Abstract
Rabbit primary dermal bacillus Calmette–Guérin (BCG) lesions were compared with reinfection BCG lesions in order to gain insight into how immune responses protect against clinical tuberculosis. As early as 3 hr, a marked infiltration of macrophages and lymphocytes occurred in the reinfection group, while very little cell infiltration occurred in the primary group. It seems that only an antigen–antibody reaction could produce such an immediate pronounced antigen-specific chemotactic effect, because very few lymphocytes are normally present in the skin. Therefore, antibodies hasten the accumulation of an expanded antigen-specific T-lymphocyte population (memory cells) at sites of bacillary lodgement. By 1–2 days, the primary and reinfection BCG lesions differed 400- to 500-fold in size. By 4–5 days, the size of the reinfection lesions had declined, while the size of the primary lesions had increased, so that, grossly, both types of lesion were similar. At 8 days in reinfection lesions and at 12 days in primary lesions, small secondary peaks in size occurred, which were probably caused by cell-mediated immune responses. In rabbits with primary BCG lesions, skin tests with Old Tuberculin were positive at 9 days, accompanied by a rise in the levels of antibodies to the secreted antigen, phosphate-specific transport protein 1, but the levels of antibodies to the constitutive antigens, purified protein derivative and heat-shock protein 65, did not increase appreciably until some time after 23 days. In tissue sections of reinfection BCG lesions, the percentage of mononuclear cells labelled, by in situ hybridization techniques, for the mRNA of monocyte chemoattractant protein 1 (MCP-1), a chemokine, peaked at 3 hr and then was down-regulated, whereas in primary lesions, this percentage was down-regulated only after 2 days. [The percentage in the tissue sections for the mRNAs of interleukins 1β and 8, as well as the proteins of MCP-1 and tumor necrosis factor alpha (TNF-α), followed a somewhat similar time-course to that of MCP-1 mRNA.] A high percentage of mononuclear cells containing the MCP-1 mRNA ‘factory’ would favour enlargement of the lesions and a low percentage would favour their regression. At 5 days, the percentage of CD4 and CD8 lymphocytes, stained by immunohistochemical techniques, and the amount of microvasculature stained similarly for vascular cell adhesion molecule 1 were higher in the reinfection group, indicating that prior immunization caused a more rapid (antigen-dependent) up-regulation of these factors. Tuberculin reactions resembled early reinfection BCG lesions in almost every factor evaluated herein. In brief, the production of chemokines began soon after BCG reinfection, peaked within a few hours and was markedly down-regulated by 24 hr, a time at which the lesions of reinfection were of maximal size. Therefore, the amount of cell infiltration was tightly controlled, probably by the variety of mechanisms listed herein.
Introduction
Tuberculosis (TB) kills more people in the world today than any other infectious disease,1 and the occurrence of multidrug-resistant strains of tubercle bacilli is increasing. More effective vaccines are urgently needed. Pulmonary TB in rabbits2,3 resembles the disease in humans4 more closely than TB in any other common laboratory animal in that cavitary lesions with bronchial spread are readily produced and caseous necrosis is common.2,3 TB lesions in guinea-pigs also show caseous necrosis, but only rarely cavitate (Dannenberg & Collins, submitted for publication).5 TB lesions in mice do not show true caseous necrosis, and never cavitate (Dannenberg & Collins, submitted for publication).5
To understand better the immune mechanisms responsible for vaccine efficacy in the rabbit model, we compared primary BCG lesions with reinfection BCG lesions with respect to (a) antibody titres; (b) interleukin-1β (IL-1β), tumour necrosis factor-α (TNF-α), monocyte chemoattractant (activating) protein 1 (MCP-1) and IL-8; (c) CD4 and CD8 lymphocytes; and (d) intercellular adhesion molecule (ICAM-1), vascular cell adhesion molecule (VCAM-1) and endothelial–leucocyte adhesion molecule (ELAM-1). To our knowledge, this study is the first to make such comparisons in this model, because histochemical procedures for lymphocyte subgroups, cytokines and vascular adhesion molecules have only been available in recent years.
We also compared tuberculin reactions with primary and reinfection bacillus Calmette–Guérin (BCG) lesions. Tuberculin reactions are produced by a small percentage of the antigens present in intact bacilli. We had hoped therefore to identify components of BCG tissue reactions that were not present in tuberculin reactions.
Materials and methods
Immunization of rabbits with BCG and tuberculin tests
Specific pathogen-free female New Zealand White rabbits (2·5–2·7 kg) were purchased from Covance Research Products, Inc., Denver, PA. The rabbits were immunized by the intradermal injection of about 5 × 106 viable log phase Tice BCG bacilli on both the right and left flanks.6,7 Twenty-four days later, they were reinfected with 5 × 106 Tice BCG also at two sites. At that time, non-immunized rabbits were similarly injected to produce primary BCG lesions.
For the tuberculin tests, a 1:30 dilution of 4 × Old Tuberculin from Lederle Laboratories, American Cyanamid Co., Pearl River, NY, was injected intradermally, usually 0·1 ml in each of two sites. Old Tuberculin (OT) contains more tuberculin-like antigens than purified protein derivative (PPD), the tuberculin used for skin-testing people. OT was preferred for testing the tuberculin sensitivity of rabbits, by Professor Max B. Lurie of the University of Pennsylvania.2
At the times listed in Fig. 1, the induration of the resulting BCG lesions and tuberculin reactions was measured with callipers, as described previously.8 Briefly, the product of length and width and ‘pinched’ double thickness (in mm), multiplied by a factor of 0·52, provided the volume of the lesion in mm3. At the end of the experiments, the rabbits were killed by intravenous sodium pentobarbital (2·5–3·0 ml, 65 mg/ml). A necropsy was carried out on each rabbit, and they were all found to be free of disease.
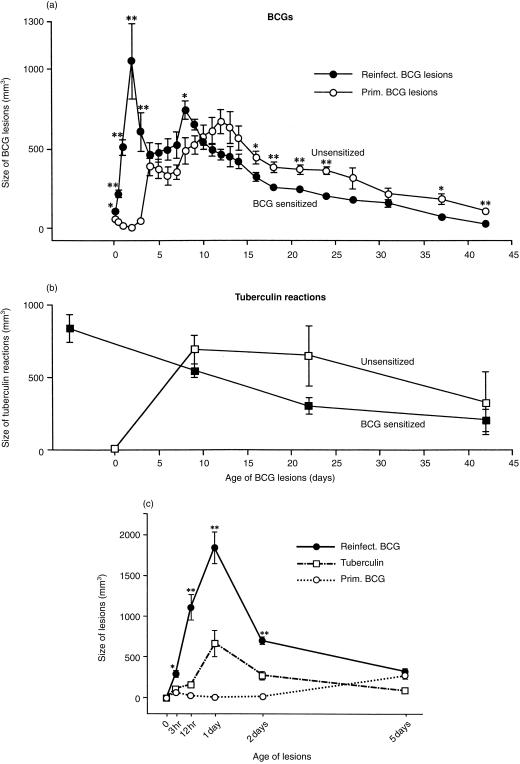
Figure 1.
(a) Size of primary BCG lesions and reinfection BCG lesions from 3 hr to 42 days in rabbits of Expt I. The reinfected rabbits had been sensitized intradermally by BCG 24 days previously. The reinfection BCG lesions were many times larger than the primary BCG lesions at 3, 12, 24 and 48 hr, a fact that was apparently initiated by an antigen–antibody reaction (see text). Also, the size of the reinfection BCG lesions reached a second peak at 8 days, whereas the primary lesions reached a similar peak at 12 days. These second peaks were apparently caused by an antigen-specific CMI/DTH reaction (see text). After the second peaks, the lesions slowly regressed. Each point represents the mean of lesions from five rabbits and its standard error. (b) Size of 2-day tuberculin reactions in rabbits of Expt I. In the reinfected host, tuberculin sensitivity was highest before challenge. This sensitivity declined thereafter, and no booster effect from the second BCG injection was apparent. In contrast, in hosts with primary BCG infections, tuberculin sensitivity was strong by 9 days and tended to remain higher than that present in the reinfected hosts, possibly because the infecting bacilli were not destroyed as readily. Each point represents the mean of five rabbits and its standard error. (c) Size of primary and reinfection BCG lesions and size of tuberculin reactions 3 hr to 5 days old in Expt II, from which tissue sections were obtained and evaluated. As in Expt I, the reinfected rabbits were sensitized intradermally by BCG 24 days previously. During these first 5 days, the size of the reinfection BCG lesions and of the tuberculin reactions followed the same pattern. Each point represents the mean of at least four lesions with its standard error. In (a) and (c), reinfection BCG lesions versus primary BCG lesions: *P < 0·05 and **P < 0·01.
Measurement of antibodies
Antibody responses were measured by enzyme-linked immunosorbent assay (ELISA) as previously described9 with 1 µg antigen per well, 1/125–1/500 dilutions of rabbit sera, swine anti-rabbit antibody conjugated to horseradish peroxidase (DAKO a/s, Glostrup, Denmark), and 3,3′,5,5′-tetramethylbenzidine solution (ICN Pharmaceuticals, Basingstoke, Hampshire, UK). Mycobacterium leprae heat-shock protein 65 (hsp 65) antigen was purified from recombinant Escherichia coli;10 a 38 000 MW antigen (phosphate-specific transport protein 1; PstS-1) from the WHO Mycobacterial Protein Bank was provided by Dr M. Singh, Genexpression, GBF, Braunschweig, Germany; PPD was obtained from Evans Medical Ltd, Langhurst, Horsham, UK.
