Abstract
Binding of mannose-binding lectin (MBL), a C-type lectin, and its associated serine proteases, MASP-1 and MASP-2, to cell surface carbohydrates activates the lectin complement pathway. As MBL plays an important role in innate immunity, it has been cloned and characterized in several species. While the pig may be used as a source of organs/tissues for xenotransplantation, little is known about its MBL, thus, we report the isolation of three monomeric forms of MBL from porcine serum. Sodium dodecyl sulphate–polyacrylamide gel electrophoresis and Coomassie staining of reduced porcine MBL revealed the presence of three monomeric forms with approximate molecular masses of 30 000, 32 000 and 34 000. Protein sequencing identified these monomeric forms as one single protein, suggesting post-translational modification. Western blot analysis demonstrated the cross-reactivity of anti-human MBL polyclonal antibody with porcine MBL. A full-length porcine liver MBL cDNA was isolated and the predicted amino acid sequence exhibited 64·9% identity with human MBL and 50·2% and 56·7% identity with rat A and C MBL, respectively. Furthermore, Northern blot analysis demonstrated the presence of a single (∼1·4–1·6 kilobase pair) transcript in porcine liver. Addition of purified porcine MBL to MBL-deficient human sera augmented N-acetylglucosamine inhibitable C3 deposition to mannan-coated plates in a dose-dependent manner. Taken together, these data demonstrate that porcine and human MBL are highly conserved, sharing structural and functional characteristics.
Introduction
The human complement system is a host defence mechanism and can be activated by three separate pathways: the classical, the alternative and the lectin pathways. The classical pathway is activated following antibody–antigen interactions leading to deposition of C1q and the activation of C1s and C1r. The alternative pathway can be triggered by a number of mechanisms, such as haemodialysis, cardiopulmonary bypass, tissue plasminogen activator and yeast cell walls (zymosan). The lectin pathway is activated upon binding of mannose-binding lectin (MBL) and its two-associated serine proteases (i.e. MASP-1 and MASP-2) to a carbohydrate ligand which then cleave C2 and C4 to form the classical C3 convertase.1,2 The three pathways merge at C3 and cleavage of C3 culminates in the activation of the terminal complement pathway and ultimately in formation of the terminal complement complex (C5b-9).3
MBL is a C-type lectin and can specifically bind to terminal-reducing sugars such as N-acetylglucosamine (GlcNAc), mannose, fructose and glucose present on micro-organisms. Human MBL circulates in the blood as large (200 000–650 000 MW) homo-oligomers consisting of 9–18 monomers of about 32 000 MW each. Each monomer consists of an amino-terminal region rich in cysteine residues, a collagen-like domain composed of 18–20 tandem repeats of a Gly-X-Y sequence, a neck region and a carboxy-terminal carbohydrate recognition domain region.4–6 Human MBL is present in a single form. However, it has been reported that rats and mice present two structurally and functionally distinct MBL proteins, MBL-A and MBL-C.6,7 MBL is believed to be an important constituent of innate immunity, with deficiencies in MBL being associated with recurrent infections in infants.8
While MBL is known to have a wide array of binding activity to carbohydrate moieties on micro-organisms, lectins are quite selective in respect to host glycoproteins. The pig may, in the near future, be a source of xenotransplantable tissues/organ, yet little is known about porcine MBL. Thus, to further the characterization of this interesting animal lectin, we have characterized porcine MBL protein and cDNA (GenBank accession number: AF164576). Porcine and human MBL display striking homology at the structural and functional levels.
