Abstract
Two plasmid DNA vectors, pCAGGS(S) encoding the genes of the major envelope protein of hepatitis B virus (HBV), and pCAGGS(S + preS2) encoding the genes of the middle envelope protein were used to study the mechanism and therapeutic potential of DNA-based immunization. Injection of these plasmids into the regenerating bilateral tibialis anterior muscle (TA) of normal C57BL/6 mice induced hepatitis B surface antigen (HBsAg)-specific humoral and cellular immune responses. Seventy-two hours after injection of pCAGGS(S), infiltrating cells including antigen-presenting dendritic cells (DC) were localized around the injection site and HBsAg was expressed by both muscle cells and infiltrating cells. Spleen DC from the mice were exposed to HBsAg for up to 32 weeks after a single injection of pCAGGS(S), because these DC induced the proliferation of HBsAg-specific memory lymphocytes in culture without exogenous HBsAg. A single injection of pCAGGS(S) or pCAGGS(S + preS2) resulted in the clearance of HBsAg in 28 out of 30 HBV-transgenic (Tg) mice. In contrast, more than 7 monthly injections of an HBsAg-based vaccine were required for the clearance of HBsAg in 6 out of 29 HBV-Tg mice. Infiltrating DC at the DNA vaccine injection site may have a role in initiating HBsAg-specific immune response, whereas the persistence of HBsAg exposed spleen DC may contribute to long-lasting immunity. This study also suggested that DNA-based vaccines may be a potent tool for treating chronic HBV carriers.
Introduction
Infection with hepatitis B virus (HBV) causes acute as well as chronic necroinflammatory liver disease and there are about 350 million chronic HBV carriers world wide. Many HBV carriers eventually develop serious complications such as liver cirrhosis or hepatocellular carcinoma, and these carriers also represent a permanent source of HBV infection.
The prospects for controlling new HBV infection depend on the availability of safe, effective, and affordable vaccines.1,2 Recently, antigen-based vaccines have been used to treat human HBV carriers3,4 and have been studied in HBV-transgenic (HBV-Tg) mice, a murine model of HBV carriage.5–8 The successful therapeutic vaccination reported in murine HBV-Tg mice might be related to the fact that hepatitis B surface antigen (HBsAg) transgene expression in these mice is blocked by methylation.9,10
Although currently available antigen-based vaccines are used for both prophylactic and therapeutic purposes, a new field of immunological research with applications to vaccine development has developed around the direct transfer of naked DNA, which leads to the in situ synthesis and accumulation of antigenic proteins followed by induction of an immune response. Immunization with plasmid DNA vectors encoding the small and middle viral envelope proteins of HBV has been shown to elicit strong and durable humoral and cell-mediated immunity in both normal mice11–15 and HBV-Tg mice,16,17 but several aspects of DNA vaccine still require further study before its wider application.
There is little information available regarding the mechanism of induction of immunity by DNA vaccines, so it is not known why a single injection of DNA vaccine can induce long-lasting immunity. Moreover, no study has made a parallel comparison of the therapeutic potential of DNA vaccines and antigen-based vaccines in human or murine HBV-carriers.
In the present study, we used a plasmid vector with a promoter designed for expression in muscle. We first constructed two plasmid HBV DNA vectors, one encoding the genes of the major envelope protein and the other encoding the genes of the middle envelope protein. Injection of these vectors into the regenerating tibialis anterior muscle (TA) induced HBsAg-specific humoral and cellular immune responses in normal C57BL/6 mice. Next, we used immunohistochemical methods to localize infiltrating cells, including antigen-presenting dendritic cells (DC), and to assess the expression of HBsAg at and around the site of injection of DNA vaccines. To evaluate the mechanism of long-lasting immunity following DNA vaccination, we analysed the function of splenic DC at 32 weeks after a single injection of DNA vaccine in C57BL/6 mice. We also compared the therapeutic potential of a DNA vaccine and the currently available antigen-based vaccine in HBV-Tg mice.
Materials and Methods
Animals
The animals used were normal C57BL/6 mice and HBV-Tg mice (official designation: 1·2HB-BS10) that were seropositive for HBsAg, hepatitis B e antigen, and HBV DNA as well as expressing HBV-related mRNAs in their tissues.18 All animals received humane care and the study was carried out with the permission of the Committee on Animal Experimentation of Ehime University School of Medicine (Ehime, Japan).
Plasmid DNA vectors
The major and the middle envelope genes of HBV were amplified by PCR from plasmid BSadr4 × 2(R), which contained two copies of the full HBV genome. Polymerase chain reaction (PCR) products were digested with EcoRI and were inserted into the multiple cloning site of the parental plasmid, pCAGGS, exactly as described by Niwa et al.19 Plasmid DNA vectors were grown in Escherichia coli HB101 (Takara, Tokyo, Japan) and were purified using a Qiagen Plasmid Purification Kit (Qiagen, Hilden, Germany).The quantity and quality of the purified plasmid DNA were assessed from the optical density at 260 and 280 nm. The insertion sites of the plasmid DNAs were confirmed using restriction enzymes and DNA sequencing. The pCAGGS(S) vector encoded the genes of the major envelope protein of HBV, whereas the vector, pCAGGS(S + preS2) encoded the genes of the middle envelope proteins.
Cell transfection, expression, and antigen production
COS I cells (SV40 transformed African green monkey kidney cells, ATCC No. CRL 1650, American Type Culture Collection, Rockville, MD) and G8 cells (mouse myoblast cells, ATCC No. CRL 1456) were transiently transfected with plasmid HBV DNA vectors using Lipofectin (Gibco, Gaithersburg, MD). Expression of HBsAg was confirmed in the these cells by immunostaining. Culture supernatants of the transfected cells were harvested every 24 hr for 15 consecutive days and the HBsAg level in each supernatant was measured.
