Abstract
The development of monocytes into macrophages is regulated by helper T cells (Th) cells and, vice versa, the differentiation of Th cells into Th1 and Th2 is regulated by macrophages. Herein we examined the role of the Th2 cytokine, interleukin-10 (IL-10), on the development of macrophages. IL-10 is known to block the expression of antigen-presenting major histocompatibility complex (MHC) II and of costimulatory B7 molecules but it induces the expression of FcRs, especially the FcγRIII (CD16). The expression of CD16 enables the macrophage to carry out antibody-dependent cell-mediated cytotoxicity (ADCC) functions. However, this differentiation step is largely undercut by the capacity of IL-10 to induce macrophage apoptosis before the process of differentiation ensues. We found that the negative effect of IL-10 on the survival of macrophages is reversed in an environment that contains immunoglobulin G (IgG). IgG, especially when immune complexed with antigen, stimulates CD16 to transmit survival signals in macrophages which enable them to complete the differentiation process into CD16+ cells. Thus, IL-10 suppresses macrophage accumulation in healthy tissues where IgG is absent, and facilitates macrophage accumulation and differentiation in tissues that contain IgG, for example inflamed tissues or tissues that present autoreactive antibodies.
Introduction
Macrophages perform a wide spectrum of functions throughout the body. They ingest foreign materials, present antigens in association with major histocompatibility complex (MHC) antigens to T lymphocytes, destroy intracellular pathogens, kill antibody-coated cells in the antibody-dependent cell-mediated cytotoxicity (ADCC) reaction, and secrete cytokines. Some of these functions are mutually exclusive and heterogeneity of macrophages has been defined on the basis of phenotype and function.1,2 Shearer2 distinguished macrophages based on the production of cytokines; interleukin (IL)-12-producing macrophages enhance the immune response whereas IL-10-producing macrophages inhibit the immune response. We distinguished MHC II+, B7+ and CD16– immunostimulatory macrophages from MHC II–, B7–, CD16+ cytotoxic macrophages, referring to them tentatively as M1 and M2 cells, respectively.1
The common ancestor of macrophages and of their more specialized subsets is the monocyte, which circulates in the blood stream. Upon extravasation or placement into tissue culture, a monocyte turns into a macrophage. In the absence of appropriate stimuli, macrophages have a short life span and undergo programmed cell death or apoptosis.3 The loss of peripheral macrophages is compensated for by monocytes entering the tissues from the blood stream. Macrophages are no longer undergoing cell division but accumulation can occur through increased recruitment from the circulation and through delay of the naturally occurring turnover, e.g. inhibition of the apoptosis mechanism. Bacterial components such as lipopolysaccharide (LPS)4 and toxic shock syndrome toxin-1 (TSSF-1) superantigen5 have been found to block macrophage apoptosis. Macrophage apoptosis is furthermore regulated by cytokines. Type 1 cytokines such as interferon-γ (IFN-γ), granulocyte–macrophage colony-stimulating factor (GM-CSF), IL-1, IL-12 and tumour necrosis factor (TNF) inhibit, whereas type 2 cytokines such as IL-4 and IL-10 enhance, macrophage apoptosis3,4,6,7. The survival of macrophages is linked to the expression of CD14, a characteristic macrophage marker and a receptor for LPS8. Apoptotic macrophages lose the phoshatidylinositol (PI)-linked surface marker.9
The present communication extends the study of cell death regulation in macrophages. The focus lies on the function of the cytokine IL-10. IL-10 has been shown to be a potent inducer of macrophage differentiation; it induces the expression of FcR, in particular of the FcγRIII (CD16) and enhances the ADCC activity of macrophages.1,10,11 However, because the cytokine induces massive apoptosis of macrophages, the inductive IL-10 function is restricted to a small fraction of apoptosis-resistant cells. We report here that in the presence of human serum or in the presence of purified human immunoglobulin G (IgG), IL-10 ceases to induce macrophage apoptosis and instead induces the survival of macrophages. Our studies attribute the survival effect to the expression of CD16, a molecule known to transmit survival signals in neutrophils.12
Materials and methods
Cell culture
Human peripheral blood mononuclear cells (PBMC) were isolated from donor blood by Ficoll-Hypaque density sedimentation. Washed cells were resuspended in RPMI-1640 (Sigma, St. Louis, MO) containing 5% toxin-free fetal calf serum (FCS, Sigma) and counted in a ultraplane improved Neubauer haemocytometer. To enrich for monocytes, 2·5 × 105 PBMC were incubated in 0·1 ml RPMI with 20% FCS for 60 min. The cells were vigorously washed to remove non-adherent cells. The cells that remained adherent were used as a source of monocytes for cell culture. At harvest time, macrophages were scraped off the culture bottom with a rubber policeman, counted, washed three times, and subjected to flow cytometric analysis.