Immunohistochemical procedures, and in situ hybridization procedures on tissue sections of the lesions (prepared in a cryostat)
These procedures were performed exactly as previously described,6,7,11 and photomicrographs were published in these references. 35S-radiolabelled anti-sense RNAs were used to visualize cytokine mRNAs in the mononuclear cells (with cytokine 35S-sense RNAs as controls).6,11
The dilutions of the primary antibodies used herein for immunohistochemistry6,7,11 were 1:500 for MCP-1, 1:200 for TNF-α, 1:50 for CD4 and CD8, 1:150 for ICAM-1, 1:8 for VCAM-1, 1:300 for ELAM-1 and 1:10 000 for von Willebrand factor. In addition, we evaluated the percentage of CD4 and CD8 cells using mouse monoclonal anti-rabbit CD4 and CD8 immunoglobulin G (IgG; both, 0·5 mg/ml, Spring Valley Laboratories, Woodbine, MD) diluted 1:50 as the primary antibody. These CD4 and CD8 monoclonal antibodies seemed to recognize each cell type in tissue sections with equal efficacy.
Quantification of mononuclear cells (MN) and microvascular endothelium in tissue sections
In each tissue section, the MN (mainly macrophages and lymphocytes) were counted microscopically in five non-necrotic sites that were densely infiltrated with cells.6 The results are presented as the percentage of the cell population that was labelled for the factor under study. Changes in the percentage of labelled cells over the 5-day period indicated whether the factor was increasing or decreasing. For example, an increase in the percentage of cells labelled for chemokine MCP-1 mRNA suggests that more MCP-1 protein would be produced and that more mononuclear cells would infiltrate the lesion. Conversely, a decrease in the percentage of cells labelled for MCP-1 mRNA suggests that mononuclear cell infiltration was being down-regulated.
Changes in the total number of cells in each type of lesion can be approximated by multiplying the lesion size shown in Fig. 1(c) by the number of MN [and polymorphonuclear cells (PMN)] per mm2 in the tissue sections (shown in Fig. 3).
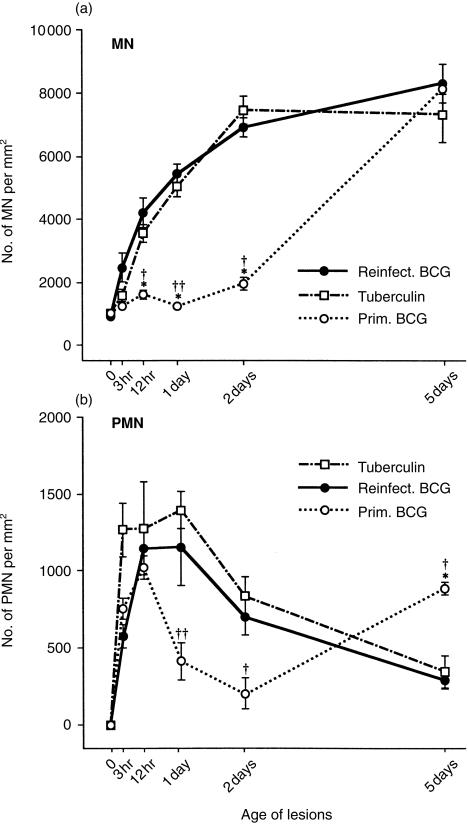
Figure 3.
Number of MN (a) and PMN (b) per mm2 of tissue section at various times in reinfection BCG lesions, in tuberculin reactions, and in primary BCG lesions. The reinfection BCG lesions and tuberculin reactions show no differences in the density of either cell type, but the primary lesions contain fewer MN per mm2 than the others at 12 hr and at 1 and 2 days. The total number of MN and PMN in each type of lesion can be estimated by combining the data depicted here with that depicted in Figure 1 (see Discussion). Each point represents the mean of four lesions with its standard error: for reinfection BCG lesions versus primary BCG lesions: *P < 0·05; and for tuberculin reactions versus primary BCG lesions: †P < 0·05 and ††P < 0·01.
The functional microvascular endothelium (detected by staining for von Willebrand factor) was also measured in the lesions. A computerized image analyser was used and the microvascular area per mm2 of tissue section was calculated, as described previously.7
Acid-fast stain for tubercle bacilli
The tubercle bacilli in the fixed–frozen sections of both primary BCG lesions and those of reinfection were stained using the Ziehl–Neelsen method.12 Specifically, the sections were incubated in carbol fuchsin for 4–16 hr at room temperature, decolorized for 2 min in acid-alcohol,12 rinsed, counterstained with methylene blue, rinsed, dehydrated through alcohols and then acetone, and mounted in Flotexx (Lerner Laboratories, Pittsburgh, PA).
Statistical analyses
Both the analysis of variance and a modified two-tailed Student's t-test were used. The means and their standard errors are shown in the table and the figures.
Results
Local cell infiltration
Figure 1(a,c) shows the size of primary and reinfection BCG lesions over a period of 42 days. Within the first 3 hr, the lesions of reinfection had increased in size, mainly due to cell infiltration. Peak size occurred at 1 or 2 days, when the primary lesions were still very small. At that time, a 400- to 500-fold difference in size was present, also due to cell infiltration. This cell infiltration was antigen-specific, and it could only have been initiated by an antigen–antibody reaction, because there are few, if any, memory T cells in skin.
By 4 and 5 days, the size of the reinfection BCG lesions had markedly decreased and the size of the primary lesions had moderately increased, so that the two types of lesion became almost the same size (Fig. 1a,c). From 5 days on, the size of the primary and reinfection BCG lesions followed the same pattern. However, the reinfection lesions showed a smaller second peak in size at 8 days, whereas the primary lesions showed this peak at 12 days. The second peak occurred earlier in the reinfection group, probably because of the booster effect of the second BCG injection on already existing cell-mediated immunity (CMI) and delayed type hypersensitivity (DTH). In both groups, after the second peaks, there was a steady decline in lesion size.
Lesion size, ulceration and healing
No apparent difference was found in the times that primary and reinfection BCG lesions ulcerated and healed. The ulceration, followed by discharge of the necrotic contents of the lesions, was a major contributor to the decrease in lesion size associated with healing. Sometimes, however, an incomplete discharge of necrotic material slowed the healing process. At 42 days, the sites of the BCG injections could still be readily discerned, but at 3 months grossly visible remnants of the lesions often could not be found, either on the skin surface or, at necropsy, on the underside of the skin (unpublished experiments).
Tuberculin reactions
Figure 1(b) shows the size of 2-day tuberculin reactions in Experiment I. In the reinfection group, there was rather strong tuberculin sensitivity initially, which declined thereafter. In the primary BCG group, tuberculin sensitivity was not present initially, was strong between day 9 and day 23 and then declined. The tuberculin sensitivity did not remain at peak levels in rabbits with either primary or reinfection BCG lesions (Fig. 1b). Once established, such sensitivity slowly declined and reinfection with BCG had no apparent booster effect.
In Experiment II, tuberculin was injected at the same time as primary and reinfection BCG and the skin lesions produced were measured for only the first 5 days (Fig. 1c). Both the BCG lesions and the tuberculin reactions increased and decreased in parallel (Fig. 1c). By 5 days, however, the local concentration of tuberculin had probably decreased substantially and no longer provided the stimulus that it had earlier.
Antibody titres
We measured the amount of antibody to three of the numerous antigens of tubercle bacilli.13–15 The first antibody was to the 38 000 MW PstS-1, which is a secreted antigen.16,17 The other two antibodies were to hsp 65 and PPD, which are constitutive, not secreted, antigens that are probably released only upon the destruction of the bacillus.16
At 2 days, antibodies against all three antigens were significantly higher (P < 0·05) in reinfected rabbits than in rabbits with primary infection (Fig. 2). With reinfection, in contrast to the tuberculin reactions, the antibody titres of all three antigens rose rapidly (Fig. 2).
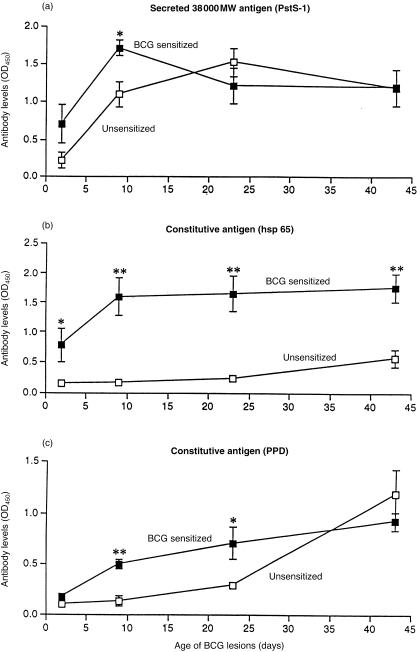
Figure 2.
Antibody levels to the secreated mycobacterial 38 000 MW antigen PstS-1 and to the constitutive antigens hsp 65 and PPD in rabbits with primary and reinfection BCG lesions. In the reinfected hosts, the second injection of BCG enhanced all existing antibody levels. In hosts with primary BCG infection, the antibody titre to the secreted 38 000 MW antigen PstS-1 became substantial by day 9, but the titres to the constitutive antigens hsp 65 and PPD rose more slowly. Each point represents the mean with its standard error from five rabbits: *P < 0·05 and **P < 0·01.
