Abstract
Secondary lymphoid tissue consists of two major populations of cells: lymphoid cells and stromal cells. It is generally accepted that these two cell populations influence each other however, factors mediating these processes are poorly understood. In this paper we characterize one of the possible means of communication between stroma and lymphocytes namely through hepatocyte growth factor/c-met receptor interactions. Hepatocyte growth factor (HGF) is a pleiotropic factor that is mainly produced by mesenchymal cells and acts on cells of epithelial origin which express the HGF receptor c-met. Here we demonstrate that biologically active HGF is constitutively produced by fibroblast-like stromal cells from human lymphoid tissues. HGF secretion from stromal cells was increased by direct contact with activated T cells. This increase was abrogated when activated T cells were separated physically from stromal cells. Using neutralizing antibody or cytokine inhibitors we provide evidence that enhancement of HGF production was due to additive effects of T-cell membrane-associated interleukin-1 (IL-1) and CD40 ligand. Finally, we also show that B lymphocytes activated with CD40L/anti-µ or phorbol 12-myristate 13-acetate (PMA) express c-met receptor. Co-culture of activated B cells with stromal cells from spleen leads to enhanced production of immunoglobulins. This can be partially inhibited by introduction of anti-HGF neutralizing antibodies to the culture system. Substitution of stromal cells with recombinant HGF did not produce enhancement of immunoglobulin secretion. On the other hand stimulation of c-met receptor with HGF leads to enhanced integrin-mediated adhesion of activated B cells to vascular cell adhesion molecule (VCAM-1) and fibronectin. On the basis of the above experiments we conclude that HGF production by fibroblast-like stromal cells can be modulated by activated T cells, thus providing signals for the regulation of adhesion of c-met expressing B cells to extracellular matrix proteins. In this way HGF may indirectly influence immunoglobulin secretion by B cells.
Introduction
The final steps in lymphocyte differentiation occur in secondary lymphoid organs where B and T lymphocytes interact with the lymphoid tissue microenvironment. Cell-to-extracellular matrix (ECM) adhesion plays a crucial role in these events.1 Hepatocyte growth factor (HGF; also known as scatter factor) is a multifunctional cytokine whose activities, apart from supporting hepatocyte growth, include stimulation of epithelial cell motility and invasiveness and the induction of angiogenesis.2 Structurally HGF is a heterodimer consisting of a 60 000 MW heavy and a 30 000 MW light chain held together by a single disulphide bond. Its receptor is a transmembrane tyrosine kinase encoded by the proto-oncogene, c-met.3 Although the main HGF targets are epithelial and endothelial cells, it has recently been shown that c-met is expressed, or can be induced, on normal B cells.4 It is also constitutively expressed by several lymphoblastoid cell lines.5
In recent studies we have shown that preparations of fibroblast-like stromal cells from human spleens can influence the differentiation of B lymphocytes as evidenced by acquisition of a plasma cell phenotype and an increase in immunoglobulin G (IgG) secretion. Our studies indicated that this effect was partially dependent on interleukin-6 (IL-6) secretion and adhesion of B cells to stromal cells via CD49d.6
Here, we show that HGF is constitutively produced by stromal cell preparations isolated from human spleen, lymph node and thymus and that its secretion can be modulated by IL-1, transforming growth factor (TGF) and soluble CD40L. We also show that T-cell membrane-bound molecules such as IL-1 and CD40L exert a similar effect. Furthermore B cells, but not T cells, when appropriately stimulated, express the HGF receptor c-met creating the potential for functional interaction between mesenchymal and lymphoid cells. These studies throw further light on the role of stromal cells and extracellular matrix in human B-cell development and function.
Materials and methods
Antibodies and reagents
Mouse monoclonal antibodies (mAb) used were: anti-CD3, UCHT-1 (IgG1; Scottish Antibody Producing Unit, Carluke, UK); anti-CD28 (Coulter Immunology, Hialeah, FL); fluorescein-conjugated anti-CD3, UCHT1 (IgG1); CD14, TUK4 (IgG2a); CD19, HD37 (IgG1; Dako Ltd, Glostrup, Denmark); CD11a, 25·3 (IgG1); CD11b 94 Mo1 (IgM); CD49a, HP2B6 (IgG1); CD49d, HP2/1 (IgG1; Coulter Immunology); anti-human CD40L (IgG1; Calbiochem-Novabiochem Coporation, La Jolla, CA); CD11c, clone #3·9 (IgG1); CD54, BBIG-E1 (IgG1; R & D Systems, Abingdon, UK). Polyclonal antibodies used were: rabbit anti-c-met; C-12 (IgG; Santa Cruz Biotechnology, Santa Cruz, CA); goat anti-human HGF antibody (IgG); goat anti-human IL-6 (IgG); goat anti-human tumour necrosis factor-α (TNF-α) (IgG; R & D Systems); anti-human IgM (Sigma, Poole, UK). Recombinant proteins used were: human IL-1 receptor antagonist (IL-1RA); HGF; vascular cell adhesion molecule (VCAM; R & D Systems); human IL-1α; IL-1β; TNF-α; TGF-β; CD40L; IL-2, IL-4, IL-6, IL-10 (PeproTech EC Ltd, London, UK).
Cell lines
The epidermoid carcinoma cell line A431 and the lung fibroblast cell line MRC-5 were obtained from the European Collection of Cell Cultures (ECACC, Salisbury, UK). The cells were propagated according to the suppliers' instructions.
Preparation and cultivation of stromal cells from human lymphoid tissue
Lymphoid organs were obtained from male and female donors age 25–55 years, where consent for multiple organ donation was granted. Organs were collected in cold saline and delivered to the laboratory within 4 hr. Stromal cells were prepared as described earlier.6 Briefly, spleens were homogenised in a loosely fitting glass homogenizer and the cell suspension obtained reconstituted to 1 × 108 nucleated cells/ml in RPMI-1640 containing 20% (v/v) fetal bovine serum (FBS; Sigma) and cultured for 1 week. After this time non-adherent cells were removed by washing with PBS. Culture of the adherent cell population was continued in RPMI-1640 medium containing 10% FBS until the adherent cells were confluent. At this stage all the cells had the morphological appearance of fibroblasts. The cells were subsequently split every 1–2 weeks and cultured for up to four passages. Cells prepared this way are referred to as stromal cells and were used in subsequent studies. Lymph node stromal cells were obtained by fibroblast outgrowth from small (1 mm3) tissue pieces. Stromal cells from all anatomical locations were allowed to reach confluence prior to use in experiments. As previously reported the cells were composed mainly of fibroblasts as assessed by their morphology and expression of specific markers.6
B-cell isolation
Splenocytes were obtained by gentle disruption of spleen tissue fragments in a sterile glass homogenizer. The cell suspension obtained was centrifuged over Ficoll-Hypaque (d = 1·077 g/cm3) (Sigma). T cells were depleted by two rounds of 2-aminoethylisothiouronium bromide (AET)–sheep red blood cell (SRBC) rosetting. Residual contaminating cells were removed by incubation of cells with anti-CD3, anti-CD14 and anti-CD56 mAb followed by incubation with goat anti-mouse IgG-coated magnetic beads and subsequent filtration through a magnetic-activated cell sorting (MACS) column (Miltenyi Biotech, Bisley, UK). The resulting B-cell population was at least 97% positive for CD19 as assessed by flow cytometry and contained less then 1% CD3 positive cells. Cell viability assessed by trypan blue was always greater then 96%.
