Abstract
Tumour cells transfected with cDNAs encoding non-self proteins were used to investigate the ability of the immune system to respond to immunogenic antigens expressed by tumours. Secreted, intracellular and surface proteins were used as model antigens, as these reflect the potential forms of tumour antigens. Syngeneic BALB/c mice injected with viable line 1 lung carcinoma or EMT6 mammary tumour cells secreting ovalbumin (OVA) or prostate-specific antigen (PSA) produced very high immunoglobulin G (IgG) antibody titres, equivalent to those of mice injected with protein in Freund's complete adjuvant (FCA). Secretion of the antigens was not necessary as tumour cells expressing a cell-surface antigen (HER-2/Neu) or an intracellular antigen – green fluorescence protein (GFP) – also generated high-titre antigen-specific IgG antibodies. In interleukin-4 (IL-4)-deficient mice, both IgG1 and IgG2a were produced in response to OVA administered in FCA, whereas in response to tumour-produced antigen, the antibodies switched from predominantly IgG1 to IgG2a, indicating that the mechanisms responsible for antibody induction differed between these forms of immunization. In contrast to the line 1 and EMT6 tumours, which are of BALB/c origin, OVA- or PSA-producing B16 melanoma cells, which are of C57BL/6 origin, failed to elicit antibody production. This was not the result of strain differences, as a similar finding was observed when the tumours were grown in (BALB/c × C57BL/6)F1 mice, but appeared to be caused by intrinsic differences in the tumours. Furthermore, co-injection of both B16/OVA and line 1 tumours resulted in production of anti-OVA antibody, indicating that B16 tumours were not immunosuppressive, but instead line 1 tumours appear to exert an adjuvant effect.
Introduction
Tumour growth may reflect either the inadequacy or the absence of an immune response. Until recently, distinguishing between these possibilities was an extremely difficult task owing to the lack of defined tumour antigens that could be used to monitor the immune responses of patients. However, the advent of novel molecular technology and improved methods of cell culture have allowed the discovery of tumour-associated antigens, particularly for melanomas. The use of cytotoxic T-lymphocyte (CTL) lines (established from patients with melanoma) to screen cDNA libraries generated from autologous tumour samples, allowed the identification of a number of tumour-associated antigens such as tyrosinase, gp100 and MelanA/MART-1.1 More recently, the use of major histocompatibility complex (MHC)–peptide tetramers have confirmed that lymph nodes (LN) of some melanoma patients contain high numbers of CD8 T cells that are specific for previously identified antigens.2 Although these antigens were identified based on T-cell responses, other tumour-associated proteins have been identified using a serological approach termed SEREX (serological analysis of recombinant cDNA expression libraries).3 This method exploits the patient's own antibody repertoire and uses immunoglobulin G (IgG) antibodies from serum to screen autologous tumour cDNA-expression libraries. Novel antigens such as NY-ESO-1 were identified by using this technique and, interestingly, proteins such as tyrosinase, which had been previously defined by CTL screening, were again detected.4 As T-cell help is required to promote high IgG antibody titres observed in these patients, these results also demonstrated that CD4 T-cell responses, as well as humoral responses to tumours, could be generated in cancer patients.
Despite this marked progress, there are still many types of tumours for which no clear antigens have been identified and for which there is little evidence of an immune response. This apparent lack of response might be attributed to many different factors. First, both central and peripheral tolerance are issues as most of the antigens expressed by cancerous cells are self-proteins shared by both tumour and normal host tissues. T cells that could potentially react to such antigens would have been eliminated in the thymus by the process of negative selection. Second, other possible tumour antigens may be largely ignored by the immune system as a result of their existence outside lymphoid organs and their inability to traffic effectively to LN.5 Third, tumours may fail to elicit inflammatory cytokines that have been suggested to provide signals important for activation of naive T cells.6–8 Unlike viral or bacterial infections, which can efficiently induce inflammatory cytokines that activate dendritic cells (DC) to process antigens and traffic to LN, tumours appear to induce these processes only poorly.9,10 Furthermore, most types of tumours lack expression of costimulatory molecules and thus are incapable of directly presenting antigen to naive T cells. Finally, tumours may also actively secrete cytokines that hinder cell-mediated responses. Many tumour cells can secrete cytokines such as transforming growth factor-β (TGF-β) and vascular endothelial growth factor (VEGF), which have been demonstrated to inhibit T-cell development and function.11,12 All of these factors would be expected to contribute to poor immune responses to tumour antigens.
The current study was designed to examine the ability of the immune system to mount a response to antigens expressed by tumours in a situation where antigen itself, in many respects, is optimal, but the other parameters characteristic of growing tumours remain the same. This investigation uses syngeneic tumours transfected with foreign antigens to examine immune responses to tumours under conditions where issues of central tolerance do not apply and responses can be easily measured. By measuring antibody responses it was possible to determine the kinetics of the response by sequentially bleeding the same animals. Furthermore, as T cells are required for the switch from immunoglobulin M (IgM) to IgG antibody production, analysis of IgG levels allowed investigation of both B-cell-dependent and T-cell-dependent responses. The results demonstrate that tumours are not necessarily immunosuppressive, but in some cases can act as potent adjuvants to promote B-cell immunity. This was true for a range of protein antigens, including secreted, intracellular and membrane-bound molecules. However, as has been observed in the clinical setting, not all tumour lines examined could promote humoral immunity.13,14 Delineation of the factors responsible for the differences in the adjuvanticity of tumours may provide important information for more effective immunotherapy of malignant disease.
Materials and methods
Mice and cell lines
BALB/cByJ (H-2d), C57BL/6 (H-2b) (BALB/cByJ × C57BL/6)F1 (H-2d/b) and interleukin-4 (IL-4)-deficient (BALB/c-Il4tm2Nnt) mice were purchased from the Jackson Laboratory (Bar Harbor, ME) and used at 2–4 months of age.
Line 1, a small-cell lung carcinoma,15 and EMT6.8, a clone of EMT6,16 a mammary carcinoma, both arose spontaneously in BALB/c mice and have been described previously. The B16F0 cell line was obtained from the American Type Culture Collection (ATCC) (Rockville, MD) and was first characterized as a spontaneously arising melanoma in C57BL/6 (H-2b) mice.17 cDNAs encoding ovalbumin (OVA) and prostate-specific antigen (PSA) were transfected into line 1 or EMT6 tumour cells using lipofection, and positive clones were isolated by limiting-dilution analysis, as previously described.18,19 Line 1 transfected with the cDNA for green fluorescent protein (GFP) was a generous gift of Dr Sandra Gollnick (Roswell Park Cancer Institute, Buffalo, NY), and EMT6 transfected with human HER-2/neu was kindly provided by Dr Pia Challita-Eid (University of Rochester, Rochester, NY). Cell lines were tested routinely for the presence of mycoplasma using the Gen-Probe detection system (Gen-Probe, San Diego, CA). Only cell lines testing negative were used in experiments.
Flow cytometric analysis of LN cell populations
BALB/c mice were injected intramuscularly (i.m.) with 1 × 105 line 1/OVA tumour cells and tumours were allowed to grow for 15–21 days. After leg diameters had reached 12–14 mm, iliac (tumour draining) and inguinal (non-tumour draining) LN were removed and dissociated into single-cell suspensions. LN cells (1 × 106) were incubated with fluorescein isothiocyanate (FITC)-conjugated antibodies to the cell-surface markers B220, CD4 and CD8 (clones RA3-6B2, RM4-5 and 53-6.72, respectively; PharMingen, San Diego, CA). FITC-conjugated rat IgG2a (PharMingen) was used as a negative control. Samples were analysed using an EPICS Elite flow cytometer (Coulter Corp., Hialeah, FL).