With primary infection, only the antibodies against the secreted 38 000 MW antigen PstS-1 rose rapidly, and reached the level found in the reinfected rabbits at 23 days (Fig. 2). Antibody levels to PPD reached such a level at 42 days, but at that time the antibody levels to hsp 65 were still far below those of the reinfected animals (Fig. 2).
These findings clearly demonstrate that, in animals previously vaccinated with BCG, circulating antibodies against both constitutive and secreted antigens were immediately available, and that these antibody levels rapidly increased upon challenge. They also demonstrate that in primary infection the initial immune response was, as expected, to the secreted antigens of the bacillus.
Amount of necrosis in the skin lesions
On day 1, in all three types of lesion the tissue damage was rather diffuse, but it was more extensive in the sensitized host. On day 5, the reinfection BCG lesions had well circumscribed caseous necrotic centres (13·2 ± 2·4 mm2), and the primary BCG lesions had smaller more diffuse necrotic centres (4·8 ± 1·4 mm2). This difference, however, was not statistically significant. The areas of necrosis were measured with a computerized image analyser.7 The tuberculin reactions showed no necrosis.
Number of bacilli in central areas of tissue sections from primary and reinfection BCG lesions
In order to estimate differences in the number of bacilli within these two types of lesion, we counted the number of acid-fast staining particles in central areas of the lesions. Most of the bacilli were in necrotic sites, but some could also be found within intact cells. Few, if any, bacilli could be found in the surrounding tuberculous granulation tissue from which we obtained all the other histological data presented in this report.
On days 1 and 2, fewer bacilli per mm2 seemed present in reinfection lesions than in primary lesions, but more lesions would have to be analysed to attain statistical significance. This apparent difference in bacillary concentration was most likely due to the marked difference in lesion size: the greater the lesion size, the smaller would be the number of bacilli per mm2. At 5 days, the primary lesions and those of reinfection were approximately the same size and appeared to contain an identical number of bacilli (about 10 bacilli per mm2).
MN and PMN
MN (macrophages, lymphocytes and activated fibroblasts) per mm2 and the PMN per mm2 were counted in the lesions' tuberculous granulation tissue that was densely infiltrated with MN and was not near sites of necrosis (where the most PMN accumulated). In these densely infiltrated areas, the number of PMN was always much less than the number of MN per mm2 (compare the y-axes in Fig. 3a,b).
Many more MN per mm2 were present in the reinfection BCG lesions than in primary BCG lesions until day 5, at which time both types of lesion contained the same number per mm2 (Fig. 3a). In the densely infiltrated areas, the number of PMN per mm2 was similar in primary and reinfection BCG lesions during the first 12 hr and then seemed to differ as shown in Fig. 3(b). PMN are thought to play little or no role in the development and healing of tuberculous lesions.2 The early PMN response depicted in Fig. 3(b) was probably due to the non-specific irritants in the vaccine, because primary and reinfection BCG lesions contained similar numbers of PMN per mm2.
Tuberculin reactions and reinfection BCG lesions showed similar numbers of MN and PMN per mm2 in the densely infiltrated areas (Fig. 3a,b). The number of PMN per mm2 seemed to peak earlier than the number of MN (Fig. 3b), which is consistent with the known early PMN infiltration in most inflammatory lesions.18
The total number of MN and PMN in each type of lesion can be estimated by combining the data in Figs 1 and 3. On day 1, the size of the reinfection BCG lesions was about 550 times that of the primary BCG lesions, and the size of the tuberculin reactions was about 140 times that of the primary BCG lesions. Thus on day 1, the number of MN and PMN in reinfection BCG lesions and in tuberculin reactions far exceeded the number of MN and PMN in primary BCG lesions. Areas near the central necroses (which contain larger numbers of PMN) were not analysed in this report.
CD4 and CD8 lymphocytes
During the first 2 days, the percentage of mononuclear cells stained for CD4 lymphocytes was about the same in the densely infiltrated areas of both primary and reinfection lesions; whereas at 5 days, this percentage was substantially higher in the reinfected group (Fig. 4a). At both 2 and 5 days, the mean percentages of mononuclear cells stained for CD8 lymphocytes was greater in the reinfection BCG lesions than in the primary lesions (Fig. 4b). At 5 days, the tuberculin reactions contained a lower percentage of CD4 and CD8 cells than did the reinfection BCG lesions, because the tuberculin had probably been destroyed or had diffused away by this time.
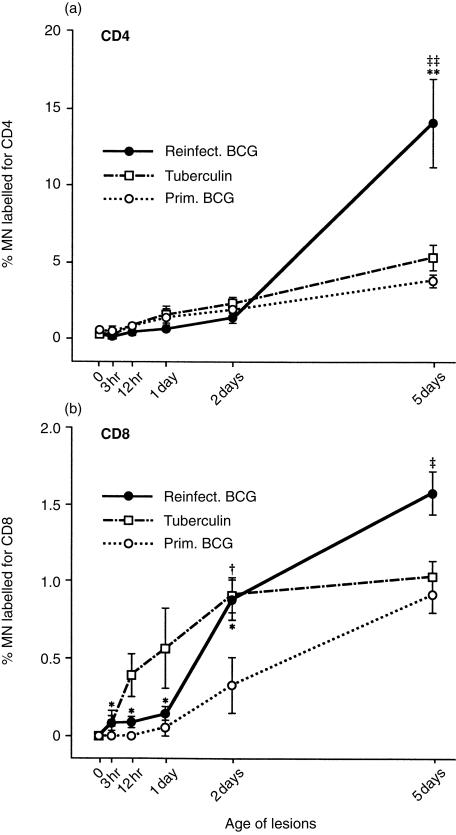
Figure 4.
Percentage of MN immunostained for (a) CD4 and (b) CD8 in the three types of lesion. At 2 days, the reinfection BCG lesions and tuberculin reactions contained a higher percentage of CD8 cells per mm2 than did the primary lesions (P < 0·05), suggesting that tuberculin sensitivity favours the production of cytotoxic T lymphocytes. Note that CD4 cells are much more numerous than CD8 cells (compare the scales on the y-axes). Each point represents the mean of four lesions with its standard error: for reinfection BCG lesions versus primary BCG lesions: *P < 0·05 and **P < 0·01; for tuberculin reactions versus primary BCG lesions: †P < 0·05; and for reinfection BCG lesions versus tuberculin reactions: ‡P < 0·05 and ‡‡P < 0·01.
In the reinfection BCG lesions at 5 days, the ratio of CD4:CD8 cells was about 8, and in the primary lesions and tuberculin reactions, this ratio was about 4 (compare the y-axes in Fig. 4a,b). Therefore, in early lesions produced by tubercle bacilli (and their tuberculin-like products), there are more CD4 cells than CD8 cells.
In another experiment, the percentage of CD4 and CD8 cells in the mononuclear cell population of the primary lesions was determined at 9, 23 and 37 days (Table 1). On those days, the percentage of CD4 cells in the primary lesions was the same as that found in 5-day reinfection lesions, but the percentage of CD8 cells was several times higher than that found in 5-day reinfection lesions (compare Table 1 with Fig. 4). By 9 days, the host with primary lesions had developed CMI and DTH, and the percentages of both CD4 and CD8 cells had increased substantially. Reinfection lesions were not evaluated beyond 5 days, so no comparisons with the primary lesions could be made at these later times.
Table 1.
CD4 and CD8 cells in mononuclear cell populations within primary BCG lesions during their development and healing*
| Age of BCG lesion | Percentage of CD4 cells | Percentage of CD8 cells | Ratio CD4/CD8 |
|---|---|---|---|
| 9 days | 13·2 + 1·2% | 8·7 + 2·1% | 1·7 + 0·5% |
| 23 days | 17·8 + 1·1% | 5·4 + 0·9% | 3·9 + 0·5% |
| 37 days | 15·7 + 2·4% | 6·3 + 1·6% | 2·9 + 0·6% |
These results are from the primary BCG lesions in the experiment described in ref. 6.
Vascular adhesion molecules: ICAM-1, VCAM-1 and ELAM-1 (E-selectin)
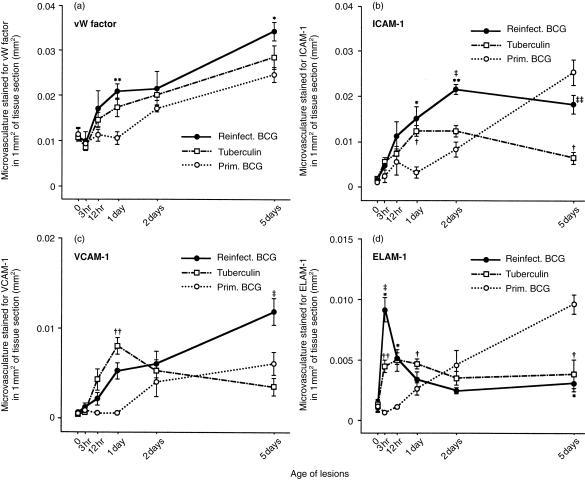
ICAM-1, VCAM-1 and ELAM-1 are major vascular endothelial adhesion molecules which react with receptors on circulating leucocytes and cause them to migrate into inflammatory sites.7,19,20 ICAM-1 aids the infiltration of PMN, monocytes and lymphocytes;20 VCAM-1 aids the infiltration of monocytes, lymphocytes and eosinophils;20 and ELAM-1 aids infiltration of PMN, monocytes and some types of lymphocyte.20 von Willebrand (vW) factor is present in almost all functional vascular endothelial cells,7,21 and immunostaining for vW factor enables us to estimate the total microvasculature in a tissue section.7,21
After 12 hr, the lesions of reinfection showed more vW-staining microvasculature per mm2 than did the primary lesions (Fig. 5a). The amount of vasculature in the tuberculin reactions resembled that in the lesions of reinfection, but was less pronounced. Therefore, the host's immune response not only increased the early cell infiltration but also increased the blood supply.