Materials and methods
Purification of MBL
MBL was purified from human and porcine sera with modifications to the previously published method.9 Briefly, sera (1 l) were precipitated with 10% polyethylene glycol 3500 (w/v) and centrifuged at 10 000 g for 10 min. The pellet was suspended in 750 ml of ice-cold TBS-TCa2+ (50 mmol/l Tris; 150 mmol/l NaCl; 0·05% Tween-20; 20 mmol/l CaCl2, pH 7·8). Following centrifugation at 10 000 g for 5 min, the supernatant was loaded onto a mannan–Sepharose column (Sigma, St Louis, MO) equilibrated with TBS-TCa2+. Following extensive washing, the proteins were eluted with phosphate-buffered saline (PBS) -TEDTA (50 mmol/l Tris; 150 mmol/l NaCl; 0·05% Tween-20; 10 mmol/l ethylenediaminetetraacetic acid, pH 7·8). The eluate was calcified (40 mmol/l) and loaded onto a maltose column (5 ml column volume); washed extensively with TBS-TCa2+ and the bound proteins were eluted with GlcNAc (100 mmol/l) in TBS-TCa2+. Immunoglobulin G (IgG) or IgM contamination was observed by Western blot analysis in the porcine, but not human MBL preparations. Porcine IgG and IgM contamination was removed by affinity chromatography using rabbit polyclonal anti-porcine IgG and IgM columns.
MBL-deficient human sera
Human serum deficient in MBL was made as previously described.10 Briefly, human serum was treated with phenylmethylsulphonyl fluoride (2 mmol/l) and loaded onto a mannan–Sepharose column. The eluate, MBL-deficient human serum, was collected and dialysed against Hanks' buffered salt solution containing calcium and magnesium, overnight at 4°. MBL-deficient human serum induced lysis of sensitized chicken red blood cells, thus demonstrating an intact classical complement pathway.10
Western blot analysis
Polyclonal antibodies against human MBL were raised by immunizing rabbits with subcutaneous purified human MBL (50 µg MBL in Titermax, Sigma). After several rounds of immunization, serum from a rabbit (R2.2) were collected 10 days after a final immunization and rabbit IgG antibodies were purified by protein G chromatography.
Porcine and human MBL were fractionated on a 9% polyacrylamide gel under non-reducing conditions. A broad-range protein standard (Bio-Rad, Hercules, CA) was used to establish the relative molecular weights of porcine and human MBL. The proteins were transferred onto nitrocellulose and the membrane was blocked with 10% non-fat dry milk (NFDM) in PBS-TB (PBS containing 0·1% Tween-20 and 0·1% bovine serum albumin) overnight. The membrane was then incubated with horseradish peroxidase-conjugated anti-human MBL polyclonal antibody (1 : 5000 dilution) in 3% NFDM in PBS-TB for 1 hr at 4°. The membrane was washed with PBS-TB and developed with the SuperSignal system (Pierce, Rockford, IL) and X-ray films (Kodak).
Peptide sequencing and mass spectroscopy
Porcine MBL (10 µg) was resolved on a 9% polyacrylamide gel under reducing conditions. Three Coomassie-stained protein bands with approximate molecular masses of 30 000, 32 000 and 34 000 were excised and subjected to internal peptide sequencing (Harvard Microchemistry Facility, Boston, MA), by microcapillary reverse-phase high-performance liquid chromatography (HPLC) nano-electrospray tandem mass spectroscopy (µlC/MS/MS) on a Finnigan LCQ quadrupole ion trap mass spectrometer (Finnigan, Piscataway, NJ).