Injection of cell lines and serum collection
Levels of OVA and PSA secreted into the supernatant were analysed by protein-specific enzyme-linked immunosorbent assays (ELISAs), as previously described.18 Levels of GFP and HER-2/neu protein in transfected tumour cells were determined by flow cytometric analysis (Table 1). Transfected tumours were injected i.m. into the left hind flank of BALB/c, C57BL/6 (BALB/c × C57BL/6)F1 or IL-4-deficient mice at cell numbers that produced tumours within 20 days. Experiments were halted at the end of the 3-week period or when the mean thigh diameter of the mouse was ≈12–14 mm. Every 3–4 days during tumour growth, blood was collected, allowed to clot and the serum removed. In control experiments, 50 µg of OVA was emulsified in Freund's complete adjuvant (FCA; Sigma, St. Louis, MO) or mixed with Alhydrogel aluminium hydroxide (Accurate Chemical, Westbury, NY) and injected i.m. For mixing experiments, 2 × 103 parental line 1 cells were mixed with 2 × 105 B16/OVA cells and injected i.m. into (BALB/c × C57BL/6)F1 mice. The cell numbers in these injections were selected to control for the different rates of in vivo growth between the two types of tumours. After 18–21 days, mice were bled and tumours removed to assay for OVA expression. To determine the amount of OVA protein being produced directly ex vivo, tumours were collagenased to form single-cell suspensions, and 1 × 107 cells were lysed in 1 ml of Nonidet P-40 (NP-40) lysis buffer. Tumour lysates were analysed by using an ELISA specific for OVA, as previously described.18 In a converse experiment, 2 × 105 parental B16 cells were co-injected with 5 × 104 line 1/OVA cells and cultured for 18–21 days. Guidelines for the humane treatment of animals were followed as approved by the University Committee on Animal Resources.
Table 1.
Amounts of antigen produced by various tumour transfectants
| Tumour cell line* | Haplotype | OVA (ng/ml)† | PSA (ng/ml)† | GFP (MFI)‡ | HER-2/neu (MFI)§ |
|---|---|---|---|---|---|
| Line 1 | H-2d | 25 | 8 | 165 | N/A |
| EMT6 | H-2d | 10 | 45 | N/A | 0·362 |
| B16 | H-2b | 5 | 16 | N/A | N/A |
Each tumour cell line represents a cell line transfected with a single antigen. In all, eight individual transfectants were utilized in this study.
Protein levels were assayed from the culture supernatant using enzyme-linked immunosorbent assay (ELISA). Culture supernatants were collected from 2 × 105 cells incubated for 48 hr in 2 ml of media.
Line 1/green fluorescent protein (GFP) tumour cells were assayed for levels of intracellular GFP by flow cytometric analysis, and the values presented represent mean fluorescent intensity (MFI).
EMT6/HER-2/neu cells were stained with mouse anti-human HER-2/neu followed by incubation with goat anti-mouse fluorescein isothiocyanate (FITC) and subjected to flow cytometric analysis.
OVA, ovalbumin.
ELISAs for the detection of mouse antibodies
OVA (Sigma), GFP (Clontech, San Diego, CA) or the extracellular domain of HER-2/neu (Genentech, San Francisco, CA) was coated onto ELISA plates at a concentration of 1 µg/ml in coating buffer (0·05 m boric acid in phosphate-buffered saline [PBS], pH 9·5). Plates were blocked with PBS containing 2% fetal bovine serum (FBS), 5 mm HEPES and 0·05% sodium azide. Dilutions of serum were prepared in the same buffer and allowed to bind to the plate. Mouse immunoglobulin was detected by using alkaline phosphatase-conjugated goat anti-mouse second-step reagents specific for IgM, total IgG, or the IgG subtypes IgG1 or IgG2a (Southern Biotechnology, Birmingham, AL). Alkaline phosphatase was detected with p-nitrophenylphosphate (Calbiochem, San Diego, CA) in diethanolamine buffer (Sigma), and colorimetric changes were read at a wavelength of 405 nm. An assay specific for anti-PSA antibodies was developed as a sandwich ELISA in which the plate was coated with 3 µg/ml of polyclonal rabbit anti-human PSA (DAKO, Carpinteria, CA), blocked with PBS containing 5% powdered non-fat milk and 0·2% Tween-20 (blotto-tween), and then PSA (Calbiochem) antigen was added at 30 ng/ml, followed by dilutions of the serum in blotto-tween. Bound antibodies were detected with horseradish peroxidase (HRP)-conjugated rabbit anti-mouse IgG (Jackson Immunoresearch, West Grove, PA) and developed using O-phenylenediamine (OPD; Sigma) as the chromagen. The absorbance at 490 nm was determined by using an ELISA reader. Results are expressed as end-point dilutions, where the end-point is the final dilution that gives an absorbance reading of twice the background level. This was performed to normalize for the different ELISAs used, and the background level was determined using a negative-control serum sample (diluted 1:20) from mice injected with untransfected parental tumour cells. Comparisons between groups were performed by using a non-parametric test (Mann–Whitney U-test).
Results
Tumour-bearing mice have an increased percentage of B220+ cells in the tumour-draining LN
To investigate how tumours may promote or prevent immune responses, a poorly immunogenic mouse lung carcinoma – line 1 – was chosen initially for study. The line 1 tumour cell line was transfected with cDNA encoding OVA to investigate the ability of the immune system to respond to potentially immunogenic antigens expressed by tumours. Our initial observations suggesting that immune effectors might be expanding in response to line 1/OVA tumours, were the enlargement and increased cellularity of the LN draining the tumour site. To further characterize these changes, the iliac (tumour draining) LN and inguinal (non-tumour draining) LN were removed from mice bearing line 1/OVA tumours. The total number of cells in the tumour-draining LN had increased by four- to fivefold. The range of cell numbers was 1·2–3·4 × 106 cells/LN in the non-tumour-draining LN with an average ±SEM of 1·9 ± 0·3 × 106 cells/LN. The tumour-draining LN, in contrast, had an average of 1·1 ± 0·1 × 107 cells/LN with a range of 7·5–15 × 106 cells/LN. Flow cytometric analysis revealed that the percentage of B220+ cells in the tumour-draining LN had increased twofold over that of the non-draining LN (Fig. 1a, 1b). The percentage of B220+ cells in the non-draining LN was comparable to the percentage of B220+ cells in a naïve LN (data not shown). This expanded percentage of B cells and the increased overall cellularity resulted in a seven- to eightfold increase in the number of B cells in the tumour-draining LN. There was a corresponding decrease in the percentage of CD4+ and CD8+ cells in these LN. However, when the expansion in total cell number was taken into account, there was a twofold increase in absolute numbers of CD4+ and CD8+ T cells (data not shown), demonstrating that while both T and B cells increased in number, the B cells were expanded preferentially.
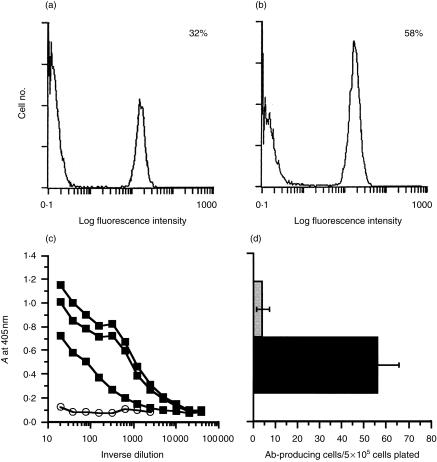
Figure 1.
