Abstract
Lymphoid aggregates (LA) develop during the proliferative phase of the menstrual cycle in the human uterine endometrium (EM). They contain mostly CD8+ T cells and B cells. As these LA are absent immediately following menses, they may arise by division of cells resident in the EM, or by division of a limited number of precursor cells that traffic into the EM during the early proliferative phase of the menstrual cycle. Alternatively, they may arise by the continuous trafficking of cells into the EM throughout the proliferative phase of the menstrual cycle. In this study we investigated the distribution and frequency of CD8+ T cells in the aggregates using expression of Vβ2 or Vβ8 as markers of clonality and Ki-67 as a marker of dividing cells. Confocal microscopic analysis of endometrial tissues showed the random distribution of CD8+ T cells within aggregates within the same sample and in aggregates from different samples. Furthermore, comparisons of the distribution of Vβ2 and Vb8 with expected values predicted from Poisson distribution values were not significantly different, suggesting that CD8+ T cells do not arise by division from single precursors. A low level of T-cell division within LAs was confirmed by positive staining for Ki-67. Dividing T cells were randomly dispersed throughout the LA and the frequency of dividing cells did not vary greatly between aggregates within the same tissue. Nearest-neighbour analysis of dividing cells showed no statistically significant deviations from a random distribution. Taken together, these results suggest that LA develop during the menstrual cycle largely by the trafficking of cells to nucleation sites within the EM, rather than by division of a limited number of precursor cells.
Introduction
The mucosal immune system of the female reproductive tract is unique compared with other mucosal sites. In particular, in order to achieve its reproductive role, the local immune system in the uterus must be tolerant to the engraftment of a semi-allogeneic conceptus. As deposition of ejaculate in the genital tract represents the highest risk of exposure to sexually transmitted diseases, this tolerance to a conceptus must be achieved without compromising overall host defence to infectious agents. The mechanisms by which these apparently contrasting requirements are accommodated remains poorly understood. However, it is clear that changes in the steroid sex hormone levels, which occur during the normal menstrual cycle, are accompanied by changes in both the immune cell composition and immune function in the human uterine endometrium (EM).
Leucocytes are a significant population in the human EM, accounting for 10–20% of all endometrial cells.1 Of the leucocyte population, ≈50% are T cells and at least two-thirds of these are CD8+ T cells. These CD8+ cells are found in three distinct locations within the EM: as scattered stromal cells; as intraepithelial lymphocytes; and in lymphoid aggregates (LA). LA develop in the stratum basalis of the human EM during the proliferative phase of the menstrual cycle, but are absent in the EM of postmenopausal women, suggesting that their development is hormonally influenced.2,3
LA usually have a B-cell core, which is surrounded by a spheroidal mass of T cells.2 The T-cell region is, in turn, surrounded by a diffuse halo of macrophages. A unique feature of these LA is that, in most endometria, the T cells are usually exclusively CD8+ T cells.2 The function of LA CD8+ cells is not known; however, it is of interest that endometrial CD8+ cells isolated from secretory-phase endometria, when LA are fully formed, are unable to mediate cytotoxic T-lymphocyte (CTL) activity following stimulation with interleukin-2 (IL-2).4 This lack of CTL function is tissue specific, as CD8+ cells from secretory-phase cervix and vaginal mucosa are active. CTL activity of CD8+ cells from postmenopausal endometria, which lack LA, do exhibit CTL activity.4 Therefore, these observations suggest that development of LA is accompanied with a concomitant reduction in CTL capacity in the uterus. It is not definitively known whether or not the LA T cells are potential cytolytic effectors or whether they represent a distinct regulatory CD8 T-cell subset.
The relative contributions of cell division and cell trafficking into the EM during the proliferative phase of the menstrual cycle to the construction of LA is not known. As direct measurement of immune cell trafficking in human subjects is not feasible, in the present study we examined the distribution of common T-cell receptor (TCR) Vβ families within and between LA in EM tissues from different patients. In addition, we evaluated the frequency and distribution of dividing CD8+ T cells detectable through expression of Ki-67.5 Our hypothesis was that if LA T cells arose by the division of a limited number of precursor cells, then: a detectable level of oligoclonality would be evident within LA T cells; that this oligoclonality would lead to clusters of Vβ+ T cells within individual LA; and subsequently this would lead to detectable differences in the frequency of T cells expressing a given Vβ TCR family between different LA in the same EM. Conversely, a lack of clustering or differences in frequency would indicate random accumulation of T cells, probably arising by the trafficking of cells into the EM during the menstrual cycle.