Porcine MBL cDNA isolation
Reverse transcription–polymerase chain reaction (RT-PCR) amplification was utilized to determine the presence of a MBL transcript in porcine liver tissue. Briefly, total RNA was purified from freshly isolated porcine liver tissue (Strategene, La Jolla, CA). Purified RNA was used as template for oligo-dT primed reverse transcription using the Access RT-PCR System (Promega, Madison, WI). Oligonucleotides for RT-PCR reactions were designed based on the conserved regions of human and bovine MBL cDNA sequences (GenBank accession numbers NM000587 and D73408). The forward (5′-tttgtggatctgacaggaaagggggtg-3′) and reverse (5′-ggagaaaagggagaaccaggtcaagga-3′) primers were synthesized. The reverse transcribed cocktail (2 µl) was used as a template for PCR amplification using 10 pmol of each primer, 1 unit of DNA Taq polymerase, 10 µmol/l of each dNTP and 5 µl of 10 × PCR buffer containing MgCl2 (total volume of 50 µl) added to the reaction mixture. PCR amplification was performed using the following conditions: a single cycle of 94° for 90 seconds followed by 30 cycles of 94° for 1 min, 55° for 2 min and 72° for 2 min and a final extension cycle of 72° for 10 min. PCR bands of the predicted size (based on human MBL sequence) were excised from low-melting-point agarose and ligated into the PGEM-T vector (Promega). Several colonies were selected and the purified plasmids were sequenced using an Automated Fluorescence DNA Sequencer (Children's Hospital Core Facility, Boston, MA). This strategy resulted in isolation of an internal 370-base pair (bp) fragment, thus necessitating application of 5′ and 3′ rapid amplification of cDNA ends (RACE). The Marathon Amplification Kit (Clontech, Palo Alto, CA) was used to obtain the 5′ and 3′ ends of the porcine liver MBL cDNA. Poly(A)+ liver RNA (1 µg) was used as the starting material for cDNA synthesis and the RACE was carried out as described by the manufacturer using 5′-ggagaaaagggagaaccaggtcaaggact-3′ and 5′-tcaccccctttcctgtcagatccacaaa-3′ as 5′ and 3′ porcine liver MBL cDNA-specific primers, respectively.
Northern blot analysis
RNA was extracted from frozen tissue and Northern blot analysis was performed according to standard procedures. Total RNA (10 µg) was resolved on a 0·9% agarose−2·2 mol/l formaldehyde gel. After hydrolysis (50 mm NaOH/10 mmol/l NaCl and neutralization in 10 mmol/l Tris–HCl, pH 7·5) RNA was transferred to a nylon membrane overnight by capillary transfer in 2 ×SSC (0·3 mol/l NaCl, 30 mmol/l sodium citrate, pH 7·0). Prehybridization was carried out in 50% formamide, 6 ×SSC, 5 ×Denhardt's solution, 1% sodium dodecyl sulphate (SDS) and 150 µg/ml of non-denatured and 150 µg/ml of denatured fish sperm DNA (Boehringer Mannheim, Germany) for 3–4 hr at 42°. A dimer of the 370-bp fragment of porcine MBL cDNA was radiolabelled using the Prime-a-Gene labelling system (Promega) and purified sequentially through two Sephadex G-50 spin columns. The probe (5 ng/ml at 2 × 109 counts per min/µg) was denatured by boiling and was hybridized overnight at 42°. The blot was washed twice in 2 ×SSC/0·1% SDS for 10 min at room temperature, followed by two washes in 0·2 SSC/0·1% SDS for 10 min at room temperature. The blot was exposed to Kodak MS autoradiography film and developed.
MBL-dependent C3 deposition enzyme-linked immunosorbent assay (ELISA)
Radioimmunoassay 96-well plates were coated with 100 µl of 0·5 mg/ml mannan in sodium carbonate/bicarbonate buffer pH 9·6 and incubated overnight at 4° as described. Plates were then washed three times with PBS buffer containing 0·5% Tween-20 and washed once with PBS buffer followed by a second wash with veronal-buffered saline (VBS: 141 mmol/l NaCl and 1·8 mmol/l sodium barbital). Five per cent MBL-deficient human serum containing purified porcine MBL (0–250 ng/ml; 100 µl) or human MBL (0–60 ng/ml; 100 µl) was added to each well and incubated for 0·5, 1, or 2 hr at 37°. Plates were then washed four times with PBS buffer containing 0·5% Tween-20. Human C3 deposition was detected by addition of 50 µl of 1 : 3000 dilution of horseradish peroxidase-conjugated goat anti-human C3 (ICN, Costa Mesa, CA) to each well and incubated at 20° for 1 hr. The plates were washed four times and 50 µl of 2,2′-azino-bis(3-ethylbenzthiazoline-6-sulphonic) acid (ABTS) was added to each well and the plates were read at 405 nm using a microplate reader (Molecular Devices, Sunnyvale CA). GlcNAc (50 mmol/l), an inhibitor of the lectin complement pathway, was added to some wells to inhibit lectin pathway activation. Non-specific background absorbance consisted of wells containing VBS buffer without sera. All ELISA experiments were performed in triplicate.
