Abstract
The interleukin-5 receptor α chain (IL-5Rα) is known to regulate the development and function of B cells and eosinophils. Although the functions of IL-5Rα cytoplasmic domain subregions have been studied extensively using cultured cell lines, this approach has limitations when studying the functions of distinct primary B-cell subpopulations and their responsiveness to IL-5. In the present study, we generated mice on an IL-5Rα null background, each expressing a mutant form of an IL-5Rα transgene ligated to a µ enhancer and VH promoter, either lacking the cytoplasmic DC3 region or substituting two proline residues for alanine (ApvA) in the membrane-proximal ppvp motif of the cytoplasmic domain. The ppvp motif, which mediates activation of JAK2/STAT5 and Btk, also contributes to c-fos, c-jun and c-myc expression. IL-5Rα null mutant mice showed impaired B-1-cell development, reduced serum immunoglobulin G3 (IgG3) and IgM, no IL-5-induced enhancement of B-cell proliferation and IL-5-induced switch recombination from the µ gene to γ1 gene; these were not recovered following the expression of the ApvA mutant. In contrast, absence of the DC3 region affected the IL-5-induced switch recombination from the µ to the γ1 gene and B-1-cell development, while IL-5-induced proliferation and IgM production were at levels similar to those of B cells expressing wild-type IL-5Rα transgene. The results clearly indicated that the ppvp motif and the DC3 region of IL-5Rα played distinct roles in B-cell proliferation and differentiation. Thus, this present approach offers new insights into the functions of the cytoplasmic subregions of IL-5Rα, in particular its carboxy-terminal region.
Introduction
Interleukin-5 (IL-5) is mainly produced by T helper type 2 (Th2) and mast cells and has pleiotropic effects on various target cells.1–4 In fact, IL-5 was originally described as a B-cell differentiation factor5 and then later recognized as a B-cell growth factor, immunoglobulin A (IgA) -enhancing factor, and eosinophil differentiation factor.6–8 In vitro analysis using recombinant IL-5 clearly shows that IL-5 is able to induce the proliferation and differentiation of B cells and eosinophils.
Among B-cell subpopulations, B-1 cells can be distinguished from B-2 cells by their expression of CD5,9 anatomical localization, self-replenishing activity and IgM VH usage.10–12 B-1 cells are the major source of IgM, IgG3 and IgA natural antibody.11,12 B-1 cells in the peritoneal cavity express IL-5 receptor (IL-5R) and respond to IL-5 with high frequency leading to IgM production, while a small proportion of conventional B (B-2) cells in spleen also express IL-5R.13,14 IL-5 has also been found to enhance IgA and IgG1 production by activated B-2 cells.15,16 Transgenic mice expressing the IL-5 gene exhibit elevated serum levels of IgM, IgA and IgE, an increase in the number of B-1 cells, autoantibody production and persistent eosinophilia.17 These studies indicate that IL-5 is deeply involved in the development and activation of B cells and eosinophils in vivo.
IL-5 exerts many biological activities by binding IL-5R, which is expressed on B cells, eosinophils and basophils.14 The IL-5R consists of two distinct membrane proteins, α and βc, each of which is a member of the cytokine receptor superfamily.18–23 IL-5Rα specifically binds IL-5 but IL-5Rα alone cannot transduce signals and has to be associated with the βc subunit in order to trigger intracellular signalling.21–23 The βc binds IL-5 in the presence of IL-5Rα, converts the receptor from low-affinity to high-affinity and transduces the signal.21,23,24 The βc is common to receptors for both IL-3 and granulocyte–macrophage colony-stimulating factor (GM-CSF), and is indispensable for signalling.25 Although the molecular mechanisms of IL-5 signal tranduction have not been fully characterized, it is known that activation of JAK2, STAT5, MAP kinase, Lyn and Btk in B cells are all critical for IL-5 signal transduction.26–29
Functional analysis of the cytoplasmic domain of IL-5Rα is relatively limited, although the role of βc cytoplasmic subregions has been examined extensively. We have shown, using in vitro mouse FDC-P1 transfectants of IL-5Rα, that the membrane-proximal domain containing the sequence Pro352 Pro353 Val354 Pro355 (ppvp motif) is indispensable for IL-5 signal transduction.29,30 Furthermore, by using alanine replacement mutants of the ppvp motif, we have shown that JAK2/STAT5 were activated following IL-5 stimulation of cells expressing the IL-5Rα mutants pAvA, AAvP, much less for ApvA mutants (where A represents an alanine substitution), while cells expressing the AAvA mutant were not activated.30 Detailed studies of the truncated cytoplasmic domain of human IL-5Rα revealed that the cytoplasmic stretch at position 346–387, containing the ppvp motif, is necessary for JAK2 binding.26 Moreover, our deletion analysis revealed that six amino acid residues in the carboxyl-terminal (DC3) region of IL-5Rα appear to be dispensable for IL-5-induced cell proliferation.29 Thus far, functional analyses of the IL-5Rα have been carried out using in vitro proliferation assays with cultured cell lines altered by conventional gene transfection experiments. It therefore remains elusive how IL-5Rα mediates B-cell function in vivo. In particular, the in vivo role of the IL-5Rα cytoplasmic domain subregions in B-cell proliferation and differentiation is still unknown. In this study, we took another approach to define the functions of the ppvp motif and DC3 region, by generating mice each expressing a mutant form of an IL-5Rα transgene; alanine substitution of proline and lacking the DC3 region, on an IL-5Rα null background and comparing these with mice expressing the wild-type (wt) IL-5Rα transgene. Our results clearly indicate that the ppvp motif and the DC3 region of the IL-5Rα play distinct roles in signalling for B-cell proliferation and differentiation.
Materials and Methods
Reagent
The following monoclonal antibodies (mAbs) were used: T21 mAb,13 which recognizes mouse IL-5Rα; Bet-2 [American Type Culture Collection (ATCC), Rockville, MD], which recognizes mouse IgM; RA3-6B2 (ATCC), which recognizes mouse B220; 2.4G2 (ATCC), which recognizes mouse FcγR; 5B11 (PharMingen, San Diego, CA), which recognizes anti-mouse IL-3Rα; 53-7.3 (ATCC), which recognizes Ly-1; M1/70 (ATCC), which recognizes mouse Mac-1; and TB13,31 which recognizes mouse IL-5. Anti-mouse CD38 mAb (CS/2)32 was kindly provided by Dr K. Miyake, Saga Medical School (Saga, Japan). Phycoerythrin (PE)-labelled streptavidin (SA-PE) was purchased from Gibco Co. Ltd. (Los Angeles, CA). Unlabelled and biotinylated goat anti-mouse IgG1 antibodies were obtained from Southern Biotechnology Associates (Birmingham, AL). Unlabelled and horseradish peroxidase (HRP)-labelled goat anti-mouse IgM antibodies and HRP-labelled streptavidin (SA-HRP) were purchased from Zymed Laboratories (San Francisco, CA). Lipopolysaccharide (LPS) was obtained from Difco Laboratories (Detroit, MI). Mouse IL-5 was prepared and purified as previously described.18 Mouse IL-3 was purchased from Dainippon Pharmaceutical Co. Ltd. (Osaka, Japan)
Construction of expression plasmid
The expression vector pSUT-1, which was constructed by insertion of cDNA encoding mouse IL-3Rα into the vector pME18Hyg, was kindly provided by Dr A. Miyajima (University of Tokyo, Japan). Plasmids, expressing mutants of mouse IL-5Rα were constructed as previously described.29 Chimeric receptors consisting of the extracellular and transmembrane domains of IL-3Rα and the cytoplasmic domain of the mutant IL-5Rα were constructed by recombinant polymerase chain reaction (PCR) methods.33 The cytoplasmic and the transmembrane domains of the mutant IL-5Rα cDNAs were amplified using 3RTM/5RIC; 5′-TCCTGCTGTGGTGGCATTTATGGACCAG-3′ and 5REND-NotI; 5′-AAGCGGCCGCAGTCCTTGCTCTTAT-3′. Part of the extracellular domain and the transmembrane domains of IL-3Rα were amplified using 3REC; 5′-AGATTCCACCATFFCCTCCT-3′ and 3RTM; 5′-CCACCACAGCAGGAGCCCA-3′. PCR products resulting from two PCR reactions were then mixed, further amplified and fused using 3REC and 5REND-NotI primers. The fragments were digested using SacI and NotI and were replaced with the SacI-NotI fragment from the pSUT-1 plasmid. All of the resultant mutated cDNAs were confirmed using DNA sequencing.
