Abstract
The invariant chain (Ii) plays a key role in regulating the antigen presentation function of major histocompatibility complex (MHC) class II molecules. Ii also influences the presentation of usually excluded endogenously synthesized proteins into the MHC class II presentation pathway. To evaluate the role of Ii in the generation of peptide–MHC class II complexes derived from endogenously synthesized proteins, we tested mutant Ii constructs in two model systems. Co-expression of wild-type Ii inhibits the presentation of hen-egg lysozyme (HEL) 35–45/Ak complex, but enhances the presentation of ovalbumin (OVA) 247–265/Ak complex from endogenously synthesized HEL or OVA precursors. The differential sensitivity of these antigens to chloroquine was consistent with their being processed in distinct compartments. Nevertheless, with a panel of Ii deletion constructs we show here that both the Ii-mediated inhibition and enhancement functions require the endosomal targeting and CLIP residues. Surprisingly, the Ii mutant lacking the endoplasmic reticulum lumenal residues 126–215, despite apparently lower expression, was at least as effective as full-length Ii in antigen presentation assays. Thus, alternative pathways exist for processing endogenously expressed antigens, and Ii-mediated inhibition and enhancement of peptide/MHC class II expression depend upon the same regions, with neither requiring the 89 C-terminal, lumenal Ii residues.
Introduction
The major histocompatibility complex (MHC) class II-associated invariant chain (Ii), a non-polymorphic Type II membrane glycoprotein, plays a key role in the MHC class II antigen presentation pathway. Upon synthesis in the endoplasmic reticulum (ER), it forms a homotrimeric complex, that serves as a scaffold for the binding of newly assembled MHC class II heterodimers.1,2 Three major functions have been ascribed to the Ii in the MHC class II pathway: (a) facilitating the proper folding of the MHC class II dimer and egress from the ER,3–5 (b) targeting of class II molecules to the endosomal pathway,6,7 and (c) inhibition of peptide binding en route to the endosomal compartments.8,9 In the endosomal compartments, the C-terminal (lumenal) and N-terminal (cytoplasmic) residues are proteolytically degraded in stepwise fashion, leaving progressively smaller fragments of the invariant chain associated with the class II molecules.10–12 The release of the final degradation product, the CLass II-associated Invariant chain Peptide (CLIP) fragment, is facilitated by the class II-like H-2M [human leucocyte antigen (HLA)-DM in human] molecule, allowing the binding of antigenic peptides.13–17
Studies in both cell lines18–24 and mice4,5,25,26 have demonstrated the importance of the invariant chain in MHC class II antigen presentation. However, the mechanisms underlying the invariant chain's effects on generation of individual peptide–MHC class II complexes remain controversial. For example, Ii is often described as responsible for the exclusion of endogenously expressed antigens from the MHC class II pathway,9,27,28 but it is becoming increasingly clear that this is not always true, and that the invariant chain is in fact required for the efficient presentation of certain endogenous epitopes.23,26,29 Additionally, presentation of epitopes within the same protein may be affected differently by the expression of the invariant chain.24,26 Taken together, these studies indicate that the Ii plays an important role in defining the repertoire of MHC–peptide complexes, increasing the presentation of some peptides while decreasing the presentation of others. The fact that different regions of the Ii are required for self-association/MHC binding, intracellular transport and proteolysis in the endosomes, raises the possibility that differential effects of the Ii on generation of peptide–MHC II complexes may require distinct Ii domains. For example, for the epitopes that are inhibited in presence of Ii because of MHC–Ii binding in the ER, the Ii endosomal targeting signal would be dispensable because absence of this signal does not affect MHC–Ii association.29,30
The regulation of endogenous antigen processing and presentation is likely to have significant implications for the normal function of the immune system. The bulk of peptides extracted from class II molecules of antigen-presenting cells (APC) appear to be derived from endogenously expressed proteins from essentially all cellular compartments;31–37 thus, presentation of endogenous antigens may be involved in initiating immune responses to viral infection or autoimmune responses. Furthermore, endogenous presentation has been found to play a role in thymic T-cell selection.38 In order to define the role of Ii in presentation of endogenously synthesized antigens by MHC class II, we undertook a functional analysis of Ii deletion mutants. Using cells transiently transfected with the Ak MHC class II, and chicken ovalbumin (OVA) or hen-egg lysozyme (HEL) as model antigens, we compared full-length and truncated forms of the murine invariant chain for their influence on presentation of either exogenously added or endogenously expressed antigens to CD4+ T cells. We show that Ii can function as an enhancer or inhibitor for presentation of specific OVA and HEL/Ak complexes, but both functions require the same N-terminal endosomal targeting and the MHC binding CLIP residues of the Ii. Interestingly, the 89 C-terminal lumenal residues of Ii were dispensable for Ii function.
Materials and methods
Cell lines, antibodies and antigens
All cell lines were maintained in RPMI-1640 medium supplemented with 10% fetal bovine serum, 2 mm glutamine, 1 mm pyruvate, 50 µm 2-mercaptoethanol, penicillin (200 U/ml), and streptomycin (200 µg/ml) at 37° in a 5% CO2/95% air atmosphere. The COS-7 (COS) cells, T-cell hybridoma BO4H9 (HEL74–88/Ab-specific) and lacZ inducible T-cell hybrids KZO (OVA247–265/Ak-specific) and KZH (HEL34–45/Ak-specific) have been described.29,39–41 The OVA247–265 and the HEL35–45 peptides recognized by KZO and KZH T cells were identified by purification and microsequencing the active fractions in cyanogen bromide and trypsin digests of OVA and HEL, respectively, and confirmed with synthetic peptides (data not shown). The hybridoma In-1 (anti-Ii)42 was the kind gift of Dr R. N. Germain (National Institutes of Health, Bethesda, MD). The hybridomas 10.2.16 (anti-Ak) and W6/32 (anti-pan-HLA) were from the American Type Culture Collection (Rockville, MD). The anti-KEPL antibody (anti-Ii) was the kind gift of Dr V. Quaranta (Scripps Institute, La Jolla, CA). Horseradish peroxidase-conjugated anti-rat and anti-rabbit antibodies were purchased from Amersham (Arlington Heights, IL). The HEL and the OVA were purchased from Sigma (St Louis, MO) and Worthington (Freehold, NJ), respectively. The CNBr digest of HEL was prepared as described.43 Synthetic OVA247–265 peptide (Pro-Asp-Glu-Val-Ser-Gly-Leu-Glu-Gln-Leu-Glu-Ser-Ile-Ile-Asn-Phe-Glu-Lys-Leu) was prepared by the Microchemical Facility at UC Berkeley.