T cells
Lymphocytes were isolated from spleens of normal donors using Ficoll-Hypaque gradient centrifugation. Monocytes were depleted by adherence to plastic at 37° for 2 hr. The cells obtained were incubated in turn with anti-CD20 mAb followed by goat anti-mouse IgG magnetic beads and filtration through a MACS column (Miltenyi Biotech). The T-cell preparations obtained contained 98% CD3+ cells.
Cell culture
RPMI-1640 medium supplemented with 2 mm l-glutamine, 100 U/ml penicillin, 100 µg/ml streptomycin and 10% FBS (Sigma) was used throughout. Cells were cultured in a humidified incubator in 5% CO2/95% air. To assess the role of HGF produced by stromal cells on B-cell IgG production a two-step assay was used. In the first step B cells were stimulated for 2 days with soluble recombinant CD40L (3 µg/ml, PeproTech) and anti-µ antibodies (Sigma). After 2 days cells were extensively washed and recultured in wells containing irradiated (5000 rad) stromal cell monolayers. The combined length of the first and second cultures was 10 days. In some experiments a two chamber Transwell culture system was used (Costar Corning, High Wycombe, UK) with 6·5 mm inserts with 0·3 µm pores. The lower well contained stromal cells, the insert B cells.
RNA isolation and reverse transcription (RT)–polymerase chain reaction (PCR)
RNA was isolated from monolayers of stromal cells grown to confluence in 25 cm2 plastic vented flasks (Costar Corning). In the case of lymphocytes, RNA was extracted from 3 × 106 cells. In both cases Triazol extraction reagent was used according to the manufacturers instruction. One µg of extracted RNA was treated with DNAseI to eliminate DNA and reverse transcribed by SuperScrip II reverse transcriptase at 42° for 70 min using oligo(dT). The PCR reaction was conducted with Taq polymerase using 1–3 µl of cDNA (1·5 mm MgCl2; annealing temperature 56°; 30–40 cycles). The primer pairs for HGF (cDNA product size 396 b, GenBank® X16323) and reduced glyceraldehyde-3-phosphate dehydrogenase (GADPH; cDNA product size 576 bp, GenBank® M33197) were purchased from R & D Systems. The GADPH PCR products obtained were resolved by electrophoresis on 1·5% (w/v) agarose gels and identified following ethidium bromide staining.
Detection of c-met protein by Western blot
Cells were washed in phosphate-buffered saline (PBS) and lysed at 4° with 100 µl/106 cells of lysis buffer (PBS containing 1% NP40, 0·5% sodium deoxycholate, 0·1% sodium dodecyl sulphate (SDS; Sigma)) and a cocktail of protease inhibitors (Merck). Protein concentrations were determined using a Bio-Rad microassay kit (Bio-Rad Laboratories Ltd, Hemel Hempstead, UK) and 10 µg/lane of total cell lysates was electrophoresed on 10% SDS–polyacrylamide gels after boiling samples for 5 min in Laemmli sample buffer. Proteins were transferred electrophoreticaly to Immobilon membranes (Millipore, Bedford, MA). After electrotransfer, the membranes were blocked with PBS–Tween buffer containing 5% (w/v) non-fat dry milk, washed with PBS–Tween buffer and incubated overnight at 4° with the primary antibody diluted in blocking buffer. Membranes were then washed, incubated with peroxidase conjugated second antibody for 2 hr and re-washed. Blotted proteins were detected using an enhanced chemiluminescence detection system (Amersham Pharmacia Biotech, Little Chalfont, UK). The appropriate synthetic peptide was added in excess to control reactions to check the specificity of the antibody.
Adhesion assay
Adhesion assays were performed in 96-well plates coated with the ECM proteins fibronectin, laminin (Sigma) and VCAM (R & D Systems) at a concentration of 20 µg/ml. Coating was performed overnight at 4°. Plates were washed with PBS and then blocked with heat denatured bovine serum albumin (BSA) at 2 mg/ml for 1·5 hr at room temperature.
Isolated splenic B lymphocytes were suspended in RPMI-1640 medium and stimulated with phorbol 12-myristate 13-acetate (PMA; 5 ng/ml) for 6 hr. After washing (three times) with serum-free culture medium the cells were pulsed with Cr51 (sodium chromate, Amersham Pharmacia Biotech) at a concentration of 300 µCi/106 cells. The labeling reaction was allowed to proceed at 37° for 1·5 hr. The cells were again washed (three times), and following treatment with different concentrations of HGF, they were added to the coated plate. The plates were centrifuged at 200 g for 5 min and the added cells allowed to adhere at 37° for 1·5 hr. The plates were then washed four times with warm medium to remove non-adherent cells. The adherent cells were finally lysed with 5% Triton-X-100 followed by counting in a 1900CA Tricarb Liquid scintillation counter (Packard, Pangbourne, UK). The percentage of adherent cells was calculated from the generated counts as follows: adherent cells (% c.p.m. input) = adherent cells c.p.m./(total input c.p.m. − spontaneous release c.p.m.) × 100. In blocking studies PMA-treated cells were incubated in antibody solution for 15 min at 37°. Tests with unstimulated B cells were run in parallel as a control. Only values exceeding at least twice background level were considered significant in all adhesion experiments.
HGF and immunoglobulin assays
Supernatants from tissue cultures were analysed for the presence of HGF using commercially available sandwich enzyme-linked immunosorbent assay (ELISA kits; R & D Systems). For IgG quantitation a standard sandwich ELISA was used as described earlier.7 All assays were performed in duplicate.
