Abstract
Delayed-type hypersensitivity reactions elicited in the footpad of ovalbumin-sensitized mice after challenge with aggregated ovalbumin on day 4 or 8 of immunization are distinct. The former was characterized by a dense mononuclear infiltrate and, macroscopically, the reaction peaked at 48 hr after antigen challenge; the latter was preceded by immediate-type reactions, reached the maximum at 24 hr and faded drastically later. Histologically, oedema and a mixed granulocytic–lymphocytic infiltrate was found at this time-point. Immunoglobulin G1 (IgG1), IgG2a and IgE antibodies were detected only in plasma obtained after 8 days of immunization. Regarding the cytokines produced by draining lymph node cells after in vitro restimulation, interleukin-4 (IL-4) and IL-10 were predominant after 4 days and interferon-γ and IL-2 after 8 days of immunization. These two types of delayed-type hypersensitivity (DTH) were used to study the influence of antibody-mediated responses on the inductive and effector phases of cell-mediated immunity. The effector phase of DTH was not affected by immediate-type reactions, as abrogation of these reactions by mediators' antagonists on day 8 or induction of passive reactions by transfer of immune serum on day 4 did not change the extent or kinetics of either type of DTH. Only transfer, before immunization, of whole or T-cell-enriched spleen cells, but not sera, from hyperimmunized donors (high antibody producers) abolished the induction of pure DTH in 4-day immunized recipient mice and changed their cytokine profile to a T helper 2 type. These results indicate that in a non-polarized immune response to a protein antigen there is initially a bias towards cell-mediated immunity, which is gradually dampened by the development of antibody-mediated immunity.
Introduction
Delayed type hypersensitivity (DTH) is an important in vivo manifestation of cell-mediated immunity. The development of DTH elicited by immunization or infection with intracellular parasites is mediated by antigen-specific T cells and involves the synthesis of activating and chemotactic cytokines, increase in vascular permeability and recruitment of antigen non-specific effector cells to the site of the reaction. The reaction can be subdivided into tuberculin-type, Jones–Mote type and contact sensitivity. Tuberculin-type hypersensitivity is normally indurated, being characterized by a prominent mononuclear infiltration and substantial fibrin deposition. A sustained response that peaks at 48 hr is maintained for several days.1 In contrast, the Jones–Mote type is erythematous, lacks induration and is characterized by a large number of polymorphonuclear leucocytes in the infiltrate. This response peaks at 12–30 hr, but declines sharply at 48 hr.2 Contact sensitivity is a cutaneous and erythematous reaction, with a mononuclear infiltrate, that peaks at 48 hr. Epidermal Langerhans' cells are crucial for sensitization and development of the reaction.3
DTH can be preceded by immediate hypersensitivity reactions, that develop within minutes to a few hours of antigen challenge and can be due to anaphylactic antibodies or immune complexes (Arthus reaction).4 Immediate and delayed-type reactions may occur simultaneously or not during the time course of an immune response. Collins and Mackaness5 have shown that in mice vaccinated with living Salmonella gallinarum an almost pure DTH was first detectable on day 4 and it was only after day 11 that a mixed Arthus and delayed-type of reactivity appeared. Subsequently, as the former reaction increased, the latter decreased. This was probably because the induction of antibodies varies inversely with the induction of DTH.6,7 However, activation-induced changes in endothelial cells and leucocytes infiltrating the site of reaction, mediated by cytokines and other mediators released in the immediate reaction, could also alter the characteristics and extent of the delayed-type reaction. In 1981, Titus and Chiller8 described a simple method to assess murine DTH to proteins. Using this method we studied in the present work the immediate and DTH reactions elicited during the time course of immune response to ovalbumin (OVA). The results showed two types of DTH at 4 and 8 days after immunization. Only the latter was preceded by immediate hypersensitivity reactions. The effect of development of antibody-mediated responses in the same site where cell-mediated reactions subsequently appear could therefore be analysed at these time-points. Furthermore, we looked at the modulation of the pure DTH obtained in recipient mice on day 4 by transfer, before immunization, of T-enriched cells or sera from hyperimmunized donors producing very high amounts of antibody.
Materials and Methods
Animals
Female DBA/2 mice were obtained from a colony at the Instituto de Ciências Biomédicas (Universidade de São Paulo, São Paulo, Brazil) and used for immunization and for transfer experiments at 7–9 weeks of age.