Plasmid DNA constructs
The Akα, Akβ, Ii and OVA cDNA constructs have been described.29 For the Δ22-Ii construct, the 300-base-pair EcoRI/HindIII fragment (containing the N-terminal 80 residues) of Ii was subcloned into the EcoRI/HindIII sites of the pSP72 vector to yield the pSP72–I80 intermediate. The 22 N-terminal residues of Ii were deleted by digesting pSP72-I80 with NcoI/SacI and ligating to oligomers 5′ CATGGAGCCAGAAAGGTGCTCCCGGGGAGCT 3′, 3′ CTCGGTCTTTCCACGAGGGCCCC 5′ to yield (Met-Glu-Pro-Glu-Arg-Cys-Ala-Arg-Gly-Ala … as the N-terminal residues) the pSP72-Δ22I80 intermediate. The 2·8-kilobase (kb) SacI/XhoI fragment of pSP72-Δ22I80 containing Ii residues 22–80 was ligated to the SacI/XhoI fragment encoding Ii residues 81–215. The 1·3-kb EcoRI/XhoI fragment encoding Ii residues 23–215 was then subcloned into the EcoRI/XhoI sites of pcDNAI vector. The full-length HEL cDNA (kind gift of Dr J. Kirsch, UC Berkeley) was subcloned into the HindIII/EcoRV sites of pcDNAI (Invitrogen, Carlsbad, CA). The cgIi, Δ81–127-Ii, Δ110–161-Ii, Δ110–130-Ii, Δ192–212-Ii and Δ126–215-Ii constructs (kind gifts of Dr N. Koch, Institut für Zoologie, Bonn, Germany) are fusions between Ii cDNA and genomic sequences and have been described.44 To ensure comparable transcription, these constructs were subcloned into the EcoRI site of pcDNAI. All plasmid DNAs were CsCl purified.
Transient transfections
The diethylaminoethyl (DEAE)-dextran transfection protocol has been described.45 Briefly, COS cells were plated out in 96-well plates with 3 × 104 cells per well or in six-well plates with 0·5 × 106−1 × 106 cells in transfection medium containing RPMI-1640/10% NuSerum (Collaborative Biomedical Products, Bedford, MA), 100 µg/ml DEAE-dextran, 100 µm chloroquine diphosphate, the indicated quantity of cDNAs for the antigens, 0·15 µg/ml MHC class II Akα, Akβ cDNAs, and with or without 0·2–0·5 µg/ml Ii DNA. After 2 hr at 37°, the transfection medium was removed and cells were incubated for 2 min with phosphate-buffered saline (PBS)/10% dimethyl sulphoxide and then placed in RPMI/10% fetal calf serum. After 48 hr the cells were assayed for T-cell stimulation in the same plates or used for protein analysis.
Chloroquine treatment
For endogenous antigens, 150 µm chloroquine was added to cells 16 hr after transfection. Cells were cultured for an additional 6–8 hr, medium was replaced and then the cells were assayed for T-cell stimulation. For exogenous antigens, 150 µm chloroquine was added together with antigen 2 days after transfection and the cells were cultured for an additional 6–8 hr. Medium was replaced and presentation to T cells was measured.
Western blot analysis
COS cells were transfected with 0·2 µg/ml of the Ii constructs in six-well plates. Two days later the cells were lysed in 0·5% nonidet P-40 (NP-40), 20 mm Tris–HCl (pH 8·0), 200 mm LiCl and 1 mm ethylenediaminetetraacetic acid and cell debris was removed by centrifugation. Lysates were resolved by 15% sodium dodecyl sulphate–polyacrylamide gel electrophoresis (SDS–PAGE) and transferred to nitrocellulose membrane. The membrane was probed with α-Ii antibody (In-1 or α-KEPL), followed by horseradish peroxidase-conjugated secondary antibody (anti-rat or anti-rabbit), and the bands were visualized using the Enhanced Chemiluminiscence kit (Amersham). Each lane represents ∼6 × 104 cell equivalents.
Immunoprecipitation analysis
COS cells were transfected with 0·15 µg/ml each of the Akα and Akβ cDNAs along with the 0·4 µg/ml of the indicated Ii constructs. Two days later the cells were washed with PBS and incubated at 37° for 30 min in prewarmed methionine-free medium. The medium was replaced with methionine-free medium containing 200 µCi/ml of 35S Expre35S35S (NEN/DuPont, Boston, MA) amino acids and the cells were incubated for another 4 hr. Cells were lysed in 0·5% NP-40, 10 mm Tris–HCl (pH 8·0), 140 mm NaCl, 1 mm phenylmethylsulphonyl fluoride (PMSF), 0·22 trypsin inhibitory units (TIU)/ml aprotinin, 5 mm iodoacetamide and 0·03% sodium azide, and cell debris was removed by centrifugation. Lysates were precleared overnight at 4° with normal rabbit serum and protein A–Sepharose (Sigma). Lysates were then divided into aliquots that were immunoprecipitated separately with the indicated antibodies and protein A–Sepharose at 4° for 1 hr. Sepharose-bound proteins were eluted by boiling in sample buffer containing 125 mm Tris–HCl (pH 6·8), 20% glycerol, 4% SDS and 0·7 m β-mercaptoethanol and analysed by 15% SDS–PAGE. Gels were fixed in 25% isopropanol/10% acetic acid and soaked in Amplify (Amersham) +4% glycerol, dried and visualized by autoradiography. Each lane represents ∼1·5 × 105 cell equivalents.
Trimerization of Ii
COS cells were transfected and labelled as above, and lysed on ice in PBS containing 0·5% NP-40, 1 mm PMSF, 0·22 TIU/ml aprotinin, 5 mm iodoacetamide and 0·2 mg/ml dithiobis (succinimidyl propionate) (DSP, Pierce, Rockford, IL). The cross-linker was quenched with 1/10 volume of 1 m lysine, and cell debris and aggregates were removed by centrifugation. Lysates were precleared and immunoprecipitated as above. Proteins were eluted with reducing (+ 0·7 m β-mercaptoethanol) or non-reducing (no β-mercaptoethanol) sample buffer.
T-cell activation assay
Antigen-specific T-cell response was assayed by measurement of the induced lacZ activity as described.41 After overnight co-culture of 1 × 105 T cells with appropriate transfected APC, in medium alone or in presence of exogenous antigens, the cells were washed once with 100 µl PBS/well. The lacZ activity in the T cells was assayed by incubating the cells in 100 µl 0·15 mm chlorophenol red β-galactopyranoside (CPRG) in PBS and 0·15% NP-40 as described.40 The reaction product generated was quantified by its absorbance at 595 nm with 655 nm as the reference wavelength. Data are presented as the averages of replicate wells, and are representative of at least two independent experiments. Error bars denote standard deviations from the mean.
Results and discussion
We chose simian COS cells for analysis of the MHC class II antigen presentation pathway because they do not express endogenous MHC class II or invariant chain (Ii).46,47 Furthermore, several studies from our own and other laboratories have shown that, like conventional APC and commonly used fibroblast cell lines, COS cells can also generate antigen–MHC class I or class II complexes that stimulate murine CD8+ or CD4+ T-cell hybrids,29,41,45,48,49 thus allowing a functional analysis of the endogenous antigen presentation pathways. Most importantly, because a large fraction of COS cells expresses transfected DNAs, their use as a model obviates problems associated with deriving and interpreting the results obtained from individual stable transfectants.