Determination of HGF activity in supernatants of stromal cell cultures
Hepatocytes were isolated from human livers by collagenase perfusion8 The hepatocytes were suspended in minimum essential medium supplemented with 10% FBS and inoculated at a density of 2 × 105 cells/well into 24 well collagen coated culture plates. After approximately 4 hr the attached cells were washed twice with PBS and refed with arginine-free Williams' E medium supplemented with 0·4 mm ornithine, 100 nm insulin, 5·5 µm hydrocortisone, 100 U/ml penicillin and 100 µg/ml streptomycin. For determination of DNA synthesis 3H-thymidine (2 µCi/ml) was added for the final 24 hr in culture. On termination of the incubation, the hepatocyte monolayers were washed three times with cold saline and twice with 10% (w/v) trichloroacetic acid containing 5 mm thymidine. The plates were then dried and extracted with 0·1 m NaOH. Incorporated counts were determined by scintillation counting in a Tricarb 1900CA beta counter (Packard).
Statistics
The data are presented as mean ±sd. All experiments described were performed on a minimum of three occasions with cultures being set up in triplicate in every experiment.
Results
Fibroblast-like cells from human spleen constitutively secrete HGF
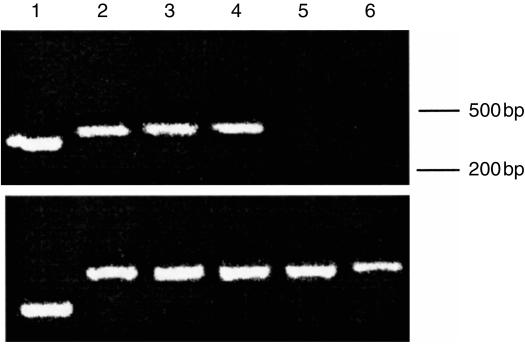
Stromal cell preparations consisting of fibroblast-like cells were tested for their ability to produce HGF. After 48 hr culture cell culture supernatants were collected and their HGF concentration was measured by ELISA. Constitutive production was observed in stromal cells derived from spleen and lymph node and the secreted levels varied depending on the stromal cell preparation (Table 1). No HGF could be detected in culture supernatants of unstimulated or PMA-stimulated lymphocytes. These results were supported by RT–PCR studies demonstrating the expression of HGF mRNA within all stromal cell population examined. In contrast HGF mRNA was not detected in lymphocyte preparations both stimulated and unstimulated (Fig. 1). To confirm the production of intact functional HGF we added the supernatant from lymph node and splenic stromal cell cultures to cultures of normal human hepatocytes. Parallel cultures were stimulated with recombinant HGF. In both cultures a dose-dependent stimulation of DNA synthesis was observed which was almost completely blocked by the addition of neutralizing anti-HGF antibodies (Table 2). Collectively these data show that fibroblast-like stromal cells from human secondary lymphoid organs secrete high levels of biologically active HGF.
Table 1.
HGF is constitutively secreted by stromal cells but not lymphocytes
| Cell preparation | HGF concentration (pg/ml supernatant, range) |
|---|---|
| Spleen stromal cells | 100–1200 |
| Lymph node stromal cells | 350–1800 |
| Lymphocytes | Not detectable |
| PMA-stimulated lymphocytes | Not detectable |
Supernatants from 48 hr confluent stromal cell cultures and from lymphocyte cultures (2 × 106 cells/ml) were collected and analysed for HGF concentration by ELISA (lower limit of detection 40 pg/ml). Results from 15 different culture preparations.
Figure 1.
HGF is expressed by human stromal cells but not lymphocytes. HGF mRNA expression in stromal cells from spleen, lymph node and MRC-5 human lung fibroblast cell line and in lymphocytes. Primers used were HGF specific (upper panel) or as control GAPDH specific (lower panel). 1, positive control template (340 bp), 2, MRC-5 fibroblasts (positive control), 3, spleen stromal cells, 4, lymph node stromal cells, 5, lymphocytes, 6, lymphocytes stimulated with PMA (10 ng/ml) for 48 hr.
Table 2.
HGF secreted by stromal cells is biologically active
| Treatment | Mean ±sd change* |
|---|---|
| Medium | 1·00 |
| Stromal cell culture supernatant 2 × dilution | 3·27 ± 0·28 |
| Stromal cell culture supernatant 4 × dilution | 2·12 ± 0·14 |
| Stromal cell culture supernatant 8 × dilution | 1·48 ± 0·16 |
| Stromal cell culture supernatant (2 × dilution)+anti-HGF IgG | 1·55 ± 0·22 |
| Stromal cell culture supernatant (2 × dilution)+control IgG | 3·39 ± 0·21 |
| Human recombinant HGF 250 pg/ml | 1·85 ± 0·26 |
| Human recombinant HGF 1000 pg/ml | 3·48 ± 0·17 |
Hepatocyte monolayers were cultured as described in Materials and methods. Stromal cell culture supernatant or other additives were added 4 hr after plating as indicated. After 24 hr the medium was removed and replaced with identical medium containing 2 µCi 3H-thymidine and cells were cultured for a further 24 hr. DNA synthesis was measured as described in Materials and methods.
Values shown are means ± sd from quadruplicate samples expressed as fold changes compared with normal medium treated control cells. Mean d.p.m./well in control samples was 6743 ± 1265 d.p.m./well. Values in table come from representative experiment repeated five times with similar results
Co-culture of stromal cells with activated T cells up-regulates HGF secretion
Since stromal cells within lymphoid organs are in close contact with lymphocytes we decided to study whether direct contact of stromal cells with lymphoid cells influences HGF secretion. In these studies isolated T cells were first activated with solid phase anti-CD3 and anti CD28 antibodies and B cells with sCD40L and anti-µ antibodies. After 2 days of culture with the above stimulants the cells were harvested, thoroughly washed and fixed with 1% paraformaldehyde and co-cultured with stromal cells for 48 hr after which supernatant was collected for HGF analysis. Unstimulated fixed T cells and B cells were used in parallel.
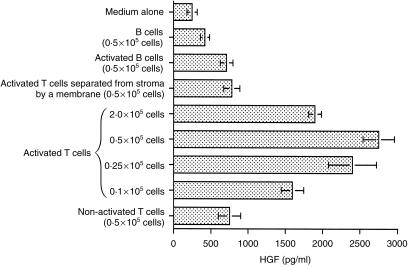
A significant increase in HGF secretion was observed in the case of coculture with activated T cells (Fig. 2). The observed increase however, was not directly related to the number of activated T cells added the response being bell-shaped. The optimum effect was observed with 0·5 × 105 cells per culture. This type of response was also noted following addition of purified recombinant CD40L and cytokines (see later Fig. 4). In contrast only a small enhancement of HGF secretion was seen when non-activated T cells or activated B cells were used. Furthermore when cells were separated by a porous nucleopore membrane in the Transwell culture system enhancement of HGF secretion was greatly reduced, the residual effect observed probably involving soluble factors. Taken together these data demonstrate that signals generated by direct cell-to-cell contact between activated T cells and stromal cells were responsible for up-regulation of HGF secretion.