Antigens and antibodies
OVA grade II and V and complete Freund's adjuvant (CFA) were obtained from Sigma Chemical Co. (St Louis, MO). Biotinylated isotype-specific goat anti-mouse antibodies and unlabelled rat anti-mouse immunoglobulin E (IgE) antibody were purchased from Southern Biotechnology Associates Inc. (Birmingham, AL). Biotinylated OVA was obtained according to a modification of the technique for immunoglobulin by Nutman.9 All the monoclonal antibodies (mAb) for cytokine assays were purified by protein G–Sepharose chromatography from hybridoma cell culture supernatants and biotinylated, as needed, in our laboratory. Biotin-labelled SXC-1 [anti-interleukin-10 (IL-10)] as well as hybridomas and recombinant standard cytokines were a gift from Dr R. L. Coffman (DNAX Research Institute, Palo Alto, CA).
Immunization protocol and skin testing
Immunization and skin testing of the animals were done as previously described.10 Briefly, six mice were injected subcutaneously (s.c.) with 100 µg of OVA (grade V) emulsified in CFA at the base of the tail. Four or eight days after immunization 30 µl of 2% aggregated OVA (grade II) was injected in one hind footpad and 30 µl of saline in the other. Footpad thickness was measured until 72 hr after challenge and the net increase was expressed as the arithmetic mean +SEM of each group. A two-way analysis of variance followed by multiple comparisons using the Tukey method11 was employed to compare the different groups.
Proliferation assay
Four or eight days after immunization, cell suspensions from inguinal and periaortic lymph nodes (LN) were prepared in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10 mm HEPES, 0·05 mm 2-mercaptoethanol, sodium pyruvate, non-essential amino acids, penicillin/streptomycin and 1% heat-inactivated normal mouse serum (DMEM-S). LN cell proliferation was measured by incorporation of [3H]thymidine (2·0 Ci/mmol, Amersham International, Amersham, UK). Triplicate preparations of cells (5 × 105/well) were incubated with OVA at 100 µg/ml for 96 hr. Tritiated thymidine (0·5 µCi/well) was added 18 hr before cell harvesting and incorporated [3H]TdR was determined by liquid scintillation spectrometry. The data were presented as the mean counts per minute (c.p.m.) incorporated by the triplicate cultures ±SD and as stimulation index, which represents the c.p.m. of [3H]TdR incorporated by stimulated LN cells divided by the c.p.m. incorporated by unstimulated cells.
Cytokine assays
For in vitro cytokine measurements, LN cells were cultured in 24-well tissue culture plates at a final concentration of 10 or 6 × 106 cells/ml in DMEM-S and stimulated with OVA (500 µg/ml). Supernatant fluids were harvested after 24 or 72 hr, respectively, and assayed for cytokine content. All the cytokines were measured by specific two-site sandwich enzyme-linked immunosorbent assay (ELISA), using the following mAb: for IL-2, JES6-IA 12 and biotinylated JES6-5H4; for interferon-γ (IFN-γ), XMG 1·2 and biotinylated AN 18; for IL-4, BVD-1D11 and biotinylated BVD6-24G2 and for IL-10, C252-2A5 and biotinylated SXC-1. Binding of biotinylated mAb was detected using streptavidin–biotinylated horseradish peroxidase complex (Amersham International) and 2-2′-azino-bis(3-ethylbenz-thiazoline-6-sulphonic acid) substrate solution (Sigma) in 0·1 m citrate buffer containing hydrogen peroxide. The sensitivity of IL-4 assays was increased by using the ELAST ELISA amplification system purchased from DuPont NEN (Wilmington, DE). Samples were quantified by comparison with standard curves of recombinant mouse cytokines. The results were expressed as the arithmetic mean of duplicate cultures +SD.
Titration of antibody isotypes by ELISA
Plasma obtained 4 or 8 days after immunization were tested for IgG1 and IgG2a antibodies using antigen-coated 96-well plates and biotinylated goat anti-mouse monospecific antisera. The reactions were developed with streptavidin–peroxidase conjugate (Jackson ImmunoResearch Laboratories Inc, West Grove, PA), O-phenylenediamine (OPD) and H2O2 and the plates were read at 450 nm on an automated ELISA reader (Dynatech, MR 5000). IgE antibodies were titrated by reverse (IgE-capture) ELISA, using rat anti-mouse IgE mAb-coated plates and biotinylated antigens.10 Four-day OVA-immunized recipient mice, transferred with hyperimmune spleen cells before immunization, were bled 3 days after antigen challenge in the footpad. Antibody-positive cut-off values were set at the mean value of preimmune plasma plus 3 SD. ELISA antibody titers represent the highest plasma dilutions giving positive reactions. The result of each group was expressed as the geometric mean of the reciprocal of individual titres +SD. The antibody responses of different groups were compared using the Mann–Whitney test.11
Treatment with antagonists of immediate hypersensitivity mediators
Groups of six mice immunized with OVA in CFA were treated intravenously (i.v.) with antagonists of vasoactive amines or platelet-activating factor (PAF) 30 min before the challenge on day 8 with aggregated OVA. Mepyramine (Sandoz, São Paulo, Brazil), methysergide (Sigma) and WEB 2170 (Boehringer, Mannheim, Germany) were kindly provided by Dr Sonia Jancar and were injected at 3, 2·5 and 5 mg/kg, respectively.