DNA immunization
Damage to the TA was produced by injecting 0·25% bupivacaine (100 µl in phosphate-buffered saline, Sigma, St. Louis, MO). Five days after bupivacaine injection, the regenerating TA was injected with plasmid DNA vectors in 100 µl of phosphate-buffered saline using a 1·0-ml syringe and a 27-g needle fitted with a collar of polyethylene tubing to limit the depth of the penetration to 2 mm. All intramuscular injections were carried out under anaesthesia.
Treatment of HBV-Tg mice with DNA-based or antigen-based vaccines
At the age of 6–7 weeks, HBV-Tg seropositive for HBsAg and HBV DNA were randomized to receive either a single intramuscular injection of a DNA vaccine (pCAGGS(S) or pCAGGS(S + preS2), or the parental plasmid, pCAGGS) or several intraperitoneal injections of an antigen-based vaccine containing 10·0 µg of HBsAg (Foundation for Microbial Diseases of Osaka University, Osaka, Japan) emulsified in complete Freund's adjuvant (CFA) at entry into the study and once in every month for 12 consecutive months. Some of the mice were only injected with CFA once a month for 12 consecutive months and other mice were left untreated, as described previously.5–8
Cell preparations
Whole spleen cells, T cells, and DC were isolated from mouse spleens as described previously.6,7,20,21 T cells were enriched by applying a T-cell recovery kit (Biotex, Lab. Inc. Alberta, Canada) to Sephadex G-10 non-adherent splenic T/B cells, from which B cells had been depleted during passage through the affinity column. DC were isolated by culturing low-density adherent spleen cells for 18 hr at 37°, after which these cells became non-adherent. Purified DC were isolated by treating Fc-receptor-negative cells with a mixture of Thy-1·2 (clone 5a-8) and Lyt-1·2 (clone CG16; Cedarline, Ontario, Canada) plus CD45R (clone RA-3-6B2; Pharmingen, San Diego, CA) and low-toxicity complement (Cedarline) to eliminate contaminating T and B cells. CD4+ T cells were isolated from mouse spleen cells using a commercial CD4 isolation kit (Biotex).
Preparation of antigen-specific memory lymphocytes
The procedure for preparation of HBsAg-specific memory lymphocytes has been described previously.6,7,21 In brief, normal C57BL/6 mice were injected twice intraperitoneally with 10 µg of HBsAg in alum at an interval of 2 weeks. Serial assessment revealed that 3 months after booster immunization, lymphocytes from HBsAg-injected mice showed proliferation when cultured with HBsAg plus professional antigen-presenting cells like spleen DC, but not when cultured with HBsAg alone or with spleen DC alone.
Cell proliferation assay and culture
Whole spleen cells, purified T cells, and CD4+ T cells were cultured with HBsAg or DC plus HBsAg for 120 hr, as described previously.6–8,20,21 The incorporation of [3H]thymidine (1 µCi/ml) during the final 12 hr of culture was measured as counts per minute (c.p.m.). HBsAg-specific T-lymphocyte proliferation was determined from the stimulation index (SI), which was calculated as the c.p.m. of cultures containing HBsAg divided by the c.p.m. of cultures without HBsAg. An SI value of > 2·0 indicated significant HBsAg-specific proliferation.
Detection of HBV-related antigens and antibody
Samples were screened for HBsAg and anti-HBs by the reverse passive haemagglutination method and passive haemagglutination method, respectively, using commercial kits (Mycell, Tokyo Institute of Immunology, Tokyo, Japan). With these methods, samples showing haemagglutination at a dilution > 22 were considered positive. HBsAg, pre S2 antigen, and anti-HBs were quantified by a chemiluminescence enzyme immunoassay using a commercial kit (Tokyo Institute of Immunology, Tokyo, Japan). The detection limits of HBsAg and anti-HBs with these methods were 0·3 ng/ml and 1·25 mIU/ml, respectively.
Histology and immunohistochemistry
Muscle tissue from the injection sites was fixed in formalin or snap frozen. Infiltrating cells were localized by haematoxylin–eosin staining. To localize HBsAg-and CD11c-positive DC, muscle specimens were incubated overnight at 4° with appropriately diluted primary antibodies as described previously.22,23 After washing in phosphate-buffered saline, the streptavidin–biotin method was employed for immunostaining. Colour was developed using true blue (KPL, Baltimore, MD) after peroxidase-conjugated streptavidin for HBsAg and using New Fuchsin (DAKO, Glostrop, Denmark) after alkaline phosphatase-conjugated streptavidin for CD11c.
Statistical analysis
Data were expressed as the mean±standard deviation (sd). Statistical analysis was done using Student's t-test, Fisher's exact test, and the Mann–Whitney U-test, as indicated. In all statistical comparisons, P < 0·05 was taken to indicate a significant difference.