Expression of Ii constructs
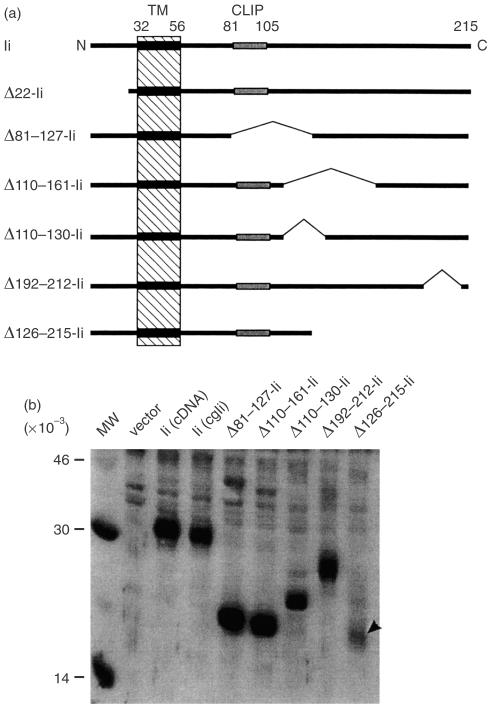
We analysed deletion mutants of Ii to delineate the structural features relevant to its function in the presentation of endogenous antigens on class II MHC. A series of Ii expression constructs deleted (Δresidues – Ii) for either lumenal or cytoplasmic residues are shown in Fig. 1(a). These deletions were chosen because previous studies had shown that the N-terminal cytoplasmic residues were required for targeting the Ii to the endosomal compartment,6,7 and the CLIP region (amino acids 81–105) was required for the Ii association with MHC class II molecules.44,49,50 Furthermore, it has also been shown that deletions in the lumenal region of Ii abrogate its function in permitting the presentation of exogenous antigens.51 As determined by Western blot analysis with monoclonal antibody (mAb) In-1 (Fig. 1b) as well as a polyclonal rabbit anti-Ii antiserum (data not shown), all the constructs were expressed in COS cells. Additionally, with the exception of Δ126–215-Ii (arrowhead), which was expressed reproducibly at a lower level, all the Ii mutants were expressed at a comparable level as single polypeptides of expected molecular weights. Initial tests in antigen presentation assays indicated that the Δ110–130, Δ110–161 and Δ192–212 mutations had little or no effect on antigen presentation (data not shown); we therefore chose to focus further study on the Δ22, Δ81–127 and Δ126–215 mutants.
Figure 1.
(a) Schematic representation of Ii-deletion constructs. Numbers refer to the amino acid sequence of the murine p31 invariant chain. TM, transmembrane region; CLIP, Class II MHC-Associated Invariant Chain Peptide. (b) Expression of Ii, deletion proteins. ‘cgIi’, the parent construct for all of the cytoplasmic deletions, is a fusion between the Ii cDNA and genomic sequences. COS cells were transiently transfected with the indicated constructs and lysed after 2 days. Lysates were resolved by SDS–PAGE, blotted to nitrocellulose, and probed with mAb In-1. The arrowhead indicates the position of the Δ126–215-Ii band. MW, molecular weight standards.
Association of Ii mutants with Ak MHC class II molecules
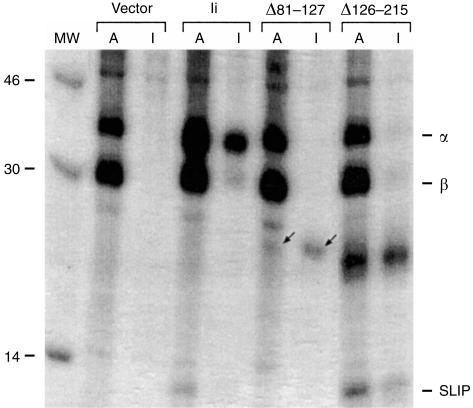
The ability of Ii to associate intracellularly with newly synthesized class II molecules plays a key role in antigen presentation.49,52 To confirm and to extend these findings in our model system, we co-transfected cells with the Aαk and Aβk constructs together with the wild-type Ii or the deletion constructs. To identify the associated proteins, lysates of metabolically labelled transfectants were immunoprecipitated with either anti-Ak (A) or anti-Ii (I) antibodies and run in adjacent lanes (Fig. 2). Immunoprecipitation with anti-Ak mAb showed that both the Aαk (∼37 kDa) and the Aβk (∼30 kDa) chains were present in all transfectants. In cells expressing Ak and the wild-type Ii, anti-Ak antibodies co-precipitated the Ii, seen as a 35-kDa band, which was also precipitated with the anti-Ii antibody. An additional ∼12 kDa band (SLIP peptide, a degradation intermediate of Ii) also co-precipitated with both anti-Ak and anti-Ii antibodies. We have previously shown that deletion of the N-terminal 22 residues of Ii had no detectable effect on association with Ak.29,30
Figure 2.
Ii deletion proteins associate intracellularly with Ak MHC class II molecules. (a) COS cells were transiently transfected with Akα, Akβ, and the indicated construct (see Fig. 1), and after 2 days were metabolically labelled with 35S-labelled methionine. Cells were lysed and divided into aliquots that were precipitated with mAb 10.2.16 (α-Ak) or mAb In-1 (α-Ii), indicated as A or I above each lane. Immunoprecipitates were run in parallel on a 15% SDS–PAGE gel and visualized by autoradiography. The specific bands corresponding to Δ81–127-Ii are indicated by arrows. The positions of Akα and Akβ subunits are labelled as α and β in the right margin. The ∼12 000 MW band corresponding to a degradation product (SLIP peptide) of the invariant chain is also indicated in the right margin. Lane labelled MW contains molecular weight standards, with the weights shown in the left margin.
Association of the Ak molecule with the Ii mutants was profoundly influenced by deletions in the lumenal region. The Δ126–215-Ii, which was expressed poorly as judged by Western blots, appeared as a clear band in both anti-Ak and anti-Ii immunoprecipitates (Fig. 2), indicating its strong association with Ak MHC. The SLIP fragment was also detectable in this sample. Interestingly, the amount of the SLIP fragment in this sample was significantly higher than that in the full-length Ii, indicating that processing of Δ126–215-Ii in the endosomal compartments may be more efficient than that of the wild-type Ii. To examine this possibility we compared the degradation rates of full-length Ii and Δ126–215-Ii by pulse-chase analysis and found that Δ126–215-Ii does indeed have a somewhat shorter half-life (∼2 hr for full-length, ∼1·25 hr for Δ126–215; data not shown). Surprisingly, the Δ81–127-Ii protein, which lacks all residues defined as the MHC class II-associated invariant chain peptide (CLIP, Fig. 1a), also retained detectable, but clearly reduced, association with Ak, as judged by a weak band at 24 000 MW, present in both anti-Ak and anti-Ii immunoprecipitates (Fig. 2, arrows). The low signal for Δ81–127-Ii in immunoprecipitates, despite good expression (see Fig. 1b), is likely due to the deletion of a methionine-rich region (8 of 14 Ii methionines). Our ability to detect association of Ii lacking the CLIP peptide, here and in previous studies with Ii-antigen fusion proteins,29 is in contrast to earlier reports that showed that deletion of the CLIP region completely ablated MHC class II-association,44,50 although others have found small amounts of association between class II molecules and CLIP-deleted Ii using milder lysis conditions.49 The reason for this discrepancy is not clear, but the residual association of Δ81–127-Ii indicates the presence of another Ak-binding region(s) in the invariant chain which can partially compensate for the loss of CLIP.53 Previous studies used different MHC class II alleles (I-E/DR3,44 DR1,50 I-Ab49), and some49,50 used Ii truncations lacking C-terminal residues, and could therefore have failed to detect additional MHC-binding sites, especially if they are MHC allele-specific. Thus, although association of Ak MHC with Ii depends predominantly upon the presence of the lumenal CLIP residues, as also observed for DR1, DR3, I-E and I-Ab MHC molecules, there appear to be one or more additional regions involved as well. The non-CLIP residues of the Ii responsible for the residual Ak association with Δ81–127-Ii remain to be defined.