Results
Purification of porcine MBL
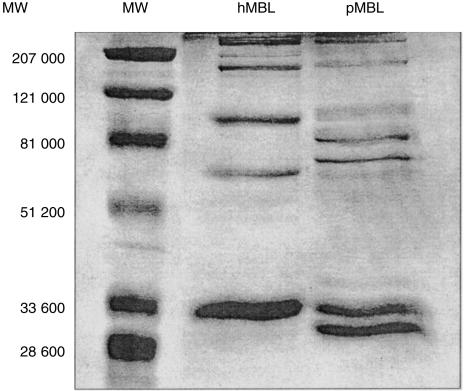
Purified porcine (2 µg) and human (2 µg) MBL were fractionated on a 9% SDS–polyacrylamide gel electrophoresis (PAGE) under partially reduced conditions (Fig. 1). Coomassie staining of human MBL demonstrated monomeric (32 000), dimeric (64 000), trimeric (96 000) and higher molecular mass forms of human MBL. Porcine MBL demonstrated three monomeric forms with approximate molecular masses of 30 000, 32 000, and 34 000.
Figure 1.
SDS–PAGE analysis of human and porcine MBL. Protein samples (2 µg each) were analysed under reduced conditions on a 9% polyacrylamide gel: molecular weight marker (MW); human MBL (hMBL); porcine MBL (pMBL).
Purified porcine (1 µg) and human (500 ng) MBL were fractionated on a 9% SDS–PAGE under non-reduced conditions (Fig. 2). Rabbit anti-human polyclonal antibody (R2.2) recognized non-reduced human and porcine MBL by Western blot analysis. Cross-reactivity of the anti-human polyclonal antibody confirmed the presence of similar epitopes between porcine and human MBL.
Figure 2.
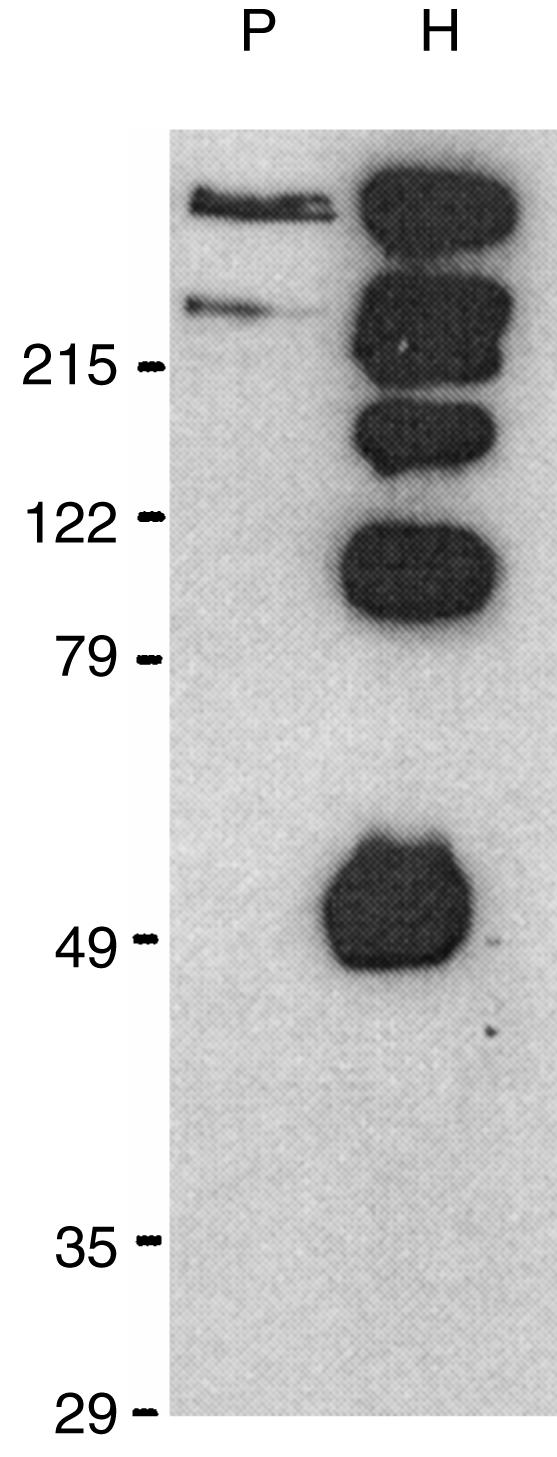
Western blot analysis of affinity-purified porcine MBL. Human (H; 500 ng) and porcine MBL (P; 1 µg) were resolved on a 9% gel via SDS–PAGE under non-reducing conditions, transferred onto a nitrocellulose membrane and reacted with horseradish peroxidase-conjugated rabbit anti-human MBL polyclonal antibody (R2.2).
Cloning of porcine MBL cDNA
In order to clone porcine MBL, an initial RT-PCR reaction was carried out on porcine liver mRNA using oligonucleotides based on the conserved cDNA regions of human and bovine MBL cDNA sequence. A 370-bp PCR band was obtained which, following nucleotide sequencing was