− (data not shown).
Table 2.
Frequency of the CD28− CD57+ phenotype within CD8+ T lymphocytes, serological status for EBV and CMV, % TCRBV families showing oligoclonality by HD and age at sampling of the 10 adult donors in this study
| Donor | CD8 cells which were CD28− CD57+ (%) | EBV | CMV | TCRBV families with with HD clonal bands (%)* | Age at sampling (years) |
|---|---|---|---|---|---|
| A1 | 24·3 | − | + | 50 | 41 |
| A2 | 7·7 | + | − | 31 | |
| A4 | 26·4 | + | + | 18 | 30 |
| A5 | 9·7 | + | − | 38 | |
| A7 | 3·6 | + | − | 4·5 | 32 |
| A11 | 43·7 | + | − | 75 | 29 |
| A12 | 43·8 | + | − | 51 | 34 |
| A14 | 3·6 | + | + | 22 | |
| A19 | 1·1 | − | − | 0 | 28 |
| A22 | 11·1 | + | − | 21 |
*For the six adult donors analysed by HD.
Discussion
Neonatal T cells express a highly diverse set of antigen-specific TCR. During aging, the clonal composition of the expressed TCR repertoire changes, becoming less diverse and more oligoclonal. The clonal expansions are commonly in the CD8+ cells, gradually accumulate during life8,33,34 and are thought to be the result of immune responses to infections with viruses, such as the herpes viruses EBV and CMV.5 However, the initial encounter with these viruses is frequently in childhood. This is the first study to analyse in detail the degree of clonal diversity of the whole expressed TCR repertoire in healthy young children.
We have previously shown that the RT-PCR-HD method is sensitive in the detection of clonal expansions to a cell frequency of approximately 1 in 50 000 cells.28 Several studies have shown that cord blood TCR expression in cord blood T cells is highly diverse.2,11 Previous reports of oligoclonal TCR expression in children have been in association either with HIV infections,35 autoimmunity,24 or immunodeficiency.36 In this study we have shown that even healthy young children can have clonotypes that are significantly expanded relative to the diverse repertoire, and that these expansions are persistent, and stable over time. In addition we have demonstrated a wide spectrum in the degree of such clonal restriction in healthy young adults, with some individuals retaining a diverse phenotype until well into the fourth decade. In keeping with previous reports of the phenotype of such T-cell expansions being CD28− C57+, we have shown that the number of CD8+ clonal expansions demonstrated by HD is tightly correlated with the percentage of CD8+ cells which have lost expression of the co-stimulatory CD28 molecule. The donor with the most highly oligoclonal pattern of TCR had a high number of EBV-specific T cells by tetramer staining and 65% of this population were CD28−. Although some data suggest that such clones may be “replenished” from the CD28+ population,37 in this study the clonotypic bands seen on HD analysis from this donor, identical to those seen in the CD8 population as a whole, were shown to be restricted to the CD28− fraction. It remains possible that these clonotypes also reside within the CD28+ subset but at a frequency which is below the limit of detection in the HD assay. In normal PBMC the expression of CD57 on CD8+ cells is tightly associated with a loss of CD28 expression,38 although this association is lost in some inflammatory T cells.24 These CD28− CD57+ clonal T-cell expansions have been suggested to be cytolytic effectors against viruses,16 although the functional role of the CD57 molecule itself is unknown.
In our group of normal adults, a highly diverse set of expressed TCR and a very low number of CD28− CD57+ cells were shown to coexist in some donors with a persistent infection by one or both of EBV and CMV. These data suggest that effective control of these viruses can be maintained for many years without the development of any clonal expansions which are large enough to be detected by HD analysis. It has been suggested that CD57+ T cells may arise in a thymus-independent manner.39 If so, it is possible that differences in the speed of thymic involution during early adulthood may affect the number of CD57+ cells produced and therefore the overall complexity of the expressed TCR repertoire.