Flow cytometry
A FACScan flow cytometer (Becton Dickinson, Mountain view, CA) was used for phenotypic analysisby fluorescence-activated cell sorting. Macrophages were examined at the time of preparation and after harvest from tissue culture by staining as previously described13 with fluoroscein-isothiocynate (FITC)-labelled or biotinylated monoclonal antibodies (mAb). The biotin label was revealed by strepavidin–phycoerythrin (Sigma). The following antibodies were used: anti-CD14 (Immunotech, Westbrook, ME); anti-CD16 (Immunotech). Macrophages were treated with these reagents in the presence of 10% heat-inactivated human serum to prevent non-specific IgG binding. Immunoglobulins matched for isotype and fluorescence label were used to determine background immunoglobulin binding. The background values were subtracted from the experimental values. Of the gated population, more than 95% of cells were CD14+.
Reagents
Antibodies against CD16 (IgG1), CD32 (IgG2b), and CD64 (IgG1) were purchased from Medarex, Ammondale, NJ. The human immunodeficiency virus (HIV) envelope antigen gp120 was a gift from D. Litman, New York University. Serum IgG was purified from heat-inactivated (30 min, 56°) human serum by passage over protein G columns according to the manufacturer's instructions (Sigma). Annexin V was purchased from Pharmingen (San Diego, CA) and used according to the manufacturer's recommendations. IL-10 was a gift from the DNAX Corporation; TNF-α was a gift from Genentech; IL-12 was a gift from Genetics Systems; anti-TNF mAb was provided by P. Kiener, Bristol Myer Squibb. LPS (Escherichia coli O127:B8) was purchased from Sigma.
Results
Monocyte-derived macrophages have a limited life span in tissue culture that can be shortened or extended by cytokines
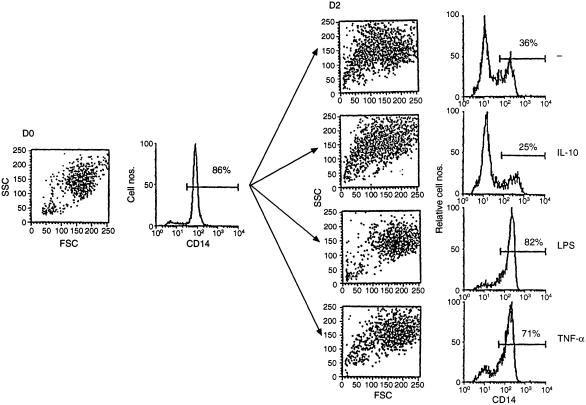
Freshly prepared human peripheral blood monocytes can be identified in the flow cytometer scattergram as a distinct population.14 Forward scatter (FSC) reflects the size of the cell whereas side scatter (SSC) reflects the degree of membrane folding. Figure 1 shows monocyte forward and side scatter characteristics; monocytes are distinct from a small contamination of lymphocytes present in the lower left corner. The great majority of cells in the monocyte population express CD14, the marker characteristic of live monocytes.9 Figure 1 shows, in addition, flow scattergrams of macrophages after 2 days in tissue culture. When cultured without exogenous cytokines, macrophages changed their morphology as previously reported;3 approximately one-third of them shrunk in size and developed the surface characteristics of smoother, deactivated cells, typical of apoptotic cells undergoing shrinking cell death. An equivalent fraction shed the CD14 marker. A separate (not shown) analysis verified that the CD14 marker was lost by those cells which had shrunk, consistent with earlier findings that apoptotic macrophages lose CD14 expression.9 The addition of IL-10 to the culture never inhibited the loss of CD14 expression; rather, it frequently enhanced it, consistent with the notion that IL-10 induces apoptosis of macrophages.3 In contrast, addition of TNF-α or of LPS blocked macrophage shrinkage and prevented the loss of CD14 expression.