Tumour-bearing mice exhibit an increased percentage of B220+ cells and high levels of anti-ovalbumin (OVA) antibodies. Non-tumour draining (a) and tumour draining (b) lymph nodes (LN) were removed from BALB/c mice 20 days after tumour inoculation. Cell numbers were determined by Trypan Blue exclusion and found to be an average of 1·9 ± 0·3 × 106 cells in the non-draining LN compared to 1·1 ± 0·1 × 107 cells in the tumour-draining LN. Total LN cells were stained with a fluorescein isothiocyanate (FITC)-conjugated anti-B220 antibody or FITC-conjugated isotype-matched control and subjected to flow cytometric analysis. The percentage of B220+ cells was determined by gating based on isotype-control staining. The histograms shown are representative of six experiments performed. (c) Sera from three individual mice injected with line 1/OVA (closed symbols) were collected on day 20 of tumour growth and assayed in an enzyme-linked immunosorbent assay (ELISA) specific for anti-OVA antibodies. Sera from mice injected with parental line 1 cells were also collected and a representative sample is shown as a negative control (open symbols). An alkaline phosphatase-conjugated goat anti-mouse immunoglobulin G (IgG) reagent was used to detect total mouse IgG specific for OVA. An ELISA representative of three performed is shown. (d) Tumour draining (black bar) and non-tumour draining (grey bar) LN cells were isolated from mice injected with line 1/OVA tumour cells for 21 days. Cells were plated in 96-well nitrocellulose microtitre plates coated with OVA protein. Cells secreting anti-OVA IgG were detected with alkaline phosphatase-conjugated goat anti-mouse IgG, and spots were enumerated using a dissecting microscope. The graph shows the mean and standard error of three separate experiments.
This marked increase in the B-cell population suggested that B cells had been activated in response to the growing line 1/OVA tumours. To examine this possibility, serum was collected from tumour-bearing mice and assayed in an ELISA specific for anti-OVA antibodies. Figure 1(c) shows representative ELISA data of sera from three individual mice injected with line 1/OVA tumours. Each of the three mice showed high levels of anti-OVA IgG antibodies, whereas serum from a representative mouse injected with parental line 1 cells did not contain OVA-specific antibodies. End-point titres in these mice ranged from 1000–10 000, suggesting that a vigorous B-cell response had been activated in response to the OVA-secreting tumours. Tumour-draining LN cells were also analysed for OVA-specific antibody-producing cells using ELISPOT assays. This type of assay has the advantage of determining the actual number of antibody-producing B cells present, rather than just the quantity of antibody made. Consistent with the serum analysis, this assay demonstrated that the frequency of OVA-specific B cells was markedly increased in the tumour-draining LN (Fig. 1d).
Line 1/OVA tumours elicit high anti-OVA antibody titres comparable to those elicited by OVA emulsified in adjuvants
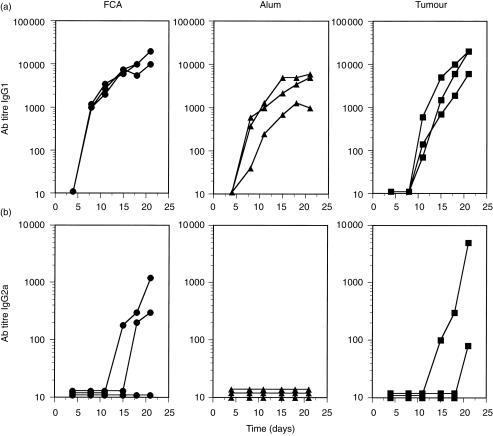
The extent of B-cell expansion in mice bearing line 1/OVA tumours was surprising, given that many reports have suggested that growing tumours suppress ongoing immune responses.11,20 To further investigate the B-cell response in tumour-bearing mice, the isotype class of the IgG antibody responses in line 1/OVA-injected mice was compared with that of mice immunized with OVA emulsified in FCA or mixed with aluminium hydroxide (alum). FCA was used because of its high potency and its induction of IgG1 antibody secretion early in the primary response with eventual switching to IgG2a, a profile consistent with a predominant T helper 1 (Th1) cell response.21 In contrast, alum induces IgG1 secretion and little or no IgG2a, an isotype pattern characteristic of T helper 2 (Th2)-type immunity.21 These two adjuvants were used to compare the kinetics, magnitude and qualitative nature of the immune response elicited by progressively growing line 1/OVA tumours to that obtained with well-characterized adjuvants. It is not possible to accurately determine the amount of OVA antigen delivered by the tumours over time but, based on the amount of antigen produced by the cells in vitro (Table 1) and the growth kinetics of the tumour, it was estimated that ≈50 µg of OVA would be an equivalent or higher dose, so this amount was used with the adjuvants. Figure 2(a) shows the IgG1 antibody titre of mice injected with OVA in FCA or in alum or with line 1/OVA cells, as a function of time. Sera from mice were collected every 3–4 days starting 4 days postinjection and continuing until 21 days after injection. As expected, mice injected with OVA in FCA or alum produced high-titre anti-OVA IgG1 antibodies, which were detectable early after immunization and increased throughout the duration of the experiment. Surprisingly, mice given a single injection of viable line 1/OVA tumour cells produced high anti-OVA IgG1 antibody responses, similar to those seen in mice injected with OVA emulsified in FCA. The kinetics of the antibody response in tumour-bearing mice were slightly delayed; anti-OVA IgG1 antibodies were not detected until days 11–15. Nevertheless, the end-point titres on day 21 in tumour-bearing mice were as high as in mice injected with OVA in FCA (geometric mean titres were ≈13 000 and 15 000, respectively). Anti-OVA IgG2a antibody titres were also examined in tumour-bearing mice and again compared to responses induced with antigen in adjuvant (Fig. 2b). Similarly to previously published results, OVA mixed in alum did not induce IgG2a synthesis at any time-point tested.21 Two out of three mice injected with OVA in FCA had produced anti-OVA IgG2a antibodies by day 15 after injection. With approximately similar kinetics, two out of three mice injected with line 1/OVA tumours also produced anti-OVA IgG2a antibodies. Therefore, the antibody response induced by OVA-expressing line 1 tumour cells appears to be similar to OVA in FCA and indeed, by this criterion, more potent than alum. In addition, the pattern of isotype switching was similar between the tumour- and FCA-immunized mice.
Figure 2.
Ovalbumin (OVA) delivered by tumour cells induces anti-OVA antibodies of the immunoglobulin G1 (IgG1) and IgG2a isotypes at a level similar to that induced by OVA in adjuvants. Three mice in each group were injected with 1 × 105 of viable line 1/OVA cells (▪), 50 µg of OVA in Freund's complete adjuvant (FCA) (•) or 50 µg of OVA in alum (▴). Mice were bled at the indicated time-points and sera were tested in an OVA-specific enzyme-linked immunosorbent assay (ELISA), using alkaline phosphatase-conjugated goat anti-mouse IgG1 (a) or alkaline phosphatase-conjugated goat anti-mouse IgG2a (b) as the detecting antibody. The graphs show the end-point titres of three individual mice at each time-point tested.