Figure 2.
Activated T cells enhance HGF production by human stromal cells. T cells isolated from human spleens were stimulated with anti-CD3/anti-CD28 antibodies for 48 hr, fixed with 1% paraformaldehyde and cultured with splenic stromal cells for 48 hr. B cells were activated with sCD40L/anti-µ for 48 hr and then treated in the same way as activated T cells. Production of HGF was determined in supernatants. Data are mean ±sd from four experiments.
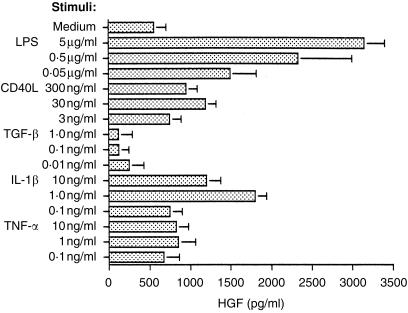
Figure 4.
IL-1β, TNF-α and sCD40L enhance HGF production by human stromal cells. Subconfluent cultures of stromal cells were cultured for 48 hr in the presence of cytokines as indicated. Lipopolysaccharide was used as a well known potent stimulant of HGF production. Data are mean±sd of four experiments.
To ascertain whether the expression of surface molecules responsible for the up-regulation of HGF secretion was dependent on the time of stimulation of T lymphocytes, peripheral blood T lymphocytes were incubated with anti-CD3/CD28 for different periods of time after which they were thoroughly washed, fixed and incubated with stromal cells for a further 48 hr. T lymphocytes enhanced HGF production as early as 6 hr after stimulation (1·5-fold increase, n = 4) and this effect persisted for up to 48 hr after activation following which it subsequently declined (data not shown). This data suggests that cell surface molecules responsible for HGF up-regulation were expressed at an early stage of T-cell activation.
Membrane-associated IL-1 and CD40L are involved in the up-regulation of HGF secretion by stromal cells
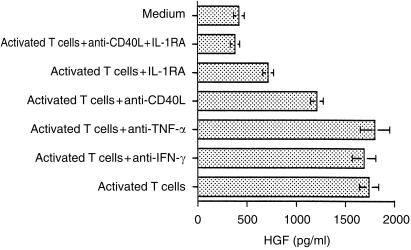
In order to identify the molecules present on activated T cells which might control up-regulation of HGF production, we first used blocking antibodies raised against CD11a, CD11b, CD11c, CD18, CD54, CD58 and CD154 (CD40L). Of these anti-CD40L antibody alone blocked the up-regulation of HGF production, however, this was not complete (see Fig. 3, data with other antibodies not shown).
Figure 3.
Anti-CD40L antibody and IL-1RA inhibit T-cell mediated enhancement of HGF production by human stromal cells. Splenic stromal cells were co-cultured for 48 hr with paraformaldehyde-fixed activated T cells in the presence or absence of anti-IFN-γ antibody (10 µg/ml) anti–CD40L antibody (5 µg/ml) anti-TNF-α antibody (10 µg/ml) and IL-1RA (2 µg/ml). HGF was assayed in cell culture supernatants by ELISA. Data are mean±sd of three experiments.
Since most of the above antibodies failed to reverse the up-regulation, we next tested the effect of specific inhibitors or neutralizing antibodies raised against cytokines. These included IL-1 receptor antagonist (IL-1RA) and neutralizing antibodies against TNF-α and interferon-γ (IFN-γ). Anti-IFN-γ and anti-TNF-α showed no effect on HGF production while recombinant IL-1RA substantially, although again not completely, blocked enhancement of HGF production. Anti-CD40L antibody together with IL-1RA completely abrogated enhanced HGF synthesis (Fig. 3). Taken together these data indicate that the increased HGF secretion noted in our cocultures can be largely accounted for by stimulatory action of cell-membrane-associated IL-1 and CD40L.
IL-1, TNF-α and sCD40L enhance HGF secretion by stromal cells
As CD40L and IL-1 exist in vivo in both membrane bound and soluble form, we studied the effect of soluble recombinant cytokines and other molecules on HGF secretion. On the basis of five independent experiments basal HGF production was enhanced by LPS, IL-1β, TNF-α and sCD40L, 5·9-, 3·1-, 1·5- and 2·1-fold, respectively. In contrast, TGF-β inhibited the release of HGF, a situation previously observed with respect to bone marrow stromal cells and dermal fibroblasts (Fig. 4).9 When combined with CD40L, TNF-α showed an additive effect while with IL-1β there was some suggestion of synergy in augmentation of HGF secretion (Table 3). On the other hand IL-2, IL-4, IL-6 and IL-10 had no effect on stromal HGF, either when tested alone or in combination with CD40L (data not shown).
Table 3.
Additive effect of sCD40L and cytokines on HGF production
| HGF secretion (pg/ml) | |||
|---|---|---|---|
| Treatment | expt 1 | expt 2 | expt 3 |
| Medium | 460 ± 150 | 580 ± 120 | 350 ± 110 |
| IL-1β (1 ng/ml) + | 4220 ± 320 | 4040 ± 110 | 3750 ± 142 |
| SCD40L (30 ng/ml) | |||
| TNF-α (10 ng/ml) + sCD40L (30 ng/ml) | 1850 ± 235 | 2290 ± 240 | 1200 ± 130 |
| sCD40L (30 ng/ml) | 1050 ± 190 | 1140 ± 190 | 820 ± 110 |
| IL-1β (1 ng/ml) | 1620 ± 240 | 1870 ± 160 | 1450 ± 180 |
| TNF-α (10 ng/ml) | 750 ± 210 | 890 ± 180 | 560 ± 90 |
Subconfluent cultures of stromal cells were cultured for 48 hr in the presence of cytokines as indicated. Data are mean ±sd of triplicate cultures. Each experiment was performed with different spleen stromal cell preparation.
To confirm the specificity of the observed effects we used a recombinant form of the natural IL-1RA. This completely neutralized the effects of exogenous IL-1β on CD40L-induced HGF secretion, production reverting to the levels induced by CD40L alone. IL-1RA also completely neutralized the effects of exogenous IL-1β in the absence of CD40L but had no effect on basal HGF production by in vitro cultured stromal cells (data not shown).