Passive transfer of immediate hypersensitivity reactions
Donor mice immunized s.c. with OVA (100 µg/animal) in CFA or injected with CFA alone were bled on day 8 or 28 after immunization. In order to induce passive immediate hypersensitivity reactions in five or six recipient mice on day 4 of immunization, 2 ml of these sera were injected i.v. in each animal. One millilitre was given 15 hr before and the other 1 hr before the challenge with aggregated OVA.
Hyperimmunization of donor mice and adoptive transfer of spleen cells or serum
For the induction of high levels of antibodies, DBA/2 mice were immunized s.c. with OVA (50 µg/animal) and aluminium hydroxide (alum – 7·5 mg/animal) as adjuvant, in the base of the tail. After 14 and 28 days, the animals were boosted s.c. with 5 µg of OVA. As a control, another group of mice was injected only with the same amount of adjuvant and received saline on day 14 and 28. Two weeks after the last booster, both groups were bled and erythrocyte-depleted cell suspensions were prepared from their spleens. One millilitre of serum or 6 × 107 spleen cells were then transferred i.v. to syngeneic recipient mice 12 hr before immunization.
Adoptive transfer of T-enriched spleen cells
Spleen T cells from hyperimmunized donor mice were enriched in three steps. First, 10 ml of a spleen cell suspension was added to a sterile glass bottle at 107 cells/ml and allowed to adhere at 37° in 5% CO2 for 1 hr. Non-adherent cells (4 × 107) were subsequently passed through a nylon wool column and after 1 hr of incubation at 37° in 5% CO2, non-adherent cells were collected drop-wise. Finally, 2 × 107 of these cells were added for 1 hr at room temperature to a plastic tissue culture bottle, previously covered with 2 mg of rabbit IgG anti-mouse immunoglobulin antibody for 24 hr. The recovered cells contained 70·9% (57·5% CD4+ 13·4% CD8+) T cells in contrast to 24·6% (19·5% CD4+ 5·1% CD8+) present in whole spleen cells, as shown by flow cytometry analysis using phycoerythrin (PE)-conjugated anti-CD4 and fluorescein isothiocyanate (FITC)-conjugated anti-CD8 mAb. Spleen cells from donor mice injected only with alum were similarly treated and contained the same proportion of T cells.
Results
Characterization of DTH reactions after 4 or 8 days of immunization
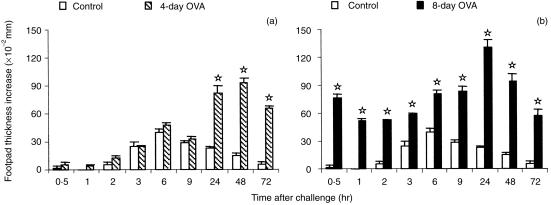
The kinetics of hypersensitivity reactions that developed 4 or 8 days after immunization of mice with OVA in CFA and following challenge with aggregated OVA was quite different. As shown in Fig. 1, a typical tuberculin-type DTH reaction was elicited on day 4, that peaked at 48 hr, with no immediate hypersensitivity reactions in the first 24 hr after challenge (Fig. 1a). In contrast, mice challenged 8 days after immunization displayed an immediate reaction at 30 min and a strong DTH at 24 hr that faded later (Fig. 1b). Histologically, the DTH reactions were also different. The former was characterized by a dense mononuclear infiltrate, while the latter exhibited oedema and a predominantly polymorphonuclear cell infiltration. In order to correlate the immediate hypersensitivity reactions with the humoral response, OVA-specific IgG1, IgG2a and IgE were measured in plasma obtained after 4 or 8 days of immunization. These antibodies were only detected in mice immunized 8 days before (antibody/titre−1: IgG1 = 1·5 × 103; IgG2a = 9 × 102; IgE = 28). Lymproliferation and cytokine production by the OVA-primed LN cells were also determined to evaluate the profile of the immune response at each time-point. Upon stimulation with OVA or concanavalin A (Con A), the LN cells obtained after 4 or 8 days of immunization showed similar proliferative responses. Thymidine incorporation in unstimulated cell cultures from 8-day immunized mice, however, was two-fold higher (data not shown). The levels of IL-4 were much higher in cultures from LN cells obtained on day 4 than on day 8, whether stimulated with OVA or Con A (Table 1). In contrast, the opposite was observed for the levels of IFN-γ, but the difference was less pronounced. IL-10 and IL-2 levels on day 4 or 8 were only different in Con A-stimulated cell cultures and followed the same pattern of IL-4 and IFN-γ, respectively.