Figure 1.
Macrophage disintegration in tissue culture is blocked by LPS and TNF but not by IL-10. PBMC monocytes were phenotyped in the flow cytometer immediately after preparation (left side) or after a 2-day culture period (right side). The SSC/FSC scattergram is shown as dot plot, CD14 expression as histogram. The proportion of CD14 positive cells is indicated above the gate. IL-10, 10 ng/ml; LPS, 1 µg/ml; IL-12, 100 U/ml; TNF-α, 10 ng/ml.
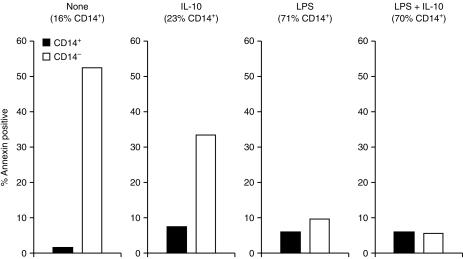
Cell shrinkage is a characteristic feature of the apoptosis pathway.15 Another apoptosis marker is the cell surface expression of Annexin V.16 Figure 2 shows that Annexin V-expressing macrophages reside in the macrophage fraction that shrunk and lost the CD14 positive phenotype, consistent with previous reports.9
Figure 2.
Annexin V is expressed on macrophages after loss of CD14. PBMC monocytes were incubated and treated as indicated for 2 days. CD14 and Annexin V expression was assessed by double-florescence flow cytometry and the proportion of Annexin positive cells in CD14 positive and negative macrophage fractions was determined. IL-10, 10 ng/ml; LPS, 1 µg/ml.
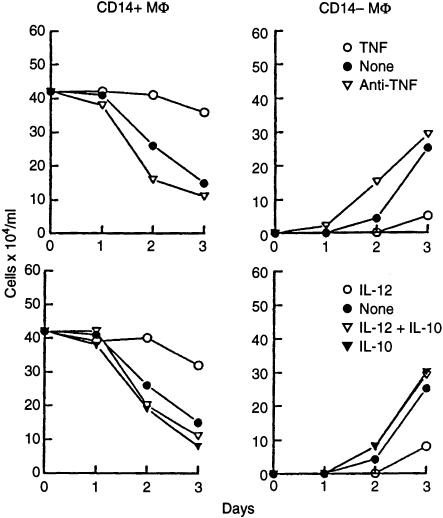
LPS, for which CD14 represents a major receptor,8 is an inducer of cytokine production by macrophages.17 Several macrophage-secreted cytokines were tested for their ability to halt macrophage decay. Consistent with reported findings, IL-1, TNF and IL-12 were found to block macrophage cell death whereas IL-10 was found to enhance it.3,4,6,7 Figure 3 shows that TNF prevents the conversion of CD14+ into CD14– cells as the fraction of CD14 negative cells no longer grows at the expense of CD14 positive cells. Endogenously produced TNF appears to have the same effect as antibody against TNF enhances the generation of CD14 negative apoptotic macrophages. IL-12 manages to prevent spontaneous macrophage death in tissue culture but fails to block cell death induction by IL-10. This observation qualifies the IL-10-induced apoptotic signalling mechanism as distinct from spontaneous apoptosis because it is less susceptible to interference than the latter.
Figure 3.
TNF-α and IL-12 abrogate the apoptotic response of macrophages. PBMC monocytes were cultured with the indicated reagents. After 1–3 days, the cells were harvested, counted and phenotyped for the expression of CD14. The absolute numbers of CD14 positive and negative macrophages were calculated and is presented. TNF, 10 ng/ml; anti-TNF mAb, 10 µg/ml; IL-12, 100 U/ml; IL-10, 10 ng/ml. The experiment represents one of three experiments with similar results.