Results
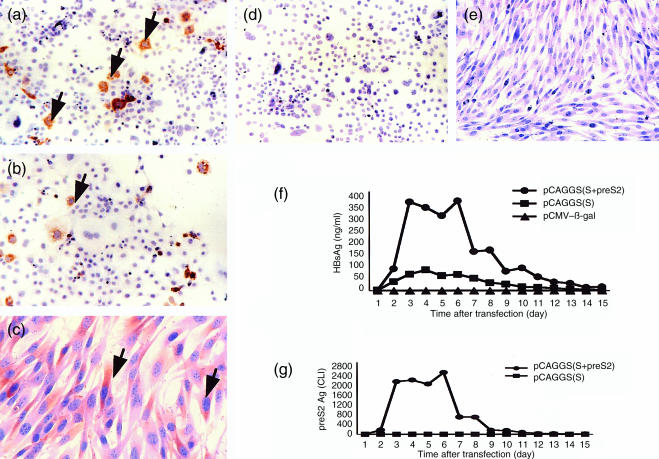
Two plasmid DNA vectors, pCAGGS(S) and pCAGGS(S + preS2), respectively, were constructed that contained the genes of the major and middle envelope proteins of HBV. To verify protein expression, COS I cells and G8 cells were transfected with these plasmids and with the parental plasmid (pCAGGS). Immunohistochemical staining confirmed the expression of HBsAg in COS I cells and G8 cells transfected with these plasmids (Fig. 1a–c), but not in cells transfected with pCAGGS (Fig. 1d, e). HBsAg was detected in the culture supernatant of COS I cells transfected with pCAGGS(S) and pCAGGS(S + preS2). PreS2 antigen was detected in the culture supernatant of COS I cells transfected with pCAGGS(S + preS2) but not in culture supernatant of COS I cells transfected with pCAGGS(S) (fig. 1f–g). The levels of HBsAg were higher in culture supernatant of COS I cells transfected with pCAGGS(S + preS2) compared with their levels in culture supernatant of COS I cells transfected with pCAGGS(S). This is possibly due to the fact that chemiluminescence enzyme immunoassay method for HBsAg detected both HBsAg and pre S2 antigen. HBsAg was also detected in the culture supernatant of G8 cells transfected with pCAGGS(S) and pCAGGS(S + preS2) (data not shown).
Figure 1.
COS I cells were transfected with pCAGGS(S) or pCAGGS(S + preS2) and G8 cells were transfected with pCAGGS(S). Expression of HBsAg was localized by immunohistochemistry. HBsAg expression was seen in COS I cells transfected with pCAGGS(S) (a) or pCAGGS (S + preS2) (b), as well as in G8 cells transfected with pCAGGS(S) (c), but was not seen in COS I cells or G8 cells transfected with the parental plasmid vector (pCAGGS) (d, e). (f) Time course of HBsAg secretion into the culture supernatant of COS I cells transfected with pCAGGS(S) or pCAGGS(S + preS2). (g) Time course of preS2 antigen secretion by COS I cells transfected with pCAGGS(S + preS2). HBsAg levels are shown as ng/ml and preS2 levels are shown as CLI (chemiluminescence intensity unit).
Humoral and cellular immune responses to HBsAg in normal C57BL/6 mice
Injection of normal C57BL/6 mice with 100·0 µg and 50·0 µg of pCAGGS(S) and pCAGGS(S + preS2) resulted in the detection of anti-HBs in the serum after only 2 weeks. In most of the mice, the maximum anti-HBs levels were seen at 8 or 12 weeks after injection. The anti-HBs level declined thereafter, but it remained detectable until 32 weeks in most of the mice. HBsAg-specific T-cell proliferation was detected in all of the normal mice injected with pCAGGS(S) that became seropositive for anti-HBs as well as in two out of five normal mice injected with pCAGGS(S) that were seronegative for anti-HBs (Table 1). On the other hand, normal C57BL/6 mice injected with the control plasmid did not show HBsAg-specific T-cell proliferation.
Table 1. HBsAg-specific cellular immune response in vaccinated normal C57BL/6 mice.
| Mouse | Numbers | Positivity (%) | SI |
|---|---|---|---|
| DNA-vaccinated normal mouse | 10 | 10 (100%) | 5·9 ± 1·2* |
| (serum anti-HBs (+ve)) | |||
| DNA-vaccinated normal mouse | 5 | 2 (40%) | 2·9, 3·1 |
| (serum anti-HBs (–ve)) | |||
Normal C57BL/6 mice were injected with a single injection of 100 µg of pCAGGS(S). Spleen cells from 10 mice seropositive for anti-HBs and 5 mice seronegative for anti-HBs were cultured with HBsAg for 120 hr. The incorporation of [3H]thymidine during the final 12 hr of culture were measured as c.p.m. HBsAg-specific T lymphocyte proliferation was determined from the stimulation index (SI), which was calculated as the c.p.m. of cultures containing HBsAg divided by the c.p.m. of cultures without HBsAg. An SI value of > 2·0 indicated significant HBsAg-specific proliferation.
Mean±SD.
Infiltration of antigen-presenting DC at the injection site and expression of HBsAg by muscle cells and infiltrating cells
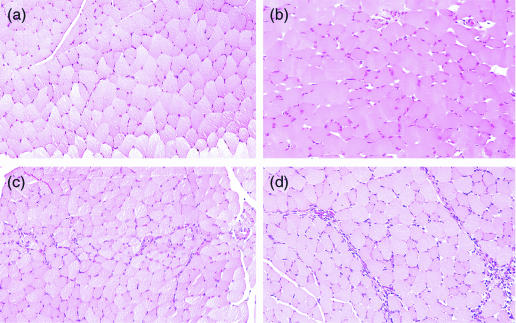
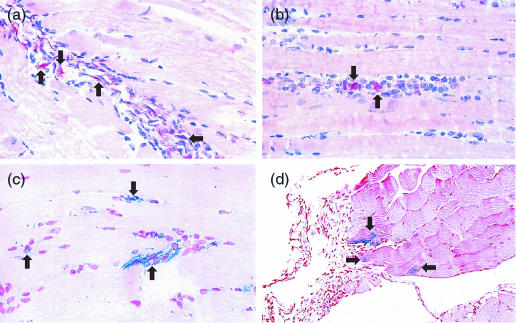
In normal mice, infiltrating cells increased over time at the injection site of pCAGGS(S) in the TA (Fig. 2a–d). CD11c-positive DC were also found among these infiltrating cells (Fig. 3a–b). HBsAg was expressed by muscle cells at the injection site(Fig. 3c), and also by infiltrating cells (Fig. 3d).
Figure 2.
Muscle samples were removed from the site of injection of the DNA vaccine before as well as 24, 48 and 72 hr after injection of pCAGGS(S), encoding the major envelope protein of HBV. Haematoxylin–eosin staining showed the localization of infiltrating cells at the site of DNA vaccination before (a), as well as 24 hr (b), 48 hr (c), and 72 hr (d) after injection.
