Abstract
Dendritic cells (DCs) are bone marrow-derived antigen-presenting cells that have an exquisite capacity to interact with T cells and modulate their responses. Little is known about porcine DCs despite the fact that they represent an important target in strategies that are aimed at modulating resistance to infection in pigs and may be of major importance in transplantation biology. We generated immature monocyte-derived porcine dendritic cells (MoDCs) directly from adherent peripheral blood cells treated with porcine granulocyte–macrophage colony-stimulating factor (GM-CSF) and interleukin-4 (IL-4). The cells were observed via electron microscopy and their phenotype was characterized using monoclonal antibodies. The functionality of the porcine MoDCs was demonstrated showing that the cells were capable of different specialized functions relevant to antigen capture and were potent stimulators in a primary allo-mixed leucocyte reaction. Treatment of the MoDCs with porcine cell line-derived necrotic factors resulted in the phenotypic and functional maturation of MoDCs. We confirmed also that monocyte-derived DCs were differentially regulated by cytokines, showing that transforming growth factor-β1 (TGF-β1) is able to redirect monocytic precursors into the differentiation pathway of Langerhans' cells presenting typical Birbeck granules. Interestingly, and in contrast to the human and murine model, we showed that the monocyte-derived porcine Langerhans'-type cells (MoLCs) were much more potent activators of allogeneic T cells than MoDCs obtained without TGF-β1.
Introduction
Dendritic cells (DCs) are professional antigen-presenting cells (APC) that are responsible for the activation of naive T cells and the generation of primary T-cell responses.1 Their specific role is to capture, process and present antigens to T cells. Immune and inflammatory signals are responsible for the migration of DCs from tissues to lymphoid organs where they initiate an immune response. These processes induce the maturation of DCs, which results in a shift from a processing to a presenting stage.
DCs are characterized by their unusual dendritic morphology, by their potent antigen-presenting capacity and by the lack of T cells, B cells, natural killer (NK) cells and monocyte-specific markers. Nevertheless, considerable functional and phenotypic heterogeneity has been documented within the DC system. It is still not clear whether the different DCs represent different stages of maturation of unique DC lineage or whether they stem from different progenitors.2
DCs are found in virtually all tissues of the body and circulate in the peripheral blood, but large-scale preparation of DCs is hampered by their very low frequency. Previous reports have described the in vitro generation of human DC using the CD34+ progenitor pool in the cord blood or by using monocyte/macrophage CD14+ blood cells.3–6 Cord blood CD34+ haematopoietic progenitor cells treated with granulocyte–macrophage colony-stimulating factor (GM-CSF) and tumour necrosis factor-α (TNF-α) differentiate into two distinct DC lineages: Langerhans' cell (LC)-type DCs and CD14+-derived DCs. Monocytes treated with GM-CSF and interleukin-4 (IL-4) rapidly become non-adherent, acquire DC morphology and down-regulate CD14 expression. LCs represent a discrete population among DCs, and the current hypothesis is that LCs arise from a specific precursor.4,7 However, it has been shown recently that LCs may originate from monocytes in response to GM-CSF, IL-4 and transforming growth factor-β1 (TGF-β1).8
Little is known about porcine DCs despite the fact that they represent an important target in strategies that are aimed at modulating resistance to infection in pigs and should have major importance in transplantation biology.
As monocytes are directly accessible from peripheral blood and seem to represent a cell population with a high developmental flexibility towards DCs, we chose to generate monocyte-derived porcine DCs (MoDCs). In the present study, we characterized the phenotype and the functionality of cells generated from adherent peripheral blood mononuclear cells (PBMC) using porcine GM-CSF and IL-4. Furthermore, we confirmed that MoDCs are differentially regulated by cytokines, showing that TGF-β1 is able to redirect monocytic precursors into the differentiation pathway of LCs.
Materials and methods
Animals
Blood samples were taken from Large White/Landrace hybrid pigs (10 to 13 weeks of age) of both sexes. Pigs were maintained in conventional conditions on a commercial diet.
Cytokines, maturation factors and cell line
Recombinant swine GM-CSF and IL-4 were prepared in our laboratory from CHO-K1 cells (ECACC no. 85051005, European Collection of Cell Culture, Salisbury, UK) transiently transfected with GM-CSF- or IL-4-coding plasmids. The supernatant from transfected cells was harvested after 24 hr, centrifuged (4800 g) and stored at −20°. The functionality of these two cytokines has been tested previously using bioassays (data not shown): IL-4-containing supernatant was shown to induce porcine lymphoblast proliferation up to a dilution of 1:2000, and GM-CSF-containing supernatant was shown to induce the differentiation of granulomacrophagic colonies (CFU-GM) from porcine bone marrow cell culture in vitro.
Porcine TNF-α and TGF-β1 were obtained from Endogen (Woburn, MA). Lipopolysaccharide (LPS) (from Salmonella abortus equi) was purchased from Sigma (St Louis, MO). Necrotic factors were obtained following four freeze–thaw cycles of the IRP porcine cell line. The IRP cell line originates from porcine kidney and has been established in-house.
Cell isolation and cultures
PBMC were isolated by using Pancoll (d = 1·077; D. Dutscher, Strasbourg, France) density-gradient centrifugation (1100 g for 30 min) of heparinized blood obtained from healthy pigs. These PBMC were plated (1 × 107 cells/3 ml per well) into 6-well plates (Corning, Brumath France) in medium (RPMI-1640 supplemented with 10% heat-inactivated fetal calf serum [FCS], 50 µmol/l of β-mercaptoethanol and antibiotics; Life Technologies, Cergy Pontoise, France) supplemented with 1% GM-CSF- and IL-4-containing supernatants. Non-adherent cells were removed after 16 hr of incubation at 39°; the adherent cells were maintained in the same culture conditions. The DC cultures were fed every 3 days with fresh medium and 1% GM-CSF- and IL-4-containing supernatants. In some cultures, 10 ng/ml of TGF-β1 was added at the time of culture initiation.