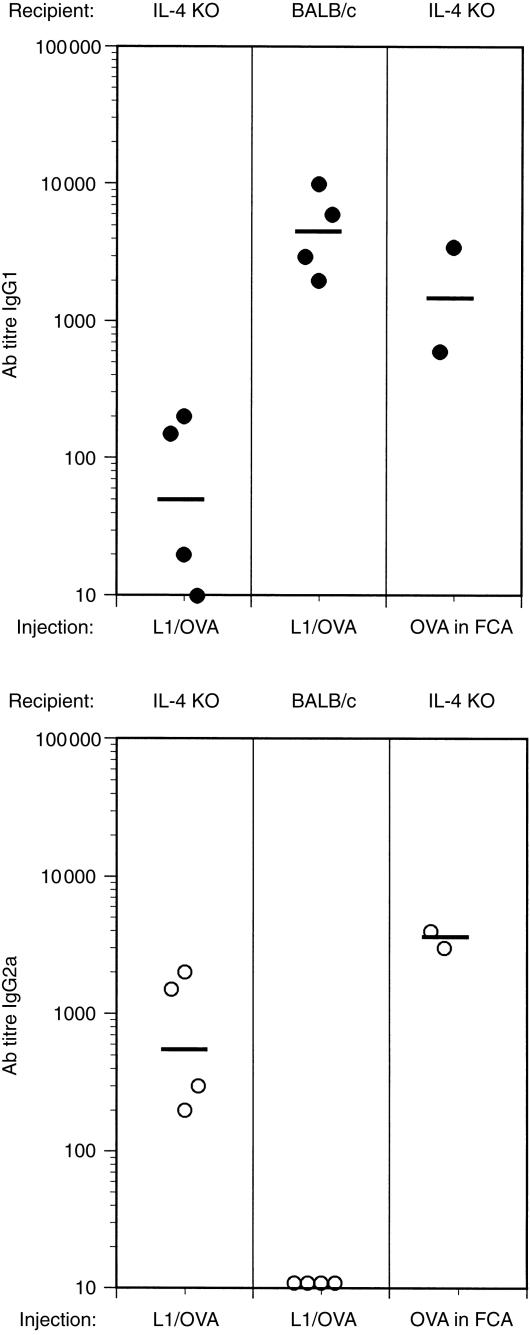
To further investigate the mechanisms involved in the generation of these high-titre anti-OVA antibodies, the antibody responses in IL-4-deficient mice were examined. IL-4 plays a major role in the production of antibodies of the IgG1 isotype.22 Therefore, we wished to determine whether IL-4 might be essential for the induction of anti-OVA antibodies. Either line 1/OVA cells or OVA in FCA was injected into IL-4 knockout mice and then sera were collected and analysed for the presence of anti-OVA antibodies of both the IgG1 and IgG2a subtypes. The results clearly demonstrate that IL-4 is not essential for eliciting an IgG antibody response when mice are immunized with OVA expressed by tumour cells or emulsified in FCA (Fig. 3). Interestingly, immunization of IL-4-deficient mice with tumour cells, although still eliciting a response, caused a marked change in the isotype of the antibodies produced. In this experiment, control BALB/c mice generated antibodies exclusively of the IgG1 isotype with titres similar to what was observed in earlier experiments (see Fig. 2) while IL-4-deficient mice produced only low titres of anti-OVA IgG1. On day 18 post-tumour inoculation, control BALB/c mice did not produce anti-OVA IgG2a in this experiment; however, there was a marked shift to production of IgG2a antibodies in IL-4-deficient mice. Immunization of the IL-4 knockout mice with OVA in FCA also resulted in high titres of IgG2a antibodies but, in contrast to the mice immunized with the line 1/OVA, high titres of IgG1 antibodies were also maintained. These results show that immunization with OVA-expressing tumour cells can elicit high-titre antigen-specific IgG antibodies in the absence of IL-4. However, the isotype of the antibodies elicited has switched from predominantly IgG1 in normal BALB/c to IgG2a in the IL-4-deficient mice. In contrast, IL-4 has less of an impact on antibody production when mice are immunized with OVA emulsified in FCA in that both IgG1 and IgG2a responses are maintained with high titres.
Figure 3.
Antibody production in response to line 1 tumours is not dependent on interleukin-4 (IL-4). IL-4-knockout (KO) mice were injected with 1 × 105 line 1/ovalbumin (OVA) tumour cells, or with 50 µg/ml of OVA in Freund's complete adjuvant (FCA) as a positive control. Mice were bled on day 18 and serum was used in an anti-OVA-specific enzyme-linked immunosorbent assay (ELISA) for immunoglobulin G1 (IgG1) (upper panel) or IgG2a (lower panel). BALB/c mice were also injected with line 1/OVA tumour cells for 18 days as a positive control for elevated production of anti-OVA IgG1 antibodies, as shown previously (Figure 2). Each point represents an individual mouse and the bars represent the geometric mean of all mice assayed. Statistically significant differences in anti-OVA IgG1 titres were observed between BALB/c mice and IL-4 KO mice injected with line 1/OVA (P = 0·02 by Mann–Whitney U-test). By the same statistical criteria, IL-4 KO mice injected with line 1/OVA tumours demonstrated significantly higher IgG2a titres than BALB/c mice injected with line 1/OVA (P = 0·02).
Transfection of BALB/c-derived tumours with different antigens can induce antigen-specific high-titre IgG antibodies
The above results demonstrated that line 1/OVA tumours could induce high titre anti-OVA antibodies and class switching to IgG isotypes. Surprisingly, these responses were equal to and sometimes surpassed those seen in mice given an injection of OVA in adjuvants. To determine if this response was unique to OVA, a strong antigen used in many experimental systems, line 1 tumours were also transfected with cDNAs encoding PSA or GFP. Although PSA and OVA are secreted proteins, GFP is an intracellular protein. Use of this antigen allowed us to determine if the protein produced by the tumour cells needs to be secreted for a response to be elicited. BALB/c mice were injected with various concentrations of tumour cell lines, and antigen-specific total IgG was assayed by ELISA, as described in the ‘Materials and methods’. Figure 4(a) shows the individual responses and geometric mean titres of mice injected with line 1 cells producing OVA, PSA or GFP. In all mice tested, line 1 tumours expressing OVA induced high anti-OVA antibody titres, similar to the results seen in Figs 1 and 2. Likewise, line 1 tumours expressing PSA induced strong anti-PSA antibody responses. Perhaps more surprisingly, line 1/GFP tumour cells also generated high-titre IgG anti-GFP antibodies. As T-cell help is required to promote B-cell isotype switching to IgG, this result suggests that intracellular tumour antigens can induce both B- and T-cell responses.
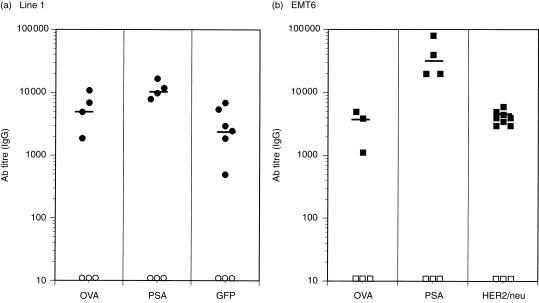
Figure 4.
Different forms of antigen delivered by H-2d-restricted tumours elicit high antigen-specific antibody titres in BALB/c mice. BALB/c mice were injected with antigen-transfected line 1 or EMT6 tumours (see Table 1). Tumours were allowed to grow for 16–21 days or until the mean leg diameter reached ≈12–14 mm. Mice were bled and the sera were assayed in antigen-specific enzyme-linked immunosorbent assays (ELISAs). Mouse antibodies were detected with horseradish peroxidase (HRP)- or alkaline phosphatase (AP)-conjugated goat anti-mouse immunoglobulin G (IgG), and the absorbance (A) was read at 490 or 405 nm, respectively. Antibody titre was determined as the inverse dilution that gave an A reading of twice background levels. This was carried out to normalize between the different ELISAs performed. Each point represents an individual mouse and the bar represents the geometric mean titre of all mice assayed. The standard error of the mean ranged between 980 and 2000 in mice injected with antigen-expressing line 1 tumours and 360–14 000 in mice injected with antigen-expressing EMT6 tumours.
To determine if the effect is unique to line 1 tumours, similar experiments were performed using another tumour model, EMT6, a spontaneously arising mammary carcinoma. As shown in Table 1, these cells secrete slightly less OVA than the line 1/OVA cell line. Additionally, to control for the secreted nature of the OVA antigen, EMT6 cells were transfected with cDNA for HER-2/neu, a cell-surface protein, and used to determine if mice could generate antibodies to surface determinants. Transfected EMT6 tumours also induced high-titre antigen-specific IgG antibodies (Fig. 4b). Interestingly, although the amount of OVA secreted by EMT6 cells was lower than the line 1 cell line, the geometric mean titre in response to EMT6/OVA tumours was as high as that observed in response to line 1/OVA tumours. Similarly, the level of human HER-2/neu on the surface of EMT6 cells appeared to be low (Table 1), yet high-titre anti-HER-2/neu antibodies were induced. The mean geometric titres of serum from mice injected with antigen-expressing EMT6 cells were between 3000 and 5000. In summary, in two different tumour models, line 1 and EMT6, it was possible to generate strong antibody responses to secreted proteins (OVA and PSA), to an intracellular protein (GFP) and to a cell-surface protein (HER-2/neu).