Figure 3.
Muscle samples were taken from the site of vaccination at 72 hr after injection of pCAGGS(S) (encoding major envelope protein of HBV) were immunohistochemically stained to localize CD11c-positive DC and HBsAg. DC were found among the infiltrating cells (arrowhead a, b). HBsAg was expressed both by the muscle cells (c) and the infiltrating cells (d).
HBsAg-pulsed DC in the spleens of DNA-vaccinated mice
Spleen DC from normal mice at 32 weeks after the injection of pCAGGS(S), but not spleen DC from untreated normal mice, induced the proliferation of T cells from DNA-vaccinated mice (Table 2a) and the proliferation of HBsAg-specific memory T cells (Table 2b) in cultures without exogenous HBsAg. These findings indicate that DC from pCAGGS(S)-vaccinated mice were already exposed to HBsAg in vivo and that no further stimulation by this antigen was needed to induce a specific cellular immune response from HBsAg-specific memory T cells. This was an antigen-specific phenomenon because spleen DC from pCAGGS(S)-injected mice did not induce the proliferation of keyhole limpet haemocyanin-specific T cells (data not shown).
Table 2. HBsAg-pulsed dendritic cells in the spleens of DNA-vaccinated mice.
| DC | T cells | HBsAg | Numbers | SI* |
|---|---|---|---|---|
| (a) | ||||
| Normal untreated mice | DNA-vaccinated mice | − | 5 | 1·0 |
| Normal untreated mice | DNA-vaccinated mice | + | 5 | 6·9 ± 1·2 |
| DNA-vaccinated mice | DNA-vaccinated mice | − | 5 | 5·3 ± 2·1 |
| DNA-vaccinated mice | DNA-vaccinated mice | + | 5 | 9·3 ± 1·2 |
| (b) | ||||
| Normal untreated mice | HBsAg-specific memory T cells | − | 5 | 1·0 |
| Normal untreated mice | HBsAg-specific memory T cells | + | 5 | 4·7 ± 0·9 |
| DNA-vaccinated mice | HBsAg-specific memory T cells | − | 5 | 4·9 ± 0·8 |
| DNA-vaccinated mice | HBsAg-specific memory T cells | + | 5 | 8·7 ± 1·5 |
DC were isolated from mouse spleens of normal untreated C57BL/6 mice or pCAGGS(S)-injected C57BL/6 mice. T cells were isolated from C57BL/6 mice at 32 weeks after a single injection of 100 µg of pCAGGS(S) (DNA-vaccinated mice). The procedure for preparation of HBsAg-specific memory T cells was described in Methods. Different cells were cultured with or without HBsAg for 120 hr. The mean cpm in cultures containing DC from normal untreated mice and T cells from DNA-vaccinated mice (a) or the mean cpm in cultures containing DC from normal untreated mice and T cells from HBsAg-specific memory T cells (b) were considered as stimulation index (SI) of 1·0. An SI value of > 2·0 indicated significant HBsAg-specific proliferation.
Mean±SD of five separate experiments.
When spleen DC from pCAGGS(S)-injected mice were cultured in the presence of recombinant mouse granulocyte–macrophage colony-stimulating factor (R & D Systems, Minneapolis, MN) for 14 days, the HBsAg level was were 12 ng/ml, 25 ng/ml, and 43 ng/ml in three separate cultures.
Therapeutic value of DNA and HBsAg-based vaccines in HBV-Tg mice
A single injection of pCAGGS(S) (50 or 100 µg/mouse) resulted in the clearance of HBsAg from 14 out of 15 HBV-Tg mice and the HBsAg level decreased in the other mouse (Table 3). Anti-HBs was detected in 4 out of 15 HBV-Tg mice at 12 weeks after vaccination (Table 3). The outcome was similar in HBV-Tg mice injected with pCAGGS (S + preS2) (Table 4). These HBV-Tg mice remained anti-HBs-positive throughout the observation period of 32 weeks (Table 4). The HBsAg level showed almost no change in HBV-Tg mice injected with pCAGGS, the parental vector (data not shown).
Table 3. Therapeutic value of vaccination of pCAGGS(S) in HBV-Tg mice.