positively identified as a MBL homologue. This PCR product corresponded to position 300–670 bp of human MBL cDNA. A full-length porcine liver MBL cDNA of 723 bp and an open reading frame of 241 amino acids was obtained (data not shown; GenBank accession number AF164576) using RACE. The deduced amino acid sequences of porcine and human MBL were aligned and demonstrated a high degree of identity (Fig. 3). Microcapillary reverse-phase HPLC nano-electrospray tandem mass spectroscopy (µlC/MS/MS) analysis confirmed that the three isoforms of pMBL present on the Coomassie-stained gel were the same protein, suggesting post-translational modification (see underlined residues in Fig. 3). Interestingly, the 30 000 band also contained a human MASP-2 homologue.
Figure 3.
Amino acid sequence alignment of human MBL (hMBL) and porcine MBL (pMBL). Shaded boxes represent amino acid residues that differ between porcine and human forms. Amino acid numbering is indicated to the right of the figure. Asterisks depict the position of cysteine residues and underlined residues represent the sequenced porcine peptide fragments.
Northern blot analysis
To investigate the presence of porcine MBL transcript(s), liver poly(A)+ RNA was subjected to Northern blot analysis. Upon hybridization of liver mRNA with a 370-bp radiolabelled porcine MBL cDNA probe, a single transcript with an approximate molecular weight of 1·4–1·6 kbp was detected (Fig. 4).
Figure 4.

Northern blot analysis of porcine liver MBL. Total RNA (10 µg) was fractionated on a 0·9% formaldehyde gel, transferred onto a membrane and hybridized with a 370-bp porcine-specific cDNA probe. A single transcript was observed. Numbers to the right of the figure of MW markers in kilobases.