Function of Ii mutants in MHC class II presentation
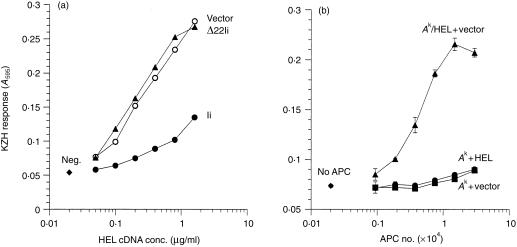
In order to examine the functional consequences of the deletions, we tested the effects of the various Ii constructs on the presentation of endogenously synthesized HEL and OVA to T-cell hybridomas by COS cells. As shown in Fig. 3(a), coexpression of HEL cDNA in Ak-expressing COS cells allowed efficient presentation of the HEL34–45/Ak epitope to KZH cells. However, coexpression of the wild-type Ii strongly inhibited this presentation, consistent with the notion that the Ii prevents the presentation of endogenously expressed antigens. Interestingly, deletion of the 22 N-terminal residues of Ii (Δ22-Ii), which removes the endosomal localization signal,6,7 completely eliminated the ability of Ii to inhibit presentation, indicating that the endosomal targeting function of the Ii is essential for this function. Furthermore, since Δ22-Ii binds to Ak as well as full-length Ii,29 this result shows that association with Ii in the ER is insufficient to inhibit MHC class II presentation of an endogenous antigen. To ensure that the presentation was via an internal route, rather than by secretion and subsequent uptake of HEL as an exogenous antigen, we mixed cells that had been separately transfected with Ak or HEL. Data shown in Fig. 3(b) confirm that only cells that were co-transfected with both Ak and HEL, but not mixtures of cells expressing HEL and Ak separately, were able to generate the HEL/Ak complex for stimulating KZH T cells.
Figure 3.
(a) Invariant chain inhibits presentation of endogenous HEL on MHC class II. COS cells were transiently transfected with cDNAs for Akα, Akβ and HEL, and either vector, Ii, or Δ22-Ii constructs. After 2 days, 1 × 105 KZH T cells were added to each well, and their activation was assayed by induced lacZ activity. Data are representative of five independent experiments. neg., no HEL cDNA. (b) Presentation of endogenous HEL on Ak requires coexpression of antigen and MHC class II in the same cell. Cells were transfected with DNAs for Akα + Akβ alone (‘Ak’), HEL alone (‘HEL’), pcDNAI alone (‘vector’), or Ak + HEL (‘Ak/HEL’), and mixed in equal numbers as indicated. Two days after transfection, KZH cells were added, and activation was determined as in (a). ‘APC Number’ indicates the total number of COS cells in each well. Data are representative of three independent experiments.
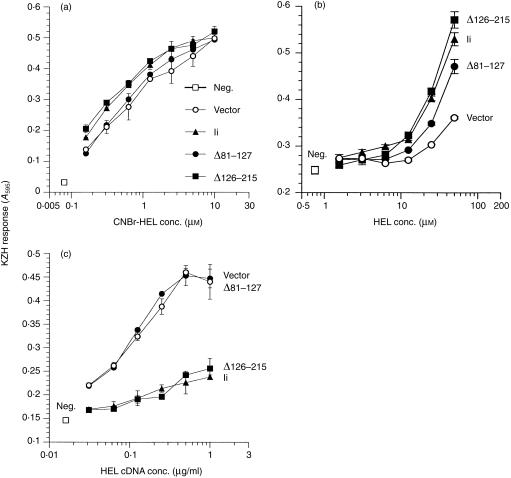
Next we tested the ability of the lumenal Ii deletions on the generation of HEL34–45/Ak. None of the constructs, including wild-type Ii, affected the presentation of exogenously added peptides in CNBr-HEL (Fig. 4a). Thus, coexpression of these Ii constructs did not influence the levels of surface expression or the functional capacity of the Ak MHC molecules. The equivalence of Ak surface levels was confirmed by flow cytometry (data not shown). Expression of intact Ii enhanced presentation of native HEL added exogenously, and surprisingly, Δ126–215-Ii was equally effective at enhancing presentation of this antigen (Fig. 4b). This is in contrast to published reports that the C-terminal domain is necessary for Ii antigen presentation function.51 The Δ81–127-Ii construct was reduced in the ability to enhance exogenous HEL presentation, consistent with its reduced MHC association. Again, expression of full-length Ii inhibited the presentation of endogenously expressed HEL to KZH (Fig. 4c), and similar to the deletion of the N-terminal cytoplasmic residues, deletion of the CLIP (in Δ81–127-Ii) also caused a failure to inhibit HEL presentation over vector-alone levels (Fig. 4b). The CLIP region was thus indispensable for inhibition of antigen presentation. Interestingly, the Δ126–215-Ii construct also inhibited HEL/Ak presentation at least as well as, and in some experiments even better than, the full-length Ii (Fig. 4b and data not shown). These results, despite the reduced expression of Δ126–215-Ii compared to the wild-type (see Fig. 1b), demonstrate that the C-terminal 89 residues of the invariant chain are not required for Ii function. The opposite effects of Ii on presentation of endogenous and exogenous HEL are probably due to processing of the antigen in different compartments depending on its route of entry into the cell (see below).
Figure 4.
Influence of deletions in the lumenal portion of Ii on presentation of the HEL/Ak complex to T cells. COS cells were transfected with Akα + Akβ plus vector or the indicated Ii construct. In (b) the transfected DNAs also included varying concentration of HEL cDNA. Two days later the transfected cells were tested as APC for stimulating KZH T cells in an overnight culture. Varying concentration of CNBr digest of HEL (a) or intact HEL (b) were added to the transfected cells to assess their exogenous antigen presentation function. Data are representative of two independent experiments. neg., no antigen added. (c) The transfected cells were tested without any further treatment. Data are representative of four independent experiments. neg., no HEL cDNA.
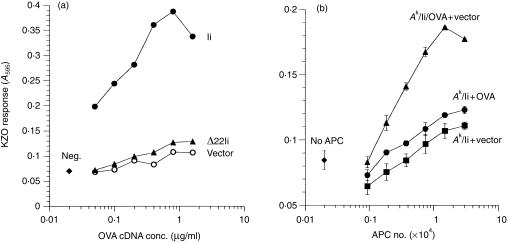
To test further the importance of the various regions of the invariant chain, we examined the effects of the Ii constructs on the presentation of a second antigen, chicken OVA. As found previously,29 and in contrast to presentation of endogenous HEL above, presentation of the OVA247–265 epitope from endogenously synthesized OVA by Ak MHC was obtained only in cells coexpressing the wild-type Ii (Fig. 5a). However, the Δ22-Ii construct, which lacks the endosomal targeting signal, was unable to increase presentation of the OVA/Ak epitope over basal levels, again indicating that the targeting function of the Ii is critical for presentation of endogenous antigens and takes precedence over its ability to associate with MHC class II molecules. Also, as for HEL, we confirmed that presentation of OVA required its synthesis in the same cells that expressed the Ak and Ii, indicating that indeed the endogenous presentation pathway was used by the cells to generate the peptide–MHC complexes (Fig. 5b).
Figure 5.