Electron microscopy
Cultured cells (sampled after 7 days of culture) were fixed in 2% glutaraldehyde, postfixed in 1% osmium tetroxide (Euromedex, Souffelweyersheim, France) and embedded in Epon 812 (Sigma). Ultrathin sections were then cut, stained with uranyl acetate and lead citrate, and examined using an electron microscope (Philips CM10; Lyon 1 Microscopy Center, Lyon, France).
Immunofluorescence analysis
Immunofluorescence analysis was performed on 5 × 105 cells. Monoclonal antibodies (mAbs) to pig CD1 (clone 76-7-4), human (hu) CD36 (CB38) and huCD68 (Y1/82 A), were obtained from PharMingen (La Jolla, CA). mAbs to pig CD11a/18 (MUC76A), CD14 (M-M9), CD11b/c (PGBL18A) and major histocompatibility complex (MHC) class I (H58A) were obtained from VMRD Inc. (Pullman, WA). mAbs to CD25 (231.3B2), CD45Ra (MIL13) and MHC class II (274 3G8) were obtained from Serotec (Oxford, UK). mAb to hup55 Fascin (55K-2) was obtained from Dako (Carpinteria, CA). In indirect assays, reactivity was detected using either fluorescein isothiocyanate (FITC)- or phycoerythrin (PE)-conjugated anti-mouse immunoglobulin G (IgG) and immunoglobulin M (IgM) (Interchim, Montlucon France). Intracellular staining was performed using the Cytofix/Cytoperm plus kit (PharMingen). Cells were analysed using a fluorescence-activated flow cytometer (FACScalibur flow cytometer; Becton-Dickinson, Mountain View, CA) calibrated with Calbrite beads (Becton-Dickinson) for FITC and PE. The distribution of debris, dead cells and any contaminating red blood cells was assessed on the basis of forward and right-angle scatter before proceeding with the analysis. A total of 10000 events were examined using a 488-nm wavelength excitation. Acquired events were analysed using Cell Quest software (Becton-Dickinson).
Endocytosis
Cells were washed in phosphate-buffered saline (PBS) and incubated with 0·1 mg/ml of FITC-dextran (DX-FITC; 40 000 molecular weight) or with 1 mg/ml of bovine serum albumin (BSA)-FITC (Sigma Chemical Co.) at 39° or 0° for different periods of time. Uptake was stopped by adding ice-cold fluorescence-activated cell sorter (FACS) buffer (PBS, 5% BSA, 0·02% sodium azide) containing 1% formol.
Formation of immune complexes (ICs)
ICs were formed by incubating 5 µg of β-galactosidase (Sigma) with anti-β-galactosidase serum from Sigma (1:100). Free β-galactosidase was eliminated by heat denaturation at 62·5°. The resulting immune complexes were then assayed by detection of the remaining β-galactosidase enzyme activity protected by antibodies, using FDG reagent (Sigma). DCs were fed with the β-galactosidase ICs or non-denaturated β-galactosidase protein (as a control) for 4 hr. Incorporated β-galactosidase was finally revealed by flow cytometry using the FDG substrate.
Mixed leucocyte reaction (MLR)
APCs were treated with mitomycin C (50 µg/ml; Sigma) for 20 min and washed three times in PBS. PBMC (5 × 104) were stimulated at different APC/effector-cell ratios for 5 days. Cultures were performed in triplicate (in 96-well plates) in 200 µl of culture medium. During the final 8 hr of incubation, each culture was labelled with 0·5 µCi of [3H]thymidine (Amersham, Les Ulis France). Cells were harvested onto glass fibre disks using a multiple cell harvester, and thymidine incorporation was determined (in counts per minute [c.p.m.]) in a liquid scintillation counter (MicroBeta counter; EG & G Wallac, Evry France). The results were expressed as index of proliferation:
Results
Generation of porcine DCs from cultured adherent PBMC
DCs were derived from adherent PBMC cultured in medium supplemented with porcine GM-CSF and IL-4. Adherent aggregates were visible on day 3. After 7 days, cultures consisted mainly of single and aggregated floating cells, presenting a typical veiled morphology. The cells obtained were evaluated by transmission electron microscopy, which revealed that the cells had a large diameter, showed pronounced protrusions and microvillous projections of their plasma membrane, and contained abundant multivacuolar and multilamellar vesicles (Fig. 1). Overall, the adherent PBMC-derived porcine cells showed ultrastructural features characteristic of DCs.
Figure 1.