Figure 5.
The microvascular area labelled immunohistochemically for (a) von Willebrand (vW) factor, (b) ICAM-1, (c) VCAM-1 and (d) ELAM-1 in the three types of lesion. The functional microvasculature (in mm2 in 1 mm2 of tissue section), that was recognized by vW staining, seems to increase more rapidly in the reinfection lesions (and in the tuberculin reactions) than in the primary lesions (a). ICAM-1 stains most of the functional microvasculature, whereas VCAM-1 and ELAM-1 stain roughly half as much (compare the scales on the y-axes). ELAM-1 shows a very early (3 hr) peak response in the lesions of the tuberculin-positive hosts. ELAM-1 is also increased at 5 days in the primary lesions. Each point represents the mean of four lesions with its standard error: for reinfection BCG lesions versus primary BCG: *P < 0·05 and **P < 0·01; for tuberculin reactions versus primary BCG lesions: †P < 0·05 and ††P < 0·01; and for reinfection BCG lesions versus tuberculin reactions: ‡P < 0·05 and ‡‡P < 0·01.
ICAM-1, VCAM-1 and ELAM-1 were up-regulated more quickly in reinfection BCG lesions (and in lesions produced by tuberculin) than in primary BCG lesions (Fig. 5), which is consistent with the rapid cell infiltration that occurred in the former two groups and the slow cell infiltration in the latter. In the primary BCG lesions, ICAM-1 and ELAM-1 (and possibly VCAM-1) continued to increase for 5 days (Fig. 5), but in the reinfection BCG lesions, only VCAM-1 seemed to continue to increase during this time (Fig. 5). ELAM-1-staining vasculature was greatest in the lesions of reinfection at 3 hr and greatest in the primary lesions at 5 days (Fig. 5).
In the tuberculin reactions, the amount of vasculature stained for these adhesion molecules in general resembled that found in the BCG lesions of reinfection, except at 5 days when the local concentration of tuberculin had probably decreased. In 1-day tuberculin reactions, the vessels staining for VCAM-1 were most numerous, suggesting that VCAM-1 was rapidly up-regulated in sensitized hosts by the tuberculin-like products of the bacilli.
When the vasculature areas stained for the three adhesion molecules were plotted as a percentage of the areas stained for vW factor, the graphs resembled those in Fig. 5(b–d) (data not presented). This finding suggests that the microvasculature stained for each of these three adhesion molecules also stained for vW factor, which is a marker for all functional vascular endothelial cells.
Cytokine mRNAs
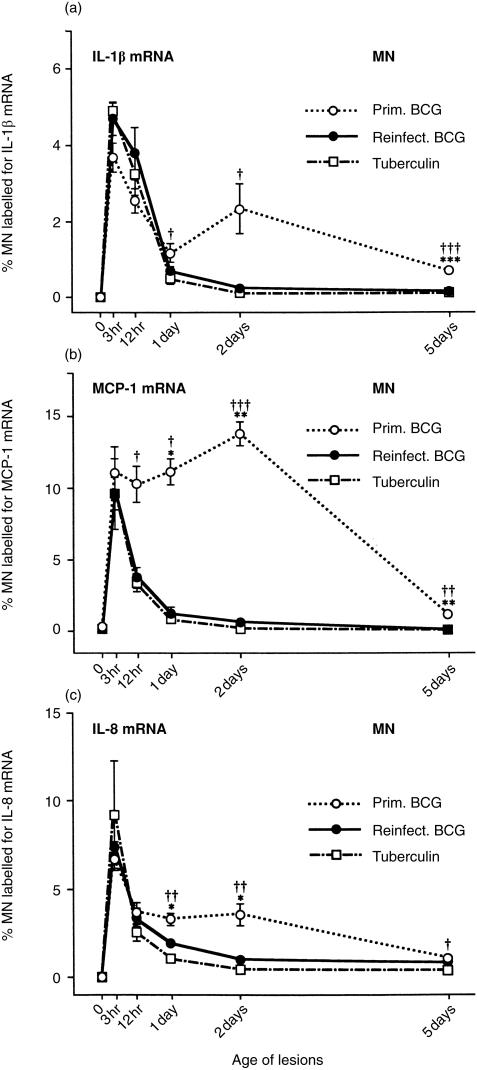
IL-1β is a primary cytokine that up-regulates other cytokines;22,23 MCP-1 is a major chemokine that attracts and activates macrophages and lymphocytes;24 and IL-8 chemoattracts PMN and lymphocytes.24
At 3 hr, the percentage of MN labelled for IL-1β mRNA, MCP-1 mRNA and IL-8 mRNA showed peak levels in all three types of lesion: primary BCG lesions, reinfection BCG lesions and tuberculin reactions (Fig. 6). Since the rabbits with primary lesions were not immunized, this 3-hr reaction was non-specific.6 The percentage of MN containing these cytokine mRNAs then rapidly declined, with the exception of MCP-1 in the primary lesions.
Figure 6.
Percentage of MN labelled for (a) IL-1β mRNA, (b) MCP-1 mRNA and (c) IL-8 mRNA in the three types of lesion. The percentage of MN containing the three cytokine mRNAs shows early peak levels at 3 hr. Then, this percentage of MN rapidly declines in BCG lesions of reinfection and in tuberculin reactions, but remains relatively high in primary BCG lesions at 2 days, especially the percentage containing MCP-1 mRNA. At 2 days, the primary lesions are growing in size, whereas the reinfection lesions and tuberculin reactions are regressing (see Figure 1c). Each point represents the mean of four lesions with its standard error: for reinfection BCG lesions versus primary BCG: *P < 0·05, **P < 0·01 and ***P < 0·001; for tuberculin reactions versus primary BCG lesions: †P < 0·05, ††P < 0·01 and †††P < 0·001.
At 2 days, the percentages of IL-1β, MCP-1 and IL-8 mRNAs in primary lesions were higher than those in lesions of reinfection, with the RNA of MCP-1 (the chemokine for MN) showing the most difference (Fig. 6). Of course, this result does not mean that the primary BCG lesions contained more MN labelled for IL-1β, MCP-1 and IL-8 mRNAs than did the reinfection BCG lesions and tuberculin reactions because the primary lesions were so much smaller in size (see Fig. 1). The percentage of chemokine-labelled cells in the lesions, however, is a good indicator of whether the lesions are expanding or contracting in size, because chemokines are major factors causing the cell infiltration.
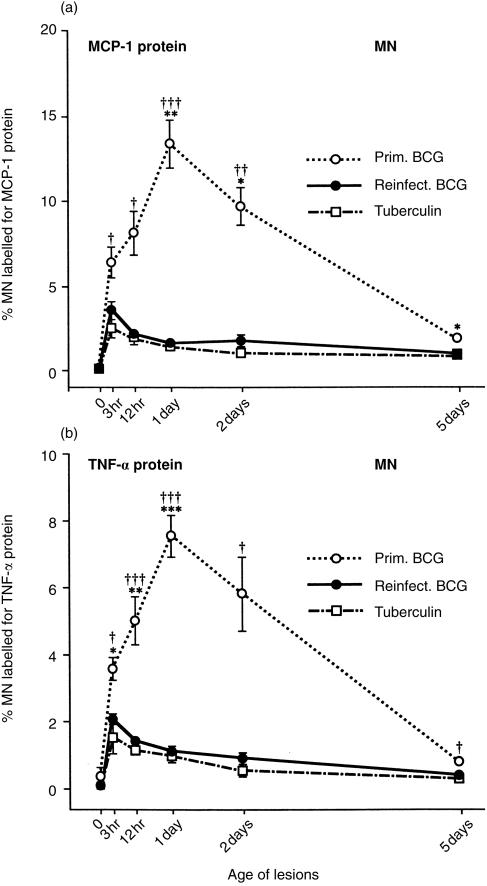
Cytokine proteins
Only antibodies to rabbit MCP-1 and TNF-α proteins were available at the time of our experiments. As stated above, MCP-1 is a major chemoattractant for monocytes and lymphocytes.24 TNF-α is a primary cytokine in that it and IL-1β help initiate the inflammatory responses.6,22–25
Among the three types of lesion, the percentage of MN stained for MCP-1 and TNF-α proteins followed the same pattern as the percentage of MN stained for MCP-1 mRNA (compare Figs 6 and 7). This finding indicates that the cells containing MCP-1 mRNA were actively producing MCP-1 protein. At 3 and 12 hr and 1 and 2 days, the percentages of MN stained for MCP-1 and TNF-α proteins were much higher in the primary BCG lesions than in those of reinfection. At 5 days, however, these percentages were much closer (Fig. 7).
Figure 7.
Percentage of MN labelled for (a) MCP-1 protein and (b) TNF-α protein in the three types of lesion. The percentage of MN containing these two cytokine proteins shows the same pattern as the percentage of MN containing MCP-1 mRNA in Figure 6, but the early peak at 3 hr in the tuberculin-sensitive hosts is much less pronounced. (The TNF-α mRNA was not labelled in these lesions.) Each point represents the mean of four lesions with its standard error: for reinfection BCG lesions versus primary BCG: *P < 0·05, **P < 0·01 and ***P < 0·001; for tuberculin reactions versus primary BCG lesions: †P < 0·05, ††P < 0·01 and †††P < 0·001.