IL-1 exists as two molecular forms (IL-1α and β) with overlapping biological activities. Both forms exhibited similar induction of HGF production and both were able to co-operate with CD40L stimulation. In addition, the effects of exogenous IL-1α were completely neutralized by addition of IL-1RA (data not shown). Together this data shows that like membrane-bound IL-1 and CD40L, soluble recombinant forms of IL-1, TNF-α and CD40L exert similar stimulatory effect on HGF secretion by stromal cell preparations from human spleen.
HGF receptor (c-met) is expressed in activated B cells but not T cells
For HGF production by stromal cells to be of biological significance in immune reactions the cells engaged in immune responses would need to express HGF receptor. To determine if this was so we isolated T and B lymphocytes from human spleen and probed them by Western blotting for the expression of high affinity HGF receptor c-met. These studies confirmed previous publications on other lymphoid tissues revealing strong c-met expression in both PMA and anti-µ/CD40L-activated B cells.4,5 In the latter case c-met expression was observed within 18 hr of stimulation, persisted for several days before finally disappearing on day 6 of culture. Weak c-met expression, reflecting most probably in vivo activation was observed in freshly isolated total splenic B cells. In contrast to previous reports incubation of B cells with HGF alone (up to 1 ng/culture) did not induce c-met expression.5 Furthermore T cells, both activated by various stimulants and non-activated, did not express c-met. (Fig. 5). Addition of HGF to B cells stimulated with CD40L/anti-µ did not have any effect on lymphocyte proliferation as measured by incorporation of 3H-thymidine. Some moderate enhancing effect of HGF on immunoglobulin secretion by CD40L/anti-µ was observed; however, it was not consistent (data not shown). These results show that although functional c-met receptor is present on B lymphocytes, the interaction with recombinant HGF alone does not have a direct major effect on lymphocyte proliferation and immunoglobulin secretion.
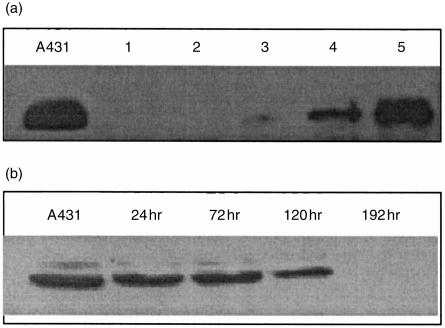
Figure 5.
HGF receptor (c-met) expression can be induced in human B cells but not T cells. (a) T and B cells were isolated from human spleen and cultured for 48 hr with or without stimuli. Then the cells were lysed and used in Western blot analysis. The blots were developed with anti-c-met antibody as described in Materials and methods. A431 (positive control) is an epidermoid carcinoma cell line which constitutively expresses c-met; 1, unstimulated T cells; 2, T cells stimulated with 10 ng/ml PMA; 3, unstimulated B cells; 4, B cells stimulated with sCD40L and anti-µ antibodies; 5, B cells stimulated with 10 ng/ml PMA. (b) Isolated B cells were stimulated with sCD40L and antiµ. Cultures were terminated at times indicated. Western blots of cell lysates were stained with anti-c-met antibodies. Lysates of A431 cell line served as positive control. The same experiment performed on four other occasions gave similar results.
HGF regulates interactions between stromal and B cells
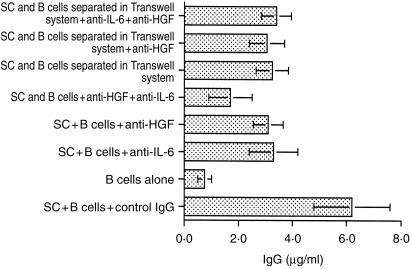
Previous studies by ourselves and others have shown that co-cultivation of splenic fibroblast-like stromal cells with appropriately stimulated B lymphocytes enhances immunoglobulin secretion.6 Here we show that the addition of neutralizing anti-HGF antibodies inhibits this enhanced immunoglobulin production (Fig. 6). An even more pronounced effect was noted when anti-HGF neutralizing antibody and anti-IL6 antibodies were used together. In contrast, anti-HGF antibodies failed to influence immunoglobulin secretion when stromal cells and B cells were separated in the Transwell system. Also addition of recombinant HGF had no effect on immunoglobulin secretion (Fig. 6). These results suggest that in this system HGF may indirectly modify immunoglobulin secretion by influencing the cell–cell adhesion processes known to occur in lymphoid tissue.10,11
Figure 6.
Antibodies to HGF partially inhibit IgG secretion in co-cultures of stromal cells and activated lymphocytes. sCD40L/anti-µ-activated splenic B cells were co-cultured on monolayers of splenic stromal cells in the presence or absence of anti-HGF, anti-IL-6 antibodies or both. Supernatant was collected and assayed for IgG secretion. Identical experiment was performed with stromal cells and B lymphocytes in two-chamber system. Data are mean of three experiments±sd. SC, stromal cells.
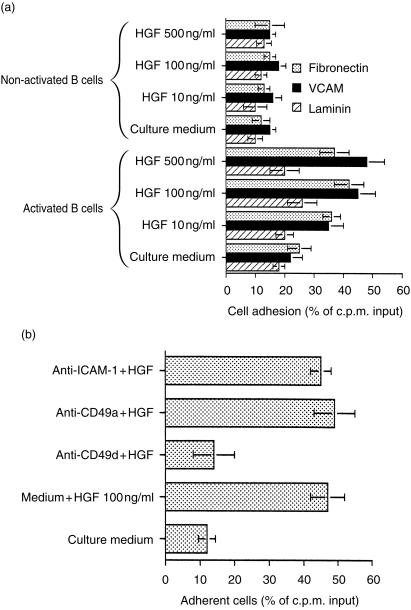
In order to test this we performed a series of experiments to establish if HGF influenced the ability of c-met expressing B lymphocytes to bind to matrix proteins. This involved studying the influence of HGF on binding of PMA-activated B lymphocytes to plastic culture plates coated with VCAM, fibronectin and laminin. Activated B cells were found to bind more effectively to both VCAM- and fibronectin-coated plates than non-activated cells and this increased in a dose-dependent manner. No increase in adhesion to laminin was observed. The small adhesion of unstimulated B cells to matrix proteins observed was not increased further by incubation with HGF (Fig. 7a).
Figure 7.
HGF enhances CD49d mediated binding of activated B cells to VCAM and fibronectin. (a) Highly purified B cells were stimulated with PMA (10 ng/ml) for 18 hr. This was followed by incubation with HGF for 1 hr at 37°. The cells were finally labelled with 51Cr and their adhesion to fibronectin-, laminin- or VCAM-1-coated plates was measured as described in Materials and methods. (b) The effect of anti-adhesion molecules on HGF enhanced binding of activated B cells to extracellular matrix proteins. Results are presented as percentage of c.p.m. input. The mean of triplicate adherent cell values ± sd of one representative experiment out of three conducted with the same results is shown.