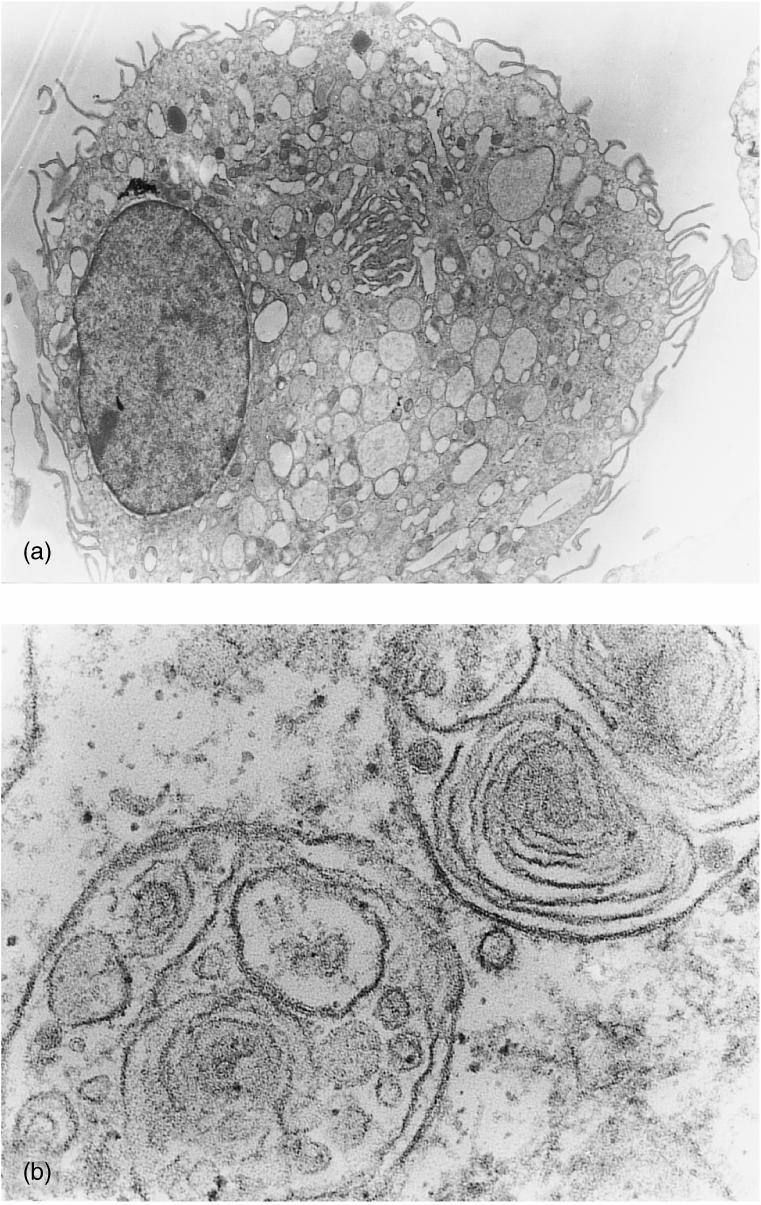
Transmission electron microscopy was used to evaluate the cellular morphological characteristics of adherent peripheral blood mononuclear cells (PBMC) after 7 days of culture in the presence of porcine granulocyte–macrophage colony-stimulating factor (GM-CSF) and interleukin-4 (IL-4). (a) Electron micrograph showing a cell with a large diameter and microvillous projection of the plasma membrane (magnification ×2600). (b) Details of the multivacuolar and multilamellar intracytoplasmic organites (magnification ×46000).
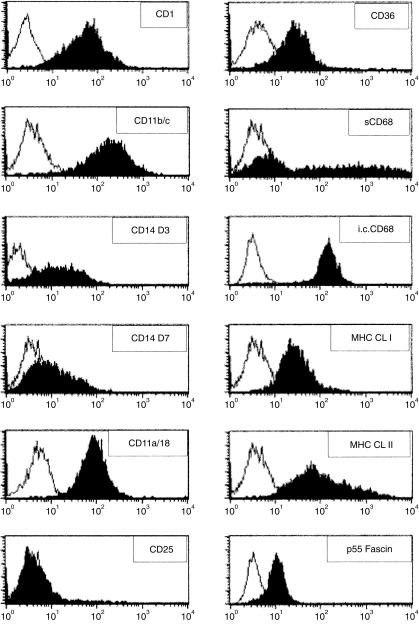
The surface phenotype of this DC-enriched cell population was analysed. The cells did not express T-, B-, or NK-cell markers (data not shown). Expression of the monocyte/macrophage marker, CD14, was assayed on days 3 and 7 after the start of the culture (Fig. 2). A marked down-regulation of its surface expression was observed, indicating the differentiation of the adherent monocytes into DCs. To assess more specifically the phenotype of these cells, several mAbs directed against surface markers were used. The results presented in Fig. 2 showed that the generated cells were MHC class I+, MHC class II+, CD1+, CD11a/18+, CD11b/c+, CD25–, CD36+, CD68+ and p55 fascin positive. Although none of the markers tested is specific for the dendritic lineage, they all have been reported to be expressed by DCs. The cells expressing the CD1 antigen are recognized by the mAb 76-7-4. This antibody was recently described as being specific to a new member of the CD1 family expressed in porcine cells and shares a high homology with the human CD1a antigen.9 The porcine cells strongly expressed β2-integrins (CD11b/c and CD18), which are important in cell–cell interactions and represent binding molecules for bacteria and LPS.10 Using an anti-human CD68 mAb, we found that the porcine DCs expressed the putative intracellular and surface CD68, which is a lysosome-associated membrane glycoprotein abundant in MoDCs.11 Surface CD68 expressed by DCs has been reported to possibly act as a ligand for E-selectin.12 The actin-bundling p55 fascin protein, which causes aggregation of F-actin into bundles in membrane protusions,13 was also detected in the porcine DCs, using an anti-human p55 mAb. p55 fascin has been reported to be expressed and restricted to mature DCs.5 On the other hand, these DCs express a putative CD1a molecule and low levels of CD14, which represent a phenotype compatible with an immature stage of differentation.14,15
Figure 2.
Flow cytometric phenotyping of the porcine dendritic-type cell. Cells were stained with control isotypes (open histogram) or with the indicated anti-human or anti-porcine monoclonal antibody (mAb) against cell surface markers (solid histogram). Intracellular (i.c.) staining was performed for the human (hu) p55 Fascin. Both i.c. and surface (s) staining were performed for CD68. Data are representative of at least three experiments performed.
Taken together, these results suggest that adherent porcine PBMC cultured with GM-CSF and IL-4 generate cells displaying all the characteristics of MoDCs. Further functional analyses are needed to define the stage of differentiation of the derived porcine MoDCs.
