Abstract
In this study, the potential of the bare skin as a non-invasive route for vaccination was examined. Following application of heat-labile enterotoxin (LT) of Escherichia coli onto bare skin of BALB/c mice, strong serum anti-LT antibody responses were observed, and mucosal immunoglobulin A (IgA) and IgG antibodies were measured in vagina washes. In addition, LT enhanced the serum and mucosal antibody and proliferative T-cell responses to the model protein antigen β-galactosidase (β-gal) when coadministered onto bare skin, highlighting its potential to exert an adjuvant effect. When a peptide representing a T-helper epitope (aa 307–319) from the haemagglutinin of influenza virus was applied onto bare skin with LT or cholera toxin (CT), it primed effectively peptide- and virus-specific T cells, as measured in vitro by the interleukin-2 (IL-2) secretion assay. LT was shown to be as immunogenic as CT. Binding activity to GM1 gangliosides was essential for effective induction of anti-CT serum and mucosal antibody responses. Finally, mice immunized onto bare skin with LT were protected against intraperitoneal challenge with a lethal dose of the homologous toxin. These findings give further support to a growing body of evidence on the potential of skin as a non-invasive route for vaccine delivery. This immunization strategy might be advantageous for vaccination programmes in Third World countries, because administration by this route is simple, painless and economical.
Introduction
The skin is part of the epithelial system of the body, which serves as an effective barrier against a potentially hostile environment. As a structural barrier, the skin keeps water and other vital substances in and foreign material out. As an immunological barrier, it serves as a first line of defence to the assault by environmental antigens or pathogens. To fulfil these functions, the skin has developed a specialized structure of unique physical and chemical composition, the stratum corneum, and possesses in the epidermis immunocompetent cells such as keratinocytes (KC) and Langerhans cells (LC).1,2 KC produce various proinflammatory cytokines such as interleukin-1 (IL-1) and tumour necrosis factor-α (TNF-α), which promote LC migration from the skin to the regional lymph nodes.3,4 The LC initiate immune responses by acting as professional antigen-presenting cells (APC), taking up and processing antigens, and subsequently presenting antigenic peptides to naive T cells in the lymph nodes.2,5 Selecting immunization routes that allow efficient uptake of antigen by APC could be advantageous for the induction of optimum immune responses.
Recent studies have demonstrated the potential of skin as a non-invasive route for administering antigens.6–9 In the case of protein antigens, the skin barrier limits the penetration of high molecular weight molecules,10 preventing their use for therapeutic purposes. However, coadministration of proteins with cholera toxin (CT) has been shown to enhance protein-specific antibody responses.6,11,12 More importantly, CT was not toxic when it was applied onto bare skin, and conferred protection against mucosal challenge with the toxin.11 CT and the closely associated heat-labile enterotoxin (LT) of Escherichia coli, have become the prototype mucosal immunogens and adjuvants in studies evaluating the potential of mucosal routes for immunization.13 Both toxins are multisubunit molecules with five non-toxic B subunits held together in a pentamer (responsible for binding to the cell membrane), surrounding a single A subunit which is responsible for toxicity. The A subunit consists of two distinct structural domains: A1, which displays the ADP-ribosyltransferase activity in the cytosol of the target cells; and A2, which mediates interaction with the B subunit.14 LT produced by certain strains of enterotoxinogenic Escherichia coli is responsible for causing ‘travellers diarrhoea' in humans, which is much less severe than the diarrhoea caused by Vibrio cholerae, but more prevalent worldwide.14,15 Anti-LT antibodies play a protective role against Escherichia coli infection in humans, and the disease itself results in significant mucosal secretory immunoglobulin A (IgA) and serum IgG anti-LT antibody responses.16 However, because LT is highly toxic in humans, its value is limited for use as a vaccine component.
Using non-invasive routes such as the skin for vaccine delivery could be advantageous for mass vaccination programmes as: (i) the use of needles is avoided, limiting the risk of infections from blood-borne pathogens such as hepatitis B virus or human immunodeficiency virus;17 (ii) it does not require trained medical personnel; and (iii) it is economical. For these reasons, we have examined the potential of the bare skin as a route for administering model protein or peptide antigens, such as the β-galactosidase (β-gal), or a synthetic peptide representing a T-helper epitope from influenza haemagglutinin, using LT as an adjuvant. In addition, we evaluated the capacity of the skin to generate protective immune responses against lethal challenge with LT.
Materials and methods
Mice
Female BALB/c mice, 6–8 weeks old at the start of the experiments were purchased from Harlan Inc (Gannat, France) and maintained in the animal facility of the Institut de Biologie Moléculaire and Cellulaire, Strasbourg.