An in vitro cell line of mouse B-cell chronic leukaemia, BCL1, was maintained in RPMI-1640 medium supplemented with 8% fetal calf serum (FCS) and 50 µm 2-mercaptoethanol (2-ME). Ten million BCL1 cells were transfected with 50 µg of expression plasmids by electroporation at 350 V and 250 µF using Gene Pulser (Bio-Rad Laboratories, Richmond, CA) as previously described.34 Transfectants were cultured for selection in a medium containing 100 µg/ml of Hygromycin (Wako Pure Chemical Industries, Osaka, Japan), and stable transfectants were established. The expression of IL-3Rα was examined by flow cytometry analysis using the anti-IL-3Rα mAb, 5B11. BCL1 and its transfectants (3 × 104 cells in a 1-ml culture) were cultured with 100–500 U/ml of IL-5 or 100–500 U/ml of IL-3 in a 24-well plate for 5 days. We measured IgM secreted in cultured supernatants by enzyme-linked immunosorbent assay (ELISA) as previously described.34
Mice
C57BL/6 mice were purchased from Japan SLC (Hamamatsu, Japan). IL-5Rα null mutant mice (IL-5Rα−/−) were generated as previously described.35 IL-5Rα−/− and wt control mice (IL-5Rα+/+) on a C57BL/6 background were maintained and bred in barrier-protected animal facilities under specific pathogen-free conditions using ventilated micro-isolator cages in the experimental animal facility at the Institute of Medical Science, University of Tokyo, Japan. The transgenic mouse line (5Rα-Tg) carrying the wt IL-5Rα gene was generated and maintained as previously described.36
The C57BL/6 transgenic mouse lines ApvA-Tg and DC3-Tg, carrying the IL-5Rα gene with residues Pro352 and Pro355 substituted to Ala and the DC3 region deleted (six amino acid residues at position 380 to 385), respectively, were generated according to procedures previously described.36 In brief, the mutated IL-5Rα cDNA29 was ligated with human Eµ enhancer and immunoglobulin VH promoter. Each transgene was microinjected into the pronuclei of fertilized eggs from a C57BL/6 mouse. To screen the transgenic mice, peripheral blood lymphocytes from each mouse were stained using fluorescein isothiocyanate (FITC) -conjugated anti-mouse IL-5Rα mAb, T21.13 Integration of the transgene was confirmed by PCR analysis using the IL-5Rα primer [5′-AGCTTTGGCAACACTGCAAGCTGA-3′ and 5′-AATGGCAAGTGGACTGAACAGCTG-3′, 670-base pair (bp) PCR fragment]. To identify transgenic mice that expressed only the transgene-derived IL-5Rα, we crossed wt-Tg, DC3-Tg and ApvA-Tg with IL-5Rα−/− mice and obtained wt-5Rα−/−, DC3-5Rα−/− and ApvA-5Rα−/− mice, respectively. IL-5Rα−/− mice were typed using a set of primers (5′-CAGGATCTCCTGTCATCTCACCTT-3′, 5′-CAGTATTTCCTATACTACAGGTCA-3′ and 5′-AATGGCAAGTGGACTGAACAGCTG-3′, 1161-bp PCR fragment for the neomycin resistance gene, and 287-bp PCR fragment of the transgene for wt IL-5Rα). All mice used in this study were maintained in the animal facility at the Institute of Medical Science, University of Tokyo, under pathogen-free conditions and were analysed at 6–8 weeks of age. All experiments were conducted according to our institution's guidelines for the care and treatment of experimental animals.
Flow cytometry
For immunofluorescence studies, mAbs were coupled to biotin (Pierce, Rockford, IL) or FITC (Sigma, St Louis, MO) according to procedures previously described.13 Cells (105−106) from the spleen and peritoneal washouts were stained using biotinylated mAb or FITC-labelled mAb for 30 min at 4°, with 2.4G2 (10 µg/ml) to avoid non-specific binding of the labelled mAbs. After washing, cells were incubated with 50 µl SA-PE for 20 min at 4°. After further washing, cells were suspended in a buffer containing 7-amino-actinomycin D (Sigma) to exclude dead cells from the analysis. Fluorescence intensity was measured using a FACScan instrument (Becton Dickinson, Mountain View, CA).
Assays for B-cell proliferation and differentiation
Mice were anaesthetized with ether and then killed. An enriched population of B cells was obtained from splenocytes by treatment with eliminating anti-Thy-1·2 mAb (Serotech, Blackthorn, Bicester, UK) and guinea-pig complement. They were cultured in 96-well flat-bottomed microtitre plates in RPMI-1640 medium supplemented with 8% heat-inactivated FCS, 2 mm glutamine, 5 µm 2-ME, penicillin (100 U/ml) and streptomycin (100 µg/ml). For the proliferation assay, splenic B cells (1 × 105 in a 200-µl culture) were stimulated with LPS (10 µg/ml), IL-5 (10–100 U/ml), CS/2 (0·5 µg/ml), or with various combinations as previously described.16,37 The proliferative response was monitored by the uptake of [3H]thymidine as previously described,37 and was expressed as mean counts per minute (c.p.m.) and the standard deviation of triplicate cultures. For determining IgM and IgG1 secretion, splenic B cells (1 × 105 in a 200-µl culture) were cultured for 7 days with LPS (1–10 µg/ml), IL-5 (10–100 U/ml), CS/2 (0·5 µg/m) or with combinations of these. After culture, the supernatants were harvested and used for the ELISA to determine amounts of IgM and IgG1.38,39 Each experiment was repeated at least three times and essentially identical results were obtained.
PCR analysis of γ1–μ reciprocal DNA recombination products
Total DNA (genomic and circular) was extracted using a QIAamp Blood Kit (Qiagen, Hilden, Germany) as described previously.39 For amplification of γ1–µ recombination products, 200 ng of total DNA from cultured or freshly isolated B cells were subjected to PCR amplification in 50 µl volumes containing LA PCR Buffer II (Takara; Kyoto, Japan), 2·5 mm MgCl2, 0·4 mm dNTP, 2·5 U LA Taq (Takara), 1 µm sense-strand 5′Sγ1 primer (5′-CACTCCTGGGTATGGAAACACATCCTAC-3′; nucleotides 262–289 in region 5′ of the Sγ1 repeats; MUSIGHANB) in combination with an anti-sense 3′Sµ primer (5′-AGCCTAACTTATCTGAGCCTAGTTCAAC-3′; nucleotides 1347–1319 in region 3′ of the Sµ repeats; MUSIGCD09). Reciprocal γ1–µ products were amplified using 35 cycles of 1 min of melting at 94° and 8 min of annealing and extension at 69°. PCR products were transferred onto nylon membranes (GeneScreen; New England Nuclear, Boston, MA) using standard procedures and then hybridized with 32P-labelled DNA fragments. As an Sγ1 probe, we used the 1·1 kb BamHI-BglII fragment (nucleotides 537–1674, MUSIGHANB) in the 5′ region of the Sγ1 repeats from pγ1E/H10.0 as previously described;39 the Sµ probe was a 99-bp PCR-amplified fragment (nucleotides 1210–1308, MUSIGCD09) starting directly downstream of the Sµ region. Blots were analysed with a Fujix BAS1000 Bioimaging Analyser (Fuji Photo Film, Tokyo, Japan).
Results
Role of the DC3 region of IL-5Rα in differentiation of BCL1 cells into IgM-secreting cells
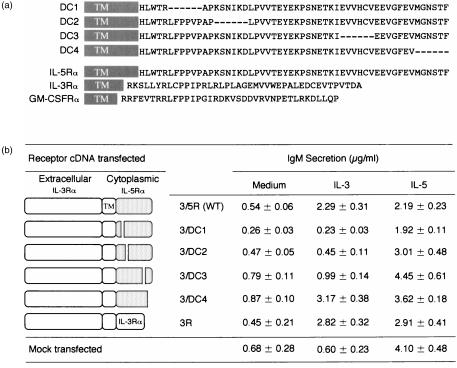
BCL1 cells respond to IL-5, but not to IL-3, by differentiating into IgM-secreting cells. As we reported,34 enforced expression of the IL-3Rα in BCL1 cells enable them to respond to IL-3 by differentiating into IgM-secreting cells. We prepared six different constructs using chimeric IL-3Rα cDNA, constructed by pairing the IL-3Rα with wt [3/5R(wt)] or deletion mutants of IL-5Rα (3/DC1 through 3/DC4), as depicted inFig. 1(a). We transfected each cDNA into BCL1 cells and established permanent transfectants. The FACS analysis using anti-IL-3Rα mAb revealed that each group of transfectants expressed chimeric IL-3Rα but expression levels varied slightly between transfectants (data not shown). In all cases, βc expression was unaltered by transfection. Each group of transfectants was cultured with either medium alone, IL-3, or IL-5. After the culture, secreted IgM levels in the culture supernatants were determined by ELISA. As shown in Fig. 1(b), all BCL1 transfectants showed IL-5-driven IgM secretion with only slight variability between transfectants. Mock-transfected BCL1 did not show IL-3-driven IgM secretion, as expected. Transfectants expressing 3/5R(wt) and 3/DC4 mounted IL-3-driven IgM secretion to an extent similar to the 3R transfectants. In contrast, the 3/DC1 and 3/DC2 transfectants did not show enhancement in IL-3-driven IgM secretion. Interestingly, the 3/DC3 transfectants showed a slight enhancement of IL-3-driven IgM secretion that was significantly lower than that of the 3/DC4 transfectants. We infer from these results that lack of the IL-5Rα DC1 and DC2 subregions diminishes IL-5-driven IgM secretion and lack of the DC3 subregion reduces IL-5-driven IgM secretion in BCL1 cells.