MBL-dependent C3 deposition ELISA
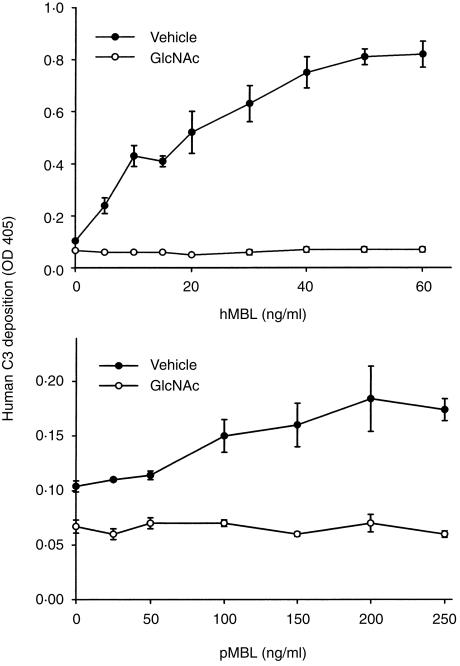
C3 deposition onto mannan-coated plates was measured by ELISA to evaluate whether porcine MBL could functionally restore lectin complement pathway activation in human MBL-deficient human sera. Addition of purified human MBL (top panel, Fig. 5) to MBL-deficient human sera restored C3 deposition in a dose-dependent manner. Maximum C3 deposition was obtained with approximately 50 ng/ml of human MBL and this is equivalent to the amount of human MBL present in human sera (1–2 µg/ml).2,11 Addition of purified porcine MBL (bottom panel, Fig. 5) was less effective than human MBL in restoring C3 deposition, but the effect was dose-dependent with maximum C3 deposition observed at approximately 200 ng/ml porcine MBL. Addition of GlcNAc (50 mmol/l) inhibited C3 deposition, demonstrating that porcine or human MBL can be inhibited by GlcNAc in this assay. Thus, these data indicate that purified porcine MBL functionally restores the lectin complement pathway in MBL-deficient human sera.
Figure 5.
Pharmacodynamics of porcine and human MBL. Various concentrations of purified human (top panel) or porcine (bottom panel) MBL were added to MBL-deficient human serum. Addition of human or porcine MBL increased C3 deposition in a dose-dependent manner. N-acetylglucosamine (GlcNAc; 50 mmol/l) inhibited C3 deposition. Values are mean±SEM of three separate experiments.
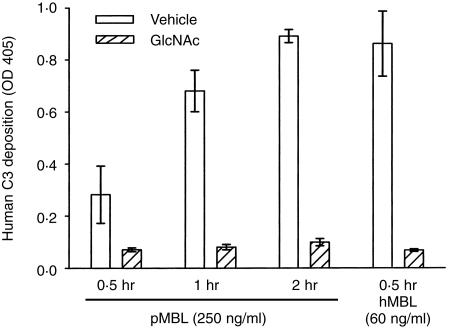
Since porcine MBL-induced C3 deposition was less, we speculated that pharmacokinetics may influence human C3 cleavage. As shown in Fig. 6, increasing the incubation period from 0·5 to 2 hr increased GlcNAc inhibitable C3 deposition. Equivalent C3 deposition was observed when MBL-deficient human serum was supplemented with human MBL (60 ng/ml) and incubated for 30 min compared with porcine MBL (250 ng/ml) supplementation and incubation for 1 or 2 hr. These data demonstrate that longer periods of incubation are required for efficient C3 deposition by porcine MBL-mediated human lectin pathway activation compared to the intact human system.
Figure 6.
Pharmacokinetics of porcine MBL. Porcine MBL (250 ng/ml) was added to MBL-deficient human sera and incubated on mannan-coated plates for 0·5, 1 or 2 hr and then analysed for human C3 deposition. C3 deposition increased with the incubation period and was similar to human MBL (60 ng/ml) incubated for 0·5 hr. N-acetylglucosamine (GlcNAc; 50 mmol/l) inhibited C3 at all time-points. Values are mean ± SEM of three separate experiments.