The engagement of the FcγIII receptor (CD16) transmits survival signals in macrophages
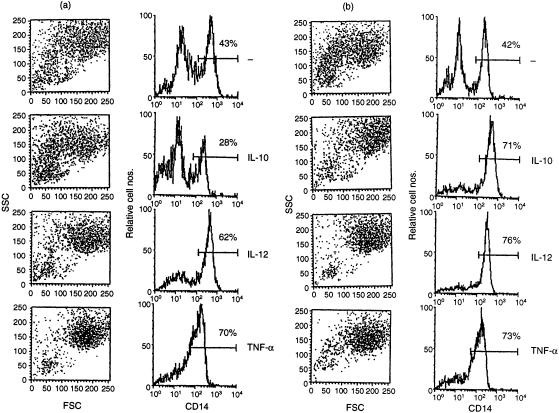
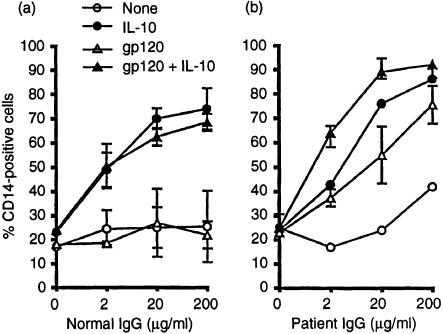
We noted that IL-10 failed to induce macrophage cell death in the presence of human serum; in fact, the cytokine prevented spontaneous macrophage death in the presence of human serum. Figure 4 shows a comparison between macrophages cultured in the presence of human or fetal calf serum. The results are virtually identical except when the cultures contained IL-10. Whereas, consistent with the observations shown above, only a small fraction of macrophages survived 2 days of culture in the presence of IL-10 and FCS, the majority of macrophages survived the treatment with IL-10 in the presence of human serum. Further analysis revealed IgG as the responsible serum component. Figure 5(a) shows that human IgG induces in the presence of IL-10 a dose-dependent refractiveness to cell death. Whereas usually approximately 80% of the macrophages lose the CD14 marker within 2 days of culture and die and only approximately one-fifth of the cells remain viable and continue to express CD14, Fig. 5(a) shows that IgG enables macrophages to retain the survival marker. IgG failed to prevent the spontaneous induction of macrophage cell death but readily blocked the strong death response induced by IL-10. Apparently, IL-10 not only induced macrophage death but also enabled macrophages to utilize an IgG-mediated survival mechanism.
Figure 4.
IL-10 inhibits rather than enhances macrophage apoptosis in the presence of human serum. PBMC monocytes were cultured with the indicated cytokines in the absence (a) or the presence (b) of heat-inactivated human serum in addition to 5% FCS. Macrophage CD14 expression and FSC/SSC scatter were assessed on day 2. IL-10, 10 ng/ml; IL-12, 100 U/ml; TNF, 10 ng/ml.
Figure 5.
Human IgG and immune complexes prevent apoptotic death of macrophages. PBMC monocytes were cultured in the presence of IgG freshly prepared from a healthy individual (left panel) or from an HIV-infected subject (right panel). Macrophage CD14 expression was assayed on day two. Open circles: control. Closed circles: IL-10 (10 ng/ml) Open triangles: gp120 (1 µg/ml). Closed triangles: IL-10 plus gp120. The results were averaged from two experiments ± sd.
We examined a connection of the IgG effect with the previously reported notion that IL-10 induces the expression of FcRs.10,11 We determined whether complex formation with antigen enhances the IgG efficacy in inducing survival signals and asked whether antibodies specifically directed against FcRs can prevent macrophage destruction.
Figure 5(b) shows an experiment with IgG purified from the serum of an acquired immune deficiency syndrome (AIDS) patient. AIDS patient serum is known to contain increased levels of immune complexes and it contains antibodies against the HIV envelope molecule, gp120.18 Adding patient IgG to macrophage cultures we found that, in contrast to normal IgG (Fig. 5a), it partially prevents the spontaneous macrophage death. The rescue effect was increased by the addition of gp120. The gp120 enhancement was seen in the presence of patient serum IgG but not in the presence of serum IgG from a healthy donor. This indicates that immune complexes formed between the envelope molecule and patient anti-gp120 enhanced the engagement of FcRs and the transmission of survival signals. Patient serum IgG synergizes much like normal human serum IgG with IL-10 in blocking macrophage death. However, gp120 enhances the synergism between IL-10 and human IgG when patient IgG is used but not when IgG from an HIV-seronegative individual is tested. Because IL-10 is known to upregulate FcR expression in macrophages,10,11 we speculated that IL-10 synergizes with IgG in the rescue of macrophages by increasing the density of IgG receptors whereas the gp120 antigen enhanced IgG efficacy by the formation of immune complexes and the more effective engagement of the FcR.