(a) Invariant chain enhances the presentation of endogenous OVA/Ak complex to T cells. COS cells were transfected with Akα + Akβ and either vector, Ii, or Δ22-Ii plus various concentrations of OVA cDNA. The KZO T cells were added 2 days after transfection, and activation was assayed by induced lacZ activity. Data are representative of five independent experiments. neg., no OVA cDNA. (b) Presentation of endogenous OVA on Ak requires coexpression of the antigen and MHC class II in the same cell. Cells were transfected with Akα + Akβ + Ii (‘Ak/Ii’), OVA alone (‘OVA’), pcDNAI alone (‘vector’), or Ak + Ii + OVA (‘Ak/Ii/OVA’), and mixed in equal numbers as indicated. Two days after transfection, KZO cells were added, and their activation was measured following overnight culture. ‘APC Number’ indicates the total number of COS cells in the wells. Data are representative of three independent experiments.
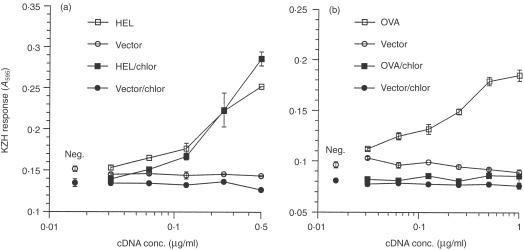
The differences in Ii inhibition/requirement in endogenous presentation of HEL and OVA suggested that the antigens may be processed in different cellular compartments. To address this, we analysed the effects of chloroquine, an inhibitor of endosomal processing, on presentation of endogenously expressed antigens. We determined that 150 µm chloroquine completely blocked presentation of exogenously added HEL and OVA (data not shown) to KZH and KZO T cells, respectively. Culturing OVA-transfected COS cells in 150 µm chloroquine completely inhibited presentation to the KZO T cell (Fig. 6a), consistent with endosomal processing. Surprisingly, presentation of endogenous HEL to the KZH T cell (Fig. 6b), as well as to the BO4H9 T cell (HEL74–88/Ab; data not shown), was unaffected by chloroquine treatment, suggesting that processing occurs in a non-endosomal compartment. Consistent with this finding, redirecting endogenous HEL to the endosomes, by expressing an Ii-HEL fusion protein,29 restored chloroquine sensitivity to both epitopes (data not shown), similar to HEL provided as a soluble extracellular protein. Thus, processing of a single epitope may occur in distinct compartments, with different requirements, depending on the route of entry to the cell (endogenous expression versus extracellular source) or the intracellular targeting of the antigen.
Figure 6.
Endogenous HEL and OVA are processed in different pathways. COS cells were transfected with Ak and varying amount of vector or HEL cDNA (a), or Ak/Ii and varying amount of vector or OVA cDNA (b), and 16 hr later were treated with or without 150 µm chloroquine for 6 hr. Presentation to KZO (a) or KZH (b) T cells was measured by induced lacZ activity. Data for HEL are representative of three independent experiments; data for OVA are representative of two independent experiments. neg., no antigen cDNA.
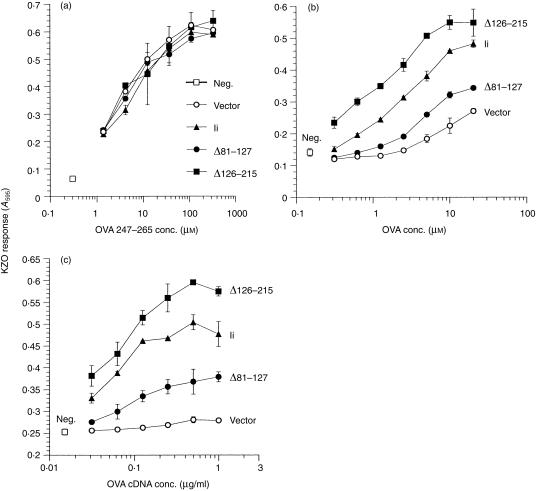
The lumenal deletions had similar effects on Ii function in enhancing the presentation of the OVA epitope as compared to inhibition of presentation of endogenous HEL. While none of the constructs had any effect on the presentation of exogenously added synthetic OVA247–265 peptide (Fig. 7a), we found that the Δ81–127 deletion, which ablated the inhibition of endogenous HEL presentation, now severely reduced the ability of Ii to enhance presentation of OVA247–265 peptide, from either exogenous (Fig. 7b) or endogenous (Fig. 7c) OVA. Remarkably, the Δ126–215-Ii deletion construct now enhanced the presentation of the OVA epitope to a level consistently above that seen for full-length Ii, for both endogenous and exogenous OVA.
Figure 7.
Influence of deletions in the lumenal portion of Ii on presentation of OVA/Ak presentation to T cells. COS cells were transfected with Akα + Akβ plus vector or the indicated Ii construct. Cells were tested for presentation of varying concentration of synthetic OVA247–265 peptide (a), exogenously added native OVA protein (b), or endogenously synthesized OVA (c), to KZO T cells following overnight culture. T-cell activation was measured by induction of lacZ activity. Data in (a) are representative of two independent experiments; (b) and (c) are representative of three independent experiments. neg., no peptide (a), OVA (b), or OVA cDNA (c).
Self-association of Ii mutants
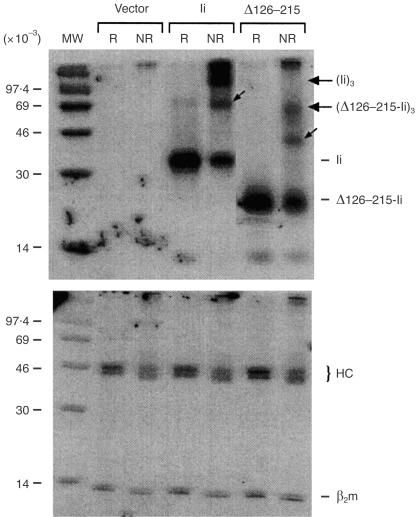
To examine further the function of the Δ126–215-Ii mutant, we tested for self-association,1 since this region was previously implicated in the trimerization of the invariant chain.50,51 COS cells transfected with either full-length Ii or Δ126–215-Ii were labelled with 35S-labelled methionine and lysed in the presence of the reducible cross-linker, Dithiobis (succinimidyl propionate) (DSP). Invariant chain complexes were precipitated with an anti-Ii mAb and eluted under reducing (R) or non-reducing (NR) conditions to determine if Ii trimers were formed. Significantly, we found evidence for trimerization of the mutant Ii, although at levels lower than those obtained with wild-type Ii (Fig. 8, upper panel). As a control for non-specific cross-linking, the same lysates were also precipitated in parallel with the α-pan-HLA antibody W6/32, which cross-reacts with the endogenous monkey class I molecules of COS cells. The W6/32 precipitates displayed no formation of multimeric complexes (Fig. 8, lower panel), showing that trimerization of the invariant chain was specific. These results indicate that function of the invariant chain can be obtained with trimer levels far below those observed with the wild-type Ii. Furthermore, in order to account for the residual self-association, there must be a secondary trimerization site in the invariant chain. It is unclear why trimerization of Δ126–215-Ii was not found in a previous study using the same construct.51 However, our results are in agreement with the analyses of class II–Ii complexes purified from cells that indicated that the natural removal of the C-terminus of Ii in the endosomes does not destroy its trimeric nature,11,54 as well as other evidence that N-terminal fragments of Ii can trimerize in the absence of the C-terminal domain.55 Taken together these findings support the existence of a secondary trimerization domain, and explain why deletion of over half of the C-terminal, lumenal Ii residues does not affect Ii function.
Figure 8.