Materials and methods
Preparation of vibratome sections
Uterine endometrial tissue was obtained with prior consent, and Institutional Review Board approval, from hysterectomy patients. All tissues were free from malignant or infectious diseases, and uterine pathology was confined to leiomyomata, adenomyomata, or prolapse uterus. The stage of the menstrual cycle of the EM was determined in accordance with accepted histological practice using haemotoxylin/eosin-stained paraffin sections.6 Evaluations were conducted by two pathologists by scoring the degree of stromal oedema and the relative frequency of glandular and stromal mitoses. Unfixed viable sections of endometrial tissue, 30–70-µm thick, were cut using a vibratome (V1000 TPI; Energy Beam Sciences, Agawam, MA). Sections were maintained in cold Leibvitz's L-15 Medium (Gibco-BRL, Paisley, Strathclyde, UK) in 96-well tissue-culture plates throughout processing.
Monoclonal antibodies (mAbs)
A panel of mAbs was used for direct and indirect immunofluorescence staining. Purified Vβ8 (clone 56C5.2) and Vβ2 (clone MPB2D5) (Beckman Coulter Immunotech, Miami, FL), were used in indirect staining. mAbs specific for CD8 (clone OKT8) and CD3 (clone OKT3) were purified from hybridoma cell culture supernatants (cell lines were obtained from the American Type Culture Collection [ATCC], Rockville, MD) using Hi-Trap protein G-Superose columns (Pharmacia LKB, Piscataway, NJ). Purified CD20 (clone B939) was obtained from Beckman Coulter Immunotech. These mAbs were labelled with cyanine 3 (Cy3) or cyanine 5 (Cy5) monofunctional reactive-dye protein-labelling kits (Amersham Pharmacia Biotech, Piscataway, NJ) according to the manufacturer's recommendations. The following fluorescein isothiocyanate (FITC)-conjugated mouse mAbs were purchased from Beckman Coulter Immunotech: CD8-FITC (clone DK25), CD3-FITC (clone UCHT1) and CD20-FITC (clone B9E9). CD45RA-FITC (clone HI100) and CD45RO-FITC (clone UCHL1) were purchased from PharMingen (San Diego, CA). Ki-67-FITC (clone Ki-S5) was purchased from Dako Corp. (Carpinteria, CA).
Triple-colour immunophenotyping
Three-colour immunofluorescence staining was performed immediately after tissue sections were cut, as previously described.2 For direct staining, 2 µg/100 µl each of FITC-, Cy3- and Cy5-labelled antibodies were added in phosphate-buffered saline (PBS)/1% bovine serum albumin (BSA)/0·1% azide – containing human immunoglobulin (6 mg/ml) to block non-specific and Fc-mediated binding (Sigma, St. Louis, MO) – and the sections were incubated overnight at 4°. Unbound antibody was removed from the sections by aspiration followed by four 20-min washes in cold PBS/1%BSA/0·1% azide. Washed sections were then fixed in the same buffer containing 2% paraformaldehyde. Stained sections were wet-mounted in Prolong™ (Molecular Probes, Eugene, OR).
Some sections were stained with two directly conjugated antibodies and one unlabelled antibody. The latter were detected using a Cy3- or Cy5-labelled goat anti-mouse second antibody (CyDye; Amersham Pharmacia Biotech). In such cases, indirect staining was completed first and then the directly conjugated antibodies were added in the presence of mouse serum block (5%).
Ki-67 and terminal deoxynucleotide transferase (TdT)-mediated nick-end labelling (TUNEL) staining of tissue sections
Proliferating and apoptosing CD8+ T cells were detected using a triple-staining method. Sections were stained with Cy3-labelled anti-CD8, as described above. Following washing in PBS, sections were permeabilized in 70% ethanol and washed in Hanks' balanced salt solution (HBSS). Apoptotic cells were detected by the TUNEL assay using incorporation of Cy5-labelled dUTP in the presence of TdT. Sections incubated without the addition of TdT acted as negative controls. Proliferating cells were detected by intracellular staining using Ki-67-FITC mAb, which is specific for a nuclear antigen present in dividing cells. This intracellular staining was carried out following extracellular staining for phenotype and then fixed as described above. Following two washes in PBS, sections were permeabilized by incubation with PBS/0·5% saponin (Sigma)/0·1% BSA/0·05% sodium azide for 20 min. Sections were then incubated for 2 hr with 2 µg of Ki-67-FITC in 100 µl of saponin solution. Sections were subsequently aspirated and washed three times (20 min each wash) with saponin solution to eliminate any unbound antibody and then fixed in 2% paraformaldehyde.
Confocal-scanning laser microscopy
Stained sections were optically sectioned using a BioRad MR1000 Confocal Scanning Laser Microscope system (Bio-Rad Laboratories, Hercules, CA), as previously described.2 Laser power, photo multiplier tube (PMT) gain and enhancement factors were determined for the FITC, Cy3 and Cy5 channels using the single fluorochrome-stained sections to ensure effective cross-channel compensation. Isotype control-stained sections showed no signal in any of the three emission detectors at these settings. Three-dimensional data sets of LA were collected for image analysis.