We have not seen expanded clonotypes within the CD4 T cells in these children, confirming previous reports that these are rare in young healthy individuals,40 although they have been reported in disease states including rheumatoid arthritis25,41 and chronic lymphocytic leukaemia,42 as well as in the elderly.43,44
The long-term consequences of a gradual accumulation of CD8+ T-cell expansions are unclear. One case of a child with X-linked severe combined immunodeficiency yet normal numbers of peripheral T cells, thought to be due to a reverse mutation, showed a highly restricted oligoclonal TCRBV repertoire, estimated to be using 1000 different TCRBV chains. Remarkably this repertoire was adequate to prevent opportunistic infection.36 Certainly CD8+ T-cell clones can be very large and might be predicted, eventually, to limit the TCR repertoire available for new immune responses. However, while a decline in immunocompetence is recognized in old age,45 the presence of T-cell clonotypes at high frequency in centenarians argues that effective T-cell immunity can be preserved, at least for life in the Western world, in the presence of such expansions.46
In conclusion, this detailed global analysis of the expressed TCR repertoire of young children and adults has extended our knowledge of the development, and the spectrum of expression, of the normal TCR repertoire. The increasing availability of reagents to identify antigen-specific T cells, combined with sensitive molecular techniques which can increasingly be performed on very small samples, will allow a far greater understanding of how the repertoire changes with age and in response to specific pathogens. In this study, we have demonstrated a wide variability in the tendency to accumulate long-lived clonal expansions, which are predominantly within the CD8+ CD28− subpopulation. We have shown that these expansions can develop in early childhood and that they persist. While the antigens recognized by such cells remain unknown, many data suggest that they arise in response to chronic viral infection and that effector memory T cells frequently reside within the CD28− population. However, we have also demonstrated that effective control of EBV and CMV infections can be maintained in the absence of such expansions, presumably mediated by a set of clones each at low precursor frequency. We suggest that studies of disease states such as autoimmune conditions, which demonstrate clonal T-cell expansions, should be interpreted with caution, given the ‘normal’ tendency for such clonotypes to arise from a young age.
Acknowledgments
We thank members of the Windeyer Institute, the children and their parents for the generous donation of blood samples. We are grateful to Dr M. Maini, Dr N. Wedderburn and Prof. R. Callard for carefully reading the manuscript. This work was funded by the Wellcome Trust (L.R.W. and A.P.) and the Medical Research Council (H.V. and P.W.); L.R.W. is a Wellcome Trust Clinician Scientist.
Abbreviations
- CDR
complementarity determining region
- CMV
cytomegalovirus
- EBV
Epstein–Barr virus
- HD
heteroduplex
- PE
phycoerythrin
- QR
quantum red
- RT
reverse transcriptase.
References
- 1.Davis MM, Bjorkman PJ. T-cell antigen receptor genes and T-cell recognition. Nature. 1988;334:301–309. doi: 10.1038/334395a0. [DOI] [PubMed] [Google Scholar]
- 2.Garderet L, Dulphy N, Douay C, et al. The umbilical cord blood αβ T-cell repertoire: characteristics of a polyclonal and naive but completely formed repertoire. Blood. 1998;91:340–6. [PubMed] [Google Scholar]
- 3.Arstila TP, Casrouge A, Baron V, Even J, Kanellopoulos J, Kourilsky P. A direct estimate of the human αβ T cell receptor diversity. Science. 1999;286:958–61. doi: 10.1126/science.286.5441.958. 10.1126/science.286.5441.958. [DOI] [PubMed] [Google Scholar]