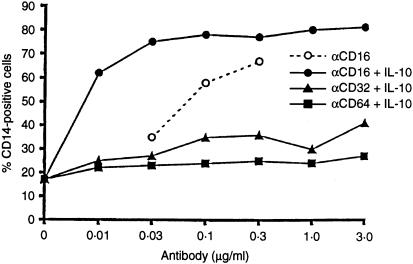
To examine the conclusion that the engagement of FcRs on the macrophage surface transmits survival signals, we incubated macrophages in the presence of IL-10 (to upregulate FcR expression) and added to the culture medium antibodies against the three major FcγRs, CD16, CD32 and CD64. Figure 6 shows that antibody against CD16 prevents the loss of CD14 in macrophage cultures in the presence of IL-10. CD16-specific antibody saves macrophages even in the absence of IL-10 (dotted line in Fig. 6) showing that the lower level of spontaneous CD16 expression qualifies macrophages for a more modest degree of CD16-mediated survival signals. The notion is consistent with the observation that immune-complexed IgG transmits survival signals without additional upregulation of Fc receptors through IL-10 (Fig. 5). Antibodies against CD32 or CD64 failed to rescue macrophages from destruction, in the absence (not shown) or in the presence of IL-10.
Figure 6.
The engagement of CD16 prevents the apoptosis of macrophages. PBMC monocytes were cultured in the presence of antibodies against CD16 (circles), CD32 (triangles), or CD64 (rectangles) in concentrations indicated. Two days later macrophages were harvested and phenotyped for CD14 expression. IL-10 (10 ng/ml) was added to all cultures except to those indicated in the dotted line.
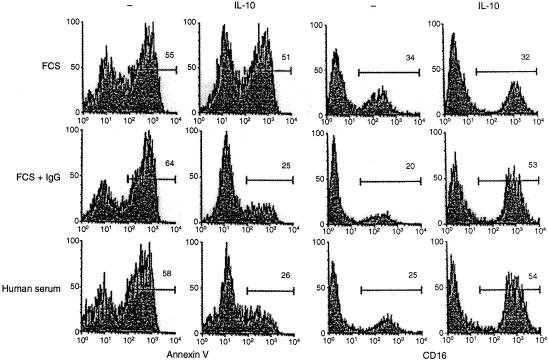
It should be noted that the IgG-mediated macrophage rescue mechanism abrogates the IL-10-mediated destruction of macrophages but not the IL-10-induced upregulation of CD16. This suggests that CD16 engagement blocks the apoptotic pathway without affecting the capacity of IL-10 to induce the differentiation of macrophages. Experiments were conducted to substantiate this assumption (Fig. 7). Most macrophages cultured with IL-10 in the absence of human IgG die and only a small fraction of cells survive and upregulate CD16; most of the cells survive and upregulate CD16 in the presence of IgG.
Figure 7.
IgG blocks the induction by IL-10 of macrophage apoptosis but not the induction of phenotypic macrophage differentiation. Adherent PBMC were cultured in the presence of heat-inactivated FCS (5%), FCS and human IgG (300 µg/ml), or heat-inactivated human serum. Two days later the cells were harvested and phenotyped for reactivity with Annexin V (left two panels) or anti-CD16 mAb. The numbers in each box represent the percentage of marker-positive cells within the indicated gates.