Antigen-expressing B16 melanoma cells do not induce antigen-specific IgG antibodies in C57BL/6 or (BALB/c × C57BL/6)F1 mice
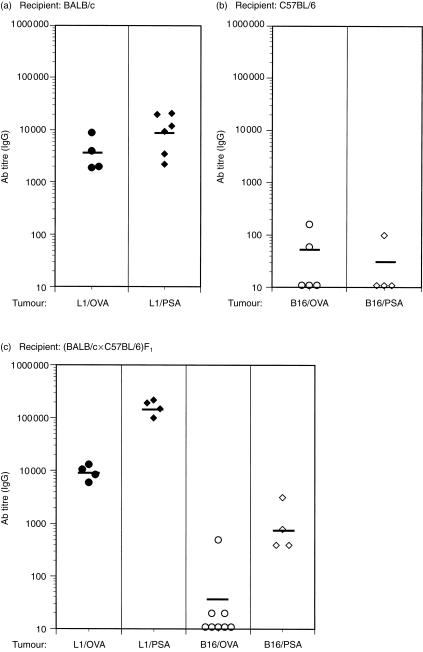
Line 1 and EMT6 cells are derived from the same strain of mouse, BALB/c. Immune responses can be significantly influenced by both MHC and background genes. It was therefore of interest to determine if these results were similar in a different strain and tumour model. The B16 melanoma cell line was employed and, as described above, transfected with cDNA encoding either OVA or PSA. As B16 is of H-2b origin, C57BL/6 mice were injected with either B16/OVA or B16/PSA tumour cells. As for previous experiments, mice were bled between 16 and 21 days post-tumour implantation, and antigen-specific antibodies were assayed using ELISA. For comparison, sera from BALB/c mice with growing line 1/OVA tumours were used as controls. Figure 5(a) again demonstrates that line 1 tumours induced antigen-specific antibodies in BALB/c mice in all animals tested. However, Fig. 5(b) shows that B16 tumours did not elicit a strong antibody response in C57BL/6 mice. For example, only two out of five mice injected with B16/OVA showed detectable levels of anti-OVA antibodies (Fig. 5b). Similarly, only one of the mice injected with B16/PSA tumours had detectable levels of anti-PSA IgG (Fig. 5b).
Figure 5.
Line 1, but not B16, tumours generate high antigen-specific antibody titres in syngeneic and semiallogeneic mice. BALB/c (a) or C57BL/6 (b) mice were injected, respectively, with line 1/ovalbumin (OVA) (•) or line 1/prostate-specific antigen (PSA) (♦), or with B16/OVA (○) or B16/PSA (◊) tumour cells. Filled symbols represent transfected line 1 cells whereas open symbols denote antigen-expressing B16 tumours. (BALB/c × C57BL/6)F1 mice (c) were also injected with line 1/OVA (•), B16/OVA (○), line 1/PSA (♦) or B16/PSA (◊). After 16–21 days of tumour growth, mice were bled, and serum samples were collected and analysed for antigen-specific immunoglobulin G (IgG) using anti-OVA- or anti-PSA-specific enzyme-linked immunosorbent assays (ELISAs). End-point titres were determined by the inverse dilution that gave an absorbance (A) reading of twice background levels. Each point represents an individual mouse and the bar depicts the geometric mean of all mice assayed. (a) Represents pooled data from two separate experiments. Similarly, the B16/OVA column in (c) represents pooled data from two separate experiments. Antibody titres in F1 mice injected with line 1/OVA tumours were significantly higher than antibody titres of mice injected with B16/OVA tumours; P = 0·008 by the Mann–Whitney U-test. Similarly, antibody titres in mice injected with line 1/PSA were significantly higher (P = 0·02) than those titres of mice injected with B16/PSA.
The different results obtained using B16 in its syngeneic host, C57BL/6, compared to those obtained with tumours in BALB/c mice, could be a result of strain differences, as documented for other experimental systems. For example, in the mouse model of leishmaniasis, Leishmania major infection of BALB/c mice promotes a Th2-type response characterized by high antibody titres, but the mice are not protected. In contrast, infection of C57BL/6 mice induces effective immunity characterized by a Th1-type response and clearance of the parasite.23 To determine if strain differences were contributing to the contrasting antibody responses observed above, an experiment was performed in which line 1/OVA or B16/OVA cells were injected into (BALB/c × C57BL/6)F1 mice. If strain differences are crucial in determining the antibody response to tumours, then F1 mice would be expected to display the responder phenotype to both line 1 and B16 tumours. Figure 5(c) shows antigen-specific antibody titres in F1 mice injected with either line 1 or B16 tumour cells. F1 mice injected with line 1/OVA or with line 1/PSA tumour cells generated high antibody titres, comparable to or higher than those seen in BALB/c mice. In contrast, B16/OVA-injected F1 mice generated weak or non-existent antibody responses to OVA, similar to those found in C57BL/6 mice. Whereas the B16/PSA-challenged F1 mice exhibited higher titres of anti-PSA antibodies than the same tumours in C57BL/6 mice, they were significantly lower than the response to PSA produced by line 1 cells. This suggests a relatively modest strain effect. Thus, while there is evidence for a strain effect of the recipient (compare the response to B16/PSA in C57BL/6 to that in the F1 mice), these experiments suggest that the tumour line itself contributes substantially to the magnitude of the antibody response.
Line 1 tumours act as adjuvants to induce anti-OVA antibodies in response to B16/OVA tumours
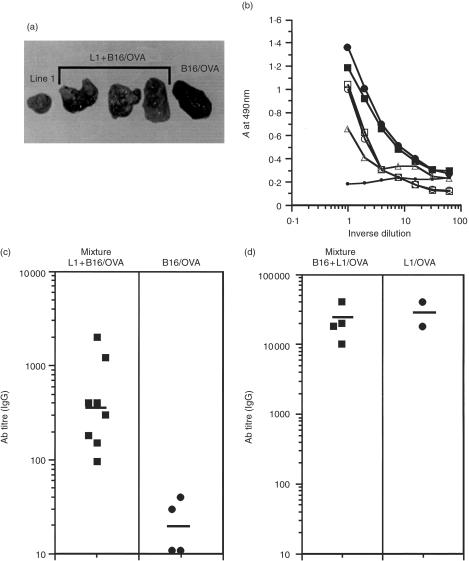
The apparent discrepancy in response to antigens expressed by different tumours could be explained by at least two distinct mechanisms. Line 1 cells might produce factors that enhance the immune response (act as an adjuvant) or, alternatively, B16 cells could produce factors that suppress a response which would normally occur. To address these possibilities, B16/OVA cells were mixed with parental line 1 cells and antibody synthesis in (BALB/c × C57BL/6)F1 mice was analysed. F1 mice were used in these experiments to allow the simultaneous growth of B16/OVA (H-2b) and line 1 (H-2d) tumours. In this protocol, the antigen is delivered by the B16 tumour and the potential adjuvant effect by the line 1 tumour. Mice were bled between days 18 and 21 and the serum assayed for anti-OVA IgG antibodies. When the mice were killed, the tumours were examined to determine that both cell lines had indeed grown. As a result of its production of melanin, growth of the B16/OVA melanoma is readily distinguishable from the non-pigmented line 1 tumour, and at the time of killing it was clear from the interspersed areas of pigmented and non-pigmented tissue that both tumour types had grown (Fig. 6a). Additionally, F1 mice were injected with B16/OVA tumours alone as a negative control. Only pigmented tissue was visible in these B16/OVA tumours (Fig. 6a). To confirm that B16/OVA cells were indeed growing and antigen was still being expressed, the amount of OVA protein expressed by B16/OVA cells injected alone and in combination with parental line 1 cells was analysed by ELISA (Fig. 6b). The amount of OVA expressed by B16/OVA tumours growing in vivo was not significantly different from the B16/OVA cells growing in vitro, suggesting that a negative variant of B16/OVA did not selectively outgrow from the original tumour (Fig. 6b). Furthermore, tumours excised from mice given a combination of line 1 and B16/OVA cells continued to express OVA at the end of the experiment. The levels of OVA in these samples were slightly lower than in the B16/OVA tumour alone owing to the concomitant growth of line 1 interspersed with B16/OVA cells (Fig. 6a, 6b). Figure 6(c) demonstrates that injection of line 1 cells in conjunction with B16/OVA induced a significant response compared to B16/OVA tumours alone. The response generated to the mixed tumour is somewhat lower than to line 1/OVA alone (compare Fig. 6c with Fig. 5c). However, the co-administration of line 1 and B16/OVA clearly had a positive effect over that of B16/OVA given alone. To determine if the B16 parental tumour could exert any suppressive effect on antibody synthesis in response to line 1/OVA tumours, F1 mice were injected with either line 1/OVA alone (as in Fig. 5c) or with a combination of line 1/OVA and parental B16 tumours. In contrast to the experiment in Fig. 6(c), the antigen was now being delivered by the line 1 tumour in order to assess any suppressive effect contributed by the B16 tumour. Figure 6(d) demonstrates that B16 tumours had no suppressive effect on anti-OVA antibody synthesis in F1 mice, and the titres in mice injected with both line 1/OVA mixed with B16 were as high as in mice injected with line 1/OVA alone. Therefore, the adjuvant effect of line 1 tumours to promote antibody synthesis is dominant over any suppressive effect that the B16 tumours may have in inhibiting the induction of antibody synthesis.