Peptide synthesis
Synthetic peptide HA: 307–319 (PKYVKQNTLKLAT) representing a promiscuous T-helper epitope from the influenza virus haemagglutinin (HA)18 was synthesized using conventional solid phase peptide synthesis and Fmoc protected amino acids. Following cleavage, the peptide was purified using preparative high performance liquid chromatography (HPLC), and characterized by HPLC and mass spectroscopy.
Immunizations
Twenty-four hours prior to immunization, mice were shaved on a restricted area of the abdomen. No visual damage to the stratum corneum was observed. Mice were injected subcutaneously with 100 µl solution of ketamine (Imalgene 1000 (15%), Merial, Lyon, France) with xylasine (2% Rompuin (9%), Bayer AG, Leverkusen, Germany), which kept the mice in a hypnotic and analgetic state for approximately 1 hr to prevent grooming. Before the application of the antigen solution, the shaved skin was hydrated with the help of a water-drenched gauze for few minutes and lightly blotted with a dry gauze prior to immunization, as previously been described.7 A volume of 40 µl of the immunizing antigen solution was then applied onto bare skin of mice over an approximately 1–2 cm2 surface area as follows:1 in experiments conducted to test the immunogenicity of LT, groups of seven to ten BALB/c mice were immunized with 100, 50 or 25 µg LT/mouse (Sigma, Poole, UK; LT was reconstituted in sterile saline prior to immunization). A booster application of the antigen preparation onto bare skin was given 35 days after priming.2 In experiments conducted to test and compare the adjuvanticity of LT, groups of 6–11 BALB/c mice were immunized as follows: (a) 100 µg β-gal (MW 540 000, Sigma); (b) a mixture of 100 µg β-gal and 100 µg LT; (c) a mixture of 100 µg β-gal and 100 µg synthetic phosphorothioate-stabilized oligonucleotide 1826 (CpG-ODN) (TCCATGACGTTCCTGACGTT, purchased from Eurogenetec, Seraing, Belgium);19 and (d) a mixture containing 100 µg β-gal, 50 µg LT and 100 µg 1826 CpG-ODN. In all cases, a booster application of antigen preparation onto bare skin was given 35 days and 84 days post-priming.3 In experiments conducted to test the immunogenicity of the influenza T-helper epitope HA: 307–319, groups of three BALB/c mice were immunized with 100 µg peptide and 50 µg LT or 50 µg CT (Sigma; CT was reconstituted in sterile saline prior to immunization) or with 100 µg peptide alone (40 µl/mouse), applied onto bare skin on days 0 and 14.
In order to compare the immunogenicity of LT and CT, and examine the role of GM1-ganglioside binding for immune stimulation, groups of 5–7 BALB/c mice were immunized with 100 µg LT or CT alone or with mixtures of 100 µg CT with threefold or sixfold w/w excess of GM1 type III ganglioside (Sigma; molar ratio; 1:168 and 1: 336, respectively) preincubated for 90 min at 37°, prior to skin application. A booster administration was given 35 days after priming.
No adverse effects, nor erythema was observed after the shaving or the immunization procedure. One hour after the application of the antigen solution onto bare skin, the inoculum was absorbed and the skin was completely dry.
LT challenge
Three weeks after the booster application of 100 µg LT onto bare skin (see above), BALB/c mice were challenged intraperitoneally with 100 µg (2 LD50 of LT in sterile saline (200 µl/mouse). Following challenge, mice were monitored daily for morbidity and mortality. For determination of LD50, groups of six to ten BALB/c mice were inoculated intraperitoneally with various concentrations of LT, and monitored for 7 days. LD50 was determined as 50 µg of LT.
Collection of vaginal washes
Vaginal washes were collected by pipetting 30 µl of sterile phosphate-buffered saline (PBS) containing 0·5% bovine serum albumin (BSA) into and out of the vaginal lumen several times and collecting the effluent. To limit the effect of oestrus cycle on local antibody responses,20 vaginal washes from 2 consecutive days were collected, pooled, centrifuged to remove particulate matter and stored at −20° until use.
Measurement of antibody responses
The presence of antibodies in the serum and vaginal washes was determined by an enzyme-linked immunosorbent assay (ELISA). Briefly, 96-well microtitre plates (Falcon, Oxnard, CA) were coated overnight with 5 µg/ml of protein or peptide antigen in 0·05 m carbonate–bicarbonate buffer, pH 9·6 at 37°. The plates were blocked with 1% of BSA in PBS + 0·05% Tween 20 (PBS-T) at 37° for 2 hr. Following washing with PBS-T, serial twofold dilutions of serum or pools of vaginal washes in PBS-T containing 0·25% BSA were made across the plate (final volume 50 µl) and plates were incubated at 37° for 1 hr. At the end of the incubation period, plates were washed with PBS-T and incubated with 50 µl/well of horseradish peroxidase-conjugated goat anti-mouse IgG (Jackson ImmunoResearch Laboratories Inc, West Grove, PA), IgA or IgG subclasses (Nordic Immunology, Tilburg, The Netherlands), Fc-specific for 1 hr at 37°. After washing with PBS-T, the final reaction was visualized by adding 150 µl/well substrate solution (10% citric phosphate buffer pH 5, + 0·04% H2O2 + 90% of a solution containing 72 ml dimethylsulphoxide + 18 ml glycerol +300 mg 3,3′,5,5′-tetramethylenebenzidine) for 15 min at 37°. The reaction was stopped with 0·25 m HCl, and absorbance was measured at 450 nm. Data are expressed as antibody titres corresponding to the reciprocal dilution giving an OD equal to 0·2.