Discussion
Cells and tissues have specific functions that they carry out after stimulation through membrane receptors. Often such receptors not only transmit signals for activation and cycle progression but also may be linked to deletion programmes that send the cell into apoptosis before a cell has a chance to differentiate and proliferate. A prototypical example is the lymphocyte. Antigen receptor ligation initiates signal cascades, which lead to cellular differentiation and proliferation when costimulatory signals are simultaneously delivered, but result in cell death if apoptosis is not stopped. Immature thymocytes do not yet possess costimulatory devices and they die after antigen receptor engagement. T cells acquire costimulatory devices as they mature. However, if costimulation is blocked or not properly engaged, antigen receptor engagement may induce the death of mature T cells as well.19,20 Apparently, activation signals can only become effective if cell death programmes are effectively cancelled.
In the present communication we confirm this concept with macrophages. Macrophages descend from monocytes. There is a steady influx of macrophages from the blood stream into the tissues. This influx must be matched by an equivalent rate of macrophage decay in tissues if excessive accumulation is to be avoided. The present study gives an example of how the body uses the cytokine IL-10 to negatively and positively regulate the size of the macrophage population. Exposed to IL-10 macrophages may respond with differentiation or with apoptosis. The cytokine induces in macrophages the expression of CD16 and has been shown to enhance the ADCC effector cell activity of macrophages.10,11,21 We show here that IL-10 initiates a macrophage deletion programme in a great majority of macrophages, permitting differentiation in only a small fraction of apoptosis-resistant macrophages. Only the interruption of the apoptosis pathway by survival signals allows macrophage differentiation to proceed. Bacterial membrane components such as LPS are biological inducers of macrophage survival and these effects may, in part, be mediated by cytokines whose release they induce. The novelty of our findings with IL-10 is that while inducing the massive death of macrophages it sets at the same time the stage for survival by enhancing the expression of FcRs which, when engaged by IgG, block the death mechanism and secure the macrophages' survival.
IgG is usually not present in tissues, suggesting that IL-10 accelerates the decay of macrophages in healthy tissues and does not facilitate the accumulation of CD16+ macrophages with ADCC effector cell capacity. Pathological conditions are known in which immunoglobulin enters tissues. Extravasation of serum components is a characteristic of the inflammatory process. Tissue-specific and polyspecific autoreactive antibodies are other examples of tissue penetration by immunoglobulins.
The presence of IL-10 and of IgG may contribute to the accumulation of macrophages at the inflammatory site specifically to the accumulation of macrophages which express CD16. It has been recognized that IL-10 induces CD16+ macrophages with ADCC effector cell potential at the expense of macrophages with antigen-presenting/costimulatory capacity.1,13,22,23 This notion seems relevant for autoimmune diseases because ADCC effector macrophages may continue to destroy antibody-tagged tissue cells even after the disappearance of inflammatory symptoms create a sense of disease remission.1
A systemic loss of antigen-presenting/costimulatory macrophages and an increase in CD16+ cytotoxic macrophages has been noted in patients with AIDS and in patients with systemic lupus erythematosus (SLE).1,2,13,22,23 Both diseases exhibit an abnormal increase of IL-10 production.2, 13,24,25,26 Both diseases are, furthermore, characterized by the presence of polyspecific autoreactive antibodies.13,27 The lymphopenia seen in advanced stages of both diseases has been attributed to the destruction of antibody-coated lymphocytes by ADCC effector cells, especially CD16+ macrophages.13,27 It is conceivable that ADCC effector cells destroy not only lymphocytes that exhibit antibodies on their surface, but also other antibody-reactive tissue cells such as endothelial cells and basal cells of the glomerulus. It has been shown in the mouse that blocking FcR signal transduction by γ-chain deletion abrogates the development of SLE-associated immune complex disease.28 γ-Chain deletion does not interfere with immune-complex deposition; it merely prevents antibody-mediated cell damage. These findings link SLE pathology to FcR function. It would seem desirable to extend these studies to other diseases with a potential pathogenic involvement of autoreactive antibodies and of the FcR.
Acknowledgments
This work was supported by a grant from the American Lupus Foundation (to M.K.H). We thank Melody Steinberg for proofreading the manuscript.