Figure 1.
(a) Cytoplasmic domains of the mutant IL-5Rα used in this study, and comparison of the amino acid sequence of cytoplasmic domains of murine IL-5Rα, IL-3Rα, and GM-CSFRα. Deleted residues are shown by hyphens. Shaded boxes with TM represent the transmembrane portion. (b) IgM secretion of BCL1 transfectants expressing the chimaeric receptors between IL-3Rα and IL-5Rα. Transfectants were incubated for 5 days in the presence of IL-3 (100 U/ml) and IL-5 (100 U/ml), and the IgM secreted in culture supernatants was titrated. The results were expressed as mean IgM (µg/ml) of triplicate cultures. Three independent clones were examined and similar results were obtained.
Proliferative response of transgenic IL-5Rα null mice expressing mutant IL-5Rα
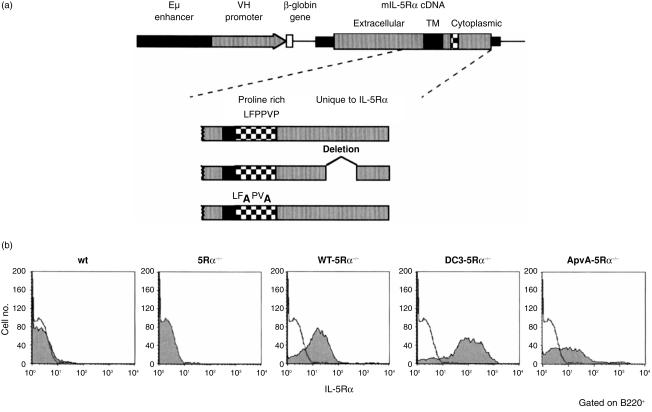
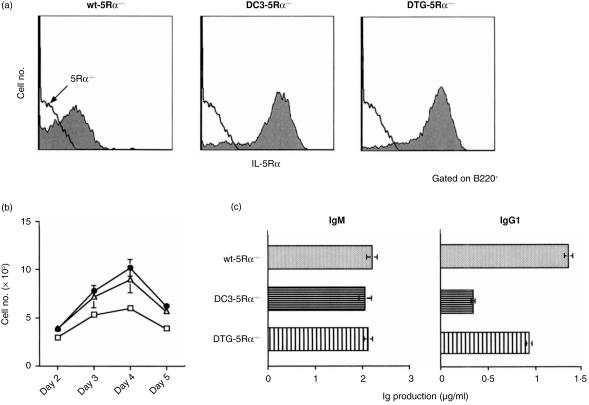
To evaluate the in vivo role of the IL-5Rα cytoplasmic subregions in B-cell proliferation and differentiation, we have generated transgenic mouse lines carrying the gene encoding either wt or mutants of IL-5Rα. As shown in Fig. 2(a), cDNA encoding wt IL-5Rα, IL-5Rα mutants lacking DC3 region (DC3), or substituting proline residues with alanine (ApvA) in the ppvp motif were ligated to human Eµ enhancer/VH promoter and used in the generation of the transgenic mouse lines. Each line of transgenic mice was born healthy and matured normally. We crossed each transgenic mouse line using IL-5Rα null (5Rα−/−) mice to obtain Tg-5Rα−/− lines. Cell surface expression of the transgene-encoding either wt, DC3 mutant, or ApvA mutant forms of the IL-5Rα was examined by flow cytometry using an anti-IL-5Rα mAb. A small proportion of wt B cells in the spleen expressed significant levels of IL-5Rα, while no IL-5Rα expression was observed in the 5Rα−/− B cells (Fig. 2b). IL-5Rα expression on splenic B cells from each mouse of the Tg-5Rα−/− lines (wt-5Rα−/−, DC3-5Rα−/− and ApvA-5Rα−/−) were higher when compared to wt B cells. Levels of mutant IL-5Rα expression in DC3-5Rα−/− B cells were higher than those found in wt-5Rα−/− B cells. ApvA-5Rα−/− B cells expressed equivalent levels of 5Rα when compared to wt-5Rα−/− B cells.
Figure 2.
(a) Diagrammatic illustrations of the transgene constructs and cytoplasmic domains of the mutant IL-5Rα used in this study. All the transgene constructs have the wt IL-5Rα extracellular domain. Deletion mutants (DC3); bars represent deleted amino acid residues. Substitution mutants (ApvA); amino acid residues indicated by bold letters have been replaced with alanine. The cDNA encoding wt or the mutated IL-5Rα were ligated with the Eµ enhancer and VH promoter and used for preparing transgenic mice. (b) Expression of IL-5Rα on spleen B cells. Spleen cells from 8-week-old mice were stained with FITC-conjugated RA3-6B2 (anti-B220 mAb) and biotinylated T21 (anti-IL-5Rα mAb) followed by SA-PE. Profiles for IL-5Rα expression of B220+ cells are shown (shaded histograms). The solid line represents the histogram of IL-5Rα–/– B cells.
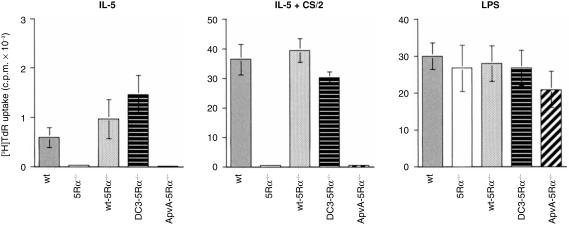
Splenic B cells from each mouse group were cultured for 3 days with either IL-5, CS/2 anti-CD38 mAb, LPS, or using combinations of these and the proliferative response was monitored using a [3H]thymidine incorporation assay. The wt B cells showed a significant proliferative response upon stimulation with IL-5 and the response was remarkably enhanced upon co-stimulation with IL-5 plus CS/2 (Fig. 3). In contrast, no IL-5-induced proliferative response was observed in IL-5Rα null (5Rα−/−) B cells. This impaired IL-5-dependent proliferative response in 5Rα−/− B cells was restored by enforced expression of both the wt IL-5Rα (wt-5Rα−/−) and mutant DC3 genes (DC3-5Rα−/−). In contrast, ApvA-5Rα−/− mouse splenic B cells showed no proliferative response to IL-5 plus CS/2. However, their response to LPS was similar in extent to that observed for splenic B cells from wt, wt-5Rα−/− and DC3-5Rα−/− mice. These results indicate that both wt and DC3 constructs restored B-cell proliferation in response to IL-5, whereas the ApvA construct did not.
Figure 3.
Proliferative response of splenic B cells stimulated with IL-5, IL-5 plus CS/2, and LPS. T-cell-depleted splenic B cells from 8-week-old wt, 5Rα–/–, wt-5Rα–/–, DC3-5Rα–/– and ApvA-5Rα–/– mice were cultured (1 × 105 cells in a 200-µl culture) for 3 days with IL-5 (10 U/ml), IL-5 (10 U/ml) plus CS/2 (0·5 µg/ml), LPS (10 µg/ml) or in their combinations. Cells were pulse-labelled with [3H]thymidine (0·2 µCi/well) for the last 8 hr of the culture. The results represent mean c.p.m. ±standard deviation of triplicate cultures.
Stimulation of the wt B cells using CS/2 has been shown to enhance IL-5Rα expression.16 So we then examined ApvA-5Rα expression levels on CS/2-stimulated ApvA-5Rα−/− B cells. Expression of ApvA-5Rα was also enhanced for 2 days following CS/2 stimulation (data not shown). We infer from these results that the impaired response of ApvA-5Rα−/− B cells to CS/2 plus IL-5 is not due to the level of ApvA-5Rα expression.