Porcine MoDCs are capable of several antigen-capture mechanisms
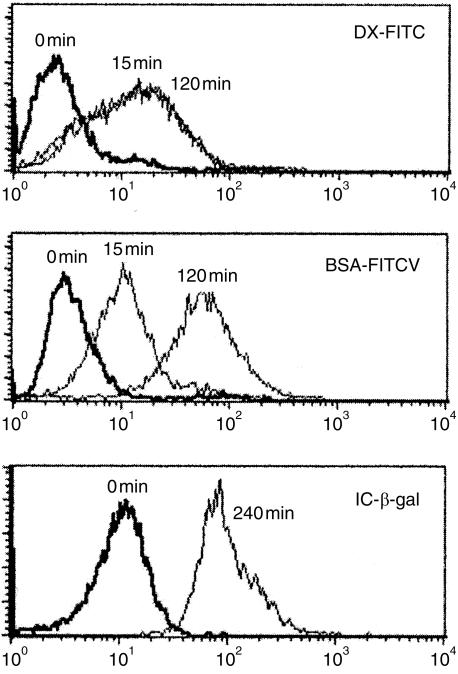
Immature DCs usually possess different specialized functions relevant to antigen uptake, including receptor-mediated endocytosis and macropinocytosis.1 We investigated whether the in vitro-derived porcine MoDCs were able to take up FITC-conjugated ligands. Figure 3 shows that porcine MoDCs were efficient at the uptake of both DX-FITC and BSA-FITC. This property were abolished when the experiments were performed at 4°.
Figure 3.
Uptake of dextran-fluorescein isothiocyanate (DX-FITC), bovine serum albumin (BSA)-FITC and β-galactosidase (β-gal) immune complexes (ICs). Monocyte-derived dendritic cells (MoDCs) were allowed to take up the different ligands for the indicated times at 39° (––) or on ice (——). The resulting fluorescence was read by flow cytometry. Results are representative of at least three experiments.
The kinetic experiments performed at short time intervals indicated that 15 min is sufficient to load the majority of DCs with DX-FITC or BSA-FITC. The fluorescent signal obtained after 15 min of DX-FITC loading was maximal and did not increase when the incubation time was extended to 2 hr (Fig. 3). In contrast, the signal intensity obtained after incubation with BSA-FITC was increased after 120 min. This observation suggests that the two ligands are taken up by different mechanisms. DX-FITC uptake has been shown to be mannose-receptor mediated and the saturated signal probably represents saturation of the mannose receptors.3 In contrast, BSA-FITC uptake is not saturated over time, suggesting that its uptake is mediated by macropinocytosis, independently of membrane receptors. Uptake of both DX-FITC and BSA-FITC was significantly increased when the incubations were carried out for a longer time-period (6–8 hr; data not shown).
Another pathway for antigen uptake by DCs is mediated trough binding of ICs to FcγR receptors.16 MoDCs were fed with β-galactosidase ICs for 4 hr and intracellular β-galactosidase activity was revealed using the fluorescent FDG substrate. Figure 3 shows that β-galactosidase ICs were efficiently internalized into the MoDCs. Therefore, the porcine MoDCs appeared to be efficient at both receptor- and non-receptor-mediated endocytosis.
MoDCs are efficient APC in primary allo-MLR
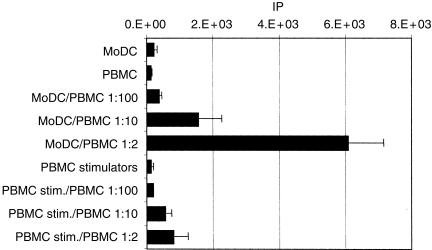
It is well established that the ability of DCs to stimulate a potent allo-MLR distinguishes them from other leucocytes.17 Immature porcine MoDCs were used on day 7 as stimulators of unprimed heterologous porcine PBMCs. As shown in Fig. 4, MoDCs were much more potent activators of allogeneic T cells than were PBMCs from the same animal. Under the same conditions, autologous MoDCs did not induce proliferation (data not shown).
Figure 4.
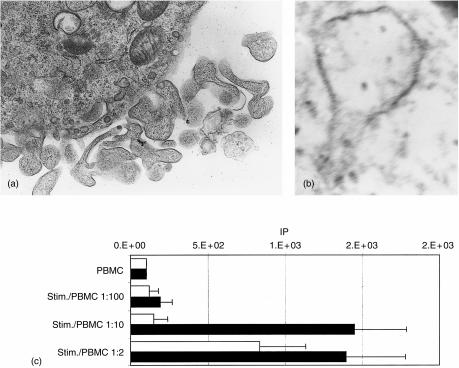
Five-day primary mixed leucocyte reaction (MLR) showing allogeneic T-lymphocyte activation in response to monocyte-derived dendritic cells (MoDCs). Proliferation was determined by [3H]thymidine incorporation. Represented are the means and SD of triplicate wells containing mitomycin-treated allogeneic MoDCs or peripheral blood mononuclear cells (PBMC) and 2 × 105 fresh responding PBMC, at the indicated ratios. MoDCs or PBMC alone were included as controls. Data shown are representative of four experiments. IP, index of proliferation (see Materials and methods).
Phenotypic and functional maturation of the porcine MoDCs can be induced in vitro
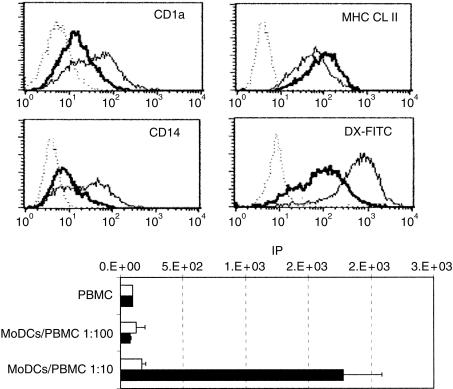
LPS, TNF-α and necrotic factors were tested for their ability to induce the maturation of MoDCs. Figure 5(a) shows the down-regulation of the CD1 and CD14 surface markers and the up-regulation of MHC class II molecules observed after 3 days of treatment with the necrotic factors. The down-modulation of CD1a and CD14 markers has been reported to occur during DC maturation.14,15 We showed here that the CD1 molecule recognized by the 76-7-4 mAb seemed to behave like the CD1a molecule expressed by human DCs.
Figure 5.