Measurement of proliferative responses
Spleens were aseptically removed and a single cell suspension was prepared in RPMI-1640 medium (Life Technologies, Gergy-Pontoise, France) supplemented with 100 IU/ml gentamicin, 2 mm l-glutamine, 25 mm HEPES, and 1% inactivated autologous mouse serum. 4 × 105 viable splenocytes were cultured in 0·2 ml volumes, in the presence of various concentrations of β-gal, for 5 days. Eighteen hours before the end of the culture, cells were pulsed with 1 µCi of [3H]thymidine (ICN, Orsay, France), and incorporation was measured using a Matrix 9600 direct beta counter (Packard, Downers Grove, IL). Results were expressed as stimulation indices (SI) of the mean counts per minute (c.p.m.) from hexaplicate cultures in the presence of antigen divided by mean c.p.m. of hexaplicate cultures with medium only. Values equal to, or higher than 2 were considered positive. Supernatants collected after 72 hr culture of 4 × 105 viable splenocytes in the presence of the HA: 307–319 peptide or heat-inactivated (1 hr at 56°) influenza virus (strain A/NT 60/68 H3N2), were tested for their ability to support the proliferation of an IL-2-dependent cell line (CTLL-2), after 31 hr culture. One µCi of [3H]thymidine was added 7 hr before the end of the culture, and incorporation was measured as above. A standard curve performed with known concentrations of rIL-2 (0–30 U/ml; PharMingen, San Diego, CA) was used as an internal control.
Statistical analysis
Comparison of levels of antibody titres and c.p.m. values between different groups, was performed using the Student t-test. P-values < 0·05 were considered as significant.
Results
Immunogenicity of various doses of LT applied onto bare skin
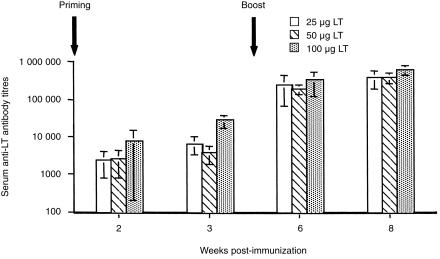
Following immunization with 25, 50 and 100 µg of LT, serum anti-LT antibody responses were detected 2 weeks after priming (Fig. 1). However, significantly higher antibody titres were obtained in sera from mice immunized with 100 µg of LT, when compared with groups of mice immunized with 50 µg (P = 0·0016) or 25 µg (P = 0·0035), 3 weeks after priming. Serum IgG antibody responses in all groups of mice were increased to comparable levels following a booster administration of LT. IgG1/IgG2a ratios in all groups of mice were 128. Anti-LT IgA (antibody titres, 1300, 2600 and 2500 for groups of mice immunized with 25, 50 and 100 µg of LT, respectively) and IgG (antibody titres, 650, 2600, 5000 for groups of mice immunized with 25, 50 and 100 µg of LT, respectively) antibodies were also detectable in vaginal washes from all groups of mice 3 weeks after the boost.
Figure 1.
Serum anti-LT IgG antibody responses following immunization onto bare skin with 25, 50 and 100 µg LT. The data represent mean serum antibody titres ±sd from groups of 7–10 BALB/c mice.
Comparison of the immunogenicity of LT and CT and requirement for GM1-binding for immune stimulation
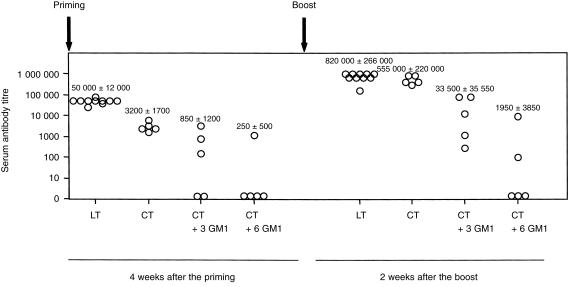
LT and CT were shown to be effective immunogens when applied onto bare skin, and no significant differences were observed in antibody titres induced after the boost (P = 0·7638) (Fig. 2).
Figure 2.
Serum anti-CT and anti-LT IgG antibody responses following skin immunization with CT, LT or mixtures of CT preincubated with three- or sixfold w/w excess of GM1 gangliosides (molar ratio; 1:168 and 1:336, respectively). Data are presented as antibody titres from individual mice in each group tested against the respective antigens CT or LT. Average values ±sd are also indicated.