Figure 1.
Hypersensitivity reactions in DBA/2 mice immunized s.c. with OVA in CFA and challenged 4 days (a) or 8 days (b) later with aggregated OVA in the footpad. Normal mice (control) were challenged in the same way for non-specific swelling. The results represent the arithmetic mean of net increase in footpad thickness of six mice ±SEM. ⋆ P < 0·05 compared with control mice.
Table 1.
Cytokines secreted by lymph node cells from 4-day or 8-day OVA-immunized mice
| OVA-primed LN cells* | ||||
|---|---|---|---|---|
| OVA | Con A | |||
| Cytokine† | 4-day | 8-day | 4-day | 8-day |
| IL-4 (pg/ml)‡ | 33·1 ± 2·8 | < 4·9 | 87·3 ± 6·2 | 23·4 ± 2·0 |
| IL-10 (U/ml)‡ | 16·1 ± 0·5 | 16·8 ± 1·1 | 31·0 ± 0·5 | 16·3 ± 0·3 |
| IFN-γ (ng/ml)‡ | 9·5 ± 0·9 | 16·4 ± 2·7 | 21·5 ± 2·5 | 28·3 ± 1·2 |
| IL-2 (ng/ml)§ | 1·6 ± 0·3 | 1·2 ± 0·2 | 14·4 ± 1·5 | 24·3 ± 1·4 |
*Culture supernatants from 4-day or 8-day OVA-primed LN cells in vitro restimulated with OVA (500 µg/ml) or Con A (5 µg/ml).
All the cytokines were assayed by two-site sandwich ELISA and the results represent the mean of duplicate cultures ±SD. The cytokine levels of unstimulated cultures were: IL-4 < 4·9; IL-10 < 3·1; IFN-γ < 1·5; IL-2 < 0·2.
IL-4, IL-10 and IFN-γ were measured in 72 hr (6 × 106 cells) supernatants.
IL-2 was quantified in 24 hr (10 × 106 cells) supernatants.
Effect of inhibition of immediate hypersensitivity reactions on DTH
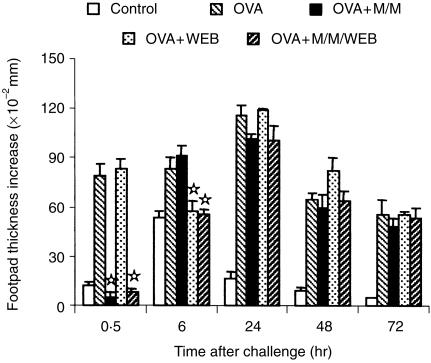
We next studied the effect of mediators released in the immediate-type reactions on the development of DTH in the same site. For this, 8-day immunized mice were treated with antagonists of histamine (mepyramine), serotonin (methysergide) and PAF (WEB 2170), just before antigen challenge in the footpad. The immediate reaction that developed at 30 min in untreated mice was completely abrogated in mice injected with mepyramine/methysergide and mepyramine/methysergide/WEB 2170, but not with WEB 2170 alone (Fig. 2). In contrast, the reaction that developed at 6 hr was only reduced to the levels of non-immunized, OVA-challenged mice in the latter two situations. These results confirmed the anaphylactic nature of the reaction peaking at 30 min, that is mainly mediated by histamine and serotonin. They also showed the participation of PAF in the reaction developed at 6 hr, which was elicited by immune complexes. Despite the inhibition of either or both types of immediate reactions, there was no change in the time course or intensity of the DTH reactions in any group.
Figure 2.
Effect of inhibition of immediate hypersensitivity reactions on DTH. Eight-day immunized mice were injected i.v. with mepyramine and methysergide (M/M), WEB 2170 (WEB) alone or together with mepyramine and methysergide (M/M/WEB) before antigen challenge in the footpad. A group of untreated OVA-immunized (OVA) and another of non-immunized (Control) mice were challenged in the same way. The results represent the arithmetic mean of net increase in footpad thickness of six mice ±SEM. ⋆P < 0·05 compared with untreated OVA-immunized mice.