References
- 1.Kummerle-Deschner JB, Hoffmann MK, Niethammer D, Dannecker GE. Pediatric rheumatology: autoimmune mechanisms and therapeutic strategies. Immunol Today. 1998;19:250–3. doi: 10.1016/s0167-5699(98)01263-8. 10.1016/s0167-5699(98)01263-8. [DOI] [PubMed] [Google Scholar]
- 2.Shearer GM. HIV-induced immunopathogenesis. Immunity. 1998;9:587–93. doi: 10.1016/s1074-7613(00)80656-1. [DOI] [PubMed] [Google Scholar]
- 3.Mangan DF, Wahl SM. Differential regulation of human monocyte programmed cell death (apoptosis) by chemotactic factors and pro-inflammatory cytokines. J Immunol. 1991;147:3408–12. [PubMed] [Google Scholar]
- 4.Mangan DF, Welch GR, Wahl SM. Lipopolysaccharide, tumor necrosis factor-alpha, and IL-1 beta prevent programmed cell death (apoptosis) in human peripheral blood monocytes. J Immunol. 1991;146:1541–6. [PubMed] [Google Scholar]
- 5.Bratton DL, May KR, Kailey JM, Doherty DE, Leung DY. Staphylococcal toxic shock syndrome toxin-1 inhibits monocyte apoptosis. J Allergy Clin Immunol. 1999;103:895–900. doi: 10.1016/s0091-6749(99)70435-5. [DOI] [PubMed] [Google Scholar]
- 6.Estaquier J, Ameisen JC. A role for T-helper type-1 and type-2 cytokines in the regulation of human monocyte apoptosis. Blood. 1997;90:1618–25. [PubMed] [Google Scholar]
- 7.Mangan DF, Robertson B, Wahl SM. IL-4 enhances programmed cell death (apoptosis) in stimulated human monocytes. J Immunol. 1992;148:1812–6. [PubMed] [Google Scholar]
- 8.Lee JD, Kato K, Tobias PS, Kirkland TN, Ulevitch RJ. Transfection of CD14 into 70Z/3 cells dramatically enhances the sensitivity to complexes of lipopolysaccharide (LPS) and LPS binding protein. J Exp Med. 1992;175:1697–705. doi: 10.1084/jem.175.6.1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Heidenreich S, Schmidt M, August C, Cullen CP, Rademaekers A, Pauels HG. Regulation of human monocyte apoptosis by the CD14 molecule. J Immunol. 1997;159:3178–88. [PubMed] [Google Scholar]
- 10.Olikowsky T, Wang ZQ, Dudhane A, Horowitz H, Conti B, Hoffmann MK. Two distinct pathways of human macrophage differentiation are mediated by interferon-γ and interleukin-10. Immunology. 1997;91:104–8. doi: 10.1046/j.1365-2567.1997.00204.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Orlikowsky T, Wang ZQ, Dudhane A, Horowitz H, Riethmuller G, Hoffmann MK. Cytotoxic monocytes in the blood of HIV type 1-infected subjects destroy targeted T cells in a CD95-dependent fashion. AIDS Res Hum Retroviruses. 1997;13:953–60. doi: 10.1089/aid.1997.13.953. [DOI] [PubMed] [Google Scholar]
- 12.Dransfield I, Buckle AM, Savill JS, McDowall A, Haslett C, Hogg N. Neutrophil apoptosis is associated with a reduction in CD16 (Fc gamma RIII) expression. J Immunol. 1994;153:1254–63. [PubMed] [Google Scholar]
- 13.Wang ZQ, Horowitz HW, Orlikowsky T, Dudhane A, Weinstein A, Hoffmann MK. Lymphocyte-reactive autoantibodies in human immunodeficiency virus type 1-infected persons facilitate the deletion of CD8 T cells by macrophages. J Infect Dis. 1998;178:404–12. doi: 10.1086/515623. [DOI] [PubMed] [Google Scholar]
- 14.Becton Dickinson. San Jose, CA: Becton Dickinson Immunocytometry Systems; 1989. FACScan Users Guide; pp. 4–5. [Google Scholar]
- 15.Rathmell JC, Thompson CB. The central effectors of cell death in the immune system. Annu Rev Immunol. 1999;17:781–828. doi: 10.1146/annurev.immunol.17.1.781. [DOI] [PubMed] [Google Scholar]