Reduced levels of IgG1 production by B cells from DC3-5Rα−/− mice
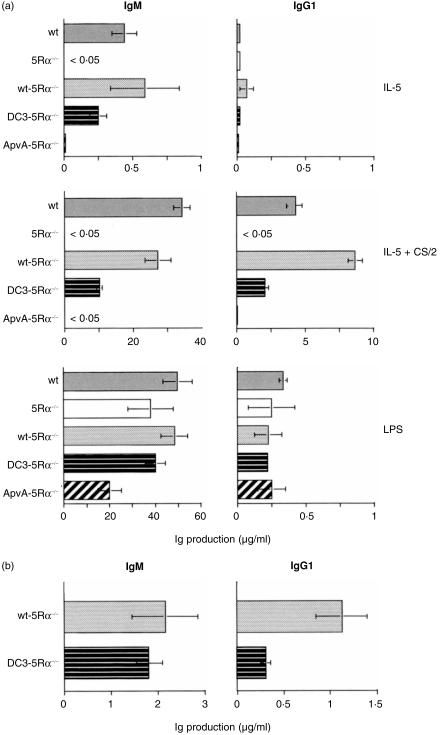
We then examined the restorative effect of the DC3 construct on impaired IL-5-driven IgM and IgG1 production in 5Rα−/− B cells. We cultured DC3-5Rα−/− B cells for 7 days with either CS/2, IL-5, IL-5 plus CS/2, or LPS. As a control, we also cultured splenic B cells from wt, 5Rα−/−, wt-5Rα−/− and ApvA-5Rα−/− mice under the same conditions. After culturing, supernatant IgM and IgG1 levels were titrated by ELISA. As shown in Fig. 4(a), splenic B cells from both wt and wt-5Rα−/− mice showed equivalent IL-5-induced IgM production, which was further enhanced in the IL-5 plus CS/2 samples. Both wt and wt-5Rα−/− B cells produced IgG1 when stimulated with CS/2 plus IL-5. The wt-5Rα−/− B cells produced higher levels of IgG1 than wt B cells. Splenic B cells from 5Rα−/− mice did not produce IgM or IgG1 even when stimulated with CS/2 plus IL-5. ApvA-5Rα−/− B cells did not show any IgM or IgG1 production upon stimulation with CS/2 plus IL-5. DC3-5Rα−/− B cells produced significant levels of IgM following stimulation with IL-5 and IL-5 plus CS/2. IgM production was slightly lower in DC3-5Rα−/− when compared to wt-5Rα−/− B cells, although this result was not statistically significant. However, DC3-5Rα−/− B cells produced significantly lower IgG1 following stimulation with IL-5 plus CS/2 than wt-5Rα−/− B cells. Results suggest that enforced expression of the DC3 gene in IL-5Rα−/− mice appears to partially restore IL-5-driven IgG1 production. B cells from each group of mice responded to LPS by producing similar levels of IgM and IgG1.
Figure 4.
Impaired IgG1 production by DC3-5Rα–/– B cells in response to IL-5 plus CS/2. (a) T-cell-depleted splenic B cells (1 × 105 in a 200-µl culture) were cultured in 96-well plates for 7 days with IL-5 (10 U/ml), IL-5 (10 U/ml) plus CS/2 (0·5 µg/ml), LPS (10 µg/ml) or in their combinations. The IgM and IgG1 concentrations in cultured supernatants were determined by isotype-specific ELISA. The results represent the mean values (µg/ml) and standard deviations of three independent cultures. (b) In the first-step culture, T-cell-depleted splenic B cells (5·0 × 105 in a 1-ml culture) in 24-well plates were cultured with CS/2 (0·5 µg/ml) plus IL-5 (100 U/ml). On days 2 and 3, cells were harvested and re-plated (2·5 × 105 in a 1-ml culture) with fresh medium containing IL-5 (100 U/ml) plus with CS/2 (0·5 µg/ml). On day 4, cells were harvested and cultured at 2·5 × 105 in a 200-µl culture in 96-well plates for 24 hr without any stimulation. After the culture, The IgG1 concentration in cultured supernatant were determined by ELISA. Results were represented as mean IgM and IgG1 (µg/ml) ± standard deviations of three independent cultures.
The numbers of viable B cells recovered on day 3 (of a 6-day culture) when culturing with IL-5 plus CS/2 were about twice those for DC3-5Rα−/− when compared to wt-5Rα−/− B cells (data not shown). Viable B-cell numbers then gradually decreased up to day 6 (data not shown). The higher growth rate of DC3-5Rα−/− B cells when compared to wt-5Rα−/− B cells in the presence of CS/2 plus IL-5 could affect IgM and IgG1 production. To ascertain whether the DC3 construct mediates a partial restoration of B-cell IgG1 production, we employed a two-step culture system. First, the splenic B cells from wt-5Rα−/− or DC3-5Rα−/− mice were cultured for 4 days (primary culture) with IL-5 plus CS/2. Culture medium was changed on days 2 and 3 to fresh medium containing IL-5 and CS/2 so that the B cells grew continuously. On day 4, we harvested the cells, adjusted the cell number and established a new set of 1-day culture (secondary culture) in the absence of IL-5 and CS/2. After the culture, we counted the total cell number and determined IgM and IgG1 secreted in each cultured supernatant. The total number of DC3-5Rα−/− B cells recovered after the second culture was comparable to that of wt-5Rα−/−B cells (data not shown). As shown in Fig. 4(b), IgM secreted in DC3-5Rα−/− B-cell culture was equivalent to IgM in wt-5Rα−/− B-cell culture (Fig. 4a, left panel). Intriguingly, IgG1 produced by DC3-5Rα−/− B cells was less than 30% of IgG1 produced by wt-5Rα−/− B cells (Fig. 4b, right panel). This reduced IgG1 production by DC3-5Rα−/− B cells was not enhanced when stimulated with CS/2 plus IL-5 in the secondary culture (data not shown). These results suggest that lack of the DC3 region in IL-5Rα reduces IL-5-induced IgG1 production without affecting IL-5-dependent IgM production.
Rescue of impaired IgG1 production of DC3-5Rα−/− B cells by means of enforced expression of wt-5Rα
It may be that DC3-5Rα−/− B cells produce lower IgG1 than wt-5Rα−/− B cells in response to CS/2 plus IL-5 owing to higher expression of relevant 5Rα on B cells. Therefore, we generated a double transgenic (DTG-5Rα−/−) mouse by means of crossing wt-5Rα mouse with DC3-5Rα−/− mouse. The 5Rα expression on DTG-5Rα−/− B cells was comparable to that on DC3-5Rα−/− B cells (Fig. 5a). The proliferative response of DTG-5Rα−/− B cells following stimulation with CS/2 plus IL-5 was comparable to that of DC-5Rα−/− B cells (Fig. 5b). IgM production by DTG-5Rα−/− B cells in the two-step culture system was also equivalent to that by DC3-5Rα−/− B cells (Fig. 5C, left panel). Importantly, IgG1 production by DTG-5Rα−/− B cells was approximately twice that produced by DC3-5Rα−/− B cells (Fig. 5c, right panel), and was about 70% of that produced by wt-5Rα−/− B cells. These results indicate that enforced expression of wt-5Rα in DC3-5Rα−/− mouse partially rescue the impaired IL-5-dependent IgG1 production observed in DC3-5Rα−/− B cells.
Figure 5.
Rescue of the impaired IgG1 production of DC3-5Rα–/– cells by the enforced expression of wt-5Rα. (a) Expression of IL-5Rα on spleen B cells of double transgenic mouse (DTG-5Rα–/–) obtained by crossing DC3-5Rα–/– mouse with wt-5Rα mouse. Spleen cells from 8-week-old mice from each line were stained with FITC-conjugated RA3-6B2 and biotinylated T21 followed by SA-PE. (b) Proliferative response of splenic B cells from wt-5Rα–/– (□), DC3-5Rα–/– (▵), and DTG-5Rα–/– (•) in response to IL-5 (100 U/ml) plus CS/2 (0·5 µg/ml). (c) IgM and IgG1 production by splenic B cells in response to CS/2 plus IL-5. Cultures were set up according to procedures as described in Fig. 4(b).