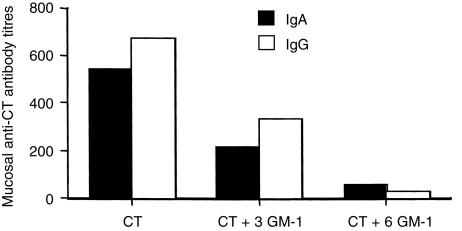
In general, GM1 binding is considered to be essential for the immunogenicity of LT and CT.2 Since LT binds to more that one type of GM1 gangliosides,14,21 the ability of GM1 (which is the receptor for CT) to block its immunogenicity was tested in vivo. Preincubation of 100 µg CT with threefold or sixfold w/w excess of GM1 ganglioside (molar ratio; 1:168 and 1:336, respectively) prior to application onto bare skin, resulted in a significant reduction of anti-CT antibody titres 4 weeks (P = 0·0027 and P = 0·0275, when three- or sixfold w/w excess of GM1 was used, respectively) after priming, and two weeks (P = 0·0088 and P = 0·0072, when three- or sixfold w/w excess of GM1 was used, respectively) after the boost (Fig. 2). Similarly, anti-CT IgA and IgG antibody responses in vaginal washes were reduced when compared to the group of mice immunized with CT alone (Fig. 3).
Figure 3.
Mucosal IgA and IgG anti-CT antibody responses following skin immunization with CT alone or with CT preincubated with three- or sixfold w/w excess of GM1 gangliosides (molar ratio; 1:168 and 1:336, respectively). The data represent antibody titres from pools of vagina washes from five to six mice, collected 2 weeks after the boost.
The effect of LT on the immune responses to protein or peptide antigens applied onto bare skin
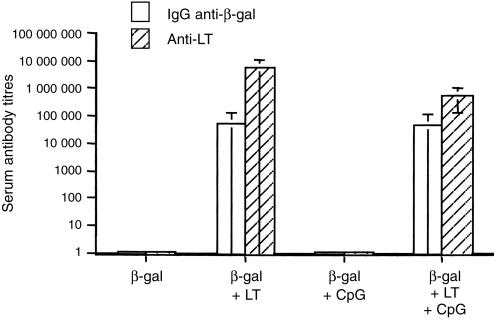
β-gal -specific antibody responses were evaluated in groups of mice immunized onto bare skin with LT or in combination with the 1826 CpG-ODN as adjuvants. As shown in Fig. 4, 3 weeks after the second boost, serum anti-β-gal antibody responses were detectable in groups of mice immunized with β-gal and LT or with a mixture of LT and 1826 CpG-ODN. Application of β-gal alone or together with the 1826 CpG-ODN did not result in any measurable antibody response. In addition, mucosal anti-β-gal IgA antibody responses were measured in vagina washes from groups of mice immunized with β-gal and LT (antibody titre, 550) or β-gal and a mixture of LT and 1826 CpG-ODN (antibody titre, 300) 3 weeks after the boost. Anti-β-gal IgG antibodies were also detectable in vagina washes of mice immunized with β-gal and LT (antibody titre, 850) 3 weeks after the boost.
Figure 4.
Serum IgG anti-β-gal and anti-LT antibody responses following three administrations of β-gal alone, or β-gal with adjuvant onto bare skin. The data represent mean serum antibody titres ±sd from groups of 6–11 BALB/c mice measured 3 weeks after the last administration.
Lymphocyte proliferation assays were performed with splenocytes collected 3 weeks after the second booster application of β-gal or β-gal and adjuvant. Results in Table 1, show that upon in vitro restimulation with various concentrations of β-gal, splenocytes from mice immunized with β-gal and a mixture of LT and 1826 CpG-ODN, proliferated significantly more than splenocytes from mice immunized with β-gal and LT (P = 0·0014, P = 0·0256 and P = 0·0004 for 0·5, 0·05 and 0·005 µg β-gal/culture, respectively). No response was detected in the groups of mice immunized with β-gal alone, or β-gal and 1826 CpG-ODN.
Table 1.
Proliferative responses of splenocytes from BALB/c mice immunized with β-gal alone, or β-gal with adjuvant onto bare skin
| Immunization procedure | ||||||||
|---|---|---|---|---|---|---|---|---|
| β-gal | β-gal + LT | β-gal + CpG | β-gal + LT + CpG | |||||
| β-gal (μg/ml) | Mean c.p.m.±SD | SI | Mean c.p.m.±SD | SI | Mean cpm±SD | SI | Mean c.p.m.±SD | SI |
| 0.5 | 1600±153 | 1.7 | 2510±618 | 3.8 | 760±73 | 1.3 | 4104±490 | 14 |
| 0.05 | 1100±181 | 1.2 | 3036±391 | 4.6 | 789±220 | 1.4 | 7400±892 | 25.3 |
| 0.005 | 961±58 | 1 | 1738±357 | 2.6 | 915±202 | 1.6 | 4274±790 | 14.6 |
| Medium | 947±199 | 665±26 | 569±214 | 292±175 | ||||
Splenocytes were cultured in the presence of increasing concentrations of β-gal, and thymidine uptake was measured after 5 days culture. The data are presented as c.p.m. ± sd from hexaplicate cultures and as SI.