Data analysis
Optical sections from each data set were selected for image analysis. Sections were selected at sufficient intervals in the Z-axis to ensure no overlap of cells (i.e. greater than 20 µm). CD8+ cells were then counted and scored for reactivity with the Vβ-specific antibodies. Ki-67- and TUNEL-positive CD8 cells were enumerated in a similar manner. Differences in Vβ frequencies within and between LA were determined by comparison of means and using the Student's t-test.
The spatial distribution of Vβ+ cells within the area of the LA was analysed by nearest-neighbour analysis (The Image Processing Tool Kit; Reindeer Games Inc., Ashville, NC). Randomly dispersed cells yield nearest-neighbour distances that conform to a Poisson distribution.7 The predicted mean for a random distribution can be described as a function of the number of cells (N) and the area (A) by the equation:
The standard deviation of the predicted mean is the square root of the mean. Significant clustering of cells results in an observed mean that is less than the predicted mean. The statistical significance of any difference in means was determined by using the Student's t-test.
Results
LA T cells are almost exclusively CD45RO+ CD45RA–
Using triple immunofluorescence staining, we evaluated the CD45RO and CD45RA expression of endometrial LA T cells in endometria from 16 patients. The LA CD8 T-cell population was consistently (> 90%) CD45RO+ (Fig. 1a) and CD45RA– (results not shown). Expression of CD45RO on T cells is generally accepted as indicating that a cell is antigen experienced. It has also been reported that antigen-experienced CD8+ cytolytic effector T cells re-express CD45RA. This low frequency of CD45RA+ CD8+ cells is consistent with the majority of LA CD8+ T cells not being cytolytic effector cells.
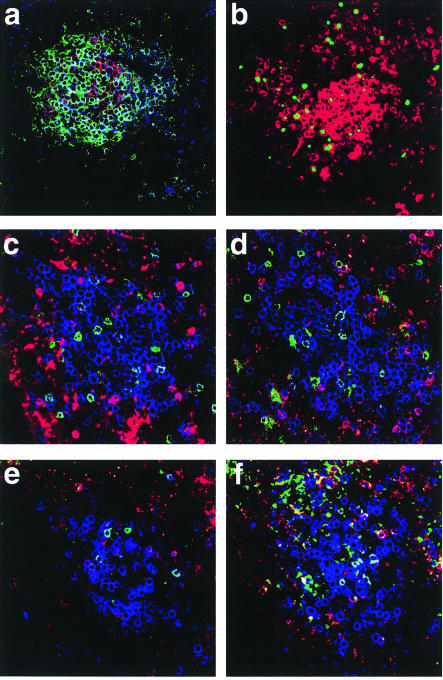
Figure 1.
Immunofluorescence phenotyping of lymphoid aggregate (LA) CD8 T cells in vibratome sections. (a) LA T cells have a memory phenotype. The section was stained with cyanine (Cy)5-conjugated anti-CD8 (blue), Cy3-conjugated anti-CD20 (red, B cells) and fluorescein isothiocyanate (FITC)-labelled (green) anti-CD45RO (memory T cells). The vast majority of CD8+ cells were also CD45RO positive (light blue and turquoise). In contrast, nearly all of the CD20+ cells were CD45RO–. This is a representative section from a single LA from one of the 10 endometria analysed. (b) Dividing and apoptotic CD8 T cells in a LA. The vibratome section was stained extracellularly with Cy3-labelled anti-CD8 (red). Proliferating cells were visualized by intracellular staining with FITC-labelled Ki-67 antibody (green) and were randomly distributed throughout the LA. Apoptotic cells were visualized following incubation with Cy5-dUTP (blue) in the presence of terminal deoxynucleotide transferase (TdT). No apoptotic cells were apparent in the LA CD8 population (absence of blue intracellular staining). Apoptotic glandular epithelial cells were present in the same vibratome sections, showing that the Cy5-dUTP incorporation detected apoptotic cells. This is a representative section from a single LA from one of the 10 endometria analysed. (c), (d), (e), (f) Vβ8 and Vβ2 expression of uterine LA CD8+ T cells. T cells in vibratome sections were immunofluorescently stained using Cy5-labelled anti-CD8 (blue) and indirectly stained using an unlabelled T-cell receptor (TCR) Vβ8-specific antibody followed by a Cy3-labelled goat anti-mouse immunoglobulin G (IgG) antibody (red), and, for TCR Vβ2, with a FITC-labelled antibody. CD8 cells dual positive for TCR Vβ8 are light blue or turquoise, whereas CD8 cells dual positive for TCR Vβ2 are purple. The distribution of TCR Vβ2- and TCR Vβ8-positive cells within two optical sections of the same LA (c) and (d) is essentially random, as it is in two other LA from the same endometrium (e) and (f).