Phenotypic and functional modification induced by treatment of the monocyte-derived dendritic cells (MoDCs) with IRP necrotic factors. (a) Five-day-old MoDCs were cultured for 4 days with (thick line) or without (thin line) IRP necrotic factor (1:2 dilution). Untreated (thin line) and necrotic factor-treated (thick line) MoDCs were stained for CD1a, CD14 and major histocompatibility complex (MHC) class II molecules and control isotypes (dotted line). Untreated (thin line) and necrotic factor-treated (thick line) MoDCs were incubated for 4 hr with dextran-fluorescein isothiocyanate (DX-FITC) at 39° or on ice (dotted line). (b) Proliferative responses of 2 × 105 porcine PBMC in response to allogeneic untreated (open histogram) or necrotic factor-treated (solid histogram) MoDCs. IP, index of proliferaion (see Materials and methods).
The down-modulation of receptor-mediated endocytosis of DCs after treatment of immature DCs with maturation stimuli has been well documented.18 To evaluate whether the necrotic factor-induced phenotypic maturation of MoDCs was accompanied with down-regulation of antigen uptake, we measured DX-FITC uptake by untreated and necrotic factor-treated MoDCs. In contrast to untreated MoDCs, DX-FITC uptake was markedly down-regulated in necrotic factor-treated MoDCs (Fig. 5a). The same phenotypic and functional alterations were obtained using LPS or TNF-α as maturation factors, but the strongest effect was mediated by the necrotic factors (data not shown).
Finally, the stimulatory properties in a primary allo-MLR of the necrotic factor-treated MoDCs were compared with those of the untreated MoDCs. Untreated MoDCs were clearly weaker in their stimulatory capacity (Fig. 5b). Therefore, treatment with necrotic factors induced MoDCs that were much more potent activators of allogeneic T cells as compared to non-treated cells.
Our data clearly demonstrate that porcine MoDCs can maturate in vitro and suggest that MoDCs derived in the presence of GM-CSF and IL-4, and without the addition of other factors, give rise to immature porcine MoDCs.
Addition of TGF-β1 induces Langerhans'-type cell derivation
Several observations suggest that monocytes can differentiate into LCs when TGF-β1 is added to the culture medium together with GM-CSF and IL-4.8 We tested whether the addition of TGF-β1 could induced porcine monocytes to differentiate into LC. After 7 days of culture in the presence of GM-CSF, IL-4 and TGF-β1, the generated cells were observed by electron microscopy. Dendritic-shaped cells with a moderate amount of Birbeck granules (resembling tennis racket-like structures) were observed (Fig. 6a, 6b), indicating a typical LC morphology. These MoLCs contained moderate amounts of Birbeck granules compared to LCs observed in vivo. We observed that the cell morphology of MoLCs was slightly different from the MoDCs generated in the absence of TGF-β, displaying shorter and thicker dendrites (Fig. 6a).
Figure 6.
Addition of transforming growth factor-β1 (TGF-β1) induces Langerhans'-type cell derivation. Electron micrographs are presented of monocyte-derived Langerhans' cells (MoLCs) showing (a) dendrite details (magnification × 10500) and (b) a Birbeck granule (magnification × 92000). (c) Proliferative responses of 2 × 105 porcine peripheral blood mononuclear cells (PBMC) to allogeneic mitomycin-treated monocyte-derived dendritic cells (MoDCs) (open histogram) or MoLCs (solid histogram) after 5 days of culture. Data shown are representative of three experiments. IP, index of proliferation (see Materials and methods).
Relative efficiency of MoDCs and MoLCs to stimulate a primary allo-MLR
It has been shown previously that DCs grown in the presence of TGF-β1 exhibit characteristics of fully immature or early DCs.19–21 Moreover, inhibition of acquisition of mature DC features by cultured LCs has been consistently observed and appears to be mediated by TGF-β1. In the present study, no phenotypic differences were found between the two types of cells by flow cytometry analysis using the mAbs described above (data not shown). When matured with necrotic factors, LPS or TNF-α, the MoLCs behaved like the MoDCs, showing down-regulated expression of CD1 and CD14 surface markers, a down-regulated DX-FITC uptake ability and an enhanced MLR activator potency (data not shown).
Surprisingly, when MoDCs and MoLCs were compared for their capacity to stimulate allogeneic PBMCs in a primary allo-MLR, it was found that porcine MoLCs were significantly more efficient for primary T-cell activation (Fig. 6c). This result suggests that TGF-β1 regulates, in a positive manner, the antigen-presenting capability of the porcine MoLCs generated.
Discussion
We have shown in the present work that adherent porcine PBMC cultured in vitro in the presence of porcine GM-CSF and IL-4 differentiate into cells displaying the morphology, the phenotype and the functions of MoDCs. In addition, phenotypic and functional modifications of the MoDCs in response to maturation stimuli allow us to predict the differentiation stage of the generated cells. We showed that the matured MoDCs presented a down-regulated CD14 and CD1 surface expression, while MHC class II molecules were up-regulated in comparison to the untreated MoDCs. The loss of CD14 and CD1a surface markers has been shown to correlate with human DC maturation.14,15 The porcine CD1 molecule (recognized by the 76-7-4 mAb in this study) is encoded by the recently cloned pCD1.1 gene and represents a new member of the CD1 family.9 It was shown (see ref. 9) that the pCD1.1 nucleotide and deduced amino-acid sequences present the highest homology with the human CD1a molecule. The results reported in the present study suggest that the expression of this porcine CD1 molecule on DCs also may be regulated like the CD1a human molecule. It is known that up-regulation of MHC class II molecules correlates with DC maturation and results from the redistribution of molecules from the intracellular vesicular compartments to the cell surface.22 Moreover, we showed that the phenotypic maturation was accompanied with a down-regulation of the receptor-mediated uptake activity and an increase of the MLR stimulatory capacity. These functional changes correlate with the shift from the processing stage to the presenting stage, which occurs during DC maturation, and have been widely characterized in the literature.18,22 In a recent report published by Basta et al., analysing the changes occurring during porcine monocyte differentiation into macrophage, the uptake of DX-FITC was used as a pH-sensitive probe.23 It was shown that during the maturation process, DX-FITC fluorescence, which is lost in the acidic endosomal vesicles, is inversely correlated with the endocytic efficiency. In contrast, in our experiment the intensity of the fluorescence signal in DX-FITC-loaded DCs seemed to be directly correlated with the uptake efficiency. This difference suggests that the DX-FITC intracellular trafficking is different between the two types of cells and that DX-FITC might escape acidic endosomal vesicles in DCs, as shown by Rodriguez et al. in a mouse DC line.24
It has been described in detail that, during the maturation process, DCs increase their level of surface costimulatory molecules such as CD40, CD80 and CD86, which provide the additional signals required to initiate T-cell activation.22 Unfortunately, we were not able to investigate the expression and up-regulation of the costimulatory molecules in the absence of porcine-specific or cross-reacting antibodies directed against these molecules. The expression of the costimulatory B7 molecules on bone-marrow-derived porcine DCs has been reported by West et al. using the human cytotoxic T-lymphocyte antigen-4 (CTLA-4)–immunoglobulin fusion protein.25 Overall, the porcine DCs appeared to behave in a similar manner to the well-characterized murine or human MoDCs and we predict that the regulation of costimulatory molecule expression on porcine DCs should be equivalent to what has been described for mouse and human models. To our knowledge, the work reported here is the first to describe both immature and mature stages of porcine DCs derived in vitro.