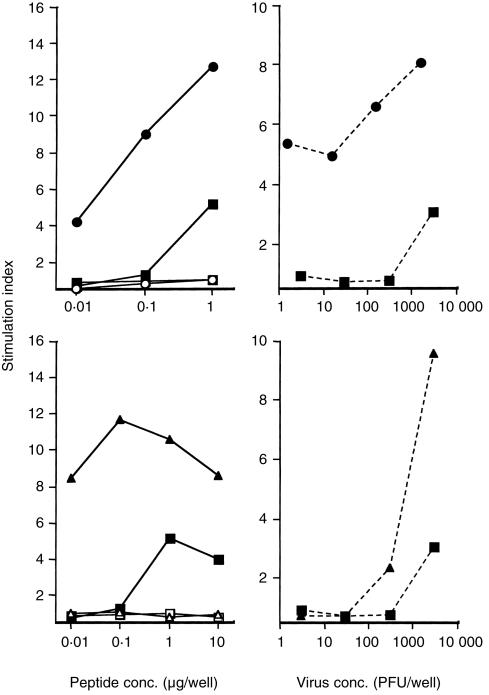
LT and CT also enhanced the immunogenicity of the T-helper epitope HA: 307–319 when they were coadministered onto bare skin. Splenocytes collected 2 weeks after the boost, proliferated in vitro in the presence of various concentrations of the homologous peptide or heat-inactivated influenza virus, as measured by the IL-2 secretion assay (Fig. 5). Splenocytes from mice immunized with peptide alone proliferated very weakly. Proliferative responses to the HA:307–319 peptide and heat-inactivated influenza virus were also measurable 14 days after a single administration of peptide with LT onto bare skin (data not shown). No anti-peptide antibody responses were measured in serum samples, suggesting that the peptide does not contain a B-cell epitope (data not shown).
Figure 5.
Immunogenicity of synthetic peptide HA: 307–319 after immunization onto bare skin with LT (top panel) and CT (bottom panel). Splenocytes collected after two administrations of peptide alone (square) or with the mucosal adjuvants LT (circle) or CT (triangle) were cultured in the presence of increasing concentrations of homologous peptide (▪, •, ▴ for mice immunized with peptide, peptide + LT, peptide + CT, respectively), irrelevant peptide (□, ○, ▵ for mice immunized with peptide, peptide + LT, peptide + CT, respectively; left top and bottom panel) or heat-inactivated influenza virus (▪, •, ▴ for mice immunized with peptide, peptide + LT, peptide + CT, respectively; right top and bottom panel). Supernatants collected after 72 hr of culture were tested for their ability to secrete IL-2 measured using the CTLL-2 cells. The data represent the mean SI values from triplicate cultures. Background values in the absence of antigen were 645 c.p.m. (for peptide and virus) after immunization with peptide alone, 774 c.p.m. and 905 c.p.m. (for peptide and virus, respectively) after immunization with peptide and LT, and 890 c.p.m. (for peptide and virus) after immunization with peptide and CT. Standard deviations in triplicate cultures were < 20%.
Protection against lethal LT challenge
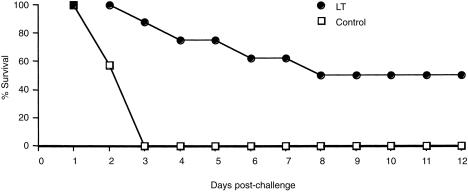
To test the potential of skin immunization for the induction of protective immune responses, a group of BALB/c mice immunized with LT onto bare skin were challenged via the intraperitoneal route with a lethal dose of LT. All immunized mice seroconverted prior to toxin challenge (mean antibody titre, 321 000).
Following intraperitoneal challenge with 2 LD50 of LT, immune mice were protected as compared to the control non-immune mice (Fig. 6). However, on day 7, fluid accumulation was observed in the peritoneal cavity, which increased the body weight of the surviving mice (data not shown), resulting in a drop of the percentage survival. Thus, it appears that the high dose of LT used for intraperitoneal challenge damaged the primary organs, and despite the presence of protective antibodies some mice finally died.
Figure 6.
Percentage survival following intraperitoneal challenge with a lethal dose of LT (100 µg), of a group of eight BALB/c mice immunized onto bare skin with LT. Control, non-immune mice (n = 10).