Low frequency of proliferation and apoptosis in LA CD8 T cells
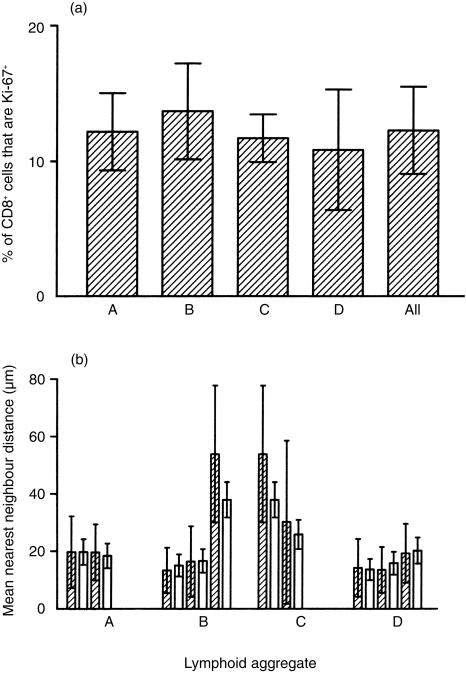
Analysis of the frequency of Ki-67+ cells in LA from proliferative-phase tissues showed a low level of CD8 T-cell division. The frequency of proliferating cells did not vary significantly between LA from the same EM (Fig. 1b and Fig. 2a). In addition, clustering (as determined using nearest-neighbour distances) in most LA conformed well to a Poisson (random) distribution. A statistically significant degree of clustering would be indicated by a reduction in the mean nearest-neighbour distance as compared to the nearest-neighbour distance predicted from a random distribution of the same number of cells within an equal area. Such a reduction in nearest-neighbour distances was not observed (Fig. 2b). An example of lack of clustering of Ki-67+ cells is shown in Fig. 1(b). In addition, apoptosis (as measured by TUNEL) in the CD8 T-cell population in these proliferative-phase tissues was extremely low (Fig. 1b). A positive control for this technique was the observation that cells in discrete regions of the epithelial glands frequently showed either TUNEL positivity or Ki-67 positivity (data not shown).
Figure 2.
Frequency and nearest-neighbour distance (cluster) analysis of dividing cells in lymphoid aggregates (LA). (a) The number of Ki-67+ cells in non-overlapping optical sections of different LA (A, B, C, D) were calculated and expressed as a percentage of the total CD8+ T cells in the section. Each bar represents the mean percentage of Ki-67+ cells ±1 SD for a single LA. The average of the mean frequency of Ki-67+ cells for aggregates A, B, C and D is shown (All). Comparison of means, either within or between LA, showed no significant differences in the frequency of Ki-67+ cells. (b) Nearest-neighbour analysis was carried out for individual non-overlapping sections from individual LA (A, B, C, D). Two bars are presented for each optical section analysed. The shaded (left) bar in each pair is the observed mean nearest-neighbour distance ±1 SD between Ki-67+ cells. The unshaded (right) bar in each pair is the predicted nearest-neighbour distance ±the predicted SD for a random distribution. Clustering of cells would be indicated by a reduction in the mean nearest-neighbour distance compared to that predicted for a random distribution.
The frequency of dividing cells was similar in LA from both early proliferative-phase and late proliferative-phase tissues, indicating that this proliferation rate was constant throughout the period of LA formation.
Distribution of Vβ2 and Vβ8 within LA CD8+ T cells
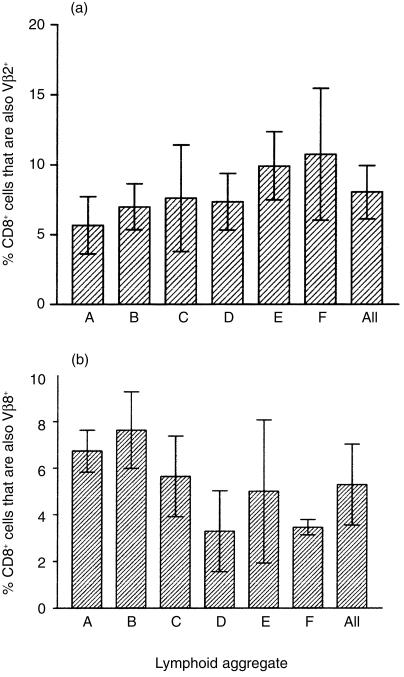
Using triple immunofluorescence staining, we examined the expression of Vβ2 and Vβ8 in LA CD8+ T cells from proliferative-phase tissues. No significant differences were found in either Vβ2 or Vβ8 frequencies between five individual LA (A to F) within the same EM (Fig. 3a), as compared to the variability of the frequencies of Vβ2 and Vβ8 found in different optical sections within the same LA (P > 0·2). Typical micrographs of one LA are shown in Fig. 1(c),1(d). Figure 1(e), 1(f) shows micrographs of Vβ2 and Vβ8 in two different LA within the same EM, as shown in Fig. 1(c),1(d). This distribution and frequency of Vβ2 and Vβ8 CD8+ T cells was consistent in LA present in the endometria from 10 individuals, irrespective of the time the sample was taken during the first 14 days of the menstrual cycle (data not shown). Statistical analysis of all of these samples showed no significant variation in Vβ2 and Vβ8 frequency (P > 0·2).