Figure 6.
Line 1 tumours act as adjuvants to induce anti-ovalbumin (OVA) antibodies in response to B16/OVA tumours. (a) Line 1 tumours grow interspersed with B16/OVA tumours at the same rate as line 1 or B16/OVA tumours injected alone. Line 1 cells (2 × 103) were mixed with B16/OVA tumour cells (2 × 105) and injected intramuscularly (i.m.) into (BALB/c × C57BL/6)F1 mice. After 18 days of tumour growth, mice were killed and tumours excised for photography. (b) B16/OVA tumours and B16/OVA mixed with line 1 cells maintain expression of OVA protein after 18 days of tumour growth in vivo. Tumour cells were injected as described above and, following growth in vivo, tumours were excised, dissociated into single-cell suspensions, lysed and assayed for levels of OVA protein by enzyme-linked immunosorbent assay (ELISA). The closed square represents B16/OVA tumour cells growing in vitro and the closed circle is a representative B16/OVA tumour grown in vivo. The open symbols represent three different line 1 + B16/OVA tumours grown in vivo and the small circle represents a line 1 tumour grown in vivo. (c) Mice injected as described above were bled at 18–21 days after tumour growth and the serum was analysed for anti-OVA antibodies of the immunoglobulin G (IgG) isotype. End-point titres were determined by the inverse dilution that gave an absorbance (A) reading of twice background levels. Each point represents an individual mouse and comprises pooled data from two separate experiments. The bar depicts the geometric mean of all mice assayed. Anti-OVA antibody titres in mice receiving a mixture of line 1 and B16/OVA tumours were significantly higher than titres in mice receiving an injection of B16/OVA alone (P = 0·007 by the Mann–Whitney U-test). (d) B16 tumours do not suppress antibody synthesis induced by line 1/OVA tumours. B16 (2 × 105) and line 1/OVA (5 × 104) tumour cells were mixed and injected intramuscularly (i.m.) into (BALB/c × C57BL/6)F1 mice. Mice were bled 18–21 days after tumour growth and the sera were analysed for anti-OVA antibodies of the immunoglobulin G (IgG) isotype. End-point titres were determined by the inverse dilution that gave an absorbance (A) reading of twice background levels. Each point represents an individual mouse and the bar depicts the geometric mean of all mice assayed.
Discussion
The current study addressed whether antigens expressed by tumours, under conditions where central tolerance does not apply, would be effective immunogens. Surprisingly, we found that in some cases (EMT6 and line 1) the transfected tumours were almost as potent as FCA at inducing humoral immunity. Furthermore, like FCA, these responses did not absolutely depend upon IL-4. Interestingly, while tumour cells expressing foreign antigens can induce antibody synthesis with titres similar to those induced with FCA, the mechanisms of action of the tumours and FCA are not identical. In the IL-4 knockout mice, OVA in FCA induced a potent antibody response with production of both IgG1 and IgG2a, in agreement with previous reports.24 Injection of line 1/OVA tumours into IL-4-deficient mice also induced antibody responses; however, the isotype of the antibodies generated was very different compared to the response in BALB/c mice. Little IgG1 was produced in the IL-4 knockout mice in response to line 1/OVA, whereas it was the predominant isotype in BALB/c mice. In contrast, the IgG2a response was elevated in IL-4-deficient mice but had not appeared by day 18 in the BALB/c mice. This is precisely what one would predict based on reports that IL-4 enhances the generation of IgG1 and down-regulates the production of IgG2a.25,26 Taken together, the results presented here suggest that tumours have a restricted adjuvant effect in which IgG1 synthesis is dependent upon IL-4, whereas FCA affects class switching through multiple pathways and is less affected by IL-4. Thus, in some cases, simply expressing a foreign antigen like OVA, GFP or PSA in the context of a growing tumour was sufficient to engender class switching and a potent antibody response. This finding was particularly interesting in light of reports of the strong immunosuppressive nature of tumours.20
The magnitude of the antibody response strongly suggests that certain tumours can act as adjuvants. This is also supported by the mixing experiments in which line 1 cells combined with OVA-expressing B16 tumours were able to engender potent antibody responses, whereas OVA-expressing B16 tumour cells alone were poorly immunogenic. These data suggest that factors produced by line 1 cells themselves, or by host cells in response to the growing line 1 tumour, enhance the antibody response. Preliminary experiments using RNAse protection assays have suggested that the cytokine milieu within the tumour microenvironment is different between the line 1 and B16 tumour systems, with RNA for the inflammatory cytokines TNF-α and interleukin-6 (IL-6) expressed in progressively growing line 1, but not B16, tumours (data not shown). Our results thus suggest that the tumours may induce inflammatory cytokines7 or provide danger signals.6 Interestingly, tumours expressing foreign antigens also have other characteristics associated with adjuvants.27 Perhaps the most prominent of these is the continual release of antigen. Indeed, in the case of tumours, the amount of antigen is not only sustained over time but increases as the tumour grows, reminiscent of antigen release during an infection. These characteristics make antigen-producing tumour cells a promising tool for further dissecting the mechanisms of adjuvant activity.