Discussion
In this report the bare skin was tested as a route for administering antigens, using LT as an immunogen and as an adjuvant. When LT was used as an immunogen, strong primary and secondary anti-LT serum antibody responses were induced, and anti-LT IgA and IgG antibodies were measured in vaginal washes. Subclasses of serum antibodies were predominantly IgG1, suggesting a T helper 2 (Th2)-type response. Findings from this study also suggest that the immunogenicity of ADP-ribosylating exotoxins when applied onto bare skin, greatly depends on their ability for binding to GM1 gangliosides, which serve as their receptor.14 This is consistent with the demonstration that their mucosal immunogenicity in part depends upon their ability for GM1-binding.22 These observations give further support to the growing body of evidence on the strong immunogenicity of ADP-ribosylating exotoxins when applied onto bare skin,1,6 and its ability to tolerate very well their high toxicity.6,7,11,12 It appears that the specific arrangement of cells and lipids in the skin precludes appreciable exchange of materials between the skin surface and the skin depth; hence the ability of the skin to tolerate high doses of the toxin without any serious side effects, as seen at the mucosal surfaces. Because LT was found to be highly immunogenic, the LT challenge mouse model was selected to evaluate whether immunization onto bare skin can induce protective immune responses against lethal systemic challenge with the toxin. Although the challenge dose of LT could be considered as an especially vigorous dose, mice were protected. This is in agreement with observations demonstrating that skin immunization can generate protective immune responses against challenge with toxins such as CT11 or tetanus toxin.7
Apart from its strong immunogenic properties, LT was shown to exert an adjuvant effect to the coadministered protein antigen β-gal. Serum and mucosal anti-β-gal antibody responses were enhanced, as well as antigen-specific proliferative responses. Similarly, LT and CT enhanced the proliferative responses to a weakly immunogenic T-helper epitope from influenza virus haemagglutinin. Given the fact that small size molecules can easily penetrate the skin barrier,10 this route might be advantageous for administering synthetic peptide vaccines. These findings are consistent and extend previous observations demonstrating the adjuvanticity of ADP-ribosylating exotoxins such as the CT, after skin immunization.6,7,12 The exact mechanism by which LT potentiates immune responses to a coadministered antigen remains to be determined.
Recently, bacterial DNA has been shown to induce B-cell proliferation, antibody secretion, and Th1-type of responses dominated by the IL-12 and interferon-γ cytokines.23,24 This was attributed to specific single-stranded oligonucleotide sequences containing unmethylated CpG dinucleotides (CpG motifs), which are far more common in bacterial DNA than in vertebrate DNA.25 A similar immunomodulating effect can be seen with synthetic ODNs containing CpG motifs.26 Given these strong immunostimulatory properties, the adjuvanticity of the synthetic CpG-ODN 182619 was compared to LT. No serum nor mucosal antibody nor proliferative responses were detectable in mice immunized with CpG-ODN and β-gal. It could be argued that when CpG-ODN is administered as a mixture with the antigen it is unlikely that both molecules will be picked up simultaneously by the same APC. Their molecular size will affect the rate of their transport, and in the particular case of CpG it has been shown that 2 hr after intradermal injection, there is a local depletion of LC.27 Perhaps conjugation of CpG-ODN with the antigen might be a more efficient way for enhancing antigen-specific immune responses, as both molecules are presented to the same APC. This is supported by recent findings demonstrating that conjugates of CpG and protein antigen are more effective in inhibiting airway eosinophilia as compared to the mixture formulation28 and result in a rapid, long-lasting and potent induction of cell-mediated and humoral immunity.29 However, when a mixture of LT and CpG-ODN were applied together with β-gal onto bare skin, proliferative responses to β-gal were significantly enhanced as compared to responses from β-gal + LT immune splenocytes. A similar synergistic effect has recently been demonstrated between CpG-ODN and the LT mutant (LTR72), when they were coadministered intranasally with a synthetic peptide of measles virus.30 Injection of ODN-CpG into the dermis enhances the expression of major histocompatibility complex class II and CD86 molecules by LC in the overlying epidermis.31 Taking into account that for efficient T-cell priming less amount of antigen is required as compared to B-cell priming, this could explain the immunopotentiating effect induced by the coadministered CpG-ODN. The fact that CpG-ODNs can be easily produced on a large scale at a minimum cost, and can induce immune activation, makes them an attractive candidate for clinical applications.
Most of the currently available vaccines are administered by parenteral routes, despite the fact that the majority of pathogens invade the host via the mucosal surfaces. In addition, intramuscular or subcutaneous immunization is (i) expensive, (ii) requires trained medical personnel, (iii) may lead to infections due to the use of contaminated needles,17 and (iv) does not stimulate mucosal immune responses required to control infection at the site of pathogen entry. The demonstration in this study, that a simple application of an antigen solution onto bare skin can result in strong antigen-specific seroconversion and mucosal immune responses, opens up the possibility for using this route as an alternative and simple way for administering vaccines.