Frequencies of IL-5-induced Sγ1–Sµ switch recombination in DC3-5Rα−/− B cells
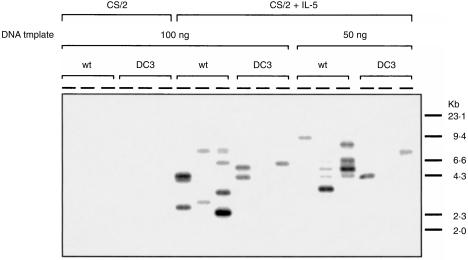
The above results suggest that lower IgG1 production by DC3-5Rα−/− B cells in response to CS/2 plus IL-5 does not simply reflect the lower expansion and differentiation of a pre-existing pool of sIgG1+ B cells. Rather, frequencies of IL-5-induced Sγ1–Sµ switch recombination in DC3-5Rα−/− B cells may be affected. To address this issue, we applied assay systems for detecting frequencies of switch recombination events regardless of subsequent proliferation by amplifying γ1–µ circular DNA according to procedures described in the Materials and methods. Either wt-5Rα−/− B cells or DC3-5Rα−/− B cells were cultured with CS/2 or CS/2 plus IL-5 for 2 days. After the culture, cells were harvested and re-cultured for 1 day in the presence of a relevant stimulus. After the culture, total DNA was prepared and amplified by semi-quantitative PCR. PCR products were hybridized with 5′Sγ1 probe or 3′Sµ probe. Three independent amplifications were performed on identical aliquots of DNA template to improve detection of rare events and assess reproducibility. As shown in Fig. 6, the stimulation of wt-5Rα−/− B cells or DC3-5Rα−/− B cells with CS/2 alone did not induce significant amplification of reciprocal Sγ1–Sµ junctions. However, γ1–µ switch circles (ranging from 2 to 10 kb) were detected in wt-5Rα−/− B cells when stimulated with CS/2 plus IL-5. Almost all of the amplified products contained both the 5′Sγ1 and 3′Sµ segments and had the expected 5′Sγ1–3′Sµ structure (data not shown), indicating that IL-5 induces µ to γ1 switch recombination in CS/2-stimulated wt-5Rα−/− B cells. Three independent amplifications using aliquots of the same DNA preparations showed multiple bands with different sizes in each replicate sample, which is consistent with amplification of multiple single-copy templates with Sµ and Sγ1 recombination breakpoints in different positions. In contrast to wt-5Rα−/− B cells, only small numbers of amplified products were detected in DC3-5Rα−/− B cells following stimulation with CS/2 plus IL-5.
Figure 6.
Impaired µ–γ1 switch recombination of DC3-5Rα–/– B cells in response to IL-5 plus CS/2. Splenic B cells (2·5 × 106 in a 5-ml culture) were cultured with CS/2 (0·5 µg/ml) or CS/2 (0·5 µg/ml) plus IL-5 (100 U/ml). On day 2, cells were harvested, the cell number was adjusted to 2·5 × 106/5 ml and the cells were re-cultured with relevant stimuli for 24 hr. On day 3, total DNA were prepared from live cells and the DNA samples (50 and 100 ng) were amplified using 5′Sγ1 and 3′Sµ primers and LA-Taq polymerase and hybridized with 5′Sγ1 probes.
Role of the IL-5Rα cytoplasmic subregion in numbers and size of B-1 cells
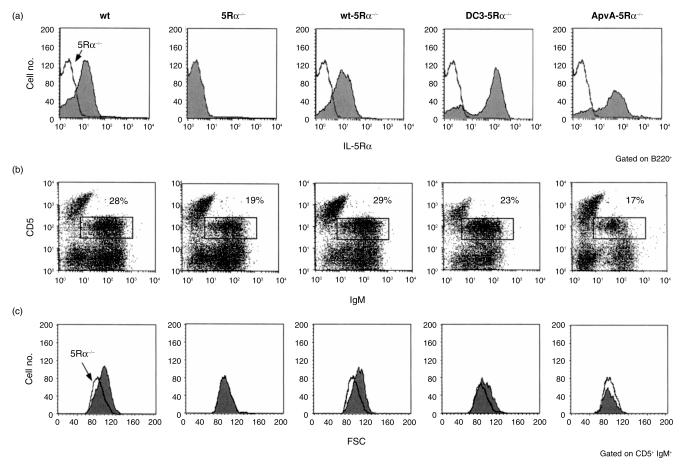
Flow cytometry analysis of spleen B cells of several wt and 5Rα−/− mice stained with antibodies to IgM, B220, CD44, BP-1, CD23, IL-7R, CD43 and CD38 did not reveal any gross differences between the two genotypes. In contrast, the total number of B cells in peritoneal washouts, particularly B1 cells of 5Rα−/−, mice was significantly lower than that in wt littermates, as previously described.35 To elucidate the role of IL-5 in development of the peritoneal B-1 cells, we performed flow cytometric analysis of B cells in the peritoneal cavity from Tg mice. Cell surface expression of transgene-encoded wt, DC3 mutant and ApvA mutant was detected on peritoneal B cells from each of the Tg-5Rα−/− lines (wt-5Rα−/−, DC3-5Rα−/− and ApvA-5Rα−/− mice, respectively) at levels significantly higher than that of wt mice in all cases. Expression of DC3 mutant and ApvA mutant was higher than wt-IL-5Rα (Fig. 7a). The proportions of B220dull CD5+ B (B-1a) cells in 5Rα−/− mice were significantly lower than those in wt mice (Fig. 7b) and the total number of B-1a cells was also less than that of wt mice (data not shown). These results were further confirmed by the decreased proportions of Mac-1+ IgMbright B cells in 5Rα−/− mice (data not shown).35 This reduced proportion of B-1a cells was fully restored in wt-5Rα−/− and partially restored in DC3-5Rα−/− mice. However, no restoration was observed in ApvA-5Rα−/− mice. Furthermore, B-1a cells in 5Rα−/− mice were smaller in size, as shown in Fig. 5(c). This was fully restored in wt-5Rα−/− and partially restored in DC3-5Rα−/− mice. Cell size of B-1a cells was not restored in ApvA-5Rα−/−. These results imply that the ppvp motif of IL-5Rα is also essential for the maintenance of cell number and size of B-1a cells.
Figure 7.
Reconstitution of B-1a cells in cell size and number from 5Rα–/– mice by introduction of wt, but not of mutant IL-5Rα. (a) Expression of IL-5Rα on B cells in peritoneal washouts obtained from 8-week-old mice was examined by staining cells with FITC-conjugated RA3–6B2 (anti-B220 mAb) and biotinylated T21 (anti-IL-5Rα mAb) followed by streptavidin-PE. Histograms represent the IL-5Rα expression on B220+ B cells. Solid line represents the histogram of IL-5Rα–/– B cells. (b) Peritoneal washouts were stained with FITC-conjugated anti-IgM mAb and biotinylated anti-Ly-1 mAb followed by SA-PE. (c) Cell size of B-1a cells represented by forward scatter of a flow cytometric analysis. The gate was set on IgM+ CD5+ (B-1a) cells. Histograms represent the relative number and size of B-1a cells. Solid line indicates the relative cell number and size of 5Rα–/– B-1a cells.
Discussion
Various cytokines stimulate not only proliferation but also differentiation of their target cells. Reconstitution studies using cloned receptor cDNAs have revealed that most receptors can mediate cytokine-triggered cellular proliferation and differentiation.40 It still remains unclear whether growth and differentiation signals can be transduced through distinct domains of the cytoplasmic regions of cytokine receptors. It is interesting to note that growth and differentiation signals have been reported to be mediated by different regions in the cytoplasmic domain of the granulocyte colony-stimulating factor receptor.41
Several groups, including us, expressed the cloned cytokine receptor in FDC-P1 that is an IL-3/GM-CSF-dependent murine myeloid precursor cell line. In all cases, the respective cytokine receptor could transduce the growth signal into cells in a cytokine-dependent manner. We have analysed functional domains in the IL-5Rα cytoplasmic region using in vitro FDC-P1 transfectants with a mutant IL-5Rα. Our results revealed that the two cytoplasmic regions of IL-5Rα, the membrane-proximal proline-rich region (DC1) containing the Pro352-Pro353-X-Pro355 (ppvp) motif and downstream of the proline-rich region (DC2), are essential for association with and activation of JAK2 and STAT5 and, consequently, downstream IL-5 signal transduction for cell proliferation.29 In contrast, the C-terminal regions (DC3 and DC4) unique to IL-5Rα appear not to be essential for IL-5-mediated B-cell proliferation, although DC3 and DC4 mutants of FDC-P1 requires higher amounts of IL-5 to induce a maximum proliferative response. We also showed that one of three proline residues in the ppvp motif is indispensable and Pro352 and Pro355 play more critical roles in IL-5-dependent cell proliferation than does Pro353. However, these approaches could not provide insights into the functions of the ppvp motif and the C-terminal regions in B-cell differentiation.