Discussion
MBL homologues have been identified in several species including monkey, cow, rabbit, rat and mouse.7,12–14 The primary sequence of human MBL has been elucidated and the four domains have been characterized based on their homology with other lectins.5 Unlike human MBL, two structurally and functionally distinct MBL isoforms, MBL-A and MBL-C, are known to be present in rats and mice.15 The ability of rat MBL-A to activate complement may be related to the cysteine content of the amino-terminal region of the MBL protein backbone.15 The amino terminus of MBL-C in rat and mouse has two cysteines, whereas MBL-A, human MBL and, as we now describe, porcine MBL have three cysteines. The presence of these cysteines allows the formation of functionally active multimeric MBL forms.15
Porcine liver MBL cDNA unveiled the striking overall structural homology of porcine MBL to human MBL. Porcine MBL shares an overall identity to rat MBL-A and -C proteins of 50·2% and 56·7%, respectively. Human (248 amino acids; Accession #4557739) and porcine MBL (241 amino acids) share an overall identity of 64·9% at the amino acid level (allowing for eight gaps). Thus, at the amino acid level, porcine MBL is more similar to human than to rat MBL. Purified porcine MBL functionally replaced human MBL and increased C3 deposition of MBL-deficient human sera in the mannan assay. C3 deposition was inhibited by GlcNAc, demonstrating that complement activation was MBL-dependent. Furthermore, these data demonstrate that GlcNAc can functionally inhibit porcine MBL.
SDS–PAGE demonstrated the presence of three monomeric forms of porcine plasma MBL under reducing conditions. The presence of three monomeric forms of porcine MBL supports the previously published observation by Storgaard and colleagues.16 However, microcapillary reverse-phase HPLC nano-electrospray tandem mass spectroscopy (µlC/MS/MS) analysis confirmed that the three isoforms of porcine MBL were the same protein, suggesting post-translational modification. These data are different from those presented by Storgaard and colleagues.16 Furthermore, we established the presence of a single (∼1·4–1·6 kbp) liver transcript. While we cannot categorically rule out the possible presence of another porcine cDNA for MBL, our data suggest that the three porcine monomeric isoforms isolated by our procedures are the same protein with post-translational modifications. We observed functional inhibition of porcine MBL-mediated lectin complement pathway activation in MBL-deficient human sera using GlcNAc, whereas a previous study demonstrated that porcine MBL did not bind to GlcNAc-agarose.16 The inability of porcine MBL to bind GlcNAc-agarose may be a result of inefficient coupling of GlcNAc to agarose. Furthermore, we used GlcNAc to elute the proteins from a maltose column that were then identified by microcapillary reverse-phase HPLC nano-electrospray tandem mass spectroscopy (µlC/MS/MS) to be porcine MBL isoforms. Thus, the porcine MBL that was isolated by our methods reveals a single protein with post-translational modifications.
Activated complement is known to induce tissue-mediated injury by several mechanisms. Although a collection of studies has demonstrated the involvement of complement in a number of disease states, the specific role of MBL still remains unknown. Identification of the important residues in the carbohydrate recognition domain of the protein could potentially unveil the structural interactions and the binding selectivity of MBL. The observation that porcine MBL can functionally activate the lectin pathway in MBL-deficient human sera may have importance in xenotransplantation or other diseases involving MBL.
Acknowledgments
We gratefully acknowledge the technical assistance of Margaret Morrissey and Kara Whoolery during the course of this study. This study was funded in part by NIH grant, HL52886.