Acknowledgments
A-S. Beignon-is supported by Biovector Therapeutics (Toulouse, France). The authors thank Dr M. Valette for providing the influenza virus, and B. Jessel and M. Meyer for animal husbandry.
References
- 1.Franz TJ, Lehman PA. The skin as a barrier: structure and function. In: Kydonieus AF, Wille JJ, editors. Biochemical Modulation of Skin Reactions: Transdermals, Topicals, Cosmetics. Boca Raton, FL: CRC Press LLC; 2000. pp. 15–33. [Google Scholar]
- 2.Bos JD, Kapsenberg ML. The skin immune system. Its cellular constituents and their interactions. Immunol Today. 1993;14:75–8. doi: 10.1016/0167-5699(86)90111-8. [DOI] [PubMed] [Google Scholar]
- 3.Wang B, Amerio P, Sauder DN. Role of cytokines in epidermal Langerhans cell migration. J Leukocyte Biol. 1999;66:33–9. doi: 10.1002/jlb.66.1.33. [DOI] [PubMed] [Google Scholar]
- 4.Stoitzner P, Zanella M, Ortner U, et al. Migration of Langerhans cells and dermal dendritic cells in skin organ cultures: augmentation by TNFα and IL-1β. J Leukocyte Biol. 1999;86:462–70. [PubMed] [Google Scholar]
- 5.Lappin MB, Kimber I, Norvall M. The role of dendritic cells in cutaneous immunity. Arch Dermatol Res. 1996;288:109–21. doi: 10.1007/BF02505819. 10.1007/s004030050032. [DOI] [PubMed] [Google Scholar]
- 6.Glenn GM, Rao M, Matyas GR, Alving CR. Skin immunisation made possible by cholera toxin. Nature. 1998;391:851. doi: 10.1038/36014. 10.1038/36014. [DOI] [PubMed] [Google Scholar]
- 7.Glenn GM, Scharton-Kersten T, Vassell R, Matyas GR, Alving CR. Transcutaneous immunisation with bacterial ADP-ribosylating exotoxins as antigens and adjuvants. Infect Immun. 1999;67:1100–6. doi: 10.1128/iai.67.3.1100-1106.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Seo N, Tokura Y, Nishijima T, Hashizume H, Furukawa F, Takigawa M. Percutaneous peptide immunization via corneum barrier-disrupted murine skin for experimental tumor immunoprophylaxis. Proc Natl Acad Sci USA. 2000;97:371–6. doi: 10.1073/pnas.97.1.371. 10.1073/pnas.97.1.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fan H, Lin Q, Morrissey GR, Khavari PA. Immunisation via hair follicles by topical application of naked DNA to normal skin. Nature Biotechnol. 1999;17:870–2. doi: 10.1038/12856. [DOI] [PubMed] [Google Scholar]
- 10.Bos JD, Meinardi MMHM. The 500 Dalton rule for the skin penetration of chemical compounds and drugs. Exp Dermatol. 2000;9:165–9. doi: 10.1034/j.1600-0625.2000.009003165.x. 10.1034/j.1600-0625.2000.009003165.x. [DOI] [PubMed] [Google Scholar]
- 11.Glenn GM, Scharton-Kersten T, Vassell R, Mallett CP, Hale TL, Alving CR. Transcutaneous immunisation with cholera toxin protects mice against lethal mucosal toxin challenge. J Immunol. 1998;161:3211–4. [PubMed] [Google Scholar]
- 12.Scharton-Kersten T, Glenn GM, Vassell R, Yu J-M, Walwender D, Alving CR. Principles of transcutaneous immunisation using cholera toxin as an adjuvant. Vaccine. 1999;17:S37–43. doi: 10.1016/s0264-410x(99)00233-9. 10.1016/s0264-410x(99)00233-9. [DOI] [PubMed] [Google Scholar]
- 13.Partidos CD. Intranasal vaccines: forthcoming challenges. Pharm Sci Techn Today. 2000;3:273–81. doi: 10.1016/s1461-5347(00)00281-9. [DOI] [PubMed] [Google Scholar]
- 14.Spangler BD. Structure and function of cholera toxin and the related Escherichia coli heat-labile enterotoxin. Microb Rev. 1992;56:622–47. doi: 10.1128/mr.56.4.622-647.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Black RE. Epidemiology of diarrheal disease: implications for control by vaccines. Vaccine. 1993;11:100–6. doi: 10.1016/0264-410x(93)90002-f. [DOI] [PubMed] [Google Scholar]
- 16.Stoll BJ, Svennerholm A-M, Gothefors L, Barum D, Huda S, Holmgen J. Local and systemic antibody responses to naturally acquired enterotoxigenic Escherichia coli diarrhoea in an endemic area. J Inf Dis. 1986;153:527–34. doi: 10.1093/infdis/153.3.527. [DOI] [PubMed] [Google Scholar]