Trimerization of Ii mutants deleted in their C-terminal lumenal region. The COS cells were transiently transfected with vector, full-length Ii, or Δ126–215-Ii and are labelled as in Figure 2. Cells were lysed in the presence of the reducible cross-linker DSP and immunoprecipitated with mAb In-1 (upper panel) or mAb W6/32, which reacts with endogenous MHC class I molecules (lower panel). The immunoprecipitates were eluted under reducing (R) or non-reducing (NR) conditions and resolved by 15% SDS–PAGE. The molecular weights of the monomeric and trimeric forms of the invariant chain are indicated in the right margin. Presumptive dimers of full-length Ii (∼65 000 MW) and Δ125–215-Ii (∼40 000 MW) are indicated by small arrows. The MHC class I heavy chain (HC) and β2-microglobulin (β2m) are indicated in the right margin (lower panel).
Although the importance of the invariant chain for the presentation of class II MHC complexes to T cells has been demonstrated in both cell lines and mice, the molecular mechanisms by which the invariant chain influences the MHC class II presentation pathway remain poorly defined. Our observations using deleted invariant chain constructs show that the same Ii regions are required for regulation of presentation of antigens from both endogenous and exogenous sources. Endosomal targeting is clearly critical, as N-terminally deleted Ii was completely non-functional, indicating that the presentation of endogenous antigens is regulated at the level of post-ER events. Although this is not surprising for presentation of exogenous antigens, which must pass through the endosomal pathway, it is not necessarily obvious that the targeting of Ii would be as critical for presentation of endogenous antigens, which might be processed in non-endosomal compartments. Likewise, the model of Ii serving to exclude endogenous peptides from class II MHC would not predict that a targeting mutant, but MHC-binding competent, invariant chain would be defective at blocking presentation of endogenous antigen. However, the failure of Δ22-Ii to inhibit the presentation of HEL/Ak complex from endogenous HEL, as well as the ability of full-length Ii to enhance presentation of OVA/Ak complex from endogenous OVA, demonstrates that binding of Ii to class II MHC is not sufficient to prevent the presentation of endogenously derived peptides.
The opposite effects of Ii on endogenous presentation of OVA point to the existence of alternative processing pathways. Endogenous OVA is processed in a compartment that is similar or identical to the endosomal pathway used for exogenous antigens, as its presentation requires endosomally targeted invariant chain and is inhibited by chloroquine. It is unclear how secreted OVA enters the endosomal pathway, as it is not via a secretion–reuptake mechanism (see Fig. 5b). It may be due to overexpression of OVA, allowing some to ‘leak’ into vesicles transiting from the ER/Golgi to the endosomes/lysosomes. However, it appears that the endosomal processing compartment can also sample proteins not otherwise targeted to the secretory or endosomal pathways, as a cytosolic form of OVA can also be presented on class II MHC in an Ii-dependent,29 TAP-independent, chloroquine-sensitive (K. Frauwirth and N. Shastri, unpublished data) fashion. Processing of endogenous HEL appears to occur in a distinct, chloroquine-resistant compartment, perhaps along the constitutive secretory pathway. This is quite surprising, as processing of exogenous HEL clearly occurs in the endosomal/lysosomal pathway; futher, redirecting HEL to the endosomes restores chloroquine sensitivity. Thus, the same epitope can be generated in different cellular compartments, depending on how the antigen is targeted. It is likely that inhibition of HEL presentation by intact Ii is primarily due to targeting of Class II molecules out of this pathway and into the endosomes, rather than by occluding the MHC peptide-binding cleft, as an N-terminally deleted Ii construct is unable to block presentation, despite normal association with Class II.
The most unexpected outcome of this study was the finding that a C-terminally truncated invariant chain, Δ126–215, was comparable in its activity to the full-length Ii protein. This was especially surprising because deletions in this region are expected to affect proper assembly50,51 and function in presentation of exogenous native proteins.51 The high level of activity of Δ126–215-Ii and the low (but detectable) activity of Δ81–127-Ii in these studies are particularly interesting in light of previously published data51 which indicate that both of these deletions completely destroy invariant chain function in antigen presentation. The reason for these dramatic disparities could be in part due to differences in experimental conditions and/or the epitopes detected by the T cells. It has been shown5,23,24,26 that expression of the invariant chain has different effects on the presentation of different epitopes, even those derived from the same protein. Another interesting explanation for these disparate effects of Ii may be related to its ability to function as a protease inhibitor as shown in vitro,56 that could, if applicable to living APC, impact on the generation of specific peptides. Thus, the Δ126–215-Ii mutant may be impermissive for presentation of some epitopes by failing to inhibit their proteolysis, while remaining permissive for presentation of other epitopes which lack internal protease sites. Alternatively, an Ii mutant defective as a protease inhibitor might increase presentation of some epitopes by enhancing cleavage at the boundaries of the peptide. Further investigation of these effects requires a quantitative and qualitative analysis of the naturally processed peptides generated as a function of Ii structure and the antigen source. These results could be useful in design of strategies for using the Ii as an agent for targeting antigens to the MHC class II pathway.57–59
Acknowledgments
We are grateful to Drs Norbert Koch and Ina Freiswinkel for gifts of the lumenal Ii deletion constructs, Ilil Carmi for defining the epitope specificity of KZH cells and other investigators for reagents. This study was supported by grants from the NIH to N.S. K.F. was a predoctoral fellow of the Howard Hughes Medical Institute.
Abbreviations
- APC
antigen-presenting cell
- CLIP
class II-associated invariant chain peptide
- ER
endoplasmic reticulum
- HEL
hen-egg lysozyme
- Ii
invariant chain
- MHC
major histocompatibility complex
- OVA
ovalbumin
References
- 1.Roche PA, Marks MS, Cresswell P. Formation of a nine-subunit complex by HLA class II glycoproteins and the invariant chain. Nature. 1991;354:392–4. doi: 10.1038/354392a0. [DOI] [PubMed] [Google Scholar]
- 2.Lamb CA, Cresswell P. Assembly and transport properties of invariant chain trimers and HLA-DR-Invariant chain complexes. J Immunol. 1992;148:3478–82. [PubMed] [Google Scholar]