Effect of development of immediate hypersensitivity reactions by passive transfer of immune serum on the effector phase of tuberculin-type DTH
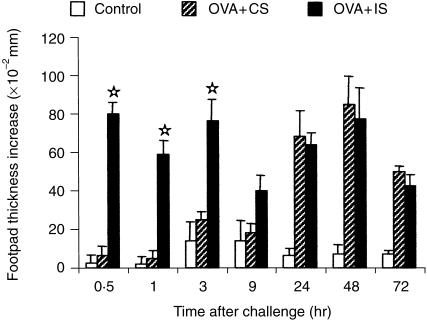
Since OVA-immunized mice challenged on day 4 express only a tuberculin-type DTH reaction, we decided to inject the animals with immune serum before antigen challenge (effector phase), in order to induce a passive immediate-type reaction in the footpad where DTH would later develop. Sera were obtained from donor mice immunized with OVA in CFA or CFA alone and bled after 8 or 28 days. Transfer of 1 ml of immune or control serum was performed 15 hr and 1 hr before challenge with aggregated OVA. Mice injected with immune serum from 8-day immunized donors developed weak immediate reactions, but had the same profile of DTH as mice injected with control serum (data not shown). As shown in Fig. 3, strong immediate reactions were detected at 30 min and 3 hr in mice that received the 28-day immune serum, and this did not modify the characteristic 48 hr peak of DTH observed in 4-day immunized mice.
Figure 3.
Effect of passive transfer of immune serum on DTH. Four-day immunized mice were injected i.v., before antigen challenge in the footpad, with 2 ml of serum from donors immunized with OVA in CFA (OVA + IS) or CFA alone (OVA + CS), 28 days before. A group of non-immunized (Control) mice was challenged in the same way. The results represent the arithmetic mean of net increase in footpad thickness of six mice ±SEM. ⋆P < 0·05 compared with OVA + CS group.
Modulation of the inductive phase of DTH by transfer of serum or cells from hyperimmunized mice
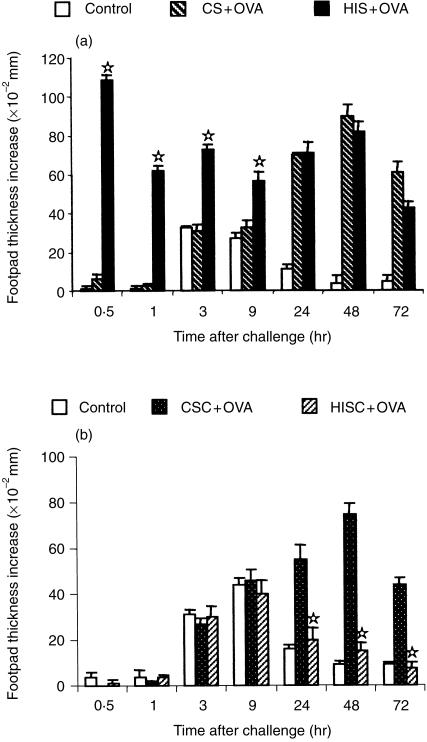
We next investigated how serum or cells derived from high OVA-specific antibody producers could modulate the induction of a cell-mediated response like DTH. Donor mice, hyperimmunized with OVA in alum as described in the Material and methods, displayed only immediate-type reactions and no DTH at all (data not shown). In addition, they produced large amounts of IgG1 (antibody/titre−1 >4×106) and of IgG2a (antibody/titre−1 >8×103) Transfer of hyperimmune serum to recipient mice followed 12 hr later by immunization with OVA in CFA resulted in development of immediate-type reactions at 30 min and 3 hr, when these mice were challenged after 4 days of immunization. The profile of the DTH reaction, however, was the same as in mice that had received serum from donors injected only with alum (Fig. 4a). In contrast, transfer of 6 × 107 spleen cells from hyperimmunized donors 12 hr before immunization of recipient mice did not induce any kind of immediate reaction, but completely suppressed the DTH reaction compared to recipients of spleen cells from alum-injected donors (Fig. 4b).
Figure 4.
Effect of transfer of serum (a) or cells (b) from hyperimmunized donors on induction of DTH. Groups of recipient mice received i.v. 2 ml of serum (HIS + OVA) or 6 × 107 spleen cells (HISC +OVA) from hyperimmune donors, before immunization. Other groups of recipients received serum (CS + OVA) or cells (CSC + OVA) from control donors. All the groups were immunized with OVA in CFA and challenged after 4 days. A group of non-immunized (Control) mice was challenged in the same way. The results represent the arithmetic mean of net increase in footpad thickness of six mice ±SEM. ⋆P < 0·05 compared with CS + OVA (a) or CSC + OVA (b) group.