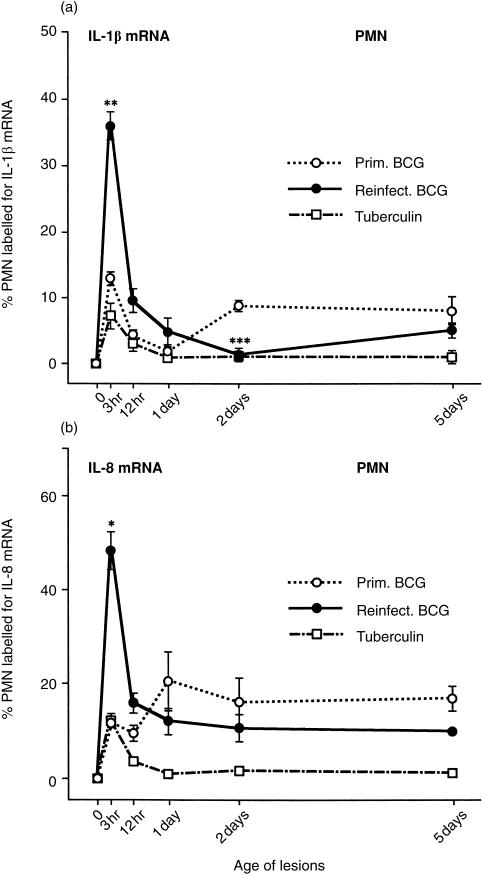
IL-1β and IL-8 mRNAs in PMN
IL-1β is a primary cytokine that tends to up-regulate other cytokines.22,23 IL-8 is a chemokine that attracts PMN into the local site and activates them.24 We did not find MCP-1 mRNA in rabbit PMN.6,11 In the Giemsa-stained frozen tissue sections (cut in a cryostat), the PMN were identified by their lobed nucleus. Young PMN without such a nucleus were often missed, unless their orange granules were prominent. Rabbit PMN are eosinophilic.
In the densely infiltrated areas of BCG lesions that were not adjacent to areas of necrosis, the percentage of PMN labelled for IL-1β and IL-8 mRNAs showed a peak at 3 hr and then regressed (except for IL-8 mRNA in the primary lesions) (Fig. 8). The 3-hr peaks were higher in reinfection BCG lesions than in primary BCG lesions, but at 2 and 5 days the percentage of PMN stained for IL-1β and IL-8 appeared to be higher in the primary lesions. In tuberculin reactions, PMN IL-1β and IL-8 mRNAs peaked at 3 hr and were low at 1, 2 and 5 days (Fig. 8).
Figure 8.
Percentage of PMN labelled for (a) IL-1β mRNA and (b) IL-8 mRNA in the three types of lesion. Similar to the MN in Figure 6, the percentage of PMN containing these cytokine mRNAs shows a 3-hr peak. Then, the percentage of labelled PMN declines in the reinfection BCG lesions and tuberculin reactions, but remains somewhat elevated in the primary BCG lesions, again similar to the MN in Figure 6. Each point represents the mean of four lesions with its standard error: for reinfection BCG lesions versus primary BCG: *P < 0·05, **P < 0·01 and ***P < 0·001.
In 3-hr lesions, the intensity of the PMN staining for IL-1β and IL-8 mRNA was strongest. Thereafter, fewer PMN stained and those that did stained more faintly.
A comparison of the percentages of PMN and the percentages of MN labelled for these cytokine mRNAs are of interest. In reinfection BCG lesions, at the 3-hr peak, IL-1β mRNA averaged 37% for PMN and 5% for MN, and IL-8 mRNA averaged 48% for PMN and 7% for MN (Figs 6 and 8). Therefore, in contrast to PMN, only a relatively small proportion of MN was engaged in IL-1β and IL-8 production, and the majority was presumably engaged in other activities. The reason for these differences may be the heterogeneity of the MN population.26
Discussion
Role of antibodies
In a cell-mediated disease, such as tuberculosis, antibodies have frequently been thought to play only a minor role in the host's defence.27,28 Yet antibodies circulate2,13–15 and plasma cells are common in the lesions.29 The studies presented here suggest a role for antibodies that has not been previously considered. We asked the question: what immune mechanism caused large numbers of cells to infiltrate at 3 hr into reinfection BCG lesions, but not into primary BCG lesions? Since very few lymphocytes are present in normal skin, this rapid antigen-specific reaction must be mediated by antibodies. Lymphocytes and antibodies are the only antigen-specific immunological host defences known to exist.
Antibodies are carried by the blood circulation and some are cytophilic (by binding to cell FC receptors). Antibodies can rapidly combine with the infecting bacilli and their antigens, causing an Arthus-type reaction and activating complement30,31 to produce C5a and the membrane-attack complex (MAC). C5a is a potent chemoattractant for granulocytes and monocytes,32 and C3a and C5a activate connective-tissue-type mast cells.33 Mast cells produce IL-16 that attracts CD4 lymphocytes.34 C5a also increases leukotriene B4 (LTB4) production in various cell types,35 and LTB4 is a major chemoattractant and cell activator.36 In addition, the activation of complement causes vascular endothelial cells to secrete chemokines37 and increase their adhesion molecules for leucocytes.37 Activated complement also has an adjuvant effect through its C3d fragment.38
Cytophilic antibodies, including IgE, are able to sensitize mast cells (and possibly other resident cells, such as fibroblasts39 and macrophages40,41). Specific antigens then cause these cells to produce and/or release permeability factors,42 the chemotaxin LTB442 and cytokines.34,42,43 Thus, antigen–antibody reactions could play a major role in the initial infiltration of macrophages, PMN and lymphocytes into reinfection BCG lesions.
References 27 and 31 provide extensive discussions on the role of antibodies in tuberculosis. These publications fail, however, to mention the role herein presented, namely, that antigen–antibody reactions accelerate CMI responses by rapidly bringing the expanded antigen-specific T-cell population to sites of bacillary lodgement.
Early lesion size and percentage of MN labelled with each specific marker
When comparing primary BCG lesions with reinfection lesions, we reported the percentages of MN labelled for each marker in many of the figures. Reporting the total number of labelled MN in the entire lesion would be meaningless because of the tremendous difference in size between the primary and reinfected BCG groups. At 1 and 2 days, the size of the reinfection lesions was 400–500 times that of the primary lesions (Fig. 1c). At 5 days, however, the sizes of the primary and reinfection lesions were approximately the same, so that the percentages of cells labelled for each marker would reflect the total number of cells labelled for that marker when lesion size was considered.
Vascular adhesion molecules: ICAM-1, VCAM-1 and ELAM-1 (E-selectin)
All three microvasculature adhesion molecules increased more rapidly in BCG lesions of reinfection (and tuberculin reactions) than in primary BCG lesions (Fig. 5). This finding is consistent with the rapid increase in cell-infiltration in the former group. Excellent overviews of the various roles of vascular adhesion molecules in inflammatory processes have been published.7,19,20,44–47
Cytokines
At 3 hr, in reinfection BCG lesions, the percentage of MN containing cytokine mRNA was at its peak (Fig. 6). Then, the size of lesions of reinfection continued to increase, reaching its peak at 1 day (Fig. 1c) when the percentage of MN with cytokine mRNA had decreased (Fig. 6). These findings indicate that the production of MN cytokines, especially MCP-1 (reflected by their mRNAs), contributes substantially to the cell infiltration that caused the 1-day peak in size in the reinfected group. The decrease of the cytokine mRNA at 1 day (Fig. 6) followed by a decrease in lesion size at 2 days (Fig. 1c) also supports the premise that cytokines, especially MCP-1, are important players in determining the amount of cell infiltration.
In contrast to the reinfection lesions, the primary BCG lesions grew slowly in size during the 5-day period of study (Fig. 1a,c), and the percentage of MN containing MCP-1 mRNA remained at peak levels for at least 2 days (Fig. 6).
What is the cause of the rapid down-regulation of cytokine production in the reinfected host? From the literature, many factors seem to be involved because all inflammatory and immune responses are carefully regulated. Specifically, high local concentrations of both antigens and certain cytokines no longer stimulate and even induce apoptosis of nearby cells (called ‘active’ apoptosis).48 FasL and TNF are involved.48 Very low concentrations of antigens (caused by the destruction of bacilli and their products) stop the production of certain cytokines and result in apoptosis of nearby cells (called ‘passive’ apoptosis).48 The withdrawal of IL-2 induces this type of apoptosis.48 Regulatory T cells,49 including T suppressor cells,50 play a distinct role. Decreases in the number and type of chemokine receptors also play a role,51–53 as do soluble cytokine receptors54 and inhibitors such as IL-1 receptor antagonist.55 Finally, numerous in vivo down-regulating substances exist: transforming growth factor-β,49,56 IL-10,49 IL-4,49 IL-6,57 prostaglandin E2,56 lipoxygenase-derived eicosanoids (e.g. 15-HpETE),58,59 inducible NO,60,61 platelet-activating factor,56 adenosine,62 catecholamines,63 immune complexes64 and annexin I (lipocortin 1).65
The role of each of these factors in the rapid down-regulation of MCP-1 mRNA in reinfection BCG lesions is not known. Evidently, by 1 day, sufficient numbers of MN are present in the reinfected group (see Fig. 1a,c), so that factors causing further mononuclear infiltration are turned off.