Figure 3.
Frequency of Vβ2 and Vβ8 T-cell receptor (TCR)-positive cells within and between lymphoid aggregates (LA) from the same endometrium. The number of Vβ2 (a) and Vβ8 (b) cells in non-overlapping optical sections of different LA (A, B, C, D, E, F) were calculated and expressed as a percentage of the total CD8+ T cells in the section. Each bar represents the mean percentage of Vβ2- or Vβ8-positive cells ±1 SD (n = 3) for a single LA. The average of the mean frequency of Vβ-positive cells for aggregates A to F is shown on the right of each figure (All). Comparison of means, either within or between LA, showed no significant differences in frequency for either Vβ2- or Vβ8-positive cells. Similar results were seen in the other nine endometria analysed.
Lack of clustering of Vβ within LA
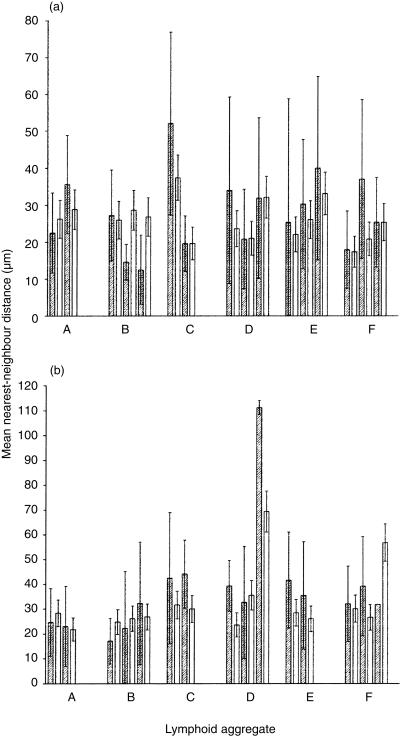
Similarly to the expression of Ki-67, if LA CD8+ T cells of a given TCR Vβ family were undergoing a significant degree of cell division, then it might be expected that some clustering of cells of that TCR Vβ family should be apparent within individual LA. The nearest-neighbour distances in LA conformed well to a Poisson (random) distribution. Nearest-neighbour analysis of the distribution of Vβ2 and Vβ8 in LA showed no statistically significant clustering of either Vβ2 (Fig. 4a) or Vβ8 (Fig. 4b). Similarly to the frequency of Vβ2 and Vβ8 CD8+ T cells in LA, described above, no significant clustering was found by nearest-neighbour analysis when comparing LA within the same EM and in those from 10 individuals, irrespective of the time the sample was taken during the first 14 days of the menstrual cycle (data not shown).
Figure 4.
Nearest-neighbour (cluster) analysis of Vβ2- and Vβ8-positive cells. Nearest-neighbour analysis was carried out for individual non-overlapping sections from individual LA (A, B, C, D, E, F). Two bars are presented for each optical section analysed. The shaded (left) bar in each pair is the observed mean nearest-neighbour distance ±1 SD between either Vβ2-positive (a) or Vβ8-positive (b) cells. The unshaded (right) bar in each pair is the predicted nearest-neighbour distance ±the predicted SD for a random distribution. Clustering of cells would be indicated by a reduction in the mean nearest-neighbour distance compared to that predicted for a random distribution. Similar results were seen in the other nine endometria analysed.
Discussion
In this study we show that LA CD8+ T cells are predominantly CD45RO+, divide infrequently and are apparently randomly dispersed within the LA. Using antibodies specific for two relatively common Vβ families we have shown that approximately equal numbers of randomly distributed CD8+ T cells, expressing these TCR, are present in different areas of the same LA. These frequencies were similar both within the same EM and in endometria from different patients. Taken together, this is evidence for the immigration of CD8+ T cells into LA from the circulation and/or stroma during progression of the proliferative phase of the menstrual cycle and not the clonal expansion of a limited number of cells within LA.
T cells in LA could originate in at least two ways. One possibility is that limited numbers of CD8+ T cells migrate and proliferate to form aggregates within endometrial sites. The other is that T cells gradually accumulate by trafficking into localized regions within the stratum basalis during the proliferative phase. The results of the present study support the latter mechanism. The distribution of CD8+ T cells expressing either Vβ2 (Fig. 3a) or Vβ8 (Fig. 3b) TCRs was random, as determined by nearest-neighbour analysis. If LA arose by division of a limited number of precursors, we would expect to see higher variability in Vβ frequencies between LA. In fact, we found similar frequencies of Vβ2 and Vβ8 in LA in the same EM and in endometrial LA from several different patients. The frequencies of these two Vβ families are consistent with those reported for CD8+ T cells in the peripheral blood.8 We observed very low levels of proliferation, as determined by staining for Ki-67, and the lack of clustering suggests that if the CD8+ T cells are dividing then it is limited to a few divisions only. These proliferative rates in uterine LA are consistent with the published studies of Tabibzadeh.3 It is possible that the extensive growth and remodelling apparent during the proliferative phase of the menstrual cycle could cause cell ‘mixing’, leading to dispersal of T cells throughout the LA. The highly structured arrangement of leucocytes within the LA makes this an unlikely scenario.