- 16.Vermes I, Haanen C, Steffens-Nakken H, Reutelingsperger C. A novel assay for apoptosis. Flow cytometric detection of phosphatidylserine expression on early apoptotic cells using fluorescein labelled Annexin V. J Immunol Methods. 1995;184:39–51. doi: 10.1016/0022-1759(95)00072-i. 10.1016/0022-1759(95)00072-i. [DOI] [PubMed] [Google Scholar]
- 17.Dentener MA, Bazil V, Von Asmuth EJ, Ceska M, Buurman WA. Involvement of CD14 in lipopolysaccharide-induced tumor necrosis factor-alpha, IL-6 and IL-8 release by human monocytes and alveolar macrophages. J Immunol. 1993;150:2885–91. [PubMed] [Google Scholar]
- 18.Levy JA. Pathogenesis of human immunodeficiency virus infection. Microbiol Rev. 1993;57:183–289. doi: 10.1128/mr.57.1.183-289.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang ZQ, Orlikowsky T, Dudhane A, Trejo V, Hoffmann MK. Macrophages may activate or destroy T cells with which they form antigen- or coreceptor-mediated cellular conjugates. Cell Immunol. 1998;189:74–82. doi: 10.1006/cimm.1998.1364. 10.1006/cimm.1998.1364. [DOI] [PubMed] [Google Scholar]
- 20.Chambers CA, Allison JP. Costimulatory regulation of T cell function. Curr Opin Cell Biol. 1999;11:203–10. doi: 10.1016/s0955-0674(99)80027-1. 10.1016/s0955-0674(99)80027-1. [DOI] [PubMed] [Google Scholar]
- 21.de Waal M, Figdor CG, Huijbens R, et al. Effects of IL-13 on phenotype, cytokine production, and cytotoxic function of human monocytes. Comparison with IL-4 and modulation by IFN- gamma or IL-10. J Immunol. 1993;151:6370–81. [PubMed] [Google Scholar]
- 22.Zwilling BS, Salkowitz J, Laufman H, Pearl D. Differences in the expression of histocompatibility antigen-DR and in anti-mycobacterial activity of monocytes from HIV-infected individuals. AIDS. 1991;5:1327–32. doi: 10.1097/00002030-199111000-00007. 10.1016/s0968-0896(97)00088-6. [DOI] [PubMed] [Google Scholar]
- 23.Dudhane A, Conti B, Orlikowsky T, Wang ZQ, Mangla N, Gupta A, Wormser GP, Hoffmann MK. Monocytes in HIV type 1-infected individuals lose expression of costimulatory B7 molecules and acquire cytotoxic activity. AIDS Res Hum Retroviruses. 1996;12:885–92. doi: 10.1089/aid.1996.12.885. [DOI] [PubMed] [Google Scholar]
- 24.Clerici M, Shearer GM. The Th1–Th2 hypothesis of HIV infection: new insights. Immunol Today. 1994;15:575–81. doi: 10.1016/0167-5699(94)90220-8. [DOI] [PubMed] [Google Scholar]
- 25.Llorente L, Zou W, Levy Y, et al. Role of interleukin 10 in the B lymphocyte hyperactivity and autoantibody production of human systemic lupus erythematosus. J Exp Med. 1995;181:839–44. doi: 10.1084/jem.181.3.839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Linker-Israeli M, Honda M, Nand R, et al. Exogenous IL-10 and IL-4 downregulate IL-6 production by SLE-derived PBMC. Clin Immunol. 1999;91:6–16. doi: 10.1006/clim.1998.4680. 10.1006/clim.1998.4680. [DOI] [PubMed] [Google Scholar]
- 27.Wang ZQ, Horowitz HW, Orlikowsky T, et al. Polyspecific self-reactive antibodies in individuals infected with human immunodeficiency virus facilitate T cell deletion and inhibit costimulatory accessory cell function. J Infect Dis. 1999;180:1072–9. doi: 10.1086/314974. 10.1086/314974. [DOI] [PubMed] [Google Scholar]
- 28.Clynes R, Dumitru C, Ravetch JV. Uncoupling of immune complex formation and kidney damage in autoimmune glomerulonephritis. Science. 1998;279:1052–4. doi: 10.1126/science.279.5353.1052. 10.1126/science.279.5353.1052. [DOI] [PubMed] [Google Scholar]