Tuberculin reactions
During the 5-day period of study presented in Figs. 1(c) to 7, each of the evaluated factors increased and decreased in a rather similar manner in both reinfection BCG lesions and tuberculin reactions. Therefore, during this early period of study, none of the factors that we evaluated in the reinfection BCG lesions showed a different pattern from those present in the tuberculin reactions. CMI and DTH in tuberculosis seem, however, to be responses to different mycobacterial antigens.66
Cytokine networks
Our studies demonstrate the rise and fall of various cytokines within primary and reinfection rabbit BCG lesions during the first 5 days. Multiple mechanisms are probably involved because cytokines are enhanced and suppressed by genetically controlled host factors, as well as by factors from micro-organisms themselves.67 We may never fully understand the in vivo cytokine network in tuberculosis, or, in fact, of any inflammatory disease. We can, however, visualize, as we have done herein, the overall cytokine pattern and thereby gain insight into how cytokines participate.
Chemokine production is influenced in vivo by cell–cell, cell–adhesion molecule, cell–extracellular matrix interactions, and other cytokines.46 For example, co-cultures of monocytes with endothelial cells produced more IL-8, MCP-1 and MIP-1α (macrophage inflammatory protein 1α) than did either cell-type alone.46 Similarly, monocyte–fibroblast co-cultures produce more MCP-1 and MIP-1α than with either cell-type alone.46 Such cell–cell interactions can also down-regulate certain chemokines,46 probably depending on the type and stage of the inflammatory lesion involved.
The initial exposure to one cytokine evidently determines the type of macrophage activation and renders the macrophage temporarily unresponsive to another type of activation by another cytokine.68 Such studies may explain the heterogeneity of macrophage functions within lesions caused by tubercle bacilli.26 Of interest is that IL-12, IFN-γ and IL-2 bind to heparin and heparan sulphate which keeps these cytokines localized near the cells that produce them (reviewed in ref. 69).
A 70-week immunohistochemical analysis of cytokines and cell phenotypes in the lungs of mice infected intraperitoneally with human-type tubercle bacilli (H37Rv) was recently published.71 These investigators found a predominance of CD4 T cells over CD8 T cells, as we did. However, the animal species, type of bacilli and times evaluated were so different from those that we used that no other comparisons could be made.
Conclusions
In BCG lesions of reinfection, the initial cell infiltration began too soon to be mediated by T cells. Antigens combining with circulating and cytophilic antibodies must have caused the infiltration.
This initial antigen-specific response brought large numbers of macrophages and lymphocytes (and also some PMN) into the sites where the bacilli were located. The infiltrating cells produced chemokines that were autocatalytic in that the accumulating leucocytes produced chemokines that attracted more leucocytes into the site.
In reinfection BCG lesions, chemokine production of MCP-1 mRNA was soon down-regulated, perhaps because the initial large infiltration of chemokine-producing cells was sufficient. In primary BCG lesions, MCP-1 mRNA down-regulation began much later (Fig. 5).
Pulmonary tuberculosis usually begins following the inhalation of a relatively few tubercle bacilli into the alveolar spaces, but in the studies described herein numerous bacilli were injected intradermally. Therefore, under natural conditions, the host would show a slower response than we observed. Nevertheless, a naturally developing pulmonary lesion would have an accelerated accumulation and activation of antigen-specific defence cells in effectively immunized hosts.
Our studies suggest that BCG immunization protects against clinical tuberculosis in the following manner. After the host inhales virulent tubercle bacilli, chemotaxins would initially be produced when bacillary antigens combine with circulating and cytophilic antibodies. These chemotaxins would cause a rapid accumulation of macrophages and antigen-specific (memory) T lymphocytes at the site of bacillary lodgement. The infiltrating cells would then produce cytokines, such as MCP-1, IL-1, TNF and IFN-γ, which increase the cell infiltration and activate the accumulating macrophages. After activation, the macrophages would kill or inhibit the tubercle bacillus and, in many cases, arrest the developing tubercle while it is still microscopic.70
Acknowledgments
We appreciate the technical assistance of Rena Ashworth during various parts of these studies, and the editorial assistance of Ilse M. Harrop. Dr Teizo Yoshimura, Laboratory of Immunobiology, National Cancer Institute, Frederick, MD, provided recombinant Bluescript plasmids containing cDNA for rabbit IL-8, rabbit MCP-1 and rabbit GRO.6,11,72 Anti-rabbit ICAM-1 and anti-rabbit VCAM-1 IgGs were supplied by Dr Myron I. Cybulsky73,74 (Department of Pathology, Toronto Hospital, University of Toronto, Ontario, Canada), and anti-rabbit ELAM-1 IgG (2.79 mg/ml) was supplied by Dr Barry A. Wolitsky75 (Department of Inflammation/Autoimmune Diseases, Hoffmann-La Roche, Inc., Nutley, NJ). In conducting research using animals, the investigators adhered to the Guide for the Care and Use of Laboratory Animals, prepared by the Committee on Care and Use of Laboratory Animals of the Institute of Laboratory Animal Resources, National Research Council (NIH Publication no. 86–23, Revised 1985). This work was supported mainly by grants AI-27165 and AI-35195 from the National Institute of Allergy and Infectious Diseases, Bethesda, MD; and partly by (a) a grant from Sequella Global Tuberculosis Foundation to Dr William R. Bishai, and (b) a grant ES-03819 for the Johns Hopkins Environmental Health Sciences Center from the National Institute of Environmental Health Sciences, Research Triangle Park, NC.
Abbreviations
- BCG
bacillus Calmette–Guérin, the attenuated Mycobacterium bovis used world-wide as a vaccine for tuberculosis
- CD
cluster of differentiation, a group of cell-surface markers (monoclonal antibodies to CD4 and CD8 are used to identify populations of helper and cytotoxic T lymphocytes, respectively)
- CMI
cell-mediated immunity
- DTH
delayed-type hypersensitivity
- hsp 65
heat-shock protein 65, a major hsp of mycobacteria
- ELAM-1
endothelial–leucocyte adhesion molecule 1 (e-selectin)
- FasL
Fas ligand (Fas is a cell death receptor)
- ICAM-1
intercellular adhesion molecule 1, a member of the immunoglobulin supergene family
- IFN-γ
interferon-γ
- IL-1β
interleukin-1β
- IL-8
interleukin-8 (same as NAP-1) – a C-X-C chemokine
- MCP-1
monocyte chemoattractant (activating) protein 1 – a C-C chemokine
- MN
mononuclear cells, mostly macrophages and lymphocytes
- PMN
polymorphonuclears (granulocytes)
- PPD
purified protein derivative of tuberculin
- PstS-1
phosphate-specific transport protein 1
- TNF-α
tumour necrosis factor-α
- VCAM-1
vascular cell adhesion molecule 1, a member of the immunoglobulin supergene family
References
- 1.Grange JM, Zumia A. Paradox of the global emergency of tuberculosis. Lancet. 1999;353:996. doi: 10.1016/S0140-6736(99)01196-4. 10.1016/s0140-6736(98)05390-2. [DOI] [PubMed] [Google Scholar]
- 2.Lurie MB. Resistance to Tuberculosis: Experimental Studies in Native and Acquired Defensive Mechanisms. Cambridge, MA: Harvard University Press; 1964. [Google Scholar]
- 3.Dannenberg Am., Jr . The rabbit model of tuberculosis. In: Bloom BR, editor. Tuberculosis: Pathogenesis, Protection and Control. Washington, DC: The American Society for Microbiology; 1994. pp. 149–479. [Google Scholar]
- 4.Dannenberg Am, Jr, Tomashefski Jf., Jr . Pathogenesis of pulmonary tuberculosis. In: Fishman AP, editor. Fishman's Pulmonary Diseases and Disorders. 3. Vol. 2. New York: McGraw-Hill Co. Inc.; pp. 2447–71. [Google Scholar]
- 5.Dannenberg Am., Jr Roles of cytotoxic delayed-type hyper-sensitivity and macrophage-activating cell-mediated immunity in the pathogenesis of tuberculosis. Immunobiology. 1994;191:461–73. doi: 10.1016/S0171-2985(11)80452-3. [DOI] [PubMed] [Google Scholar]