Additional studies revealed that monoclonal antibodies directed against CD49d completely inhibited adhesion to matrix proteins. In contrast, no inhibition was obtained with CD49a antibody (Fig. 7b). Furthermore, there was no up-regulation of CD49d expression after treatment of B cells with HGF. These observations suggest that HGF enhances B-cell adhesion by activation of CD49d molecules.
Discussion
It is now well established that stromal cell preparations can influence the development and function of B cells in vitro.6,12–14 These effects can be mediated in part by cell–cell contact and the production by stromal cells of cytokines such as IL-6. It has recently been reported that another stromal-cell-derived mediator, namely HGF, may have a role in B-cell biology. In brief, HGF is produced by stromal cells and follicular dendritic-cell enriched preparations from human tonsils4 and B lymphocytes in germinal centres, certain Burkitt lymphoma and myeloma cell lines express functional c-met, the receptor for HGF.5
The aim of the present studies was to establish whether stromal cell preparations from secondary human lymphoid tissues other then tonsil can produce HGF and to determine if this was influenced by interaction with other immunocompetent cells. Finally, we wanted to establish the possible effect of HGF on B-cell functions.
The first report drawing attention to the possible role of HGF in the immune system, was that by Delaney et al.15 in which they demonstrated a stimulatory effect of HGF on immunoglobulin secretion in cultures of mouse splenocytes. c-met receptor expression on B cells was, however, not studied and the effect of HGF on other cell subpopulations not excluded. Subsequently, it was shown that functional c-met receptor is expressed on activated B cells,4,5 bone marrow mononuclear cells,9 thymocytes,16 as well as activated monocytes17 and neutrophils.18 The stimulatory effect of HGF on memory T-cell adhesion was also reported; however, c-met receptor could not be found suggesting the existence of another functional HGF receptor, different from c-met.19
In the present studies we have extended previous observations by providing extensive data on the cellular and molecular basis of HGF secretion by human stromal cells and its possible relevance to B-cell survival and function. In brief, we have first shown that HGF is secreted by stromal cells from secondary lymphoid organs other than tonsils and hence are readily available in the microenvironment of secondary lymphoid tissue in general. Second, HGF secretion from stromal cell preparations can be up-regulated by activated T cells and this up-regulation is mediated by CD40L and IL-1. Finally, our findings confirm and extend the results obtained by Delaney et al.15 and van der Voort et al.4 with c-met-transfected lymphoma cell lines and show that c-met–HGF interactions are essential in mediating activated cell adhesion and indirectly, immunoglobulin secretion.
Our studies on the influence of lymphocytes on HGF production by stromal cells clearly demonstrate that activated T cells, but not B cells, enhance its release in a contact-dependent manner. As human T cells are known to adhere to fibroblasts via CD2–CD58 and CD11a–CD18–CD54 interactions20 we have investigated the role of these and other cell surface interactions on HGF release by stromal cells. Of the antibodies tested, antibody to CD40L alone inhibited T-cell enhanced HGF secretion, suggesting the involvement of the CD40–CD40L signalling pathway in this process. Furthermore as cytokines such as, for example IL-1, granulocyte–macrophage colony-stimulating factor (GM-CSF) and IL-4 have been shown to modulate HGF production in vitro by stromal cells9 and membrane-associated cytokines such as IL-1, TNF and IFN-γ have been detected on activated T cells,21,22 we investigated the effects on HGF secretion of neutralizing antibodies or cytokine inhibitors for a number of these cytokines. These studies revealed that neutralization of surface IL-1 greatly impaired the ability of fixed activated T cells to enhance HGF production by stromal cells. Finally, application of both IL-1RA and anti-CD40L antibody almost completely prevented the increase in HGF secretion thus showing an intimate interplay between IL-1 and CD40L in vitro.
Recognizing that cytokines may be released from fixed T cells23 further experiments were performed to establish the role of cell–cell contact versus soluble factors in the observed phenomenon. These studies revealed that sCD40L and recombinant IL-1β- and TNF-α-enhanced HGF secretion. Furthermore the effects of sCD40L and IL-1β appeared to be synergistic, thus mimicking observations in other systems.24–27 Interestingly, the synergistic effects observed with IL-1 and CD40L were not found with combinations of CD40L and TNF-α where only additive effect was observed. However, as TNF-α and CD40L belong to the same superfamily of ligands it might be that they induce overlapping sets of signal transduction pathways.28 Currently, the molecular mechanisms behind the additive action of CD40L and IL-1 are not well understood. It has, however, been recently established that both CD40- and IL-1-mediated signalling, leading to nuclear factor-κB (NF-κB) activation uses the same member of TNF receptor-associated factor (TRAF) family proteins, namely TRAF6·29
For HGF production to be of relevance in the immune system its receptor c-met would have to be expressed by cells within the lymphoid tissue microenvironment. The present studies confirm and extend recent reports indicating that this indeed is the case with c-met being expressed by immature B cells, in vitro-activated B cells and B cells within germinal centres (centroblasts strongly, centrocytes weakly) as well as by haematopoietic cell precursors.4,5,9 Because CD40L and anti-µ ligation, used in this work to stimulate B cells, mimics processes of germinal centre reaction, it is likely that c-met is strongly expressed early after stimulation and then its expression declines as B lymphocytes differentiate to centrocytes stage and later to antibody-secreting cells. The disappearance of the receptor may be a result of either shedding or internalization and remains to be established.