In contrast to studies that utilized one-colour immunohistochemistry and show human leucocyte antigen (HLA)-DR expression by LA cells,9 we have shown (by triple staining) little expression of HLA-DR by LA CD8+ T cells. However, high levels of HLA-DR expression by LA B cells and macrophages have been found previously.2 In addition, expression of the early activation marker CD69 has been detected on some LA and stromal CD8+ T cells (G. R. Yeaman, unpublished; ref. 10). The expression of CD69 may be the result of endothelial contact during migration, as has been suggested for the accumulation of CD69+ cells in rheumatoid arthritic joints.11 Taken together with our data showing a high frequency of CD45RO-expressing cells, these findings indicate that the majority of LA T cells are of an activated memory T-cell population.
It is unclear at present what factors are responsible for the specific trafficking of CD8+ T cells. Our preliminary data suggest that these cells are CCR5+. One of the ligands for this receptor is macrophage inflammatory protein-1α (MIP-1α), which has been shown to be responsible, in a murine graft-versus-host disease model, for infiltration of CD8+ T cells into the liver.12 Furthermore, MIP-1α is known to attract CD8+, rather than CD4+, memory T cells.13 The presence of CCR5 and lack of CXCR4 (also shown in our preliminary studies) indicates that these cells could be Tc2 cells, as defined by Dutton et al.14 Studies by others on the immigration of T cells into trauma-induced skin blisters have shown the random accumulation of CD45RO+ T cells.15 Blood vessels within the LA have been shown to express the appropriate adhesion molecules for transmigration of these CD8+ T cells.16,17
Our previous studies have shown that the size of the LA within the stratum basalis of the EM increases with progression of the menstrual cycle, reaching a maximum by the end of the proliferative phase of the cycle.2 Taken together with the absence of LA in postmenopausal endometria, these data indicate that the development of LA is hormonally influenced. Whether this (presumably) oestradiol effect is mediated by a direct or indirect control of chemokine production or adhesion molecule expression, or both, remains to be determined. Our hypothesis therefore is that memory CD8+ T cells begin to accumulate in small numbers at the start of the menstrual cycle by trafficking from the circulation. The initial stimulus for this accumulation is unclear, but could be via chemokines (e.g. MIP-1α) and through the expression of endothelial adhesion molecules, e.g. platelet cell adhesion molecule-1 (PECAM-1) and intracellular adhesion molecule-1 (ICAM-1),18–20 still expressed at high levels in some pockets following production of high levels of tumour necrosis factor-α (TNF-α) during the secretory-phase cycle of the previous menstrual cycle.21–25 The continuous trafficking of CD8+ T cells into the LA would be caused by cytokines produced by other cells, e.g. B cells that are found within the LA. The low level of proliferation seen within the LA is probably a result of the antigen-independent turnover of memory cells.26,27 Little apoptosis is found to occur in proliferative-phase endometria which, together with continuous immigration, results in a progressive increase in size of the LA.2 Maintenance of CD8+ T-cell viability may result from interaction with extracellular matrix components or stromal fibroblasts.28
The function of LA and the CD8+ T cells within them is unknown. Previous studies by our group have shown that endometrial CD8+ T cells have inducible cytolytic activity during the proliferative phase of the cycle, which is absent during the secretory phase.4 However, that mature CTL are present in the EM during the proliferative phase of the cycle is unlikely as they contain little or no perforin (G. R. Yeaman, unpublished; ref. 29). Alternatively, this CD8+ memory T-cell population could be a regulatory subset. By analogy with the graft-versus-host models described above, these cells could be involved in preventing CTL-mediated destruction of a semiallogeneic conceptus following implantation. This potential mechanism is currently under investigation. Further studies on the expression of specific addressins and chemokines mediating the recruitment of these CD8+ T cells will more fully define the origins and functions of the LA T cells.