| HBsAg (ng/ml) | Anti-HBs (mIU/ml) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Before | 2 weeks | 4 weeks | 8 weeks | 12 weeks | 32 weeks | Before | 2 weeks | 4 weeks | 8 weeks | 12 weeks | 32 weeks | |
| (a) 100 µg/mouse | ||||||||||||
| Female HBV-Tg mice | ||||||||||||
| No.1 | 50 | < 0·3 | < 0·3 | < 0·3 | < 0·3 | < 0·3 | < 1·25 | < 1·2 | < 1·25 | < 1·25 | < 1·25 | < 1·25 |
| No.2 | 48 | < 0·3 | < 0·3 | < 0·3 | < 0·3 | < 0·3 | < 1·25 | < 1·25· | < 1·25 | 11 | 82 | 65 |
| No.3 | 52 | < 0·3 | < 0·3 | < 0·3 | < 0·3 | < 0·3 | < 1·25 | < 1·25 | < 1·25 | 17 | 69 | 56 |
| No.4 | 29 | < 0·3 | < 0·3 | < 0·3 | < 0·3 | < 0·3 | < 1·25 | < 1·25 | < 1·25 | < 1·25 | < 1·25 | < 1·25 |
| No.5 | 57 | < 0·3 | < 0·3 | < 0·3 | < 0·3 | ND | < 1·25 | < 1·25 | < 1·25 | < 1·25 | < 1·25 | ND |
| Male HBV-Tg mice | ||||||||||||
| No.1 | 120 | < 0·3 | 76 | 45 | ND | < 0·3 | < 1·25 | < 1·25 | < 1·25 | < 1·25 | ND | < 1·25 |
| No.2 | 210 | 68 | 45 | ND | < 0·3 | < 0·3 | < 1·25 | < 1·25 | < 1·25 | ND | < 1·25 | < 1·25 |
| No.3 | 208 | < 0·3 | 98 | 48 | ND | < 0·3 | < 1·25 | < 1·25 | < 1·25 | < 1·25 | ND | < 1·25 |
| No.4 | 105 | < 0·3 | < 0·3 | < 0·3 | < 0·3 | < 0·3 | < 1·25 | < 1·25 | < 1·25 | < 1·25 | 5·0 | < 1·25 |
| No.5 | 154 | < 0·3 | 52 | 25 | < 0·3 | < 0·3 | < 1·25 | < 1·25 | < 1·25 | < 1·25 | 7·0 | < 1·25 |
| (b) (50 µg/mouse): Female HBV-Tg mice | ||||||||||||
| No.1 | 56 | < 0·3 | < 0·3 | < 0·3 | < 0·3 | < 0·3 | < 1·25 | < 1·25 | < 1·25 | < 1·25 | < 1·25 | < 1·25 |
| No.2 | 52 | < 0·3 | < 0·3 | < 0·3 | < 0·3 | < 0·3 | < 1·25 | < 1·25 | < 1·25 | < 1·25 | < 1·25 | < 1·25 |
| No.3 | 54 | < 0·3 | < 0·3 | < 0·3 | < 0·3 | < 0·3 | < 1·25 | < 1·25 | < 1·25 | < 1·25 | < 1·25 | < 1·25 |
| No.4 | 50 | < 0·3 | < 0·3 | < 0·3 | < 0·3 | < 0·3 | < 1·25 | < 1·25 | < 1·25 | < 1·25 | < 1·25 | < 1·25 |
| No.5 | 56 | < 0·3 | 12 | 8 | 7 | ND | < 1·25 | < 1·25 | < 1·25 | < 1·25 | < 1·25 | ND |
At the age of 6–7 weeks, 15 HBV-Tg mice (female 10 and male 5) were injected with a single intramuscular injection of 100 µg of pCAGGS(S) (a) or 50 µg of pCAGGS(S) (b). Serum HBsAg and anti-HBs levels were evaluated before and at 2, 4, 8, 12 and 32 weeks after vaccination by chemiluminescence enzyme immunoassay method. The detection limits of HBsAg and anti-HBs by these methods were 0·3 ng/ml and 1·25 mIU/ml, respectively.ND, not determined.
Table 4. Therapeutic value of vaccination of pCAGGS(S + preS2) in HBV-Tg mice.
| HBsAg (ng/ml) | Anti-HBs (mIU/ml) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Before | 2 weeks | 4 weeks | 8 weeks | 12 weeks | 32 weeks | Before | 2 weeks | 4 weeks | 8 weeks | 12 weeks | 32 weeks | |
| (a) 100 µg/mouse | ||||||||||||
| Female HBV-Tg mice | ||||||||||||
| No.1 | 52 | < 0·3 | < 0·3 | < 0·3 | < 0·3 | < 0·3 | < 1·25 | 20·0 | 25·0 | 32 | 156 | 124 |
| No.2 | 50 | < 0·3 | < 0·3 | < 0·3 | < 0·3 | < 0·3 | < 1·25 | 18 | 21 | 24 | 148 | 72 |
| No.3 | 51 | < 0·3 | < 0·3 | < 0·3 | < 0·3 | < 0·3 | < 1·25 | < 1·25 | < 1·25 | < 1·25 | < 1·25 | < 1·25 |
| No.4 | 62 | 28 | 26 | 29 | 25 | 24 | < 1·25 | < 1·25 | < 1·25 | < 1·25 | < 1·25 | < 1·25 |
| No.5 | 58 | < 0·3 | < 0·3 | < 0·3 | < 0·3 | ND | < 1·25 | < 1·25 | < 1·25 | < 1·25 | < 1·25 | ND |
| Male HBV-Tg mice | ||||||||||||
| No.1 | 202 | 54 | < 0·3 | < 0·3 | < 0·3 | < 0·3 | < 1·25 | < 1·25 | < 1·25 | < 1·25 | < 1·25 | < 1·25 |
| No.2 | 260 | 68 | < 0·3 | < 0·3 | < 0·3 | < 0·3 | < 1·25 | < 1·25 | < 1·25 | < 1·25 | < 1·25 | < 1·25 |
| No.3 | 278 | 75 | < 0·3 | < 0·3 | < 0·3 | < 0·3 | < 1·25 | < 1·25 | < 1·25 | < 1·25 | < 1·25 | < 1·25 |
| No.4 | 150 | 52 | < 0·3 | < 0·3 | < 0·3 | < 0·3 | < 1·25 | < 1·25 | < 1·25 | < 1·25 | < 1·25 | < 1·25 |
| No.5 | 280 | 80 | < 0·3 | < 0·3 | < 0·3 | < 0·3 | < 1·25 | < 1·25 | < 1·25 | < 1·25 | < 1·25 | < 1·25 |
| (b) 50 µg/mouse: Female HBV-Tg mice | ||||||||||||
| No.1 | 56 | 30 | < 0·3 | < 0·3 | < 0·3 | < 0·3 | < 1·25 | < 1·25 | < 1·25 | < 1·25 | < 1·25 | < 1·25 |
| No.2 | 53 | 35 | < 0·3 | < 0·3 | < 0·3 | < 0·3 | < 1·25 | < 1·25 | < 1·25 | < 1·25 | < 1·25 | < 1·25 |
| No.3 | 54 | < 0·3 | < 0·3 | < 0·3 | < 0·3 | < 0·3 | < 1·25 | < 1·25 | < 1·25 | < 1·25 | 21 | 68 |
| No.4 | 51 | < 0·3 | < 0·3 | < 0·3 | < 0·3 | < 0·3 | < 1·25 | < 1·25 | < 1·25 | < 1·25 | < 1·25 | < 1·25 |
| No.5 | 57 | < 0·3 | < 0·3 | < 0·3 | < 0·3 | ND | < 1·25 | < 1·25 | < 1·25 | < 1·25 | < 1·25 | ND |
At the age of 6–7 weeks, 15 HBV-Tg mice (female 10 and male 5) were injected with a single intramuscular injection of 100 µg of pCAGGS(S + preS2) (a) or 50 µg of pCAGGS(S + preS2) (b). Serum HBsAg and anti-HBs levels were evaluated before and at 2, 4, 8, 12 and 32 weeks after vaccination by chemiluminescence enzyme immunoassay method. The detection limits of HBsAg and anti-HBs by these methods were 0·3 ng/ml and 1·25 mIU/ml, respectively.ND, not determined.