Why do some tumours, like EMT6 and line 1, engender such strong antibody responses, whereas others, such as B16, do not? Originally, we had suspected this to be a reflection of the hosts for the tumours. EMT6 and line 1 are of BALB/c origin whereas B16 arose in a C57BL/6 mouse. BALB/c often is considered a strain with propensity for development of Th2 responses, whereas the C57BL/6 strain is thought to have a predilection for Th1 responses, as illustrated by their responses to parasitic infections.28 The antibody responses in (BALB/c × C57BL/6)F1 mice would be expected to exhibit the high-responder phenotype.23 However, the F1 experiments suggest that whereas there may be a slight effect of the strain, the magnitude of the response is determined to a greater extent by the tumour itself. One might envision several possible mechanisms for this difference. As mentioned above, preliminary data suggests that these different tumour types may produce distinct cytokine profiles, or they may generate different inflammatory responses in the host. Other possibilities are the characteristics of cell death induced within the tumour microenvironment, which may serve as danger signals to antigen-presenting cells (APC).29 It remains to be determined which, if any, of these explanations is correct. Nevertheless, these results are reminiscent of the heterogeneous results obtained in the clinical setting in which some patients produce substantial antibody responses whereas others exhibit weak or undetectable responses. For example, recent studies have shown that ≈50% of patients with HER-2/neu-positive breast cancers have HER-2/neu-specific antibody.13 Similarly, Stockert et al. reported that eight out of 15 stage IV melanoma patients whose tumours expressed the NY-ESO-1 antigen produced antibody to this antigen.14 These results suggest that whereas a fairly high percentage of tumours can engender antibody responses, there is still considerable variability among tumours, even of similar phenotype, to induce humoral immunity. These immune responses measured in cancer patients to their own tumours are complicated by the potential lack of strong tumour antigens, by the genetic differences among patients and by the unknown differences among individual tumours. In our experiments we have controlled for the lack of a strong tumour antigen by providing a foreign antigen, and controlled for genetic heterogeneity by using genetically defined strains of mice. Even with these issues removed from consideration, there were still striking differences between line 1 and EMT6 tumour systems compared to B16 tumours in their abilities to generate humoral immunity, even within the F1 mice. Although it is not yet clear what mechanisms are responsible for these differences, they do illustrate the multifaceted nature of antitumour responses. Clearly, immunogenic determinants are essential, but other factors capable of activating immunity are also important.
It is also intriguing that even when a foreign antigen is present and the tumour is capable of generating an impressive antibody response, the tumours can still grow progressively. This leads one to consider whether the antibody generated is protective or detrimental. A recent study demonstrated that serum antibodies were generated against the spontaneous mammary adenocarcinoma, TS/A, in BALB/c mice. Interestingly, it was also shown that BALB/c mice deficient for B cells (and incapable of producing antibodies) generated a greater level of protective immunity against this tumour than did normal mice.30 These data suggest that B cells may suppress effective antitumour immunity. In contrast, in other experimental models of malignancy, antibodies directed against tumour antigens have been shown to be protective. In these cases, tumour growth or metastases could be abrogated by immunization with the tumour antigen31 or by passive transfer of monoclonal antibodies (mAbs) against antigen-expressing B16 tumours.32 The mechanism of action of these antibodies was shown to be antibody-dependent cell-mediated cytotoxicity (ADCC)31 and was shown to require Fc receptors,33 illustrating the importance of the form of the antigen in determining the effectiveness of the antibody response.
In the clinical setting, the prognostic value of antibody responses has not been rigorously determined and remains controversial. Conceptually, one might argue that the presence of antibodies in patients indicates the lack of tolerance to these tumour antigens, and thus would be a beneficial sign. However, one might envision that such antibodies could be detrimental in eliminating tumours, particularly if the target antigen is not a cell-surface antigen, by eliminating antigen before it can be presented to generate effector T cells. This scenario would be consistent with reports of blocking or enhancing antibodies that acted to increase tumour growth.34,35 At least one clinical study, in which the presence of antibodies to p53 was found to correlate with a poor prognosis,36 would be consistent with this view. Large amounts of antibody being produced might effectively bind free antigen, preventing antigen uptake by APC, such as DC, which are effective in generating Th1-type responses that may be the most favourable for destroying tumour cells.37 Clearly, this and previous studies30 demonstrate that CD4 T-cell responses are being generated, as evidenced by the efficient switching to IgG antibody production. In this light, it is also possible that such antibodies might not be functionally significant themselves, but might instead reflect a skewed Th2-dominated response and thus be an example of immune deviance, which may not be protective in the case of tumours.38 Finally, as many immune responses consist of a blend of both humoral and cell-mediated immunity, perhaps a critical factor is the balance of these responses. If true, then the presence or absence of an antibody response by itself would not be expected to be predictive.
Whatever the clinical significance, the ability of tumours to act as adjuvants can also have consequences for direct practical applications. First, advances in molecular biology and genomics have led to the identification of large numbers of genes whose products are poorly characterized. Using cDNA expressed in tumours, we have been able to efficiently make hybridomas that produce mAbs specific for the cDNA product (data not shown). Such antibodies are extremely useful for characterizing the expression of the protein in different cell types and in determining subcellular expression levels and, of equal importance, they can also serve as tools to elucidate the function of these molecules. Second, elucidation of the characteristics that make certain tumours strong adjuvants could provide important information for generating effective antitumour immunity. Additional experiments are underway to distinguish between these differences.
Acknowledgments
The authors gratefully acknowledge the expert technical assistance of Sebold Torno, the assistance of Dr Robert Rose in the statistical analysis, and the generosity of Dr Sandra Gollnick (Roswell Park Cancer Institute) and Dr Pia Challita-Eid for the kind gifts of reagents. This work was supported by USPHS grants CA28332 and CA70218. D.B. and T.F. were supported in part by USPHS training grant T32AI07285 and C.W. by USPHS training grant T32CA09363.
Abbreviations
- DC
dendritic cells
- FCA
Freund's complete adjuvant
- GFP
green fluorescent protein
- LN
lymph nodes
- OVA
ovalbumin
- PSA
prostate-specific antigen
References
- 1.Boon T, Coulie PG, Van den Eynde B. Tumor antigens recognized by T cells. Immunol Today. 1997;18:267–8. doi: 10.1016/s0167-5699(97)80020-5. 10.1016/s0167-5699(97)01071-2. [DOI] [PubMed] [Google Scholar]
- 2.Pittet MJ, Valmori D, Dunbar, et al. High frequencies of naive Melan-A/MART-1-specific CD8 (+) T cells in a large proportion of human histocompatibility leukocyte antigen (HLA) -A2 individuals. J Exp Med. 1999;190:705–15. doi: 10.1084/jem.190.5.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sahin U, Tureci O, Pfreundschuh M. Serological identification of human tumor antigens. Cur Opin Immunol. 1997;9:709–16. doi: 10.1016/s0952-7915(97)80053-2. [DOI] [PubMed] [Google Scholar]