- 4.Pantaleo G, Demarest JF, Soudeyns H, et al. Major expansion of CD8+ T cells with a predominant Vβ usage during the primary immune response to HIV. Nature. 1994;370:463–7. doi: 10.1038/370463a0. [DOI] [PubMed] [Google Scholar]
- 5.Wang EC, Moss PA, Frodsham P, Lehner PJ, Bell JI, Borysiewicz LK. CD8highCD57+ T lymphocytes in normal, healthy individuals are oligoclonal and respond to human cytomegalovirus. J Immunol. 1995;155:5046–56. [PubMed] [Google Scholar]
- 6.Callan MF, Steven N, Krausa P, et al. Large clonal expansions of CD8+ T cells in acute infectious mononucleosis. Nat Med. 1996;2:906–11. doi: 10.1038/nm0896-906. [DOI] [PubMed] [Google Scholar]
- 7.Hingorani R, Choi I, Akolkar P, Gulwani-Akolkar B, Pergolizzi R, Silver J, Gregersen PK. Clonal predominance of T cell receptors within the CD8+CD45RO+ subset in normal human subjects. J Immunol. 1993;151:5762–9. [PubMed] [Google Scholar]
- 8.Posnett DN, Sinha R, Kabak S, Russo C. Clonal populations of T cells in normal elderly humans: the T cell equivalent to ‘benign monoclonal gammopathy’. J Exp Med. 1994;179:609–18. doi: 10.1084/jem.179.2.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Morley JK, Batliwalla FM, Hingorani R, Gregersen PK. Oligoclonal CD8+ T cells are preferentially expanded in the CD57+ subset. J Immunol. 1995;154:6182–90. [PubMed] [Google Scholar]
- 10.Azuma M, Phillips JH, Lanier LL. CD28– T lymphocytes. Antigenic and functional properties. J Immunol. 1993;150:1147–59. [PubMed] [Google Scholar]
- 11.Shen DF, Doukhan L, Kalams S, Delwart E. High-resolution analysis of TCRβ-chain repertoires using DNA heteroduplex tracking: generally stable, clonal CD8+ expansions in all healthy young adults. J Immunol Methods. 1998;215:113–21. doi: 10.1016/s0022-1759(98)00066-0. 10.1016/s0022-1759(98)00066-0. [DOI] [PubMed] [Google Scholar]
- 12.Sallusto F, Lenig D, Forster R, Lipp M, Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 1999;401:708–12. doi: 10.1038/44385. 10.1038/44385. [DOI] [PubMed] [Google Scholar]
- 13.Pilling D, Akbar AN, Bacon PA, Salmon M. CD4+ CD45RA+ T cells from adults respond to recall antigens after CD28 ligation. Int Immunol. 1996;8:1737–42. doi: 10.1093/intimm/8.11.1737. [DOI] [PubMed] [Google Scholar]
- 14.Maini MK, Gudgeon N, Wedderburn LR, Rickinson AB, Beverley PC. Clonal Expansions in Acute EBV Infection Are Detectable in the CD8 and not the CD4 Subset and Persist with a Variable CD45 Phenotype. J Immunol. 2000;165:5729–37. doi: 10.4049/jimmunol.165.10.5729. [DOI] [PubMed] [Google Scholar]
- 15.Monteiro J, Batliwalla F, Ostrer H, Gregersen PK. Shortened telomeres in clonally expanded CD28–CD8+ T cells imply a replicative history that is distinct from their CD28+CD8+ counterparts. J Immunol. 1996;156:3587–90. [PubMed] [Google Scholar]
- 16.Weekes MP, Wills MR, Mynard K, Hicks R, Sissons JG, Carmichael AJ. Large clonal expansions of human virus-specific memory cytotoxic T lymphocytes within the CD57+ CD28– CD8+ T-cell population. Immunology. 1999;98:443–9. doi: 10.1046/j.1365-2567.1999.00901.x. 10.1046/j.1365-2567.1999.00901.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kern F, Khatamzas E, Surel I, Frommel C, Reinke P, Waldrop SL, Picker LJ, Volk HD. Distribution of human CMV-specific memory T cells among the CD8pos. subsets defined by CD57, CD27, and CD45 isoforms. Eur J Immunol. 1999;29:2908–15. doi: 10.1002/(SICI)1521-4141(199909)29:09<2908::AID-IMMU2908>3.0.CO;2-8. 