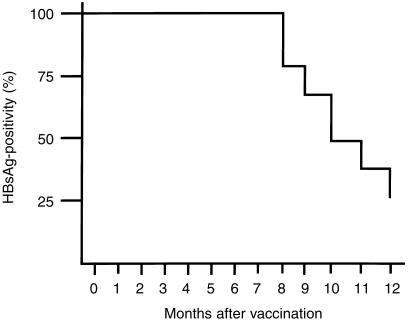
The changes of HBsAg positivity in 29 HBV-Tg mice injected with the HBsAg-based vaccine are shown in Fig. 4. None of the mice became negative for HBsAg until 7 months after the start of therapy. Six of the 29 mice became negative for HBsAg after receiving 8 monthly vaccinations and 21 were negative after 12 months. There was no alteration of the HBsAg titre in HBV-Tg mice injected with CFA alone or in control HBV-Tg mice (data not shown).
Figure 4.
HBsAg-based vaccine therapy in HBV-Tg mice. Twenty-nine HBV-Tg mice were assigned to receive injections of a vaccine containing 10 µg of HBsAg emulsified in CFA at the start of the study and then once a month for 12 consecutive months. No HBV-Tg mice became negative for HBsAg until 7 months after the start of vaccination. Six of the 29 HBV-Tg mice became negative for HBsAg after receiving eight vaccinations with HBsAg in CFA, and 21 mice became negative for HBsAg after 12 months.
The CD4+ T-cell response against HBsAg in HBV-Tg mice vaccinated with pCAGGS(S) or HBsAg-based vaccines are shown in Table 5. Both of these vaccines induced the HBsAg-specific proliferation of CD4+ T cells in the mice.
Table 5. HBsAg-specific CD4 + T cell responses in vaccinated HBV-Tg.
| Mouse | Numbers | Positivity (%) | SI* |
|---|---|---|---|
| DNA-vaccinated HBV-Tg mice | 5 | 5 (100%) | 4·1 ± 0·4 |
| (anti-HBs(+ve)) | |||
| HBsAg-vaccinated HBV-Tg mice | 5 | 5 (100%) | 3·6 ± 0·5 |
| (anti-HBs(+ve)) | |||
HBV-Tg were randomized to receive either a single intramuscular injection of 100 µg of pCAGGS(S) or several intraperitoneal injections of 10·0 µg of HBsAg in CFA at entry and once in every month for 12 consecutive months. CD4+ T cells were isolated from spleens of HBV-Tg mice seropositive for anti-HBs in the sera using an affinity column (Biotex). CD4+ T cells and spleen DC were cultured without or with HBsAg for 120 hr. HBsAg-specific T lymphocyte proliferation was determined from the stimulation index (SI), which was calculated as the cpm of cultures containing HBsAg divided by the c.p.m. of cultures without HBsAg. An SI value of > 2·0 indicated significant HBsAg-specific proliferation.
Mean±SD of five separate experiments.
Discussion
We produced two HBV DNA-encoding plasmid vectors, pCAGGS(S) and pCAGGS(S + preS2) that expressed their gene products in muscle tissue. COS I cells and G8 cells transiently transfected with these two vectors expressed and produced HBsAg, with higher levels of HBsAg being detected after injection of pCAGGS(S + preS2) (Fig. 1).
Detection of anti-HBs is generally being used to assess the HBsAg-specific immune response due to DNA vaccination. However, two of the HBV DNA-vaccinated normal C57BL/6 mice were negative for anti-HBs, but showed an HBsAg-specific cellular immune response (Table 1). Davis et al. have shown that HBV DNA-vaccinated mice simultaneously secrete HBsAg and produce anti-HBs.24,25 Due to this, low levels of anti-HBs may not be detectable in some DNA-vaccinated mice due to circulating HBsAg. Under this circumstances, it is important to always evaluate the HBsAg-specific cellular immune response, in addition to serum anti-HBs level.
Muscle cells and infiltrating mononuclear cells were found to express HBsAg both at and around the site of DNA vaccination. CD11c-positive DC were also found at these locations. Although the expression of HBsAg on DC in the muscle tissue has not been confirmed in this model, DC located near the injection site may take up HBsAg secreted by the muscle cells. Indeed, DC may undergo direct transfection by these plasmids.26 Whatever the mechanism involved, according to the general principles of antigen presentation, DC should capture HBsAg and migrate to regional lymph nodes or secondary lymphoid tissues like the spleen to induce an HBsAg-specific immune response. A single injection of DNA vaccine has been reported to induce a long-lasting HBsAg-specific immune response. We also detected anti-HBs for up to 32 weeks after a single injection of DNA vaccine in some mice. HBsAg-specific memory lymphocytes may be a source of anti-HBs in HBV DNA-vaccinated mice, but it is important to clarify how these memory lymphocytes are activated in vivo to produce anti-HBs. DC isolated at 32 weeks after a single injection of pCAGGS(S) had already been exposed to HBsAg because these DC were able to induce the proliferation of HBsAg-specific memory lymphocytes when cultured in the absence of HBsAg (Table 2b). We also detected HBsAg in the cultures of purified splenic DC from pCAGGS(S)-injected mice. Although the underlying mechanism needs to studied in detail, the persistence of HBsAg in the spleens of DNA-vaccinated mice may have had a cardinal role.