To overcome this problem, we used BCL1 cells that express high-affinity IL-5Rα and respond to IL-5, but not to IL-3, resulting in IgM-secreting cells. Enforced expression of IL-3Rα in BCL1 enabled them respond to IL-3 for IgM secretion.34 We established BCL1 transfectants expressing 3R, 3/5R(wt) and 3/DC1 through 3/DC4. Each group of BCL1 transfectants expressed relevant IL-3Rα to a similar extent (data not shown). All BCL1 transfectants showed IL-5-driven IgM secretion with a slight variability (Fig. 1b). Stimulation indices (IL-5-driven IgM versus IgM in medium control) of 3/DC1, 3/DC2, 3/DC3 and 3/DC4 were 7·4, 6·4, 5·6 and 4·2, respectively. Although 3/DC1 transfectants produced relatively lower IgM in response to IL-5, the 3/DC1 receptor expression on the transfectants was stable. Furthermore, the βc expression was not altered by transfection of the 3/DC1 gene. Moreover, 3/DC1 transfectants proliferated and survived in a similar manner to other transfectants. These results suggest that lower levels of IL-5-driven IgM secretion by transfectants expressing chimaeric 3/5R such as 3/DC1 do not reflect the inherent toxicity of chimaeric receptors or an enhanced rate of turnover.
The 3/5R(wt) and 3/DC4 transfectants mounted IL-3-driven IgM secretion, while the 3/DC3 transfectants showed a significantly lower IL-3-stimulated IgM secretion than 3/DC4 transfectants. Stimulation indices of IL-3-driven IgM secretion, of 3/5R(wt), 3/DC3 and 3/DC4 were 4·0, 1·2 and 3·6, respectively. These results suggest that lack of the DC3 region of IL-5Rα affects IL-5-driven IgM secretion in BCL1 cells. Since 3/DC3 transfectants grew autonomously, the addition of IL-3 to the culture did not affect their cell growth (data not shown). The explanation for the slight variability in the IL-5 or IL-3 data may lie in part with the fact that ectopic expression of 3/DC transgene might compete for available βc with IL-5Rα, thus affecting the response to IL-5 or IL-3.
We generated wt-5Rα−/−, DC3-5Rα−/− and ApvA- 5Rα−/− mice by means of crossing IL-5Rα-Tg, DC3-Tg and ApvA-Tg mice with IL-5Rα null mutant mice. In each group of mice, peripheral B cells expressed IL-5Rα with higher density than that of wt mouse. Among wt-5Rα−/−, DC3-5Rα−/− and ApvA-5Rα−/− lines, there were no significant differences regarding cellularity of peripheral blood, bone marrow and spleen cells. Splenic B cells of ApvA-5Rα−/− failed to respond with IL-5 for proliferation, and IgM and IgG1 secretion (Figs 3 and 4). CS/2-stimulated splenic B cells from ApvA-5Rα−/− mouse expressed a higher density of relevant IL-5Rα than did wt-5Rα−/− B cells and responded to LPS to a similar extent to the wt-5Rα−/− B cells. We infer from these results that ApvA-5Rα is apparently defective in transmitting most IL-5 signals. Intriguingly, B cells in the IL-5Rα null mouse expressing the DC3 gene (DC3-5Rα−/−) responded well to IL-5 for proliferation (Fig. 3). DC3-5Rα−/− B cells seemed to produce low levels of IgM compared with wt B cells (statistically not significant) following stimulation with CS/2 plus IL-5 (Fig. 4a). In other experiments, DC3-5Rα−/− B cells produced IgM to a similar extent to wt B cells. DC3-5Rα−/− B cells produced significantly and reproducibly lower IgG1 in response to CS/2 plus IL-5 than wt-5Rα−/− B cells (Fig. 4a). This was further confirmed by a two-step culture system (Fig. 4b right panel) in which IgM production by DC3-5Rα−/− B cells was equivalent to that of wt-5Rα−/− B cells (Fig. 4b. left panel).
DC3-5Rα−/− B cells showed higher expression of cell surface DC3-5Rα than wt-5Rα on wt-5Rα−/− B cells (Fig. 2b) and proliferated better in response to IL-5 than wt-5Rα−/− B cells (Fig. 3). We attempted to analyse various DC3-5Rα−/− lines whose DC3-5Rα expressions were variable, but as a whole we could examine only one line of DC3-5Rα−/− mice. To examine effects of the overexpression of DC3-5Rα in the transgenic mice on suppression of the µ to γ1 switching by the exaggerated proliferative signals, we generated a DTG-5Rα−/− line whose 5Rα expression on B cells was comparable to that of DC3-5Rα−/− B cells (Fig. 5a). This gave no indication of the relative proportions or densities of wt-5Rα−/− and DC3-5Rα−/− species. DTG-5Rα−/− B cells responded to IL-5 to induce a proliferative response better than DC3- 5Rα−/− B cells (Fig. 5b). Intriguingly, stimulation of DTG-5Rα−/− B cells with CS/2 plus IL-5 induced a higher level of IgG1 production than that found in DC3-5Rα−/− B cells and a lower level than in wt-5Rα−/− B cells (Fig. 5c). We infer from these results a partial, rather than a complete, restorative effect for IgG1 secretion in DTG3-5Rα−/− B cells. This might be explained by limiting levels of the βc subunit.
As IL-5-induced IgG1 production by CS/2-stimulated B cells accompanies IgH switch recombination from µ to γ1,39 the DC3 region may be required for inducing IgH switch recombination. We examined frequencies of µ to γ1 switch recombination in B cells stimulated with CS/2 plus IL-5. As shown in Fig. 6, DC3-5Rα−/− B cells showed significantly lower frequencies of µ to γ1 switch recombination than wt-5Rα−/− B cells. These results support the notion that distinct subregions of the cytoplasmic domain of IL-5Rα play a role in B-cell growth when compared to differentiation. The C-terminal DC3 region unique to IL-5Rα and distinct from IL-3Rα and GM-CSFRα may play important roles in transducing IL-5 signal for µ to γ1 switch recombination and IgG1 production.
B-1 cells can be distinguished from conventional B (B-2) cells by their surface antigen expression, anatomical localization, self-renewing activity, expression of distinct VH phenotype, activation status of STAT3 and Btk requirement for their development.42–52 In the mouse, the subset of self-renewing B-1 cells is categorized as either B-1a cells, which express CD5, or B-1b cells, which are very similar to B-1a cells but do not express CD5.46 Controlling the numbers of these intrinsically activated, self-regenerating B-1 cells may regulate the level of natural antibodies. As we reported, IL-5Rα is highly expressed on all B-1 cells in the peritoneal cavity and to a lesser extent on a small proportion of splenic B cells.13,53 IL-5-Tg mice have propagated B-1a cells in the spleen.17,54 The IL-5Rα−/− mice show a decrease in B-1 cells from the peritoneal cavity and lamina propria.35,55 Moreover, sIgA+ B-1 cells from the effector site, but not sIgA+ B-2 cells from the inductive site, were significantly reduced in the effector tissue of IL-5Rα−/− and IL-5−/− mice.55,56 These results suggest that IL-5 is a preferred and important cytokine for B-1-cell development and maintenance. In this report, we demonstrated that B-1 cells in IL-5Rα−/− mice were smaller in size (Fig. 7). Furthermore, the cytoplasmic domain of IL-5Rα, particularly the ppvp motif, was important in the maintenance of total cell number and size of B-1 cells. Taking account of all these results, the IL-5 and IL-5R system plays an important role in the expansion of progenitor cells already committed to differentiate into B-1 cells and in B-1-cell activation. It is not clear what molecular mechanisms are underlined in B-1-cell development and how IL-5 maintains the number and size of B-1 cells.
An inherent problem of transgenic studies is the dependency of transcription on the location of transgene integration. Some results could therefore be explained by transcriptional differences. In conclusion, we demonstrated using mice which each expressed a mutant form of an IL-5Rα transgene in IL-5Rα null background that the ppvp motif of IL-5Rα is essential for IL-5-induced proliferation and differentiation of B-2 cells and B-1-cell development in vivo. In contrast, the IL-5Rα C-terminal region, in particular the DC3 subregion, is important for both IL-5-induced IgM production and the µ to γ1 switch recombination in B-2 cells, but is dispensable for IL-5-induced B-cell proliferation.
Acknowledgments
We are grateful to Drs A. Miyajima, K. Miyake, and T. Yokota for providing valuable reagents and to Mr G. Maxwell for critical reading of the manuscript. This study was supported in part by a special grant, the Priority Core Research Grant on Molecular Pathogenesis and Immunointervention of Immune Disorders, from the Ministry of Education, Science, Sports and Culture of Japan and by the research fund from the Meiji Milk Product Co. Ltd.