- 3.Layet C, Germain RN. Invariant chain promotes egress of poorly expressed, haplotype-mismatched class II major histocompatibility complex AαAß dimers from the endoplasmic reticulum/cis-Golgi compartment. Proc Natl Acad Sci USA. 1991;88:2346–50. doi: 10.1073/pnas.88.6.2346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Viville S, Neefjes J, Lotteau V, Dierich A, Lemeur M, Ploegh H, Benoist C, Mathis D. Mice lacking the MHC Class II-associated invariant chain. Cell. 1993;72:635–48. doi: 10.1016/0092-8674(93)90081-z. [DOI] [PubMed] [Google Scholar]
- 5.Bikoff EK, Huang LY, Episkopou V, van Meerwijk J, Germain RN, Robertson EJ. Defective major histocompatibility complex class II assembly, transport, peptide acquisition, and CD4+ T cell selection in mice lacking invariant chain expression. J Exp Med. 1993;177:1699–712. doi: 10.1084/jem.177.6.1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bakke O, Dobberstein B. MHC class II-associated invariant chain contains a sorting signal for endosomal compartments. Cell. 1990;63:707–16. doi: 10.1016/0092-8674(90)90137-4. [DOI] [PubMed] [Google Scholar]
- 7.Lotteau V, Teyton L, Peleraux A, Nilsson T, Karlsson L, Schmid SL, Quaranta V, Peterson PA. Intracellular transport of class II MHC molecules directed by invariant chain. Nature. 1990;348:600–5. doi: 10.1038/348600a0. [DOI] [PubMed] [Google Scholar]
- 8.Roche PA, Cresswell P. Invariant chain association with HLA-DR molecules inhibits immunogenic peptide binding. Nature. 1990;345:615–8. doi: 10.1038/345615a0. [DOI] [PubMed] [Google Scholar]
- 9.Teyton L, O'Sullivan D, Dickson PW, Lotteau V, Sette A, Fink P, Peterson PA. Invariant chain distinguishes between the exogenous and endogenous antigen presentation pathways. Nature. 1990;348:39–44. doi: 10.1038/348039a0. [DOI] [PubMed] [Google Scholar]
- 10.Roche PA, Cresswell P. Proteolysis of the class II-associated invariant chain generates a peptide binding site in intracellular HLA-DR molecules. Proc Natl Acad Sci USA. 1991;88:3150–4. doi: 10.1073/pnas.88.8.3150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Amigorena S, Webster P, Drake J, Newcomb J, Cresswell P, Mellman I. Invariant chain cleavage and peptide loading in major histocompatibility complex class II vesicles. J Exp Med. 1995;181:1729–41. doi: 10.1084/jem.181.5.1729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Riese RJ, Wolf PR, Bromme D, Natkin LR, Villadangos JA, Ploegh HL, Chapman HA. Essential role for cathepsin S in MHC class II-associated invariant chain processing and peptide loading. Immunity. 1996;4:357–66. doi: 10.1016/s1074-7613(00)80249-6. [DOI] [PubMed] [Google Scholar]
- 13.Riberdy JM, Newcomb JR, Surman MJ, Barbosa JA, Cresswell P. HLA-DR molecules from an antigen-processing mutant cell line are associated with invariant chain peptides. Nature. 1992;360:474–7. doi: 10.1038/360474a0. [DOI] [PubMed] [Google Scholar]
- 14.Sette A, Ceman S, Kubo RT, et al. Invariant chain peptides in most HLA-DR molecules of an antigen-processing mutant. Science. 1992;258:1801–4. doi: 10.1126/science.1465617. [DOI] [PubMed] [Google Scholar]
- 15.Morris P, Shaman J, Attaya M, Amaya M, Goodman S, Bergman C, Monaco JJ, Mellins E. An essential role for HLA-DM in antigen presentation by class II major histocompatibility molecules. Nature. 1994;368:551–4. doi: 10.1038/368551a0. [DOI] [PubMed] [Google Scholar]
- 16.Sherman MA, Weber DA, Jensen PE. DM enhances peptide binding to class II MHC by release of invariant chain-derived peptide. Immunity. 1995;3:197–205. doi: 10.1016/1074-7613(95)90089-6. [DOI] [PubMed] [Google Scholar]
- 17.Fung-Leung WP, Surh CD, Liljedahl M, Pang J, Leturcq D, Peterson PA, Webb SR, Karlsson L. Antigen presentation and T cell development in H2-M-deficient mice. Science. 1996;271:1278–81. doi: 10.1126/science.271.5253.1278. [DOI] [PubMed] [Google Scholar]
- 18.Stockinger B, Pessara U, Lin RH, Habicht J, Grez M, Koch N. A role of Ia-associated invariant chains in antigen processing and presentation. Cell. 1989;56:683–9. doi: 10.1016/0092-8674(89)90590-4. [DOI] [PubMed] [Google Scholar]
- 19.Peterson M, Miller J. Invariant chain influences the immunological recognition of MHC class II molecules. Nature. 1990;345:172–4. doi: 10.1038/345172a0. [DOI] [PubMed] [Google Scholar]
- 20.Bertolino P, Forquet F, Pont S, Koch N, Gerlier D, Rabourdin-Combe C. Correlation between invariant chain expression level and capability to present antigen to MHC class II-restricted T cells. Int Immunol. 1991;3:435–43. doi: 10.1093/intimm/3.5.435. [DOI] [PubMed] [Google Scholar]
- 21.Nadimi F, Moreno J, Momburg F, Heuser A, Fuchs S, Adorini L, Hammerling GJ. Antigen presentation of hen egg-white lysozyme but not of ribonuclease A is augmented by the major histocompatibility complex class II-associated invariant chain. Eur J Immunol. 1991;21:1255–63. doi: 10.1002/eji.1830210524. [DOI] [PubMed] [Google Scholar]
- 22.Peterson M, Miller J. Antigen presentation enhanced by the alternatively spliced invariant chain gene product p41. Nature. 1992;357:596–8. doi: 10.1038/357596a0. [DOI] [PubMed] [Google Scholar]
- 23.Humbert M, Bertolino P, Forquet F, Rabourdin-Combe C, Gerlier D, Davoust J, Salamero J. Major histocompatibility complex class II-restricted presentation of secreted and endoplasmic reticulum resident antigens requires the invariant chains and is sensitive to lysosomotropic agents. Eur J Immunol. 1993;23:3167–72. doi: 10.1002/eji.1830231219. [DOI] [PubMed] [Google Scholar]
- 24.Momburg F, Fuchs S, Drexler J, Busch R, Post M, Hammerling GJ, Adorini L. Epitope-specific enhancement of antigen presentation by invariant chain. J Exp Med. 1993;178:1453–8. doi: 10.1084/jem.178.4.1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Elliott EA, Drake JR, Amigorena S, Elsemore J, Webster P, Mellman I, Flavell RA. The invariant chain is required for intracellular transport and function of major histocompatibility complex class II molecules. J Exp Med. 1994;179:681–94. doi: 10.1084/jem.179.2.681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bodmer H, Viville S, Benoist C, Mathis D. Diversity of endogenous epitopes bound to MHC class II molecules limited by invariant chain. Science. 1994;263:1284–6. doi: 10.1126/science.7510069. [DOI] [PubMed] [Google Scholar]
- 27.Dodi AI, Brett S, Nordeng T, Sidhu S, Batchelor RJ, Lombardi G, Bakke O, Lechler RI. The invariant chain inhibits presentation of endogenous antigens by a human fibroblast cell line. Eur J Immunol. 1994;24:1632–9. doi: 10.1002/eji.1830240727. [DOI] [PubMed] [Google Scholar]
- 28.Long EO, LaVaute T, Pinet V, Jaraquemada D. Invariant chain prevents the HLA-DR-restricted presentation of a cytosolic peptide. J Immunol. 1994;153:1487–94. [PubMed] [Google Scholar]
- 29.Sanderson S, Frauwirth K, Shastri N. Expression of endogenous peptide-major histocompatibility complex class II complexes derived from invariant chain-antigen fusion proteins. Proc Natl Acad Sci USA. 1995;92:7217–21. doi: 10.1073/pnas.92.16.7217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Anderson MS, Swier K, Arneson L, Miller J. Enhanced antigen presentation in the absence of the invariant chain endosomal localization signal. J Exp Med. 1993;178:1959–69. doi: 10.1084/jem.178.6.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rudensky AY, Preston-Hurlburt P, Hong S-C, Barlow A, Janeway CAJ. Sequence analysis of peptides bound to MHC class II molecules. Nature. 1991;353:622–7. doi: 10.1038/353622a0. [DOI] [PubMed] [Google Scholar]