Transfer of T-enriched spleen cells from hyperimmunized donors suppresses the induction of DTH and changes the cytokine profile in recipient mice
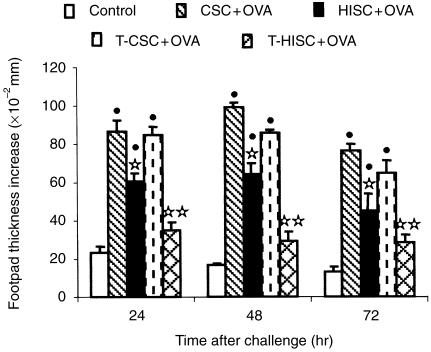
Spleen cells from donor mice hyperimmunized with OVA in alum or injected only with alum were then enriched with T cells and 4 × 107 cells were injected i.v. into recipient mice 12 hr before immunization with OVA in CFA. After challenge on day 4, we observed that transfer of whole hyperimmune spleen cells induced a partial but significant suppression of DTH, while transfer of the same number of T-enriched cells completely abrogated the reactions from 24 to 72 hr (Fig. 5). At this time-point, the mice were bled and their LN cells were restimulated in vitro with OVA for 72 hr. Recipients of OVA-specific, T-enriched cells produced much more IL-4 and IL-10 than those of OVA-specific, whole spleen cells compared with the respective control recipients (Table 2). In contrast, the levels of IFN-γ in recipients of T-enriched cells from hyperimmunized mice were almost three-fold lower than those found in recipients of whole spleen cells. Regarding antibody production (data not shown), recipients of whole or T-enriched, hyperimmune spleen cells showed higher antibody titres compared to their respective controls, but OVA-specific IgG2a increased less (around 30-fold) than IgE (150-fold) and IgG1 (more than 250-fold).
Figure 5.
DTH in recipient mice injected i.v. with 4 × 107 whole (HISC +OVA) or T-enriched (T-HISC +OVA) spleen cells from hyperimmunized donors. Other recipient mice received whole (CSC + OVA) or T-enriched (T-CSC + OVA) spleen cells from control donors. All the groups were immunized with OVA in CFA and challenged after 4 days. A group of non-immunized (Control) mice was challenged in the same way. The results represent the arithmetic mean of net increase in footpad thickness of four to six mice ±SEM. •P < 0·05 compared with Control group; ⋆P < 0·05 compared with CSC + OVA group; ⋆⋆P < 0·05 compared with T-CSC + OVA group.
Table 2.
Cytokines secreted by lymph node cells from OVA-immunized recipient mice transferred with spleen cells from hyperimmunized donor mice
| OVA-primed LN cells* | ||||
|---|---|---|---|---|
| Cytokine‡ | W-Control† | W-Immune† | T-Control† | T-Immune† |
| IL-4 (pg/ml)§ | 15·2 ± 0·4 | 29·2 ± 3·7 | 18·6 ± 5·5 | 61·1 ± 3·4 |
| IL-10 (U/ml)§ | 12·5 ± 0·7 | 19·3 ± 0·3 | 15·4 ± 1·2 | 35·1 ± 3·1 |
| IFN-γ (ng/ml)§ | 28·8 ± 1·3 | 44·3 ± 2·2 | 25·1 ± 0·1 | 15·5 ± 0·5 |
*Culture supernatants of LN cells obtained from 4-day OVA-immunized recipient mice 3 days after OVA footpad challenge.
Recipient mice were transferred i.v., before immunization, with whole (W-) or T-enriched (T-) spleen cells obtained from donor mice injected with alum (Control) or hyperimmunized with OVA in alum (Immune).
All the cytokines were assayed by two-site sandwich ELISA and the results represent the mean of duplicate cultures ±SD. The cytokine levels of unstimulated cultures were: IL-4 < 4·9; IL-10 < 3·1; IFN-γ < 1·5.
IL-4, IL-10 and IFN-γ were measured in supernatants of 6 × 106 LN cells restimulated with OVA for 72 hr.
Discussion
In this work, we have demonstrated that antibody-mediated responses do not have any effect upon the inductive and effector phases of cell-mediated immunity, but T cells from high antibody producers can suppress the inductive phase. For this, we used two time-points in the course of the immune response to OVA, one after 4 days of immunization, when a pure DTH reaction was obtained after antigen challenge, resembling the tuberculin-type reaction,1 and another after 8 days, when the DTH was more like the Jones–Mote reaction,2 with an earlier onset and preceded by immediate hypersensitivity reactivity. The histological aspect of the reactions, with a prominent mononuclear cell infiltrate on day 4 and a predominantly granulocytic infiltrate plus oedema on day 8, also characterized them as such. Cher and Mosmann12 have shown that a Jones–Mote-type DTH can be obtained by local adoptive transfer of several mouse T helper type 1 (Th1), but not Th2, clones and IFN-γ is one of the effector cytokines in this reaction.13 In this sense, our results show that in vitro antigen stimulation of LN cells draining the DTH site induced relatively more IFN-γ than IL-4, when a Jones–Mote-like reaction was elicited on day 8 of immunization, and more IL-4 than IFN-γ on day 4.