References
- 1.Arai KI, Lee F, Miyajima A, Miyatake S, Arai N, Yokota T. Cytokines: coordinators of immune and inflammatory responses. Annu Rev Biochem. 1990;59:289–300. doi: 10.1146/annurev.bi.59.070190.004031. [DOI] [PubMed] [Google Scholar]
- 2.Kishimoto T, Taga T, Akira S. Cytokine signal transduction. Cell. 1994;76:253–62. doi: 10.1016/0092-8674(94)90333-6. [DOI] [PubMed] [Google Scholar]
- 3.Paul WE, Seder RA. Lymphocyte responses and cytokines. Cell. 1994;76:241–50. doi: 10.1016/0092-8674(94)90332-8. [DOI] [PubMed] [Google Scholar]
- 4.Taniguchi T. Cytokine signaling through nonreceptor protein tyrosine kinases. Science. 1995;268:251–5. doi: 10.1126/science.7716517. [DOI] [PubMed] [Google Scholar]
- 5.Takatsu K, Tominaga A, Harada N, Matsumoto M, Takahashi T, Kikuchi Y, Yamaguchi N. T Cell-Replacing Factor (TRF) /Interleukin 5 (IL-5): Molecular and Functional Properties. Immunol Rev. 1988;102:107–35. doi: 10.1111/j.1600-065x.1988.tb00743.x. [DOI] [PubMed] [Google Scholar]
- 6.Takatsu K. Interleukin-5 receptor. Curr Opin Immunol. 1992;3:299–306. doi: 10.1016/0952-7915(92)90080-x. [DOI] [PubMed] [Google Scholar]
- 7.Sonoda E, Matsumoto R, Hitoshi Y, et al. Transforming growth factor beta induces IgA production and acts additively with interleukin 5 for IgA production. J Exp Med. 1989;170:1415–20. doi: 10.1084/jem.170.4.1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sanderson CJ, Campbell HD, Young IG. Molecular and cellular biology of eosinophil differentiation factor (interleukin-5) and its effects on human and mouse B cells. Immunol Rev. 1988;102:29–50. doi: 10.1111/j.1600-065x.1988.tb00740.x. [DOI] [PubMed] [Google Scholar]
- 9.Kantor AB. The development and repertoire of B-1 cells (CD5 B cells) Immunol Today. 1991;12:389–91. doi: 10.1016/0167-5699(91)90136-H. [DOI] [PubMed] [Google Scholar]
- 10.Herzenberg LA, Stall AM, Lalor PA, Sidman C, Moore WA, Parks DR, Herzenberg LA. The Ly-1 B cell lineage. Immunol Rev. 1986;93:81–102. doi: 10.1111/j.1600-065x.1986.tb01503.x. [DOI] [PubMed] [Google Scholar]
- 11.Hardy RR, Hayakawa K. CD5 B cells, a fetal B cell lineage. Adv Immunol. 1994;55:297–339. doi: 10.1016/s0065-2776(08)60512-x. [DOI] [PubMed] [Google Scholar]
- 12.Kocks C, Rajewsky K. Stable expression and somatic hypermutation of antibody V regions in B-cell developmental pathways. Annu Rev Immunol. 1989;7:537–59. doi: 10.1146/annurev.iy.07.040189.002541. [DOI] [PubMed] [Google Scholar]
- 13.Hitoshi Y, Yamaguchi N, Mita S, Sonoda E, Takaki S, Tominaga A, Takatsu K. Distribution of IL-5 receptor-positive B cells. Expression of IL-5 receptor on Ly-1 (CD5)+ B cells. J Immunol. 1990;144:4218–25. [PubMed] [Google Scholar]
- 14.Takatsu K, Takaki S, Hitoshi Y. Interleukin-5 and its receptor system: implications of the immune system and inflammation. Adv Immunol. 1994;57:145–90. doi: 10.1016/s0065-2776(08)60673-2. [DOI] [PubMed] [Google Scholar]
- 15.Matsumoto R, Matsumoto M, Mita S, et al. Interleukin-5 induces maturation but not class-switching of surface IgA-positive B cells into IgA-secreting cells. Immunology. 1989;66:32–8. [PMC free article] [PubMed] [Google Scholar]
- 16.Yasue T, Nishizumi H, Aizawa S, et al. A critical role of Lyn and Fyn for B cell responses to CD38 ligation and interleukin 5. Proc Natl Acad Sci USA. 1997;94:10307–11. doi: 10.1073/pnas.94.19.10307. 10.1073/pnas.94.19.10307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tominaga A, Takaki S, Koyama N, et al. Transgenic mice expressing a B cell growth and differentiation factor gene (interleukin 5) develop eosinophilia and autoantibody production. J Exp Med. 1991;173:429–37. doi: 10.1084/jem.173.2.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mita S, Tominaga A, Hitoshi Y, et al. Characterization of high-affinity receptors for interleukin 5 on interleukin 5-dependent cell lines. Proc Natl Acad Sci USA. 1989;86:2311–15. doi: 10.1073/pnas.86.7.2311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yamaguchi N, Hitoshi Y, Mita S, Hosoya Y, Murata Y, Kikuchi Y, Tominaga A, Takatsu K. Characterization of the murine interleukin 5 receptor by using a monoclonal antibody. Int Immunol. 1990;2:181–7. doi: 10.1093/intimm/2.2.181. [DOI] [PubMed] [Google Scholar]
- 20.Hitoshi Y, Yamaguchi N, Korenaga M, Mita S, Tominaga A, Takatsu K. In vivo administration of antibody to murine IL-5 receptor inhibits eosinophilia of IL-5 transgenic mice. Int Immunol. 1991;3:135–9. doi: 10.1093/intimm/3.2.135. [DOI] [PubMed] [Google Scholar]
- 21.Murata Y, Takaki S, Migita M, Kikuchi Y, Tominaga A, Takatsu K. Molecular cloning and expression of the human interleukin 5 receptor. J Exp Med. 1992;175:341–51. doi: 10.1084/jem.175.2.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Takaki S, Tominaga A, Hitoshi Y, Mita S, Sonoda E, Yamaguchi N, Takatsu K. Molecular cloning and expression of the murine interleukin-5 receptor. EMBO J. 1990;9:4367–74. doi: 10.1002/j.1460-2075.1990.tb07886.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Takaki S, Mita S, Kitamura T, Yonehara S, Yamaguchi N, Tominaga A, Miyajima A, Takatsu K. Identification of the second subunit of the murine interleukin-5 receptor: interleukin-3 receptor-like protein, AIC2B is a component of the high affinity interleukin-5 receptor. EMBO J. 1991;10:2833–8. doi: 10.1002/j.1460-2075.1991.tb07832.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Takaki S, Murata Y, Kitamura T, Miyajima A, Tominaga A, Takatsu K. Reconstitution of the functional receptors for murine and human interleukin 5. J Exp Med. 1993;177:1523–9. doi: 10.1084/jem.177.6.1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miyajima A, Mui A, Ogorochi T, Sakamaki K. Receptors for granulocyte-macrophage-colony stimulating factor, interleukin-3, and interleukin-5. Blood. 1993;82:1960–8. [PubMed] [Google Scholar]
- 26.Ogata N, Kouro T, Yamada A, Koike M, Hanai N, Ishikawa T, Takatsu K. JAK2 and JAK1 constitutively associate with an interleukin-5 (IL-5) receptor alpha and beta c subunit, respectively, and are activated upon IL-5 stimulation. Blood. 1998;91:2264. [PubMed] [Google Scholar]
- 27.Sato S, Katagiri T, Takaki S, et al. IL-5 receptor-mediated tyrosine phosphorylation of SH2/SH3-containing proteins and activation of Bruton's tyrosine and Janus 2 kinases. J Exp Med. 1994;180:2101–10. doi: 10.1084/jem.180.6.2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Alam R, Pazdrak K, Stafford S, Forsythe P. The interleukin-5/receptor interaction activates Lyn and Jak2 tyrosine kinases and propagates signals via the Ras-Raf-1-MAP kinase and the Jak-STAT pathways in eosinophils. Int Arch Allergy Immunol. 1995;107:226–8. doi: 10.1159/000236985. [DOI] [PubMed] [Google Scholar]
- 29.Takaki S, Kanazawa H, Shiiba M, Takatsu K. A critical cytoplasmic domain of the interleukin-5 (IL-5) receptor alpha chain and its function in IL-5-mediated growth signal transduction [see comments] Mol Cell Biol. 1994;14:7404–13. doi: 10.1128/mcb.14.11.7404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kouro T, Kikuchi Y, Kanazawa H, et al. Critical proline residues of the cytoplasmic domain of the IL-5 receptor alpha chain and its function in IL-5-mediated activation of JAK kinase and STAT5. Int Immunol. 1996;8:237–45. doi: 10.1093/intimm/8.2.237. [DOI] [PubMed] [Google Scholar]