- 32.Rudensky AY, Preston-Hurlburt P, Al-Ramadi BK, Rothbard J, Janeway CAJ. Truncation variants of peptides isolated from MHC class II molecules suggest sequence motifs. Nature. 1992;359:429–31. doi: 10.1038/359429a0. [DOI] [PubMed] [Google Scholar]
- 33.Chicz RM, Urban RG, Lane WS, Gorga JC, Stern LJ, Vignali DAA, Strominger JL. Predominant naturally processed peptides bound to HLA-DR1 are derived from MHC-related molecules and are heterogenous in size. Nature. 1992;358:764–8. doi: 10.1038/358764a0. [DOI] [PubMed] [Google Scholar]
- 34.Hunt DF, Henderson RA, Shabanowitz J, et al. Characterization of peptides bound to the class I MHC molecule HLA-A2.1 by mass spectrometry. Science. 1992;255:1261–3. doi: 10.1126/science.1546328. [DOI] [PubMed] [Google Scholar]
- 35.Hunt DF, Michel H, Dickinson TA, et al. Peptides presented to the immune system by the murine class II major histocompatibility complex molecule I-Ad. Science. 1992;256:1817–20. doi: 10.1126/science.1319610. [DOI] [PubMed] [Google Scholar]
- 36.Newcomb JR, Cresswell P. Characterization of endogenous peptides bound to purified HLA-DR molecules and their absence from invariant chain-associated alpha beta dimers. J Immunol. 1993;150:499–507. [PubMed] [Google Scholar]
- 37.Harris PE, Maffei A, Colovai AI, Kinne J, Tugulea S, Suciu-Foca N. Predominant HLA-class II bound self-peptides of a hematopoietic progenitor cell line are derived from intracellular proteins. Blood. 1996;87:5104–12. [PubMed] [Google Scholar]
- 38.Oukka M, Cohen-Tannoudji M, Tanaka Y, Babinet C, Kosmatopoulos K. Medullary thymic epithelial cells induce tolerance to intracellular proteins. J Immunol. 1996;156:968–75. [PubMed] [Google Scholar]
- 39.Shastri N, Gammon G, Miller A, Sercarz EE. Ia molecule-associated selectivity in T cell recognition of a 23-amino-acid peptide of lysozyme. J Exp Med. 1986;164:882–96. doi: 10.1084/jem.164.3.882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sanderson S, Shastri N. LacZ inducible peptide/MHC specific T-hybrids. Int Immunol. 1994;6:369–76. doi: 10.1093/intimm/6.3.369. [DOI] [PubMed] [Google Scholar]
- 41.Karttunen J, Shastri N. Measurement of ligand induced activation in single viable T-cells using the lacZ reporter gene. Proc Natl Acad Sci USA. 1991;88:3972–6. doi: 10.1073/pnas.88.9.3972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Koch N, Koch S, Hammerling GJ. Ia invariant chain detected on lymphocyte surfaces by monoclonal antibody. Nature. 1982;299:644–5. doi: 10.1038/299644a0. [DOI] [PubMed] [Google Scholar]
- 43.Shastri N, Miller A, Sercarz EE. The expressed T cell repertoire is hierarchical: the precise focus of lysozyme-specific T cell clones is dependent upon the structure of the immunogen. J Molec Cell Immunol. 1984;1:369–79. [PubMed] [Google Scholar]
- 44.Freisewinkel IM, Schenck K, Koch N. The segment of invariant chain that is critical for association with major histocompatibility complex class II molecules contains the sequence of a peptide eluted from class II polypeptides. Proc Natl Acad Sci USA. 1993;90:9703–6. doi: 10.1073/pnas.90.20.9703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shastri N, Gonzalez F. Endogenous generation and presentation of the OVA peptide/Kb complex to T-cells. J Immunol. 1993;150:2724–36. [PubMed] [Google Scholar]
- 46.Sekaly R, Tonnelle C, Strubin M, Mach B, Long E. Cell Surface Expression of Class II Histocompatibility Antigens Occurs in the Absence of the Invariant Chain. J Exp Med. 1986;164:1490–504. doi: 10.1084/jem.164.5.1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Miller J, Germain R. Efficient Cell Surface Expression of Class II MHC Molecules in the Absence of Associated Invariant Chain. J Exp Med. 1986;164:1478–89. doi: 10.1084/jem.164.5.1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Brichard V, Van Pel A, Wolfel T, Wolfel C, De Plaen E, Lethe B, Coulie P, Boon T. The tyrosinase gene codes for an antigen recognized by autologous cytolytic T lymphocytes on HLA-A2 melanomas. J Exp Med. 1993;178:489–95. doi: 10.1084/jem.178.2.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Romagnoli P, Germain RN. The CLIP region of invariant chain plays a critical role in regulating major histocompatibility complex class II folding, transport and peptide occupancy. J Exp Med. 1994;180:1107–13. doi: 10.1084/jem.180.3.1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bijlmakers ME, Benaroch P, Ploegh HL. Mapping functional regions in the lumenal domain of the class II-associated invariant chain. J Exp Med. 1994;180:623–30. doi: 10.1084/jem.180.2.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bertolino P, Staschewski M, Trescol-Biemont M-C, et al. Deletion of a C-terminal sequence of the Class II-associated invariant chain abrogates invariant chains oligomer formation and class II antigen presentation. J Immunol. 1995;154:5620–9. [PubMed] [Google Scholar]
- 52.Romagnoli P, Layet C, Yewdell J, Bakke O, Germain RN. Relationship between invariant chain expression and major histocompatibility complex class II transport into early and late endocytic compartments. J Exp Med. 1993;177:583–96. doi: 10.1084/jem.177.3.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhong G, Castellino F, Romagnoli P, Germain RN. Evidence that binding site occupancy is necessary and sufficient for effective major histocompatibility complex (MHC) class II transport through the secretory pathway redefines the primary function of class II-associated invariant chain peptides (CLIP) J Exp Med. 1996;184:2061–6. doi: 10.1084/jem.184.5.2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Newcomb JR, Cresswell P. Structural analysis of proteolytic products of MHC class II-invariant chain complexes generated in vivo. J Immunol. 1993;151:4153–63. [PubMed] [Google Scholar]
- 55.Ashman JB, Miller J. A role for the transmembrane domain in the trimerization of the MHC class II-associated invariant chain. J Immunol. 1999;163:2704–12. [PubMed] [Google Scholar]
- 56.Katunuma N, Kakegawa H, Matsunaga Y, Saibara T. Immunological significances of invariant chain from the aspect of its structural homology with the cystatin family. FEBS Lett. 1994;349:265–9. doi: 10.1016/0014-5793(94)00657-1. [DOI] [PubMed] [Google Scholar]
- 57.Nakano N, Rooke R, Benoist C, Mathis D. Positive selection of T cells induced by viral delivery of neopeptides to the thymus. Science. 1997;275:678–83. doi: 10.1126/science.275.5300.678. 10.1126/science.275.5300.678. [DOI] [PubMed] [Google Scholar]
- 58.Wang RF, Wang X, Atwood AC, Topalian SL, Rosenberg SA. Cloning genes encoding MHC class II-restricted antigens: mutated CDC27 as a tumor antigen. Science. 1999;284:1351–4. doi: 10.1126/science.284.5418.1351. 10.1126/science.284.5418.1351. [DOI] [PubMed] [Google Scholar]
- 59.Scott D, Addey C, Ellis P, James E, Mitchell MJ, Saut N, Jurcevic S, Simpson E. Dendritic cells permit identification of genes encoding MHC class II-restricted epitopes of transplantation antigens. Immunity. 2000;12:711–20. doi: 10.1016/s1074-7613(00)80221-6. [DOI] [PubMed] [Google Scholar]