- 17.Kane A, Lloyd J, Zaffran M, Simonsen L, Kane M. Transmission of hepatitis B, hepatitis C and human immunodeficiency viruses through unsafe injections in the developing world: model-based regional estimates. Bull WHO. 1999;77:801–7. [PMC free article] [PubMed] [Google Scholar]
- 18.O'Sullivan D, Arrhenius T, Sidney J, et al. On the interaction of promiscuous antigenic peptides with different DR alleles. Identification of common structural motifs. J Immunol. 1991;147:2663–9. [PubMed] [Google Scholar]
- 19.Davis HL, Weeranta R, Waldschmidt TJ, Tygrett L, Schorr J, Krieg AM. CpG DNA is a potent enhancer of specific immunity in mice immunised with recombinant hepatitis B surface antigen. J Immunol. 1998;160:870–6. [PubMed] [Google Scholar]
- 20.Gallichan WS, Rosenthal KL. Effects of estrous cycle on local immune responses and protection of intranasally immunized mice against HSV type II infection in the genital tract. Virology. 1996;224:487–97. doi: 10.1006/viro.1996.0555. 10.1006/viro.1996.0555. [DOI] [PubMed] [Google Scholar]
- 21.Holmgren J, Lindblad H, Fredman P, Svennerholm L, Myrvold A. Comparison of receptors for cholera and Escherichia coli enterotoxin in human intestine. Gastroenterology. 1985;89:27–35. doi: 10.1016/0016-5085(85)90741-3. [DOI] [PubMed] [Google Scholar]
- 22.De Haan L, Verweij WR, Feil IK, Holtrop M, Hol WGJ, Agsteribbe E, Wilschut J. Role of GM1 binding in the mucosal immunogenicity and adjuvant activity of the Escherichia coli heat-labile enterotoxin and its B subunit. Immunology. 1998;94:424–30. doi: 10.1046/j.1365-2567.1998.00535.x. 10.1046/j.1365-2567.1998.00535.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Krieg AM, Yi AK, Matson S, Waldschmidt TJ, Bishop GA, Teasdale R, Koretzky GA, Klinman DM. CpG motifs in bacterial DNA trigger direct B-cell activation. Nature. 1995;374:546–9. doi: 10.1038/374546a0. [DOI] [PubMed] [Google Scholar]
- 24.Klinman D, Yi AK, Beaucage SL, Conover J, Krieg AM. CpG motifs expressed by bacterial DNA rapidly induce lymphocytes to secret IL-6, IL-12 and INF-γ. Proc Natl Acad Sci, USA. 1996;93:2879–83. doi: 10.1073/pnas.93.7.2879. 10.1073/pnas.93.7.2879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bird AP. CpG-rich islands and the function of DNA methylation. Nature. 1986;321:209–13. doi: 10.1038/321209a0. [DOI] [PubMed] [Google Scholar]
- 26.Roman M, Martin-Orozco E, Goodman JS, et al. Immunostimulatory DNA sequences function as T helper 1 promoting adjuvants. Nature Med. 1997;3:849–54. doi: 10.1038/nm0897-849. [DOI] [PubMed] [Google Scholar]
- 27.Ban E, Dupre L, Hermann E, et al. CpG motifs induce Langerhans cell migration in vivo. Int Immunol. 2000;12:737–45. doi: 10.1093/intimm/12.6.737. 10.1093/intimm/12.6.737. [DOI] [PubMed] [Google Scholar]
- 28.Shirota H, Sano K, Kikuchi T, Tamura G, Shirato K. Regulation of murine airway eosinophilia and Th2 cells by antigen-conjugated CpG oligodeoxynucleotides as a novel antigen-specific immunomodulator. J Immunol. 2000;164:5575–82. doi: 10.4049/jimmunol.164.11.5575. [DOI] [PubMed] [Google Scholar]
- 29.Tighe H, Takabayashi K, Schwaetz D, et al. Conjugation of protein to immunostimulatory DNA results in a rapid, long-lasting and potent induction of cell-mediated and humoral immunity. Eur J Immul. 2000;30:1939–47. doi: 10.1002/1521-4141(200007)30:7<1939::AID-IMMU1939>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 30.Olszewska W, Partidos CD, Steward MW. Anti-peptide antibody responses following intranasal immunisation: effectiveness of mucosal adjuvants. Infect Immun. 2000;68:4923–9. doi: 10.1128/iai.68.9.4923-4929.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jacob T, Walker PS, Krieg AM, Udey MC, Vogel JC. Activation of cutaneous dendritic cells by CpG containing oligonucleotides: a role for dendritic cells in the augmentation of Th1 responses by immunostimulatory DNA. J Immunol. 1998;161:3042–9. [PubMed] [Google Scholar]