- 31.Harada N, Takahashi T, Matsumoto M, et al. Production of a monoclonal antibody useful in the molecular characterization of murine T-cell-replacing factor/B-cell growth factor II. Proc Natl Acad Sci USA. 1987;84:4581–5. doi: 10.1073/pnas.84.13.4581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yamashita Y, Miyake K, Kikuchi Y, Takatsu K, Noda S, Kosugi A, Kimoto M. A monoclonal antibody against a murine CD38 homologue delivers a signal to B cells for prolongation of survival and protection against apoptosis in vitro: unresponsiveness of X-linked immunodeficient B cells. Immunology. 1995;85:248–55. [PMC free article] [PubMed] [Google Scholar]
- 33.Higuchi R. Recombinant PCR. In: Innis MA, Geldand ADH, Shinski JJ, White TJ, editors. PCR Protocols, a Guide to Methods and Application. San Diego: Academic Press; 1990. pp. :177–83. [Google Scholar]
- 34.Shiiba M, Takaki S, Takatsu K. Interleukin-3 (IL-3) and granulocyte-macrophage colony-stimulating factor (GM-CSF) can induce differentiation of chronic B cell leukemia expressing the alpha subunit of IL-3 and GM-CSF receptor. Int Arch Allergy Immunol. 1996;111:12–15. doi: 10.1159/000237406. [DOI] [PubMed] [Google Scholar]
- 35.Yoshida T, Ikuta K, Sugaya H, et al. Defective B-1 cell development and impaired immunity against Angiostrongylus cantonensis in IL-5R alpha-deficient mice. Immunity. 1996;4:483–94. doi: 10.1016/s1074-7613(00)80414-8. [DOI] [PubMed] [Google Scholar]
- 36.Koike M, Kikuchi Y, Tominaga A, Takaki S, Akagi K, Miyazaki J, Yamamura K, Takatsu K. Defective IL-5-receptor-mediated signaling in B cells of X-linked immunodeficient mice. Int Immunol. 1995;7:21–30. doi: 10.1093/intimm/7.1.21. [DOI] [PubMed] [Google Scholar]
- 37.Kikuchi Y, Yasue T, Miyake K, Kimoto M, Takatsu K. CD38 ligation induces tyrosine phosphorylation of Bruton tyrosine kinase and enhanced expression of interleukin 5-receptor alpha chain: synergistic effects with interleukin 5. Proc Natl Acad Sci USA. 1995;92:11814–18. doi: 10.1073/pnas.92.25.11814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Uehara S, Hitoshi Y, Numata F, Makino M, Howard M, Mizuochi T, Takatsu K. An IFN-gamma-dependent pathway plays a critical role in the pathogenesis of murine immunodeficiency syndrome induced by LP-BM5 murine leukemia virus. Int Immunol. 1994;6:1937–47. doi: 10.1093/intimm/6.12.1937. [DOI] [PubMed] [Google Scholar]
- 39.Mizoguchi C, Uehara S, Akira S, Takatsu K. IL-5 induces IgG1 isotype switch recombination in mouse CD38-activated sIgD-positive B lymphocyte. J Immunol. 1999;162:2812–19. [PubMed] [Google Scholar]
- 40.Ihle JN. Cytokine receptor signaling. Nature. 1995;377:591–4. doi: 10.1038/377591a0. [DOI] [PubMed] [Google Scholar]
- 41.Fukunaga R, Ishizaka-Ikeda E, Nagata S. Growth and differentiation signals mediated by different regions in the cytoplasmic domain of granulocyte colony-stimulating factor receptor. Cell. 1993;74:1079–87. doi: 10.1016/0092-8674(93)90729-a. [DOI] [PubMed] [Google Scholar]
- 42.Hayakawa K, Hardy RR, Parks DR, Herzenberg LA. The ‘Ly-1B’ cell subpopulation in normal immunodeficient, and autoimmune mice. J Exp Med. 1983;157:202–18. doi: 10.1084/jem.157.1.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Waldschmidt TJ, Kroese FG, Tygrett IT, Conrad DH, Lynch RJ. The expression of B cell surface receptors. III. The murine low-affinity IgE Fc receptor is not expressed on Ly 1 or ‘Ly 1-like’ B cells. Int Immunol. 1991;3:305–15. doi: 10.1093/intimm/3.4.305. [DOI] [PubMed] [Google Scholar]
- 44.Hayakawa K, Hardy RR, Honda M, Herzenberg LA, Steinberg SD, Herzenberg LA. Ly-1 B cells: functionally distinct lymphocytes that secrete IgM autoantibodies. Proc Natl Acad Sci USA. 1984;81:2494–8. doi: 10.1073/pnas.81.8.2494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mercolino TJ, Arnold LW, Haughton G. Phosphatidylcholine is recognized by a series of Ly-1+ murine B cell lymphomas specific for erythrocyte membranes. J Exp Med. 1986;163:155–65. doi: 10.1084/jem.163.1.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tornberg UC, Holnberg D. B-1a and B-1b and B-2 cells display unique VHDHJH repertoires formed at different stages of ontogeny and under different selection pressures. EMBO J. 1995;14:1680–9. doi: 10.1002/j.1460-2075.1995.tb07157.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Murakami M, Tsubata T, Okamoto M, Shimizu A, Kumagai S, Imura H, Honjo T. Antigen-induced apoptotic cell death of Ly-1 B cells responsible for autoimmune disease in transgenic mice. Nature. 1992;357:77–80. doi: 10.1038/357077a0. [DOI] [PubMed] [Google Scholar]
- 48.Pillai S. The chosen few? Positive selection and the generation of naive B lymphocytes. Immunity. 1999;10:493–502. doi: 10.1016/s1074-7613(00)80049-7. [DOI] [PubMed] [Google Scholar]
- 49.Clarke SH, Arnold LW. B-1 cell development: evidence for an uncommitted immunoglobulin (Ig) M+ B cell precursor in B-1 cell deficiency. J Exp Med. 1998;187:1325–34. doi: 10.1084/jem.187.8.1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Karras JG, Wang Z, Huo L, Howard RG, Frank DA, Rothstein TL. Signal transduction and activator of transcription-3 (STAT3) is constitutively activated in normal, self-renewing B-1 cells, but only inducibly expressed in conventional B lymphocytes. J Exp Med. 1997;185:1035–42. doi: 10.1084/jem.185.6.1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Khan WN, Alt FW, Gerstein RM, et al. Defective B cell development and function in Btk-deficient mice. Immunity. 1995;3:283–99. doi: 10.1016/1074-7613(95)90114-0. [DOI] [PubMed] [Google Scholar]
- 52.Kerner JD, Appleby MW, Mohr RN, Chien S, Rawlings DJ, Maliszewski CR, Witte ON, Perlmutter RM. Impaired expansion of mouse B cell progenitors lacking Btk. Immunity. 1995;3:301–12. doi: 10.1016/1074-7613(95)90115-9. [DOI] [PubMed] [Google Scholar]
- 53.Takatsu K, Takaki S, Hitoshi Y, Mita S, Katoh S, Yamaguchi N, Tominaga A. Cytokine receptors on Ly-1 B cells. IL-5 and its receptor system. Ann New York Acad Sci. 1992;651:241–58. doi: 10.1111/j.1749-6632.1992.tb24619.x. [DOI] [PubMed] [Google Scholar]
- 54.Katoh S, Bendig MM, Kanai Y, Shultz LD, Hitoshi Y, Takatsu K, Tominaga A. Maintenance of CD5+ B cells at an early developmental stage by interleukin-5: evidence from immunoglobulin gene usage in interleukin-5 transgenic mice. DNA Cell Biol. 1993;12:481–91. doi: 10.1089/dna.1993.12.481. [DOI] [PubMed] [Google Scholar]
- 55.Hiroi T, Yanagida M, Iijima H, Iwatani K, Yoshida T, Takatsu K, Kiyono H. Deficiency of IL-5 receptor alpha-chain selectively influences the development of the common mucosal immune system independent IgA-producing B-1 cell in mucosa-associated tissues. J Immunol. 1999;162:821–8. [PubMed] [Google Scholar]
- 56.Bao S, Beagley KW, Murray AM, Caristo V, Matthaei KI, Young IG, Husband AJ. Intestinal IgA plasma cells of the B1 lineage are IL-5 dependent. Immunology. 1998;94:181–8. doi: 10.1046/j.1365-2567.1998.00512.x. [DOI] [PMC free article] [PubMed] [Google Scholar]