- 6.Sugisaki K, Dannenberg Am, Jr, Abe Y, Tsuruta J, Su W-J, Said W, Feng L, Yoshimura T, Converse PJ, Mounts P. Nonspecific and immune-specific up-regulation of cytokines in rabbit dermal tuberculous (BCG) lesions. J Leukoc Biol. 1998;63:440–50. doi: 10.1002/jlb.63.4.440. [DOI] [PubMed] [Google Scholar]
- 7.Abe Y, Sugisaki K, Dannenberg Am., Jr Rabbit vascular endothelial adhesion molecules: ELAM-1 is most elevated in acute inflammation, whereas VCAM-1 and ICAM-1 predominate in chronic inflammation. J Leukoc Biol. 1991;60:692–703. doi: 10.1002/jlb.60.6.692. [DOI] [PubMed] [Google Scholar]
- 8.Dannenberg Am, Jr, Ando M, Shima K. Macrophage accumulation, division, maturation and digestive and microbicidal capacities in tuberculous lesions. III. The turnover of macrophages and its relation to their activation and antimicrobial immunity in primary BCG lesions and those of reinfection. J Immunol. 1972;109:1109–21. [PubMed] [Google Scholar]
- 9.Das S, Cheng SH, Lowrie DB, Walker KB, Mitchison DA, Vallishayee RS, Narayanan PR. The pattern of mycobacterial antigen recognition in sera from Mantoux-negative individuals is essentially unaffected by bacille Calmette-Guérin (BCG) vaccination in either South India or London. Clin Exp Immunol. 1992;89:402–6. doi: 10.1111/j.1365-2249.1992.tb06970.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Billingham MEJ, Carney S, Butler R, Colston MJ. A mycobacterial 65-kDa heat shock protein induces antigen-specific suppression of adjuvant arthritis, but is not itself arthritogenic. J Exp Med. 1990;171:339–44. doi: 10.1084/jem.171.1.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tsuruta J, Sugisaki K, Dannenberg Am, Jr, Yoshimura T, Abe Y, Mounts P. The cytokines NAP-1 (IL-8), MCP-1, IL-1 beta, and GRO in rabbit inflammatory skin lesions produced by the chemical irritant sulfur mustard. Inflammation. 1996;20:293–318. doi: 10.1007/BF01488205. [DOI] [PubMed] [Google Scholar]
- 12.Bartholomew JW. Stains for microorganisms in sections. In: Clark G, editor. Staining Procedures. 4. Baltimore: Williams & Wilkins; 1981. pp. 441–73. [Google Scholar]
- 13.Laal S, Samanich KM, Sonnenberg MG, Zolla-Pazner S, Phadtare JM, Belisle JT. Human humoral responses to antigens of Mycobacterium tuberculosis: Immunodominance of high-molecular-mass antigens. Clin Diag Lab Immunol. 1997;4:49–56. doi: 10.1128/cdli.4.1.49-56.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Samanich KM, Belisle JT, Sonnenberg MG, Keen MA, Zolla-Pazner S, Laal S. Delineation of human antibody responses to culture filtrate antigens of Mycobacterium tuberculosis. J Infect Dis. 1998;178:1534–8. doi: 10.1086/314438. 10.1086/314438. [DOI] [PubMed] [Google Scholar]
- 15.Barksdale L, Kim K-S. Mycobacterium. Bacteriol Rev. 1977;41:217–372. doi: 10.1128/br.41.1.217-372.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sethna KB, Mistry NF, Dholakia Y, Antia NH, Harboe M. Longitudinal trends in serum levels of mycobacterial secretory (30 kD) and cytoplasmic (65 kD) antigens during chemotherapy of pulmonary tuberculosis patients. Scand J Infect Dis. 1998;30:363–9. doi: 10.1080/00365549850160657. [DOI] [PubMed] [Google Scholar]
- 17.Wiker HG, Michell SL, Hewinson RG, Spierings E, Nagai S, Harboe M. Cloning, expression and significance of MPT53 for identification of secreted proteins of Mycobacterium tuberculosis. Microbial Pathogenesis. 1999;26:207–19. doi: 10.1006/mpat.1998.0267. 10.1006/mpat.1998.0267. [DOI] [PubMed] [Google Scholar]
- 18.Majno G, Joris I. Cells, Tissues and Disease: Principles of General Pathology. Cambridge, MA: Blackwell Science, Inc.; 1996. The inflammatory exudate; pp. 415–27. [Google Scholar]
- 19.Cotran RS, Mayadas-Norton T. Endothelial adhesion molecules in health and disease. Pathol Biol. 1998;46:164–70. [PubMed] [Google Scholar]
- 20.Pigott R, Power C. The Adhesion Molecule FactsBook. London: Academic Press; 1993. [Google Scholar]
- 21.Wagner DD, Bonfanti R. Von Willebrand factors and the endothelium. Mayo Clin Proc. 1991;66:621–7. doi: 10.1016/s0025-6196(12)60522-9. [DOI] [PubMed] [Google Scholar]
- 22.Kupper TS. Immune and inflammatory processes in cutaneous tissues. Mechanisms and speculations. J Clin Invest. 1990;86:1783–9. doi: 10.1172/JCI114907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kupper TS, Groves RW. The interleukin-1 axis and cutaneous inflammation. J Invest Dermatol. 1995;105:62S–66S. doi: 10.1111/1523-1747.ep12316087. [DOI] [PubMed] [Google Scholar]
- 24.Vaddi K, Keller M, Newton RC. The Chemokine FactsBook. London: Academic Press; 1997. [Google Scholar]
- 25.Callard RE, Gearing AJH. The Cytokine FactsBook. London: Academic Press; 1994. [Google Scholar]
- 26.Suga M, Dannenberg Am, Jr, Higuchi S. Macrophage functional heterogeneity in vivo: Macrolocal and microlocal macrophage activation, identified by double-staining tissue sections of BCG granulomas for pairs of enzymes. Am J Pathol. 1980;99:305–24. [PMC free article] [PubMed] [Google Scholar]
- 27.Glatman-Freedman A, Casadevall A. Serum therapy for tuberculosis revisited: Reappraisal of the role of antibody-mediated immunity against Mycobacterium tuberculosis. Clin Microbiol Rev. 1998;11:514–32. doi: 10.1128/cmr.11.3.514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reggiardo Z, Middlebrook G. Failure of passive serum transfer of immunity against aerogenic tuberculosis in rabbits. Proc Soc Exp Biol Med. 1974;145:173–5. doi: 10.3181/00379727-145-37771. [DOI] [PubMed] [Google Scholar]
- 29.Converse PJ, Dannenberg Am, Jr, Estep JE, Sugisaki K, Abe Y, Schofield BH, Pitt MLM. Cavitary tuberculosis produced in rabbits by aerosolized virulent tubercle bacilli. Infect Immun. 1996;6:4776–87. doi: 10.1128/iai.64.11.4776-4787.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Majno G, Joris I. Cells. Tissues, and Disease. Principles of General Pathology. Cambridge. MA: Blackwell Science; 1996. pp. 530–2. [Google Scholar]
- 31.Rich AR. The Pathogenesis of Tuberculosis. 2. Springfield, IL: Charles C. Thomas; 1951. Mechanism of acquired resistance (continued). The role of antibodies, phagocytes, lymphoid cells and ‘acquired tissue immunity.’; pp. 570–613. [Google Scholar]
- 32.Cooper NR. Biology of the complement system. In: Gallin JI, Snyderman R, Fearon DT, Haynes BF, Nathan C, editors. Inflammation: Basic Principles and Clinical Correlates. 3. Philadelphia: Lippincott Williams & Wilkins; 1999. pp. 281–315. [Google Scholar]
- 33.Erdei A, Kerekes K, Pecht I. Role of C3a and C5a in the activation of mast cells. Exp Clin Immunogenet. 1997;14:16–18. [PubMed] [Google Scholar]
- 34.Rumsaeng V, Cruikshank WW, Foster B, Prussin C, Kirshenbaum AS, Davis TA, Kornfeld H, Center DM, Metcalfe DD. Human mast cells produce the CD4+ T lymphocyte chemoattractant factor, IL-16. J Immunol. 1997;59:2904–10. [PubMed] [Google Scholar]
- 35.Crooks SW, Stockley RA. Leukotriene B4. Int J Biochem Cell Biol. 1998;30:173–8. doi: 10.1016/s1357-2725(97)00123-4. 10.1016/s1357-2725(97)00123-4. [DOI] [PubMed] [Google Scholar]
- 36.Lam BK, Austen KF. Leukotrienes: biosynthesis, release, and actions. In: Gallin JI, Goldstein IM, Snyderman R, editors. Inflammation: Basic Principles and Clinical Correlates. 2. New York: Raven Press Ltd; 1992. pp. 139–47. [Google Scholar]
- 37.Czermak BJ, Lentsch AB, Bless NM, Schmal H, Friedl HP, Ward PA. Role of complement in in vitro and in vivo lung inflammatory reactions. J Leukoc Biol. 1998;64:40–8. doi: 10.1002/jlb.64.1.40. [DOI] [PubMed] [Google Scholar]
- 38.Dempsey PW, Allison ME, Akkaraju S, Goodnow CC, Fearon DT. C3d of complement as a molecular adjuvant: Bridging innate and acquired immunity. Science. 1996;271:348–50. doi: 10.1126/science.271.5247.348. [DOI] [PubMed] [Google Scholar]
- 39.Smith RS, Smith TJ, Blieden TM, Phipps RP. Fibroblasts as sentinel cells. Synthesis of chemokines and regulation of inflammation. Am J Pathol. 1997;151:317–22. [PMC free article] [PubMed] [Google Scholar]
- 40.Gosset P, Tillie-Leblond I, Oudin S, Parmentier O, Wallaert B, Joseph M, Tonnel A-B. Production of chemokines and proinflammatory and antiinflammatory cytokines by human alveolar macrophages activated by IgE receptors. J All Clin Immunol. 1999;103:289–97. doi: 10.1016/s0091-6749(99)70504-x. [DOI] [PubMed] [Google Scholar]