Two approaches were used to correlate the extent and time course of the two types of DTH with the presence or absence of immediate-type reactions in the same site during the first hours after antigen challenge. First, antagonists of mediators that play an important role in these reactions14,15 were used in mice challenged on day 8 to effectively abolish anaphylactic (30 min) and Arthus (6 hr) reactions. Second, passive immediate-type reactions were induced in mice challenged on day 4 by transfer of immune serum. None of these procedures led to any change in the evolution over time of either type of DTH, indicating that activation of effector cells and release of mediators responsible for macroscopically detectable, immediate-type reactions do not appear to have any influence over the development of delayed-type reactions in the site of antigen challenge. Ptak et al.16 have reported that very small amounts of monoclonal IgE antibody or serotonin, which were unable to produce a macroscopically measurable early reaction, were still able to initiate a delayed reaction mediated by adoptive transfer of late-acting effector DTH T cells. In addition, the inability of high doses of IgE or IgG1 to mediate DTH initiation was caused by local release of large inhibitory amounts of histamine. Since 50 mg/kg of methysergide had been used to inhibit footpad swelling and localization of effector cells at DTH sites,17 it is possible that in our experiments, where 2·5 mg/kg of the drug was used, some serotonin was left active after the treatment of 8-day immunized mice, which could still account for the initiation of DTH. However, it seems unlikely that the prominent early reaction induced by transfer of immune serum in 4-day immunized mice would not affect at all the intensity or time course of the DTH reaction, if local release of histamine in large amounts could inhibit not only the DTH T-cell function of isolated, transferred cells, but also DTH initiation in actively sensitized animals.
In fact, even transfer of hyperimmune serum, which induced the highest early reaction of all, could not change the profile of the DTH reaction in 4-day immunized recipient mice. The transfer of spleen cells from high antibody producers 12 hr before immunization, in contrast, completely inhibited the development of DTH in similar recipients. Such an effect seems to be mediated by Th2 cells, since OVA-specific, T-enriched spleen cells were more suppressive than the same number of whole spleen cells and mice that received the former cells showed a different cytokine profile, synthesizing more IL-4 and IL-10 and less IFN-γ. In support of this interpretation, change in cytokine profile in recipients of hyperimmune spleen cells resulted in a great enhancement of IgG1 (mainly) and IgE antibody production, that was positively regulated by IL-4. These results indicate that cytokine effects, which occurred before initial exposure to antigen, dramatically altered the type of CD4+ T-cell subset that underwent expansion following encounter with antigen. Rizzo et al.18 have shown that Th1 and Th2 clones interact in vivo to determine the outcome of antibody and DTH responses. The result of this interaction is a decrease in the magnitude of DTH as compared to the response seen when only a Th1 clone is present. In addition, it is known that IL-4 and IL-10 synergize in the inhibition of the effector function of Th1 cells, as demonstrated in a tuberculin-type DTH response to Leishmania major.19 Therefore, in our system, the transferred cells from hyperimmunized mice could also act by modulating the effector stage of DTH reactivity through the release of these cytokines.
We have recently obtained new data using OVA-specific, spleen T cells expanded in vitro with antigen and IL-2 and selected with antibody-coated magnetic beads. The results showed that neutralization of IL-4 by treatment with mAb at the same time as transfer of these cells to and immunization of recipient mice abolished the suppression of the inductive phase of DTH and the recipients developed typical DTH reactions when challenged with antigen after 4 days (Jacysyn, Abrahamsohn and Macedo, manuscript in preparation).
It should also be mentioned here that challenge of OVA-immunized mice after 10, 12, or 14 days resulted in the elicitation of more vigorous immediate-type reactions, while the delayed-type reactions faded (data not shown).
Together these data suggest that in an ordinary, non-polarized immune response there is a bias towards cell-mediated immunity at the beginning of the response, which is gradually controlled by the development of antibody-mediated immunity. This control is mainly exerted at the inductive phase by Th2-derived cytokines, but not by elements of antibody-mediated effector mechanisms, and leads to changes in the characteristics and manifestation of cell-mediated reactions during the time course of the immune response to protein antigens.
Acknowledgments
J.F.J. was in receipt of a fellowship from CNPq and CAPES.