Acknowledgments
The authors would like to thank Vincent Memoli MD, Jorge Gonzalez MD, Alan Schned MD, Peter Seery and Maryalice Achbach, of the Department of Pathology, for invaluable assistance with tissue retrieval and classification. In addition we would like thank Barry Smith MD, Joan Barthold MD, Misty Blanchette-Porter MD, Jackson Beecham MD, John Currie MD, Leslie Demars MD, Paul Hanissian MD, John Ketterer MD, Benjamin Mahlab MD, Paul Manganiello MD, Eric Sailer MD, Susan Steffan MD and William Young MD, of the Department of Obstetrics and Gynecology, and operating room nurses Jeannette Sawyer, Tracy Stokes, Fran Reinfrank and Jaclyn Logren. We also thank Karen Carter, Kris Ramsey, Tamara Krivit and Laura Wolf for clinical support. Confocal-scanning laser microscopy was performed in the Herbert C. Englert Cell Analysis Laboratory, which was established with a grant from the Fannie E. Rippel Foundation, and is supported in part by the core grant of the Norris Cotton Cancer Center (CA-23108). The research reported here is supported by National Institutes of Health Program Project grant (AI34478) to Dr Charles R. Wira.
References
- 1.Givan AL, White HD, Stern JE, Colby E, Gosselin EJ, Guyre PM, Wira CR. Flow cytometric analysis of leukocytes in the human female reproductive tract: comparison of fallopian tube, uterus, cervix, and vagina. Am J Reprod Immunol. 1997;38:350. doi: 10.1111/j.1600-0897.1997.tb00311.x. [DOI] [PubMed] [Google Scholar]
- 2.Yeaman GR, Guyre PM, Fanger MW, et al. Unique CD8+ T cell-rich lymphoid aggregates in human uterine endometrium. J Leukoc Biol. 1997;61:427. [PubMed] [Google Scholar]
- 3.Tabibzadeh S. Proliferative activity of lymphoid cells in human endometrium throughout the menstrual cycle. J Clin Endocrinol Metab. 1990;70:437. doi: 10.1210/jcem-70-2-437. [DOI] [PubMed] [Google Scholar]
- 4.White HD, Crassi KM, Givan AL, Stern JE, Gonzalez JL, Memoli VA, Green WR, Wira CR. CD3+ CD8+ CTL activity within the human female reproductive tract: influence of stage of the menstrual cycle and menopause. J Immunol. 1997;15:3017. [PubMed] [Google Scholar]
- 5.Gerdes J, Lemke H, Baisch H, Wacker HH, Schwab U, Stein H. Cell cycle analysis of a cell proliferation-associated human nuclear antigen defined by the monoclonal antibody Ki-67. J Immunol. 1984;133:1710. [PubMed] [Google Scholar]
- 6.Noyes RW, Hertig AT. Dating the endometrial biopsy. Fertil Steril. 1955;1:3. doi: 10.1016/j.fertnstert.2019.08.079. [DOI] [PubMed] [Google Scholar]
- 7.Russ JC, Dehoff RT. Practical Stereology. 2. New York: Plenum Press; 2001. [Google Scholar]
- 8.Clarke GR, Humphrey CA, Lancaster FC, Boylston AW. The human T cell antigen receptor repertoire: skewed use of V beta gene families by CD8+ T cells. Clin Exp Immunol. 1994;96:364. doi: 10.1111/j.1365-2249.1994.tb06568.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tabibzadeh S. Evidence of T-cell activation and potential cytokine action in human endometrium. J Clin Endocrinol Metab. 1990;71:645. doi: 10.1210/jcem-71-3-645. [DOI] [PubMed] [Google Scholar]
- 10.Chen CK, Huang SC, Chen CL, Yen MR, Hsu HC, Ho HN. Increased expressions of CD69 and HLA-DR but not of CD25 or CD71 on endometrial T lymphocytes of nonpregnant women. Hum Immunol. 1995;42:227. doi: 10.1016/0198-8859(94)00105-y. 10.1016/0198-8859(94)00105-y. [DOI] [PubMed] [Google Scholar]
- 11.Iannone F, Corrigall VM, Kingsley GH, Panayi GS. Evidence for the continuous recruitment and activation of T cells into the joints of patients with rheumatoid arthritis. Eur J Immunol. 1994;24:2706. doi: 10.1002/eji.1830241120. [DOI] [PubMed] [Google Scholar]
- 12.Murai M, Yoneyama H, Harada A, et al. Active participation of CCR5 (+) CD8 (+) T lymphocytes in the pathogenesis of liver injury in graft-versus-host disease. J Clin Invest. 1999;104:49. doi: 10.1172/JCI6642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Taub DD, Conlon K, Lloyd AR, Oppenheim JJ, Kelvin DJ. Preferential migration of activated CD4+ and CD8+ T cells in response to MIP-1 alpha and MIP-1 beta. Science. 1993;260:355. doi: 10.1126/science.7682337. [DOI] [PubMed] [Google Scholar]