Necrotic cell lines have been recently described as maturation factors for DCs.26,27 Among the different maturation factors tested, the necrotic factors obtained from the IRP porcine cell line seemed to be the most effective. Despite the fact that immature MoDCs expressed low levels of CD14 and strongly expressed β2 integrin receptors, LPS seemed to represent a weaker maturation signal, suggesting that the supernatant of the necrotic IRP cell line may contain a combination of several factors involved in DC maturation. It is of interest that the role of necrotic factors in DC maturation has been challenged and may, in fact, be related to the presence of mycoplasma in the cell lines.28 We cannot rule out this possibility as the IRP cell line used in the present work was not assayed for mycoplasma contamination.
LCs are DCs that are present in the epidermis, bronchi and mucosae. Little is known about their lineage of origin. TGF-β1 has been shown to play an important role in their biology,29,30 and in vitro studies of human or murine haematopoietic progenitor cells revealed that precursor LCs generated in response to TGF-β1 stimulation expressed monocyte-lineage marker molecules.31,32 Recently, Geissmann et al. demonstrated that TGF-β1 in the presence of GM-CSF and IL-4 could induce the differentiation of human monocytes into Langerhans'-type cells.8 We showed in the present work that, in the same manner, porcine TGF-β1 added to porcine GM-CSF and IL-4 drove in vitro differentiation of pig monocytes into Langerhan'-type cells that displayed moderate amounts of Birbeck granules.
It has been reported that the addition of TGF-β1 in vitro promotes the differentiation of fully immature DCs and inhibits acquisition of mature DC features.19–21 Geissmann et al. have shown that Langerhans'-type cells derived from monocytes in the presence of TGF-β1 express almost exclusively intracellular class II antigens and a low level of costimulatory molecules.19 Furthermore, these Langerhans'-type cells did not mature in response to inflammatory stimuli but needed cognate stimulus induced by CD40 to fully mature. In the present study, the phenotype of the porcine MoLCs was not distinguishable from the phenotype of the MoDCs, when studied using our panel of mAbs. Treatment of the MoLCs with maturation factors resulted in the same phenotype and functional modifications to the ones reported here for the MoDCs. We cannot exclude that a more detailed analysis of intracellular MHC class II localization, costimulatory molecule expression and cytokine production by our MoLCs would reveal some differences that distinguished them from the MoDCs. Surprisingly, we observed that the MoLCs were more potent activators in primary allo-MLR than the MoDCs. This observation suggests that TGF-β1 promotes the differentiation of MoLCs with enhanced antigen-processing or costimulatory functions. Additionally, Riedl et al. have shown that TGF-β1 reduced TNF-α-induced Fas expression on LCs derived from CD34+ progenitors, protecting them against Fas-mediated apoptosis.33 We can hypothesize that a reduced suceptibility to Fas-mediated apoptosis of the stimulator MoLCs cells would account for the observed result. Additional work is required to clarify the effect of TGF-β1 on the differentiation of porcine MoLCs.
Despite the fact that pigs represent an important food-animal group and, owing to their similarities with humans, constitute an animal model in medical research, little is known about the cellular immune responses in this species. In particular, access to the porcine CD8 T-cell-mediated immune response would be of great help in vaccine design and xenotransplantation studies. Moreover, the pig presents a original T-lymphocyte compartment containing sizeable, unconventional DP (CD4+ CD8+), DN (CD4– CD8–) and γδ T-cell subsets,34–37 rendering this species a suitable model for elucidating the potential role of these T cells in immune defences. Analysis of the in vitro pig CD8 T-cell response is hampered, in part, by the difficulty of generating APC in an outbred context. We showed in this report that DCs can be easily generated from individual pigs. Moreover, we demonstrated that these porcine DCs can be antigen loaded in different ways. It is now widely accepted that DCs are endowed with cross-presentation capacity38,39 and can therefore present exogenous antigen in combination with class I molecules. We emphasize that these porcine DCs represent an important tool for in vitro studies.
Acknowledgments
We thank M. Bublot (Virogenetics, New York, USA), L. Fischer, B. Charley (INRA, Jouy-en-Josas, France) and their teams for the plasmid constructs expressing the porcine cytokines. J. Estabel and J. M. Exbrayat (General Biological Laboratory, Lyon Catholic University, France) are gratefully acknowledged for the electron microscopy analyses.