- 4.Old LJ, Chen YT. New paths in human cancer serology [comment] J Exp Med. 1998;187:1163–7. doi: 10.1084/jem.187.8.1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Starzl TE, Zinkernagel RM. Antigen localization and migration in immunity and tolerance. New Eng J Med. 1998;339:1905–13. doi: 10.1056/NEJM199812243392607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Matzinger P. Tolerance, danger, and the extended family. Annu Rev Immunol. 1994;12:991–1045. doi: 10.1146/annurev.iy.12.040194.005015. [DOI] [PubMed] [Google Scholar]
- 7.Medzhitov R, Janeway Ca., Jr Innate immune recognition and control of adaptive immune responses. Sem Immunol. 1998;10:351–3. doi: 10.1006/smim.1998.0136. 10.1006/smim.1998.0136. [DOI] [PubMed] [Google Scholar]
- 8.Curtsinger JM, Schmidt CS, Mondino A, Lins DC, Kedl RM, Jenkins MK, Mescher MF. Inflammatory cytokines provide a third signal for activation of naive CD4+ and CD8+ T cells. J Immunol. 1999;162:3256–62. [PubMed] [Google Scholar]
- 9.Chaux P, Favre N, Bonnotte B, Moutet M, Martin M, Martin F. Tumor-infiltrating dendritic cells are defective in their antigen-presenting function and inducible B7 expression. A role in the immune tolerance to antigenic tumors. Adv Exp Med Biol. 1997;417:525–8. doi: 10.1007/978-1-4757-9966-8_86. [DOI] [PubMed] [Google Scholar]
- 10.Gabrilovich DI, Corak J, Ciernik IF, Kavanaugh D, Carbone DP. Decreased antigen presentation by dendritic cells in patients with breast cancer. Clin Cancer Res. 1997;3:483–90. [PubMed] [Google Scholar]
- 11.Chouaib S, Asselin-Paturel C, Mami-Chouaib F, Caignard A, Blay JY. The host-tumor immune conflict: from immunosuppression to resistance and destruction. Immunol Today. 1997;18:493–7. doi: 10.1016/s0167-5699(97)01115-8. 10.1016/s0167-5699(97)01115-8. [DOI] [PubMed] [Google Scholar]
- 12.Gabrilovich D, Ishida T, Oyama T, Ran S, Kravtsov V, Nadaf S, Carbone DP. Vascular endothelial growth factor inhibits the development of dendritic cells and dramatically affects the differentiation of multiple hematopoietic lineages in vivo. Blood. 1998;92:4150–66. [PubMed] [Google Scholar]
- 13.Cheever MA, Disis ML, Bernhard H, et al. Immunity to oncogenic proteins. Immunol Rev. 1995;145:33–59. doi: 10.1111/j.1600-065x.1995.tb00076.x. [DOI] [PubMed] [Google Scholar]
- 14.Stockert E, Jager E, Chen YT, et al. A survey of the humoral immune response of cancer patients to a panel of human tumor antigens [see comments] J Exp Med. 1998;187:1349–54. doi: 10.1084/jem.187.8.1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yuhas JM, Pazmino NH, Proctor JO, Toya RE. A direct relationship between immune competence and the subcutaneous growth rate of a malignant murine lung tumor. Cancer Res. 1974;34:722–8. [PubMed] [Google Scholar]
- 16.Rockwell SC, Kallman RF, Fajardo LF. Characteristics of a serially transplanted mouse mammary tumor and its tissue-culture-adapted derivative. J Natl Cancer Inst. 1972;49:735–49. [PubMed] [Google Scholar]
- 17.Fidler IJ. Biological behavior of malignant melanoma cells correlated to their survival in vivo. Cancer Res. 1975;35:218–24. [PubMed] [Google Scholar]
- 18.Pulaski BA, Yeh KY, Shastri N, Maltby KM, Penney DP, Lord EM, Frelinger JG. Interleukin 3 enhances cytotoxic T lymphocyte development and class I major histocompatibility complex ‘representation’ of exogenous antigen by tumor-infiltrating antigen-presenting cells. Proc Natl Acad Sci USA. 1996;93:3669–74. doi: 10.1073/pnas.93.8.3669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wei C, Storozynsky E, McAdam AJ, et al. Expression of human prostate-specific antigen (PSA) in a mouse tumor cell line reduces tumorigenicity and elicits PSA-specific cytotoxic T lymphocytes. Cancer Immunol Immunother. 1996;42:362–8. doi: 10.1007/s002620050295. 10.1007/s002620050295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kiessling R, Wasserman K, Horiguchi S, Kono K, Sjoberg J, Pisa P, Petersson M. Tumor-induced immune dysfunction [see comments] Cancer Immunol Immunother. 1999;48:353–62. doi: 10.1007/s002620050586. 10.1007/s002620050586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grun JL, Maurer PH. Different T helper cell subsets elicited in mice utilizing two different adjuvant vehicles: the role of endogenouse interleukin 1 in proliferative responses. Cell Immunol. 1989;121:134–45. doi: 10.1016/0008-8749(89)90011-7. [DOI] [PubMed] [Google Scholar]
- 22.Snapper CM, Finkelman FD, Paul WE. Regulation of IgG1 and IgE production by interleukin 4. Immunol Rev. 1988;102:51–75. doi: 10.1111/j.1600-065x.1988.tb00741.x. [DOI] [PubMed] [Google Scholar]
- 23.Reiner SL, Locksley RM. The regulation of immunity to Leishmania major. Annu Rev Immunol. 1995;13:151–77. doi: 10.1146/annurev.iy.13.040195.001055. [DOI] [PubMed] [Google Scholar]
- 24.Brewer JM, Conacher M, Satoskar A, Bluethmann H, Alexander J. In interleukin-4-deficient mice, alum not only generates T helper 1 responses equivalent to freund's complete adjuvant, but continues to induce T helper 2 cytokine production. Eur J Immunol. 1996;26:2062–6. doi: 10.1002/eji.1830260915. [DOI] [PubMed] [Google Scholar]
- 25.Snapper CM, Paul WE. Interferon-gamma and B cell stimulatory factor-1 reciprocally regulate Ig isotype production. Science. 1987;236:944–7. doi: 10.1126/science.3107127. [DOI] [PubMed] [Google Scholar]
- 26.Fiorentino DF, Bond MW, Mosmann TR. Two types of mouse T helper cell. IV. Th2 clones secrete a factor that inhibits cytokine production by Th1 clones. J Exp Med. 1989;170:2081–95. doi: 10.1084/jem.170.6.2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chedid L. Adjuvants of immunity. Annal Inst Pasteur – Immunologie. 1985;136D:283–91. doi: 10.1016/s0769-2625(85)80113-6. [DOI] [PubMed] [Google Scholar]
- 28.Hsieh CS, Macatonia SE, O'Garra A, Murphy KM. T cell genetic background determines default T helper phenotype development in vitro. J Exp Med. 1995;181:713–21. doi: 10.1084/jem.181.2.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bonnotte B, Favre N, Moutet M, Fromentin A, Solary E, Martin M, Martin F. Role of tumor cell apoptosis in tumor antigen migration to the draining lymph nodes. J Immunol. 2000;164:1995–2000. doi: 10.4049/jimmunol.164.4.1995. [DOI] [PubMed] [Google Scholar]
- 30.Qin Z, Richter G, Schuler T, Ibe S, Cao X, Blankenstein T. B cells inhibit induction of T cell-dependent tumor immunity. Nature Med. 1998;4:627–30. doi: 10.1038/nm0598-627. [DOI] [PubMed] [Google Scholar]
- 31.Bright RK, Shearer MH, Kennedy RC. Immunization of BALB/c mice with recombinant simian virus 40 large tumor antigen induces antibody-dependent cell-mediated cytotoxicity against simian virus 40-transformed cells. An antibody-based mechanism for tumor immunity. J Immunol. 1994;153:2064–71. [PubMed] [Google Scholar]
- 32.Hara I, Takechi Y, Houghton AN. Implicating a role for immune recognition of self in tumor rejection: passive immunization against the brown locus protein. J Exp Med. 1995;182:1609–14. doi: 10.1084/jem.182.5.1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Clynes R, Takechi Y, Moroi Y, Houghton A, Ravetch JV. Fc receptors are required in passive and active immunity to melanoma. Proc Natl Acad Sci USA. 1998;95:652–6. doi: 10.1073/pnas.95.2.652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hellstrom KE, Hellstrom I. Lymphocyte-mediated cytotoxicity and blocking serum activity to tumor antigens. Adv Immunol. 1974;18:209–77. doi: 10.1016/s0065-2776(08)60311-9. [DOI] [PubMed] [Google Scholar]
- 35.Manson LA. Anti-tumor immune responses of the tumor-bearing host: the case for antibody-mediated immunologic enhancement. Clin Immunol Immunopath. 1994;72:1–8. doi: 10.1006/clin.1994.1099. [DOI] [PubMed] [Google Scholar]
- 36.Houbiers JG, van der Burg SH, van de Watering LM, Tollenaar RA, Brand A, van de Velde CJ, Melief CJ. Antibodies against p53 are associated with poor prognosis of colorectal cancer. Br J Cancer. 1995;72:637–41. doi: 10.1038/bjc.1995.386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Handel-Fernandez ME, Cheng X, Herbert LM, Lopez DM. Down-regulation of IL-12, not a shift from a T helper-1 to a T helper-2 phenotype is responsible for impaired IFN-g production in mammary tumor-bearing mice. J Immunol. 1997;158:280–6. [PubMed] [Google Scholar]
- 38.Kobayashi M, Kobayashi H, Pollard RB, Suzuki F. A pathogenic role of Th2 cells and their cytokine products on the pulmonary metastasis of murine B16 melanoma. J Immunol. 1998;160:5869–73. [PubMed] [Google Scholar]