- 41.Ying S, Barata LT, Meng Q, Grant JA, Barkans J, Durham SR, Kay AB. High-affinity immunoglobulin E receptor (FcεRI)-bearing eosinophils, mast cells, macrophages and Langerhans cells in allergen-induced late-phase cutaneous reactions in atopic subjects. Immunology. 1998;93:281–8. doi: 10.1046/j.1365-2567.1998.00418.x. 10.1046/j.1365-2567.1998.00418.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nilsson G, Costa JJ, Metcalfe DD. Mast cells and basophils. In: Gallin JI, Snyderman R, Fearon DT, Haynes BF, Nathan C, editors. Inflammation: Basic Principles and Clinical Correlates. 3. Philadelphia: Lippincott Williams & Wilkins; 1999. pp. 97–117. [Google Scholar]
- 43.Bradding P. Human mast cell cytokines. Clin Exp Allergy. 1996;26:13–19. doi: 10.1111/j.1365-2222.1996.tb00051.x. [DOI] [PubMed] [Google Scholar]
- 44.Hamann A. Adhesion Molecules and Chemokines in Lymphocyte Trafficking. Amsterdam: Harwood Academic Publishers; 1997. [Google Scholar]
- 45.Pober JS, Cotran RS. Immunologic interactions of T lymphocytes with vascular endothelium. Adv Immunol. 1991;50:261–302. doi: 10.1016/s0065-2776(08)60827-5. [DOI] [PubMed] [Google Scholar]
- 46.Smith RE, Hogaboam CM, Strieter RM, Lukacs NW, Kunkel SL. Cell-to-cell and cell-to-matrix interactions mediate chemokine expression: an important component of the inflammatory lesion. J Leukoc Biol. 1997;62:612–19. doi: 10.1002/jlb.62.5.612. [DOI] [PubMed] [Google Scholar]
- 47.Bochner BS. Cellular adhesion and its antagonism. J Allergy Clin Immunol. 1997;100:581–5. doi: 10.1016/s0091-6749(97)70158-1. [DOI] [PubMed] [Google Scholar]
- 48.Lenardo M, Chan FK, Hornung F, McFarland H, Siegel R, Wang J, Zheng L. Mature T lymphocyte apoptosis – Immune regulation in a dynamic and unpredictable antigenic environment. Annu Rev Immunol. 1999;17:221–53. doi: 10.1146/annurev.immunol.17.1.221. [DOI] [PubMed] [Google Scholar]
- 49.Mason D, Powrie F. Control of immune pathology by regulatory T cells. Curr Opin Immunol. 1998;10:649–55. doi: 10.1016/s0952-7915(98)80084-8. [DOI] [PubMed] [Google Scholar]
- 50.Shreedhar VK, Pride MW, Sun Y, Kripke ML, Strickland FM. Origin and characteristics of ultraviolet-B radiation-induced suppressor T lymphocytes. J Immunol. 1998;161:1327–35. [PubMed] [Google Scholar]
- 51.Mantovani A, Allavena P, Vecchi A, Sozzani S. Chemokines and chemokine receptors during activation and deactivation of monocytes and dendritic cells and in amplification of Th1 versus Th2 responses. Int J Clin Lab Res. 1998;28:77–82. doi: 10.1007/s005990050023. 10.1007/s005990050023. [DOI] [PubMed] [Google Scholar]
- 52.Penton-Rol G, Polentarutti N, Luini W, Borsatti A, Mancinelli R, Sica A, Sozzani S, Mantovani A. Selective inhibition of expression of the chemokine receptor CCR2 in human monocytes by IFN-γ. J Immunol. 1998;160:3869–73. [PubMed] [Google Scholar]
- 53.Fantuzzi L, Borghi P, Ciolli V, Pavlakis G, Belardelli F, Gessani S. Loss of CCR2 expression and functional response to monocyte chemotactic protein (MCP-1) during the differentiation of human monocytes: Role of secreted MCP-1 in the regulation of the chemotactic response. Blood. 1999;94:875–83. [PubMed] [Google Scholar]
- 54.Heaney ML, Golde DW. Soluble receptors in human disease. J Leukoc Biol. 1998;64:135–46. doi: 10.1002/jlb.64.2.135. [DOI] [PubMed] [Google Scholar]
- 55.Arend WP, Malyak M, Guthridge CJ, Gabay C. Interleukin 1 receptor antagonist. Role in biology. Annu Rev Immunol. 1998;16:27–55. doi: 10.1146/annurev.immunol.16.1.27. [DOI] [PubMed] [Google Scholar]
- 56.Fadok VA, Bratton DL, Konowal A, Freed PW, Westcott JY, Henson PM. Macrophages that have ingested apoptotic cells in vitro inhibit proinflammatory cytokine production through autocrine/paracrine mechanisms involving TGF-β, PGE2, and PAF. J Clin Invest. 1998;101:890–8. doi: 10.1172/JCI1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Barton BE. IL-6: Insights into novel biological activities. Clin Immunol Immunopathol. 1997;85:16–20. doi: 10.1006/clin.1997.4420. 10.1006/clin.1997.4420. [DOI] [PubMed] [Google Scholar]
- 58.Serhan CN, Drazen JM. Antiinflammatory potential of lipoxygenase-derived eicosanoids: a molecular switch at 5 and 15 positions? J Clin Invest. 1997;99:1147–8. doi: 10.1172/JCI119268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ferrante JV, Huang ZH, Nandoskar M, Hii CST, Robinson BS, Rathjen DA, Poulos A, Morris CP, Ferranti A. Altered responses of human macrophages to lipopolysaccharide by hydroperoxy eicosatetraenoic acid, hydroxy eicosatetraenoic acid, and arachidonic acid: inhibition of tumor necrosis factor production. J Clin Invest. 1997;99:1445–52. doi: 10.1172/JCI119303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Peng H-B, Spiecker M, Liao JK. Inducible nitric oxide: an autoregulatory feedback inhibitor of vascular inflammation. J Immunol. 1998;161:1970–6. [PubMed] [Google Scholar]
- 61.Taylor-Robinson AW. Inhibition of IL-2 production by nitric oxide: a novel self-regulatory mechanism for Th1 cell proliferation. Immunol Cell Biol. 1997;75:167–75. doi: 10.1038/icb.1997.23. [DOI] [PubMed] [Google Scholar]
- 62.Zidek Z. Adenosine–cyclic AMP pathways and cytokine expression. Eur Cytokine Netw. 1999;10:319–28. [PubMed] [Google Scholar]
- 63.Bergquist J, Tarkowski A, Ewing A, Ekman R. Catecholaminergic suppression of immunocompetent cells. Immunol Today. 1998;19:562–7. doi: 10.1016/s0167-5699(98)01367-x. 10.1016/s0167-5699(98)01367-x. [DOI] [PubMed] [Google Scholar]
- 64.Clynes R, Maizes JS, Guinamard R, Ono M, Takai T, Ravetch JV. Modulation of immune complex-induced inflammation in vivo by the coordinate expression of activation and inhibitory Fc receptors. J Exp Med. 1999;189:179–85. doi: 10.1084/jem.189.1.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Euzger HS, Flower RJ, Goulding NJ, Perritti M. Differential modulation of annexin I binding sites on monocytes and neutrophils. Mediators Inflam. 1999;8:53–62. doi: 10.1080/09629359990720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pais TF, Silva RA, Smedegaard B, Appelberg R, Andersen P. Analysis of T cells recruited during delayed-type hypersensitivity to purified protein derivative (PPD) versus challenge with tuberculosis infection. Immunol. 1998;95:69–75. doi: 10.1046/j.1365-2567.1998.00561.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wilson M, Seymour R, Henderson B. Bacterial perturbation of cytokine networks. Infect Immun. 1998;66:2401–9. doi: 10.1128/iai.66.6.2401-2409.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Erwig L-P, Kluth DC, Walsh GM, Rees AJ. Initial cytokine exposure determines function of macrophages and renders them unresponsive to other cytokines. J Immunol. 1998;161:1983–8. [PubMed] [Google Scholar]
- 69.Hasan M, Najjam S, Gordon MY, Gibbs RV, Rider CC. IL-12 is a heparin-binding cytokine. J Immunol. 1999;162:1064–70. [PubMed] [Google Scholar]
- 70.Dannenberg Am., Jr Lurie's tubercle-count method to test TB vaccine efficacy in rabbits. Frontiers in Bioscience. 1998;3:c27–33. doi: 10.2741/a261. On the Internet. http://www.bioscience.org/1998/v3/c/dannenbe/list.htm. [DOI] [PubMed] [Google Scholar]
- 71.Mustafa T, Phyu S, Nilsen R, Jonsson R, Bjune G. In situ expression of cytokines and cellular phenotypes in the lungs of mice with slowly progressive primary tuberculosis. Scand J Immunol. 2000;51:548–56. doi: 10.1046/j.1365-3083.2000.00721.x. 10.1046/j.1365-3083.2000.00721.x. [DOI] [PubMed] [Google Scholar]
- 72.Yoshimura T, Yuhki N. Neutrophil attractant/activation protein-1 and monocyte chemoattractant protein-1 in rabbit: cDNA cloning and their expression in spleen cells. J Immunol. 1991;146:3483–8. [PubMed] [Google Scholar]
- 73.Cybulsky MI, Gimbrone Ma., Jr Endothelial expression of a mononuclear leukocyte adhesion molecule during atherogenesis. Science. 1991;251:788–91. doi: 10.1126/science.1990440. [DOI] [PubMed] [Google Scholar]
- 74.Richardson M, Hadcock SJ, DeReske M, Cybulsky MI. Increased expression in vivo of VCAM-1 and E-selectin by the aortic endothelium of normolipemic and hyperlipemic diabetic rabbits. Arterioscler Thromb. 1994;14:760–9. doi: 10.1161/01.atv.14.5.760. [DOI] [PubMed] [Google Scholar]
- 75.Olofsson AM, Arfors K-E, Ramezani L, Wolitzky BA, Butcher EC, von Andrian UH. E-selectin mediates leukocyte rolling in interleukin-1-treated rabbit mesentery venules. Blood. 1994;84:2749–58. [PubMed] [Google Scholar]