10.1002/(sici)1521-4141(199909)29:09<2908::aid-immu2908>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 18.Hamann D, Baars PA, Rep MH, Hooibrink B, Kerkhof-Garde SR, Klein MR, van Lier RA. Phenotypic and functional separation of memory and effector human CD8+ T cells. J Exp Med. 1997;186:1407–18. doi: 10.1084/jem.186.9.1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Callan MF, Tan L, Annels N, et al. Direct visualization of antigen-specific CD8+ T cells during the primary immune response to EBV in vivo. J Exp Med. 1998;187:1395–402. doi: 10.1084/jem.187.9.1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Butz EA, Bevan MJ. Massive expansion of antigen-specific CD8+ T cells during an acute virus infection. Immunity. 1998;8:167–75. doi: 10.1016/s1074-7613(00)80469-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Callan MF, Annels N, Steven N, Tan L, Wilson J, McMichael AJ, Rickinson AB. T cell selection during the evolution of CD8+ T cell memory in vivo. Eur J Immunol. 1998;28:4382–90. doi: 10.1002/(SICI)1521-4141(199812)28:12<4382::AID-IMMU4382>3.0.CO;2-Z. 10.1002/(sici)1521-4141(199812)28:12<4382::aid-immu4382>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 22.Maryanski JL, Jongeneel CV, Bucher P, Casanova JL, Walker PR. Single-cell PCR analysis of TCR repertoires selected by antigen in vivo: a high magnitude CD8 response is comprised of very few clones. Immunity. 1996;4:47–55. doi: 10.1016/s1074-7613(00)80297-6. [DOI] [PubMed] [Google Scholar]
- 23.Sourdive DJ, Murali-Krishna K, Altman JD, et al. Conserved T cell receptor repertoire in primary and memory CD8 T cell responses to an acute viral infection. J Exp Med. 1998;188:71–82. doi: 10.1084/jem.188.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wedderburn LR, Maini MK, Patel A, Beverley PCL, Woo P. Molecular fingerprinting reveals non-overlapping T cell oligoclonality between an inflamed site and peripheral blood. Int Immunol. 1999;11:535–43. doi: 10.1093/intimm/11.4.535. 10.1093/intimm/11.4.535. [DOI] [PubMed] [Google Scholar]
- 25.Schmidt D, Goronzy JJ, Weyand CM. CD4+ CD7– CD28– T cells are expanded in rheumatoid arthritis and are characterized by autoreactivity. J Clin Invest. 1996;97:2027–37. doi: 10.1172/JCI118638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wagner UG, Koetz K, Weyand CM, Goronzy JJ. Perturbation of the T cell repertoire in rheumatoid arthritis. Proc Natl Acad Sci USA. 1998;95:14447–52. doi: 10.1073/pnas.95.24.14447. 10.1073/pnas.95.24.14447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wack A, Montagna D, Dellabona P, Casorati G. An improved PCR-heteroduplex method permits high-sensitivity detection of clonal expansions in complex T cell populations. J Immunol Methods. 1996;196:181–92. doi: 10.1016/0022-1759(96)00114-7. 10.1016/0022-1759(96)00114-7. [DOI] [PubMed] [Google Scholar]
- 28.Maini MK, Wedderburn LR, Hall F, Wack A, Casorati G, Beverley PCL. A comparison of two techniques for the molecular tracking of specific T cell responses; CD4+ human T cell clones persist in a stable hierarchy but at a lower frequency than clones in the CD8+ population. Immunology. 1998;94:529–35. doi: 10.1046/j.1365-2567.1998.00556.x. 10.1046/j.1365-2567.1998.00556.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Grunewald J, Janson CH, Wigzell H. Biased expression of individual T cell receptor V gene segments in CD4+ and CD8+ human peripheral blood T lymphocytes. Eur J Immunol. 1991;21:819–22. doi: 10.1002/eji.1830210342. [DOI] [PubMed] [Google Scholar]
- 30.Davey MP, Meyer MM, Munkirs DD, Babcock D, Braun MP, Hayden JB, Bakke AC. T-cell receptor variable beta genes show differential expression in CD4 and CD8 T cells. Hum Immunol. 1991;32:194–202. doi: 10.1016/0198-8859(91)90056-f. [DOI] [PubMed] [Google Scholar]
- 31.Hawes GE, Struyk L, van den Elsen PJ. Differential usage of T cell receptor V gene segments in CD4+ and CD8+ subsets of T lymphocytes in monozygotic twins. J Immunol. 1993;150:2033–45. [PubMed] [Google Scholar]