References
- 1.Matsushita M. The lectin pathway of the complement system. Microbiol Immunol. 1996;40:338–343. doi: 10.1111/j.1348-0421.1996.tb01156.x. [DOI] [PubMed] [Google Scholar]
- 2.Turner MW. Mannose-binding lectin (MBL) in health and disease. Immunobiology. 1998;199:327–39. doi: 10.1016/S0171-2985(98)80037-5. [DOI] [PubMed] [Google Scholar]
- 3.Esser AF. The membrane attack complex of complement. Assembly, structure and cytotoxic activity. Toxicology. 1994;87:229–47. doi: 10.1016/0300-483x(94)90253-4. [DOI] [PubMed] [Google Scholar]
- 4.Hoppe HJ, Reid KB. Trimeric C-type lectin domains in host defence. Structure. 1994;2:1129–33. doi: 10.1016/S0969-2126(94)00115-4. [DOI] [PubMed] [Google Scholar]
- 5.Ezekowitz RA, Day LE, Herman GA. A human mannose-binding protein is an acute-phase reactant that shares sequence homology with other vertebrate lectins [published erratum appears in J Exp Med 1991 Sep 1; 174 (3): 753] J Exp Med. 1988;167:1034–46. doi: 10.1084/jem.167.3.1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sastry K, Herman GA, Day L, Deignan E, Bruns G, Morton CC, Ezekowitz RAB. The human mannose-binding protein gene. Exon structure reveals its evolutionary relationship to a human pulmonary surfactant gene and localization to chromosome 10. J Exp Med. 1989;170:1175–89. doi: 10.1084/jem.170.4.1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wada M, Ozaki K, Itoh N, Yamashina I, Kawasaki T. Two forms of messenger RNA encoding rat liver mannan-binding protein are generated by differential utilization of polyadenylation sites of one transcript. J Biochem (Tokyo) 1990;108:914–7. doi: 10.1093/oxfordjournals.jbchem.a123314. [DOI] [PubMed] [Google Scholar]
- 8.Super M, Thiel S, Lu J, Levinsky RJ, Turner MW. Association of low levels of mannan-binding protein with a common defect of opsonisation. Lancet. 1989;2:1236–9. doi: 10.1016/s0140-6736(89)91849-7. [DOI] [PubMed] [Google Scholar]
- 9.Tan SM, Chung MCM, Kon OL, Thiel S, Lee SH, Lu J. Improvements on the purification of mannan-binding lectin and demonstration of its Ca2+-independent association with a C1s-like serine protease. Biochem J. 1996;319:329–32. doi: 10.1042/bj3190329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Collard CD, Vakeva A, Morrissey MA, et al. Complement activation following oxidative stress: Role of the lectin complement pathway. Am J Pathol. 2000;156:1549–56. doi: 10.1016/S0002-9440(10)65026-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Super M, Levinsky RJ, Turner MW. The level of mannan-binding protein regulates the binding of complement-derived opsonins to mannan and zymosan at low serum concentrations. Clin Exp Immunol. 1990;79:144–50. doi: 10.1111/j.1365-2249.1990.tb05170.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mogues T, Ota T, Tauber AI, Sastry KN. Characterization of two mannose-binding protein cDNAs from rhesus monkey (Macaca mulatta): stucture and evolutionary implications. Glycobiology. 1996;6:543–50. doi: 10.1093/glycob/6.5.543. [DOI] [PubMed] [Google Scholar]
- 13.Kawai T, Suzuki Y, Eda S, et al. Cloning and characterization of a cDNA encoding bovine mannan-binding protein. Gene. 1997;186:161–5. doi: 10.1016/s0378-1119(96)00664-6. 10.1016/s0378-1119(96)00664-6. [DOI] [PubMed] [Google Scholar]
- 14.Kawai T, Suzuki Y, Eda S, Ohtani K, Kase T, Sakamoto T, Uemura H, Wakamiya N. Molecular and biological characterization of rabbit mannan-binding protein (MBP) Glycobiology. 1998;8:237–44. doi: 10.1093/glycob/8.3.237. 10.1093/glycob/8.3.237. [DOI] [PubMed] [Google Scholar]
- 15.Wallis R, Drickamer K. Molecular determinants of oligomer formation and complement fixation in mannose-binding proteins. J Biol Chem. 1999;274:3580–9. doi: 10.1074/jbc.274.6.3580. [DOI] [PubMed] [Google Scholar]
- 16.Storgaard P, Nielsen EH, Andersen O, et al. Isolation and characterization of porcine mannan-binding proteins of different size and ultrastructure. Scand J Immunol. 1996;43:289–96. doi: 10.1046/j.1365-3083.1996.d01-39.x. [DOI] [PubMed] [Google Scholar]