The present experiments also assessed the therapeutic potential of DNA-based versus HBsAg-based vaccines. So far, we have conducted six placebo-controlled trials of HBsAg-based vaccination in HBV-Tg mice.5–8 Consistent with our previous observations, monthly injection of HBsAg-based vaccine for 7 consecutive months did not achieve HBsAg-negativity in any of our HBV-Tg mice (Fig. 4). Only 6 of the 29 (21·0%) HBV-Tg mice became negative for HBsAg after receiving 8 monthly injections of HBsAg in CFA (Fig. 4). At the end of 12 months, however, 21 out of 29 HBV-Tg mice (70%) were negative for HBsAg. In sharp contrast, most of the HBV-Tg mice (28 out of 30) became negative for HBsAg at 2–4 weeks after a single injection of DNA vaccine (Tables 3 and 4). DNA-vaccinated HBV-Tg mice not only showed a rapid anti-HBs response, but these mice revealed an HBsAg-specific CD4+ T-cell proliferative response (Table 5).
In summary, we showed that DNA vaccines encoding HBV envelope protein genes can efficiently elicit both humoral and cellular immune responses in normal mice. The presence of HBsAg-pulsed DC in the spleen at 32 weeks after a single injection of DNA vaccine and the production of HBsAg by spleen DC indicate that these cells may play a cardinal role in long-lasting HBsAg-specific immune responses following a single injection of DNA vaccine. Finally, a DNA-based vaccine may be a better therapeutic choice for the treatment of HBV carriers than an antigen-based vaccine.
Acknowledgments
The authors are grateful to Prof. K.-I. Yamamura, Institute of Molecular Embryology and Genetics, Kumamoto University Medical School, Kumamoto, Japan for generously supplying HBV-Tg mice. We are also grateful to Dr Jun-Ichi Miyazaki, Department of Nutrition and Physiological Chemistry, Osaka University Medical School, Osaka, Japan for kindly providing us with the pCAGGS expression vector. We are also indebted to Dr Kazunori Kajino, Cancer Institute, Tokyo, Japan for providing the HBV genome. The help of Mr Kenji Tanimoto, Ehime University, Ehime, Japan in immunohistochemistry is also appreciated. This study was supported in part by a grant-in-aid from The Suzuken Memorial Foundation, Nagoya, Japan.
Abbreviations
- CFA
complete Freund's adjuvant
- c.p.m.
counts per minute
- DC
dendritic cells
- HBV
hepatitis B virus, HBsAg, hepatitis B surface antigen
- sd
standard deviation
- TA
tibialis anterior muscle
- Tg
transgenic.
References
- 1.Peters M, Vierling J, Grenshwin ME, Millich D, Chisari FV, Hoofnagle JH. Immunology and the liver. Hepatology. 1991;13:977–94. [PubMed] [Google Scholar]
- 2.Bloom BS, Hillman AL, Fendrick AM, Schwartz JS. A reapprisal of hepatitis B virus vaccination strategies using cost-effectiveness analysis. Ann Intern Med. 1993;118:298–306. doi: 10.7326/0003-4819-118-4-199302150-00009. [DOI] [PubMed] [Google Scholar]
- 3.Pol S, Driss F, Michel M, Nalpes B, Berthelot P, Brechot C. Specific vaccine therapy in chronic hepatitis B infection. Lancet. 1994;344:342. doi: 10.1016/s0140-6736(94)91384-6. (Letter) [DOI] [PubMed] [Google Scholar]
- 4.Wen Y-M, Wu X-H, Hu D-C, Zhang Q-P, Guo S-Q. Hepatitis B vaccine and anti-HBs complex as approach for vaccine therapy. Lancet (Letter) 1995;345:1575–6. doi: 10.1016/s0140-6736(95)91126-x. [DOI] [PubMed] [Google Scholar]
- 5.Akbar SMF, Kajino K, Tanimoto T, Kurose K, Masumoto T, Michitaka K, Horiike N, Onji M. Placebo-controlled trial of vaccination with hepatitis B virus surface antigen in hepatitis B virus transgenic mice. J Hepatol. 1997;26:131–7. doi: 10.1016/s0168-8278(97)80019-0. [DOI] [PubMed] [Google Scholar]
- 6.Akbar SMF, Horiike N, Onji M. Prognostic importance of antigen-presenting dendritic cells during vaccine therapy in a murine hepatitis B virus carrier. Immunology. 1999;96:98–108. doi: 10.1046/j.1365-2567.1999.00668.x. 10.1046/j.1365-2567.1999.00668.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Akbar SMF, Abe M, Masumoto T, Horiike N, Onji M. Mechanism of action of vaccine therapy in murine hepatitis B virus carrier: vaccine-induced activation of antigen presenting dendritic cells. J Hepatol. 1999;30:755–64. doi: 10.1016/s0168-8278(99)80125-1. [DOI] [PubMed] [Google Scholar]
- 8.Akbar SMF, Yamamoto K, Abe M, et al. Potent synergistic effect of sho-saiko-to (TJ-9), a herbal medicine during vaccine therapy in a murine model of hepatitis B virus (HBV) -carrier. Eur J Clin Invest. 1999;29:786–92. doi: 10.1046/j.1365-2362.1999.00533.x. 10.1046/j.1365-2362.1999.00533.x. [DOI] [PubMed] [Google Scholar]
- 9.Pourcelli C, Tiollais P, Farza H. Transcription of the S gene in transgenic mice is associated with hypomethylation at specific sites and with DNase I sensitivity. J Virol. 1990;64:931–5. doi: 10.1128/jvi.64.2.931-935.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kadchouel M, Farza H, Simon D, Tiollais P, Pourcel C. Maternal inhibition of hepatitis B surface antigen gene expression in transgenic mice correlates with de novo methylation. Nature. 1987;329:454–6. doi: 10.1038/329454a0. [DOI] [PubMed] [Google Scholar]