Recently, we have established that fibroblast-like stromal cells from human spleen enhance antibody secretion by anti-CD40/anti-µ-stimulated B cells6 and that this was partially dependent on IL-6 secretion and their very late antigen-4 (VLA-4; CD49d)-mediated interaction with B cells. In this paper we show that addition of anti-HGF neutralizing antibody to co-cultures of stromal cells and B lymphocytes partially prevented enhancement of immunoglobulin secretion, indicating the role for HGF in this system. However, when stromal cells were replaced by HGF enhanced production of immunoglobulin by activated B cells was not observed. There was also no effect on cell proliferation. Likewise, when stromal cells and B lymphocytes were separated by a microporous membrane in a two-chamber culture system addition of neutralizing anti-HGF antibody to the system did not modify immunoglobulin secretion in comparison with control cultures not containing this antibody. These observations suggested that HGF may influence immunoglobulin production by some indirect means, thus prompting us to study its effect on the adhesion of B cells to stroma. In this paper we show that the adhesion of PMA-activated B cells to VCAM-1 and fibronectin is enhanced by HGF in a dose-dependent fashion and this can be blocked by antibody to CD49d. Because HGF itself had no effect on CD49d expression by activated B cells (data not shown) we believe that HGF acts on B cells by activating VLA-4 integrins. These observations support previous findings by van der Voort et al.4 showing that HGF can regulate adhesion of c-met-transfected Namalwa cell line to ECM proteins such as VCAM and fibronectin and fit in with previous studies by ourselves indicating that antibodies to CD49d inhibit enhanced immunoglobulin production noted in human splenic stromal cell–B-cell cultures.6
For HGF to be of relevance in vivo would also require it to be available within lymphoid microenvironments. The present studies clearly indicate that like their tonsil counterparts4 stromal preparations from spleen and lymph node produce substantial amounts of HGF. Moreover this production can be enhanced by membrane-bound as well as soluble CD40L and IL-1. It is entirely feasible that in a local microenvironment the levels of stromal-cell-derived HGF produced following interaction with activated T cells is sufficient to generate the enhanced interactions of B cells with stromal tissue and extramatrix proteins. In this connection it should also be noted that enhanced levels of HGF have been reported in the blood, bone marrow, plasma and pleural fluid of patients suffering from lymphomas.30,31
Based upon these observations we would hypothesize that the following series of interactions may take place in secondary lymphoid tissue. Following activation by antigen, T cells up-regulate their membrane expression of CD40L and IL-1. The T cells in turn interact with stromal cells and this leads to enhanced local secretion of HGF. Stromal cell derived HGF binds to the c-met receptors on activated B cells thus promoting B-cell survival. Such interaction may facilitate germinal centre formation. In this respect it remains to be established if the small number of T cells found in germinal centres in vivo would be sufficient to achieve the effects observed with relatively high number of T cells in vitro. It is possible however, that soluble IL-1, TNF-α and CD40L can amplify the effect of relatively small numbers of cells. Finally, we are also aware that while we have attempted to recreate events which might occur in vivo our model obviously does not entirely mimic this situation.
Another possible physiological role for HGF in lymphoid tissue would be to prevent apoptotic death of c-met-expressing cells. In this connection it is interesting to note that we have recently shown that c-met-expressing Burkitt lymphoma cell lines can be rescued from apoptotic death induced by DNA-damaging agents by preincubation with HGF. While this is an attractive idea our preliminary data however, failed to show any effect of HGF on the apoptosis of normal activated B cells (G. Skibinski et al., submitted for publication)
In conclusion, our in vitro data presented here suggests another paracrine pathway by which stromal cell product may be involved in the regulation of immune response in secondary human lymphoid tissues. Up-regulation of HGF secretion can be achieved by contact with CD40L and membrane IL-1 expressing activated T cells resulting in increased adhesion of appropriately activated B cells. Because these cells remain in close contact in the lymphoid tissue environment this sequence is potentially operative in vivo.
Acknowledgments
This study was funded by the Wellcome Trust. We are grateful to our clinical colleagues and transplant co-ordinators for supplying human lymphoid tissue. We also thank Jeane Maingay and Katherine Sangster for help with Western blotting and Joanne Haley for PCR experiments.
References
- 1.Hynes RO. Integrins, versatility, modulation and signaling in cell adhesion. Cell. 1992;69:11–25. doi: 10.1016/0092-8674(92)90115-s. [DOI] [PubMed] [Google Scholar]
- 2.Boros P, Miller CM. Hepatocyte growth factor; a multifunctional cytokine. Lancet. 1995;435:293–5. doi: 10.1016/s0140-6736(95)90279-1. [DOI] [PubMed] [Google Scholar]
- 3.Bottaro DP, Rubin JS, Faletto DL, Chan AM, Kmiecik TE, Van de Woude GF, Aaronson SA. Identification of the hepatocyte growth factor receptor as the c-met proto-oncogene product. Science. 1991;251:802–4. doi: 10.1126/science.1846706. [DOI] [PubMed] [Google Scholar]
- 4.van der Voort R, Taher TEI, Keehnen MJ, Smit L, Groenink M, Pals ST. Paracrine regulation of germinal centre B cell adhesion through the c-Met–hepatocyte growth factor/scatter factor pathway. J Exp Med. 1997;185:2121–31. doi: 10.1084/jem.185.12.2121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Weimar IS, de Jong D, Muller EJ, Nakamura T, van Gorp JMHH, De Gast GC, Gerritsen WR. Hepatocyte growth factor/scatter factor promotes adhesion of lymphoma cells to extracellular matrix molecules via α4β1 and α5β1 integrins. Blood. 1997;89:990–00. [PubMed] [Google Scholar]