Abbreviations
- APC
antigen-presenting cells
- DC
dendritic cell
- DX
dextran
- MLR
mixed leucocyte reaction
- MoDC
monocyte-derived DC
- MoLC
monocyte-derived Langerhans' cell
References
- 1.Cella M, Sallusto F, Lanzavecchia A. Origin, maturation and antigen presenting function of dendritic cells. Curr Opin Immunol. 1997;9:10–6. doi: 10.1016/s0952-7915(97)80153-7. [DOI] [PubMed] [Google Scholar]
- 2.Vandenabeele S, Wu L. Dendritic cell origins: puzzles and paradoxes. Immunol Cell Biol. 1999;77:411–9. doi: 10.1046/j.1440-1711.1999.00857.x. 10.1046/j.1440-1711.1999.00857.x. [DOI] [PubMed] [Google Scholar]
- 3.Caux C, Massacrier C, Vanbervliet B, Dubois B, Durand I, Cella M, Lanzavecchia A, Banchereau J. CD34+ hematopoietic progenitors from human cord blood differentiate along two independent dendritic cell pathways in response to granulocyte–macrophage colony-stimulating factor plus tumor necrosis factor alpha: II. Functional analysis. Blood. 1997;90:1458–70. [PubMed] [Google Scholar]
- 4.Caux C, Vanbervliet B, Massacrier C, et al. CD34+ hematopoietic progenitors from human cord blood differentiate along two independent dendritic cell pathways in response to GM-CSF + TNF alpha. J Exp Med. 1996;184:695–706. doi: 10.1084/jem.184.2.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bender A, Sapp M, Schuler G, Steinman RM, Bhardwaj N. Improved methods for the generation of dendritic cells from nonproliferating progenitors in human blood. J Immunol Methods. 1996;196:121–35. doi: 10.1016/0022-1759(96)00079-8. 10.1016/0022-1759(96)00079-8. [DOI] [PubMed] [Google Scholar]
- 6.Schuler G, Romani N. Generation of mature dendritic cells from human blood. An improved method with special regard to clinical applicability. Adv Exp Med Biol. 1997;417:7–13. [PubMed] [Google Scholar]
- 7.Strunk D, Egger C, Leitner G, Hanau D, Stingl G. A skin homing molecule defines the Langerhans' cell progenitor in human peripheral blood. J Exp Med. 1997;185:1131–6. doi: 10.1084/jem.185.6.1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Geissmann F, Prost C, Monnet JP, Dy M, Brousse N, Hermine O. Transforming growth factor beta1, in the presence of granulocyte/macrophage colony-stimulating factor and interleukin 4, induces differentiation of human peripheral blood monocytes into dendritic Langerhans' cells. J Exp Med. 1998;187:961–6. doi: 10.1084/jem.187.6.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chun T, Wang K, Zuckermann FA, Gaskins HR. Molecular cloning and characterization of a novel CD1 gene from the pig. J Immunol. 1999;162:6562–71. [PubMed] [Google Scholar]
- 10.Ammon C, Meyer SP, Schwarzfischer L, Krause SW, Andreesen R, Kreutz M. Comparative analysis of integrin expression on monocyte-derived dendritic cells. Immunology. 2000;100:364–9. doi: 10.1046/j.1365-2567.2000.00056.x. 10.1046/j.1365-2567.2000.00056.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ebner S, Lenz A, Reider D, Fritsch P, Schuler G, Romani N. Expression of maturation/migration-related molecules on human dendritic cells from blood and skin. Immunobiology. 1998;198:568–87. doi: 10.1016/S0171-2985(98)80079-X. [DOI] [PubMed] [Google Scholar]
- 12.Holness CL, da Silva RP, Fawcett J, Gordon S, Simmons DL. Macrosialin, a mouse macrophage-restricted glycoprotein, is a member of the lamp/lgp family. J Biol Chem. 1993;268:9661–6. [PubMed] [Google Scholar]
- 13.Yamashiro S, Yamakita Y, Ono S, Matsumura F. Fascin, an actin-bundling protein, induces membrane protrusions and increases cell motility of epithelial cells. Mol Biol Cell. 1998;9:993–1006. doi: 10.1091/mbc.9.5.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Duperrier K, Eljaafari A, Dezutter-Dambuyant C, et al. Distinct subsets of dendritic cells resembling dermal DCs can be generated in vitro from monocytes, in the presence of different serum supplements. J Immunol Methods. 2000;238:119–31. doi: 10.1016/s0022-1759(00)00147-2. 10.1016/s0022-1759(00)00147-2. [DOI] [PubMed] [Google Scholar]
- 15.Rouard H, Leon A, Klonjkowski B, et al. Adenoviral transduction of human ‘clinical grade’ immature dendritic cells enhances costimulatory molecule expression and T-cell stimulatory capacity. J Immunol Methods. 2000;241:69–81. doi: 10.1016/s0022-1759(00)00214-3. 10.1016/s0022-1759(00)00214-3. [DOI] [PubMed] [Google Scholar]
- 16.Regnault A, Lankar D, Lacabanne V, et al. Fc gamma receptor-mediated induction of dendritic cell maturation and major histocompatibility complex class I-restricted antigen presentation after immune complex internalization. J Exp Med. 1999;189:371–80. doi: 10.1084/jem.189.2.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thomas R, Davis LS, Lipsky PE. Comparative accessory cell function of human peripheral blood dendritic cells and monocytes. J Immunol. 1993;151:6840–52. [PubMed] [Google Scholar]
- 18.Sallusto F, Cella M, Danieli C, Lanzavecchia A. Dendritic cells use macropinocytosis and the mannose receptor to concentrate macromolecules in the major histocompatibility complex class II compartment: downregulation by cytokines and bacterial products. J Exp Med. 1995;182:389–400. doi: 10.1084/jem.182.2.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Geissmann F, Revy P, Regnault A, et al. TGF-beta 1 prevents the noncognate maturation of human dendritic Langerhans' cells. J Immunol. 1999;162:4567–75. [PubMed] [Google Scholar]