- 32.Tan LC, Gudgeon N, Annels NE, et al. A re-evaluation of the frequency of CD8+ T cells specific for EBV in healthy virus carriers. J Immunol. 1999;162:1827–35. [PubMed] [Google Scholar]
- 33.Monteiro J, Hingorani R, Choi IH, Silver J, Pergolizzi R, Gregersen PK. Oligoclonality in the human CD8+ T cell repertoire in normal subjects and monozygotic twins: implications for studies of infectious and autoimmune diseases. Mol Med. 1995;1:614–24. [PMC free article] [PubMed] [Google Scholar]
- 34.Batliwalla F, Monteiro J, Serrano D, Gregersen PK. Oligoclonality of CD8+ T cells in health and disease: aging, infection, or immune regulation? Hum Immunol. 1996;48:68–76. doi: 10.1016/0198-8859(96)00077-8. 10.1016/0198-8859(96)00077-8. [DOI] [PubMed] [Google Scholar]
- 35.Niehues T, Horneff G, Knipp S, Adams O, Wahn V. Treatment-resistant expansion of CD8+CD28– cells in pediatric HIV infection. Pediatr Res. 2000;47:418–21. doi: 10.1203/00006450-200003000-00022. [DOI] [PubMed] [Google Scholar]
- 36.Bousso P, Wahn V, Douagi I, et al. Diversity, functionality, and stability of the T cell repertoire derived in vivo from a single human T cell precursor. Proc Natl Acad Sci USA. 2000;97:274–8. doi: 10.1073/pnas.97.1.274. 10.1073/pnas.97.1.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Posnett DN, Edinger JW, Manavalan JS, Irwin C, Marodon G. Differentiation of human CD8 T cells: implications for in vivo persistence of CD8+ CD28– cytotoxic effector clones. Int Immunol. 1999;11:229–41. doi: 10.1093/intimm/11.2.229. 10.1093/intimm/11.2.229. [DOI] [PubMed] [Google Scholar]
- 38.Kern F, Ode-Hakim S, Vogt K, Hoflich C, Reinke P, Volk HD. The enigma of CD57+CD28– T cell expansion – anergy or activation? Clin Exp Immunol. 1996;104:180–4. doi: 10.1046/j.1365-2249.1996.d01-635.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mackall CL, Gress RE. Thymic aging and T cell regeneration. Immunol Rev. 1997;160:91–102. doi: 10.1111/j.1600-065x.1997.tb01030.x. [DOI] [PubMed] [Google Scholar]
- 40.Maini M, Casorati G, Dellabonna P, Wack A, Beverley PCL. T cell clonality in immune responses. Immunol Today. 1999;20:262–6. doi: 10.1016/s0167-5699(99)01472-3. 10.1016/s0167-5699(99)01472-3. [DOI] [PubMed] [Google Scholar]
- 41.Bowman SJ, Geddes GC, Corrigall V, Panayi GS, Lanchbury JS. Large granular lymphocyte expansions in Felty's syndrome have an unusual phenotype of activated CD45RA+ cells. Br J Rheumatol. 1996;35:1252–5. doi: 10.1093/rheumatology/35.12.1252. 10.1093/rheumatology/35.12.1252. [DOI] [PubMed] [Google Scholar]
- 42.Serrano D, Monteiro J, Allen SL, et al. Clonal expansion within the CD4+CD57+ and CD8+CD57+ T cell subsets in chronic lymphocytic leukemia. J Immunol. 1997;158:1482–9. [PubMed] [Google Scholar]
- 43.Schwab R, Szabo P, Manavalan JS, Weksler ME, Posnett DN, Pannetier C, Kourilsky P, Even J. Expanded CD4+ and CD8+ T cell clones in elderly humans. J Immunol. 1997;158:4493–9. [PubMed] [Google Scholar]
- 44.Wack A, Cossarizza A, Heltai S, Barbieri D, D'Addato S, Fransceschi C, Dellabona P, Casorati G. Age-related modifications of the human αβ T cell repertoire due to different clonal expansions in the CD4+ and CD8+ subsets. Int Immunol. 1998;10:1281–8. doi: 10.1093/intimm/10.9.1281. 10.1093/intimm/10.9.1281. [DOI] [PubMed] [Google Scholar]
- 45.Wick G, Grubeck-Loebenstein B. Primary and secondary alterations of immune reactivity in the elderly: impact of dietary factors and disease. Immunol Rev. 1997;160:171–84. doi: 10.1111/j.1600-065x.1997.tb01037.x. [DOI] [PubMed] [Google Scholar]
- 46.Franceschi C, Monti D, Sansoni P, Cossarizza A. The immunology of exceptional individuals: the lesson of centenarians. Immunol Today. 1995;16:12–6. doi: 10.1016/0167-5699(95)80064-6. [DOI] [PubMed] [Google Scholar]