- 11.Geissler M, Schirmbeck R, Reimann J, Blum HE, Wands JR. Cytokine and hepatitis B virus DNA co-immunizations enhance cellular and humoral responses to the middle but not to the large hepatitis B virus surface antigen in mice. Hepatology. 1998;28:202–9. doi: 10.1002/hep.510280126. [DOI] [PubMed] [Google Scholar]
- 12.Chow Y-H, Huang W-L, Chi W-K, Chu Y-D, Tao M-H. Improvement of hepatitis B virus DNA vaccines by plasmids coexpressing hepatitis B surface antigen and interleukin-2. J Virol. 1997;71:169–78. doi: 10.1128/jvi.71.1.169-178.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Geissler M, Tokushige K, Chante CC, Zurawski VR, Wands JR. Cellular and humoral immune response to hepatitis B virus structural proteins in mice after DNA-based immunization. Gastroenterology. 1997;112:1307–20. doi: 10.1016/s0016-5085(97)70145-8. [DOI] [PubMed] [Google Scholar]
- 14.Michel M-L, Davis HL, Schileef M, Mancini M, Tiollais P, Whalen RG. DNA-mediated immunization to the hepatitis B surface antigen in mice: aspects of the humoral response mimic hepatitis B viral infection in humans. Proc Natl Acad Sci USA. 1995;92:5307–11. doi: 10.1073/pnas.92.12.5307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Davis HL, Mancini M, Michel M-L, Whalen RG. DNA-mediated immunization to hepatitis B surface antigen: longevity of primary response and effect of boost. Vaccine. 1996;14:910–5. doi: 10.1016/0264-410x(95)00255-y. 10.1016/0264-410x(95)00255-y. [DOI] [PubMed] [Google Scholar]
- 16.Mancini M, Hadchouel M, Davis HL, Whalen RG, Tiollais P, Michel M-L. DNA-mediated immunization in a transgenic mouse model of the hepatitis B surface antigen chronic carrier state. Proc Natl Acad Sci USA. 1996;93:496–501. doi: 10.1073/pnas.93.22.12496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Davis HL, Millan CLB, Mancini M, et al. DNA-based immunization against hepatitis B surface antigen (HBsAg) in normal and HBsAg-transgenic mice. Vaccine. 1997;15:849–52. doi: 10.1016/s0264-410x(96)00267-8. 10.1016/s0264-410x(96)00267-8. [DOI] [PubMed] [Google Scholar]
- 18.Araki K, Miyazaki J, Hino O, Tomita N, Chisaka O, Matsubara K, Yamamura K. Expression and replication of hepatitis B virus genome in transgenic mice. Proc Natl Aca Sci USA. 1989;86:207–11. doi: 10.1073/pnas.86.1.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Niwa H, Yamamura K-I, Miyazaki J-I. Efficient selection for high expression transfectants with a novel eukaryotic vector. Gene. 1991;108:192–200. doi: 10.1016/0378-1119(91)90434-d. [DOI] [PubMed] [Google Scholar]
- 20.Akbar SMF, Inaba K, Onji M. Upregulation of MHC class II antigen on dendritic cells from hepatitis B virus transgenic mice by γ-interferon: abrogation of immune response defect to a T-cell-dependent antigen. Immunology. 1996;87:519–27. doi: 10.1046/j.1365-2567.1996.516576.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Akbar SMF, Onji M, Inaba K, Yamamura K, Ohta Y. Low responsiveness of hepatitis B virus transgenic mice in antibody response to T-cell-dependent antigen: defect in antigen presenting activity of dendritic cells. Immunology. 1993;78:468–73. [PMC free article] [PubMed] [Google Scholar]
- 22.Tanimoto K, Akbar SMF, Michitaka K, Onji M. Immunohistochemical localization of antigen presenting cells in liver from patients with primary biliary cirrhosis; highly restricted distribution of CD83 positive activated dendritic cells. Pathol Res Pract. 1999;195:157–62. doi: 10.1016/S0344-0338(99)80028-4. [DOI] [PubMed] [Google Scholar]
- 23.Chen S, Akbar SMF, Tanimoto K, Ninomiya T, Iuchi H, Michitaka K, Horiike N, Onji M. Absence of CD83-positive mature and activated dendritic cells at cancer nodules from patients with hepatocellular carcinoma: relevance to hepatocarcinogenesis. Cancer Lett. 1999;148:48–57. doi: 10.1016/s0304-3835(99)00312-2. [DOI] [PubMed] [Google Scholar]
- 24.Davis HL, Michel M-L, Whalen RG. DNA-based immunization induces continuous secretion of hepatitis B surface antigen and high levels of circulating antibody. Hum Mol Genet. 1993;2:1847–51. doi: 10.1093/hmg/2.11.1847. [DOI] [PubMed] [Google Scholar]
- 25.Davis HL, Michel M-L, Mancini M, Schleef M, Whalen RG. Direct gene transfer in skeletal muscle: plasmid DNA-based immunization against the hepatitis B virus surface antigen. Vaccine. 1994;12:1503–9. doi: 10.1016/0264-410x(94)90073-6. [DOI] [PubMed] [Google Scholar]
- 26.Akbari O, Panjwani N, Garcia S, Tascon R, Lowrie D, Stockinger B. DNA vaccination: transfection and activation of dendritic cells as key events for immunity. J Exp Med. 1999;189:169–77. doi: 10.1084/jem.189.1.169. [DOI] [PMC free article] [PubMed] [Google Scholar]