- 20.Bonham CA, Lu L, Banas RA, Fontes P, Rao AS, Starzl TE, Zeevi A, Thomson AW. TGF-beta 1 pretreatment impairs the allostimulatory function of human bone marrow-derived antigen-presenting cells for both naive and primed T cells. Transpl Immunol. 1996;4:186–91. doi: 10.1016/s0966-3274(96)80015-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yamaguchi Y, Tsumura H, Miwa M, Inaba K. Contrasting effects of TGF-beta 1 and TNF-alpha on the development of dendritic cells from progenitors in mouse bone marrow. Stem Cells. 1997;15:144–53. doi: 10.1002/stem.150144. [DOI] [PubMed] [Google Scholar]
- 22.Winzler C, Rovere P, Rescigno M, et al. Maturation stages of mouse dendritic cells in growth factor-dependent long-term cultures. J Exp Med. 1997;185:317–28. doi: 10.1084/jem.185.2.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Basta S, Knoetig SM, Spagnuolo-Weaver M, Allan G, McCullough KC. Modulation of monocytic cell activity and virus susceptibility during differentiation into macrophages. J Immunol. 1999;162:3961–9. [PubMed] [Google Scholar]
- 24.Rodriguez A, Regnault A, Kleijmeer M, Ricciardi-Castagnoli P, Amigorena S. Selective transport of internalized antigens to the cytosol for MHC class I presentation in dendritic cells. Nat Cell Biol. 1999;1:362–8. doi: 10.1038/14058. 10.1038/14058. [DOI] [PubMed] [Google Scholar]
- 25.West KA, al-Alwan MM, Colp PE, Rowden G. Characterization of porcine dendritic cells grown in vitro. Transplant Proc. 1999;31:666–7. doi: 10.1016/s0041-1345(98)01740-0. 10.1016/s0041-1345(98)01740-0. [DOI] [PubMed] [Google Scholar]
- 26.Gallucci S, Lolkema M, Matzinger P. Natural adjuvants: endogenous activators of dendritic cells. Nat Med. 1999;5:1249–55. doi: 10.1038/15200. 10.1038/15200. [DOI] [PubMed] [Google Scholar]
- 27.Sauter B, Albert ML, Francisco L, Larsson M, Somersan S, Bhardwaj N. Consequences of cell death: exposure to necrotic tumor cells, but not primary tissue cells or apoptotic cells, induces the maturation of immunostimulatory dendritic cells. J Exp Med. 2000;191:423–34. doi: 10.1084/jem.191.3.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Salio M, Cerundolo V, Lanzavecchia A. Dendritic cell maturation is induced by mycoplasma infection but not by necrotic cells. Eur J Immunol. 2000;30:705–8. doi: 10.1002/1521-4141(200002)30:2<705::AID-IMMU705>3.0.CO;2-P. 10.1002/(sici)1521-4141(200002)30:02<705::aid-immu705>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 29.Borkowski TA, Letterio JJ, Farr AG, Udey MC. A role for endogenous transforming growth factor beta 1 in Langerhans' cell biology: the skin of transforming growth factor beta 1 null mice is devoid of epidermal Langerhans' cells. J Exp Med. 1996;184:2417–22. doi: 10.1084/jem.184.6.2417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Borkowski TA, Letterio JJ, Mackall CL, Saitoh A, Wang XJ, Roop DR, Gress RE, Udey MC. A role for TGF-beta1 in Langerhans' cell biology. Further characterization of the epidermal Langerhans' cell defect in TGF-beta1 null mice. J Clin Invest. 1997;100:575–81. doi: 10.1172/JCI119567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Strobl H, Riedl E, Scheinecker C, Bello-Fernandez C, Pickl WF, Rappersberger K, Majdic O, Knapp W. TGF-beta 1 promotes in vitro development of dendritic cells from CD34+ hemopoietic progenitors. J Immunol. 1996;157:1499–507. [PubMed] [Google Scholar]
- 32.Zhang Y, Zhang YY, Ogata M, Chen P, Harada A, Hashimoto S, Matsushima K. Transforming growth factor-beta1 polarizes murine hematopoietic progenitor cells to generate Langerhans' cell-like dendritic cells through a monocyte/macrophage differentiation pathway. Blood. 1999;93:1208–20. [PubMed] [Google Scholar]
- 33.Riedl E, Strobl H, Majdic O, Knapp W. TGF-beta 1 promotes in vitro generation of dendritic cells by protecting progenitor cells from apoptosis. J Immunol. 1997;158:1591–7. [PubMed] [Google Scholar]
- 34.Lunney JK, Pescovitz MD. Phenotypic and functional characterization of pig lymphocyte populations. Vet Immunol Immunopathol. 1987;17:135–44. doi: 10.1016/0165-2427(87)90134-6. [DOI] [PubMed] [Google Scholar]
- 35.Saalmuller A, Reddehase MJ, Buhring HJ, Jonjic S, Koszinowski UH. Simultaneous expression of CD4 and CD8 antigens by a substantial proportion of resting porcine T lymphocytes. Eur J Immunol. 1987;17:1297–301. doi: 10.1002/eji.1830170912. [DOI] [PubMed] [Google Scholar]
- 36.Saalmuller A, Pauly T, Höhlich BJ, Pfaff E. Characterization of porcine T lymphocytes and their immune response against viral antigens. J Biotechnol. 1999;73:223–33. doi: 10.1016/s0168-1656(99)00140-6. 10.1016/s0168-1656(99)00140-6. [DOI] [PubMed] [Google Scholar]
- 37.Binns RM. The null/gamma delta TCR+ T-cell family in the pig. Vet Immunol Immunopathol. 1994;43:69–77. doi: 10.1016/0165-2427(94)90122-8. [DOI] [PubMed] [Google Scholar]
- 38.Bachmann MF, Lutz MB, Layton GT, Harris SJ, Fehr T, Rescigno M, Ricciardi-Castagnoli P. Dendritic cells process exogenous viral proteins and virus-like particles for class I presentation to CD8+ cytotoxic T lymphocytes. Eur J Immunol. 1996;26:2595–600. doi: 10.1002/eji.1830261109. [DOI] [PubMed] [Google Scholar]
- 39.Brossart P, Bevan MJ. Presentation of exogenous protein antigens on major histocompatibility complex class I molecules by dendritic cells: pathway of presentation and regulation by cytokines. Blood. 1997;90:1594–9. [PMC free article] [PubMed] [Google Scholar]