- 6.Skibinski G, Skibinska A, Stewart GD, James K. Enhancement of terminal B lymphocyte differentiation in vitro by fibroblast-like stromal cells from human spleen. Eur J Immunol. 1998;28:3940–8. doi: 10.1002/(SICI)1521-4141(199812)28:12<3940::AID-IMMU3940>3.0.CO;2-L. 10.1002/(sici)1521-4141(199812)28:12<3940::aid-immu3940>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 7.Skibinski G, Hoffmann P, Radbruch A, James K. Organ culture of human lymphoid tissue. II. Marked differences in cytokine production and proliferation between slice and suspension cultures of human spleen. J Immunol Methods. 1997;205:115–25. doi: 10.1016/s0022-1759(97)00058-6. 10.1016/s0022-1759(97)00058-6. [DOI] [PubMed] [Google Scholar]
- 8.Strom SC, Jirtle RL, Jones PS, et al. Isolation, culture and transplantation of human hepatocytes. J Natl Canc Invest. 1982;68:771–7. [PubMed] [Google Scholar]
- 9.Takai K, Hara J, Matsumoto K, Hosoi G, Osugi Y, Tawa A, Okada S, Nakamura T. Hepatocyte growth factor is constitutively produced by human bone marrow stromal cells and indirectly promotes hematopoiesis. Blood. 1997;89:1560–5. [PubMed] [Google Scholar]
- 10.Liakka KA. The integrin subunits alpha 2, alpha 3, alpha 4, alpha 5, alpha 6, alpha V, beta 1 and beta 3 in fetal, infant and adult human spleen as detected by immunohistochemistry. Differentiation. 1994;56:183–90. doi: 10.1046/j.1432-0436.1994.5630183.x. [DOI] [PubMed] [Google Scholar]
- 11.Yoshida K, Kaji M, Tajahashi T, van den Berg TK, Dijkstra CD. Host origin of follicular dendritic cells induced in the spleen of SCID mice after transfer of allogeneic lymphocytes. Immunology. 1995;84:117–26. [PMC free article] [PubMed] [Google Scholar]
- 12.Lindhout E, van Eijk M, van Pel M, Lindeman J, Dinant HJ, de Groot C. Fibroblast-like synoviocytes from rheumatoid arthritis patients have intrinsic properties of follicular dendritic cells. J Immunol. 1999;162:5949–56. [PubMed] [Google Scholar]
- 13.Roldan E, Rodriguez C, Navas G, Parra C, Brieva JA. Cytokine network regulation and terminal maturation of human bone marrow cells capable of spontaneous and high rate Ig secretion rate in vitro. J Immunol. 1992;149:2367–71. [PubMed] [Google Scholar]
- 14.Kim H-S, Zhang E, Klyushenkova E, Choi YS. Stimulation of germinal center B lymphocyte proliferation by an FDC-like cell line, HK. J Immunol. 1995;153:2951–8. [PubMed] [Google Scholar]
- 15.Delaney B, Koh WS, Yang KH, Strom SC, Kaminski NE. Hepatocyte growth factor enhances B cell activity. Life Sci. 1993;53:89–93. doi: 10.1016/0024-3205(93)90654-l. [DOI] [PubMed] [Google Scholar]
- 16.Tamura S, Sugawara T, Tokoro Y, Taniguchi H, Fukao K, Nakauchi H, Takahama Y. Expression and function of c-met, a receptor for hepatocyte growth factor, during T cell development. Scand J Immunol. 1998;47:296–301. doi: 10.1046/j.1365-3083.1998.00324.x. 10.1046/j.1365-3083.1998.00324.x. [DOI] [PubMed] [Google Scholar]
- 17.Beilmann M, Odenthal M, Jung W, Van de Woude GF, Dienes HP, Schirmacher P. Neo-expression of the c-met/hepatocyte growth factor–scatter factor receptor in gene activated monocytes. Blood. 1997;90:4450–8. [PubMed] [Google Scholar]
- 18.Mine S, Tanaka Y, Suematu M, Aso M, Fujosaki T, Yamada S, Eto S. Hepatocyte growth factor is a potent trigger of neutrophil adhesion through rapid activation of lymphocyte function-associated antigen-1. Lab Invest. 1998;78:1395–404. [PubMed] [Google Scholar]
- 19.Adams DH, Harvath L, Bottaro DP, et al. Hepatocyte growth factor and macrophage inflammatory protein1b: structurally distinct cytokines that induce rapid cytoskeletal changes and subset-preferential migration in T cells. Proc Natl Acad Sci USA. 1994;91:7144–8. doi: 10.1073/pnas.91.15.7144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Abraham D, Lupoli S, McWhirter A, Plater-Zyberk C, Piela TH, Korn JH, Olsen I, Black C. Expression and function of surface-antigens on scleroderma fibroblasts. Arthritis Rheum. 1991;34:1164–72. doi: 10.1002/art.1780340913. [DOI] [PubMed] [Google Scholar]
- 21.Rezzonico R, Burger D, Dayer J-M. Direct contact between T lymphocytes and human dermal fibroblasts or synoviocytes down-regulates types I and III collagen production via cell-associated cytokines. J Biol Chem. 1998;273:18720–8. doi: 10.1074/jbc.273.30.18720. [DOI] [PubMed] [Google Scholar]
- 22.Assenmacher M, Scheffold A, Segura-Checa JA, Miltenyi S, Radbruch A. Specific expression of surface interferon-gamma on interferon-gamma producing T cells from mouse and man. Eur J Immunol. 1996;26:263–7. doi: 10.1002/eji.1830260141. [DOI] [PubMed] [Google Scholar]
- 23.Sebbag M, Parry SL, Brennan FM, Feldmann M. Cytokine stimulation of T lymphocytes regulates their capacity to induce monocyte production of tumor necrosis factor alpha but not IL-10. Possible relevance to pathophysiology of rheumatoid arthritis. Eur J Immunol. 1997;27:624–32. doi: 10.1002/eji.1830270308. [DOI] [PubMed] [Google Scholar]
- 24.Galy AHM, Spits H. CD40 is functionally expressed on human thymic epithelial cells. J Immunol. 1992;149:775–82. [PubMed] [Google Scholar]
- 25.Rissoan MC, van Kooten C, Chomarat P, Galibert L, Durand I, Thivolet-Bejui F, Miossec P, Banchereau J. The functional CD40 antigen of fibroblasts may contribute to the proliferation of rheumatoid synovium. Clin Exp Immunol. 1996;106:481–90. doi: 10.1046/j.1365-2249.1996.d01-858.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dechanet J, Grosset C, Taupin JL, Merville P, Banchereau J, Ripoche J, Moreau JF. CD40 ligand stimulates proinflammatory cytokine production by human endothelial cells. J Immunol. 1997;159:5640–7. [PubMed] [Google Scholar]
- 27.van Kooten C, van der Linde X, Woltman AM, van Es LA, Daha MR. Synergistic effect of interleukin-1 and CD40L on the activation of human renal tubular epithelial cells. Kidney Int. 1999;56:41–51. doi: 10.1046/j.1523-1755.1999.00514.x. 10.1046/j.1523-1755.1999.00514.x. [DOI] [PubMed] [Google Scholar]
- 28.Smith CA, Farrah T, Goodwin RG. The TNF receptor superfamily of cellular and viral proteins: activation, costimulation and death. Cell. 1994;76:965–2. doi: 10.1016/0092-8674(94)90372-7. [DOI] [PubMed] [Google Scholar]
- 29.Lomaga MA, Yeh W-C, Sarosi I, et al. TRAF6 deficiency results in osteoporosis and defective interleukin-1, CD40 and LPS signaling. Genes Devel. 1999;13:1015–24. doi: 10.1101/gad.13.8.1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nakamura S, Gohda E, Matsuo Y, Minowada J. Significant amount of hepatocyte growth factor detected in blood and bone marrow of leukemia patients. Br J Haematol. 1994;87:640–2. doi: 10.1111/j.1365-2141.1994.tb08330.x. [DOI] [PubMed] [Google Scholar]
- 31.Eagles G, Warn A, Ball RY, et al. Hepatocyte growth factor/scatter factor is present in most pleural effusion fluids from cancer patients. Brit J Cancer. 1996;73:377–81. doi: 10.1038/bjc.1996.64. [DOI] [PMC free article] [PubMed] [Google Scholar]