References
- 1.Alexander J, Curtis J. Development of delayed hypersensitivity response in Mycobacterium lepraemurium infections in resistant and susceptible mice. Immunology. 1979;36:563–7. [PMC free article] [PubMed] [Google Scholar]
- 2.Richerson HB, Dvorak HF, Lestowitz S. Cutaneous basophilic hypersensitivity: a new interpretation of Jones-Mote reaction. J Immunol. 1969;103:1431–4. [PubMed] [Google Scholar]
- 3.Toews GB, Bergstresser PR, Streilein JW. Epidermal Langerhans cell density determines whether contact sensitivity or unresponsiveness follows skin painting with DNFB. J Immunol. 1980;124:445–53. [PubMed] [Google Scholar]
- 4.Crowle AJ. Delayed hypersensitivity in the mouse. Adv Immunol. 1975;20:197–265. doi: 10.1016/s0065-2776(08)60209-6. [DOI] [PubMed] [Google Scholar]
- 5.Collins FM, Mackaness GB. Delayed hypersensitivity and Arthus reactivity in relation to host resistance in Salmonella-infected mice. J Immunol. 1968;101:830–45. [PubMed] [Google Scholar]
- 6.Parish CR, Liew FY. Immune response to chemically modified flagellin. III. Enhanced cell-mediated immunity during high and low zone tolerance to flagellin. J Exp Med. 1972;135:298–311. doi: 10.1084/jem.135.2.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Silver J, Benacerraf B. Dissociation of T cell helper function and delayed hypersensitivity. J Immunol. 1974;113:1872–5. [PubMed] [Google Scholar]
- 8.Titus RG, Chiller JM. A simple and effective method to assess murine delayed type hypersensitivity to proteins. J Immunol Meth. 1981;45:65–78. doi: 10.1016/0022-1759(81)90094-6. [DOI] [PubMed] [Google Scholar]
- 9.Nutman TB. Measurement of polyclonal immunoglobulin synthesis using ELISA. In: Coligan JE, Margulies AM, Shevach EM, Strober W, editors. Current Protocols in Immunology. New York: Wiley; 1991. pp. 7.12.1–6. [DOI] [PubMed] [Google Scholar]
- 10.Ferreira AP, Faquim ES, Abrahamsohn IA, Macedo MS. Immunization with Ascaris suum extract impairs T cell functions in mice. Cell Immunol. 1995;162:202–10. doi: 10.1006/cimm.1995.1070. 10.1006/cimm.1995.1070. [DOI] [PubMed] [Google Scholar]
- 11.Zar JH. Biostatistical Analysis. New York: Prentice Hall; 1984. [Google Scholar]
- 12.Cher DJ, Mosmann TR. Two types of murine helper T cell clone. II. Delayed-type hypersensitivity is mediated by TH1 clones. J Immunol. 1987;138:3688–94. [PubMed] [Google Scholar]
- 13.Fong TAT, Mosmann TR. The role of IFN-γ in delayed type hypersensitivity mediated by TH1 clones. J Immunol. 1989;143:2887–93. [PubMed] [Google Scholar]
- 14.Movat HZ. The Inflammatory Reaction. New York: Elsevier; 1985. [Google Scholar]
- 15.Barnes PJ, Chung KF, Page CP. PAF as a mediator of allergic diseases. J Allergy Clin Immunol. 1988;81:919–34. doi: 10.1016/0091-6749(88)90952-9. [DOI] [PubMed] [Google Scholar]
- 16.Ptak W, Geba GP, Askenase PW. Initiation of delayed-type hypersensitivity by low doses of monoclonal IgE antibody. Mediation by serotonin and inhibition by histamine. J Immunol. 1991;146:3929–36. [PubMed] [Google Scholar]
- 17.Askenase PW, Metzler CM, Gershon RK. Localization of leucocytes in sites of delayed-type hypersensitivity and in lymph nodes: dependence on vasoactive amines. Immunology. 1982;47:239–46. [PMC free article] [PubMed] [Google Scholar]
- 18.Rizzo LV, DeKruyff RH, Umetsu DT, Caspi RR. Regulation of the interaction between Th1 and Th2 T cell clones to provide help for antibody production in vivo. Eur J Immunol. 1995;25:708–16. doi: 10.1002/eji.1830250312. [DOI] [PubMed] [Google Scholar]
- 19.Powrie F, Menon S, Coffman RL. Interleukin-4 and interleukin-10 synergize to inhibit cell-medited immunity in vivo. Eur J Immunol. 1993;23:2223–9. doi: 10.1002/eji.1830230926. [DOI] [PubMed] [Google Scholar]