- 14.Cerwenka A, Morgan TM, Harmsen AG, Dutton RW. Migration kinetics and final destination of type 1 and type 2, CD8 effector cells predict protection against pulmonary virus infection. J Exp Med. 1999;189:423. doi: 10.1084/jem.189.2.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pitzalis C, Kingsley GH, Covelli M, Meliconi R, Markey A, Panayi GS. Selective migration of the human helper-inducer memory T cell subset: confirmation by in vivo cellular kinetic studies. Eur J Immunol. 1991;21:369. doi: 10.1002/eji.1830210218. [DOI] [PubMed] [Google Scholar]
- 16.Tabibzadeh S, Kong QF, Babaknia A. Expression of adhesion molecules in human endometrial vasculature throughout the menstrual cycle. J Clin Endocrinol Metab. 1994;79:1024. doi: 10.1210/jcem.79.4.7962270. [DOI] [PubMed] [Google Scholar]
- 17.Tabibzadeh SS, Poubouridis D. Expression of leukocyte adhesion molecules in human endometrium. Am J Clin Pathol. 1990;93:183. doi: 10.1093/ajcp/93.2.183. [DOI] [PubMed] [Google Scholar]
- 18.Kelly FD, Tawia SA, Rogers PA. Immunohistochemical characterization of human endometrial microvascular basement membrane components during the normal menstrual cycle. Hum Reprod. 1995;10:268. doi: 10.1093/oxfordjournals.humrep.a135927. [DOI] [PubMed] [Google Scholar]
- 19.Tawia SA, Beaton LA, Rogers PA. Immunolocalization of the cellular adhesion molecules, intercellular adhesion molecule-1 (ICAM-1) and platelet endothelial cell adhesion molecule (PECAM), in human endometrium throughout the menstrual cycle. Hum Reprod. 1993;8:175. [PubMed] [Google Scholar]
- 20.Iruela-Arispe ML, Rodriguez-Manzaneque JC, Abu-Jawdeh G. Endometrial endothelial cells express estrogen and progesterone receptors and exhibit a tissue specific response to angiogenic growth factors. Microcirculation. 1999;6:127. [PubMed] [Google Scholar]
- 21.Tabibzadeh S, Satyaswaroop PG, von Wolff M, Strowitzki T. Regulation of TNF-alpha mRNA expression in endometrial cells by TNF-alpha and by oestrogen withdrawal. Mol Hum Reprod. 1999;5:1141. doi: 10.1093/molehr/5.12.1141. 10.1093/molehr/5.12.1141. [DOI] [PubMed] [Google Scholar]
- 22.Tabibzadeh S, Zupi E, Babaknia A, Liu R, Marconi D, Romanini C. Site and menstrual cycle-dependent expression of proteins of the tumour necrosis factor (TNF) receptor family, and BCL-2 oncoprotein and phase-specific production of TNF alpha in human endometrium. Hum Reprod. 1995;10:277. doi: 10.1093/oxfordjournals.humrep.a135928. [DOI] [PubMed] [Google Scholar]
- 23.Garcia FU, Chen HL, Yang Y, Pace JL, Hu XL, Hunt JS. Tumor necrosis factor-alpha mRNA and protein in endometrial tumors: analysis by in situ hybridization and immunocytochemistry. Hum Pathol. 1994;25:1324. doi: 10.1016/0046-8177(94)90093-0. [DOI] [PubMed] [Google Scholar]
- 24.Hunt JS, Chen HL, Hu XL, Tabibzadeh S. Tumor necrosis factor-alpha messenger ribonucleic acid and protein in human endometrium. Biol Reprod. 1992;47:141. doi: 10.1095/biolreprod47.1.141. [DOI] [PubMed] [Google Scholar]
- 25.Tabibzadeh S. Ubiquitous expression of TNF-alpha/cachectin immunoreactivity in human endometrium. Am J Reprod Immunol. 1991;26:1. doi: 10.1111/j.1600-0897.1991.tb00692.x. [DOI] [PubMed] [Google Scholar]
- 26.Rocha B, Dautigny N, Pereira P. Peripheral T lymphocytes: expansion potential and homeostatic regulation of pool sizes and CD4/CD8 ratios in vivo. Eur J Immunol. 1989;19:905. doi: 10.1002/eji.1830190518. [DOI] [PubMed] [Google Scholar]
- 27.Bruno L, von Boehmer H, Kirberg J. Cell division in the compartment of naive and memory T lymphocytes. Eur J Immunol. 1996;26:3179. doi: 10.1002/eji.1830261251. [DOI] [PubMed] [Google Scholar]
- 28.Rich S, Van Nood N, Lee HM. Role of alpha 5 beta 1 integrin in TGF-beta 1-costimulated CD8+ T cell growth and apoptosis. J Immunol. 1996;157:2916. [PubMed] [Google Scholar]
- 29.Hameed A, Fox WM, Kurman RJ, Hruban RH, Podack ER. Perforin expression in endometrium during the menstrual cycle. Int J Gynecol Pathol. 1995;14:143. doi: 10.1097/00004347-199504000-00008. [DOI] [PubMed] [Google Scholar]