Abstract
Synthetic lipopeptides based on bacterial lipoprotein are efficient activators for monocytes/macrophages inducing the release of interleukin (IL)-1, IL-6, tumour necrosis factor-α (TNF-α), reactive oxygen/nitrogen intermediates, and the translocation of nuclear factor κB (NFκB). In this report we investigate the signal transduction pathways involved in leucocyte activation by the synthetic lipopeptide N-palmitoyl-S-[2,3-bis(palmitoyloxy)-(2R,S)-propyl]-(R)-cysteinyl-seryl-(lysyl)3-lysine (P3CSK4). We show that P3CSK4 activates mitogen-activated protein (MAP)-kinases ERK1/2 and MAP kinase (MAPK)-kinases MEK1/2 in bone-marrow-derived macrophages (BMDM) and in the macrophage cell line RAW 264·7. Additionally, we could detect differences between the P3CSK4 and lipopolysaccharide (LPS)-induced phosphorylation of MAP kinases: Different levels in phosphorylation were found both in kinetics and dose–response using RAW 264·7 cells or BMDM from BALB/c and LPS responder mice (C57BL/10ScSn) or LPS non-responder mice (C57BL/10ScCr). The lipopeptide activated the MAPK-signalling cascade in both LPS responder and non-responder macrophages, whereas LPS induced the MAPK signalling pathway only in macrophages derived from LPS responder mice. An approximately 70% decrease of lipopeptide induced NFκB translocation and an about 50% reduction of nitric oxide (NO) release was observed in the presence of anti-CD14. These data correspond to the reduction of phosphorylation of ERK1/2 after stimulation with P3CSK4 in the presence of anti-CD14 antibodies. Inhibition of MEK1/2 by PD98059 completely reduced the lipopeptide-induced phosphorylation of ERK1/2 indicating that MEK1/2 are solely responsible for the phosphorylation of the downstream-located MAP kinases ERK1/2.
Introduction
Synthetic lipopeptides derived from bacterial lipoprotein constitute potent B-lymphocyte and macrophage/monocyte activators in vitro1–3 inducing the release of a broad range of cytokines and reactive oxygen and nitrogen intermediates. In vivo they function as immunoadjuvants in parenteral, nasal and oral immunization either in combination or after covalent linkage with antigens, non- or low immunogenic substances or toxins.4–8 Synthetic lipopeptides are well defined and can be synthesized easily and reproducibly9–11 in large amounts under quality assurance conditions. They are exhibiting an excellent purity, lack of toxicity, and long-term stability and can substitute for Freund's adjuvant with respect to efficiency; they lack side-effects and inflammatory reactions.7,12,13 Recent investigations indicate that lipopeptides bind to distinct membrane-localized compounds (Toll-like receptor (TLR)2, CD14);14,15 on the other hand they are, within minutes, distributed throughout the cell including the nucleus.16 This suggests two modes of action: a direct influence by targeting the nucleus which is favoured by ‘built in’ nuclear localization sequences, and binding to distinct membrane compounds leading to the induction of signal transduction pathways similar as known for lipopolysaccharide (LPS). We focused our studies on the effect of lipopeptides on the mitogen-activated protein (MAP) kinase cascades. Until now, five distinct evolutionary conserved MAP kinase signalling cascades have been identified. These pathways include the mitogenic ERK1/2 cascade,17 the stress activated JNK and p38 MAP kinase cascades,18 and the ERK3 and ERK5 pathways. All MAP kinase pathways are organized in a conserved three-kinase architecture consisting of a MAP kinase, a MAP kinase kinase (MAP kinase activator), and a MAP kinase kinase kinase which activates the MAP kinase activator.19 As the ERK1/2 cascade17 plays a significant role in mitogenesis, differentiation, cellular transformation, regulation of cell motility, and further cellular events20–29 we studied the influence of N-palmitoyl-S-[2,3-bis(palmitoyloxy)-(2R,S)-propyl]-(R)-cysteinyl-seryl-(lysyl)3-lysine (P3CSK4) on this signal transduction pathway. Our results indicate (1) the activation of the ERK1/ERK2 signalling pathway by the lipopeptide and (2) clear differences between LPS and P3CSK4 with respect to the induction of the ERK1/ERK2 signal transduction pathway by using macrophages derived from LPS responder and non-responder mice. In addition our investigations clearly indicate that P3CSK4-dependent nitric oxide (NO) induction and nuclear factor κB (NFκB) translocation is due to a CD14-dependent process.
Materials and Methods
Reagents
Anti-CD14 polyclonal antibody (CD14 (M-20): sc-6999) was purchased from Santa Cruz Biotechnology, Inc., CA; P3CSK4 was prepared by chemical synthesis as described previously10 and was obtained from EMC, Tübingen, Germany. LPS from Salmonella abortus equi was a kind gift from C. Galanos, Freiburg, Germany.
Mice
Six to 10-week-old BALB/c, C57BL/10 ScCr, and C57BL/10 ScSn mice were obtained from the breeding facilities of the Max-Planck-Institut für Immunbiologie, Freiburg, Germany.
Macrophage cell lines
The murine macrophage cell line RAW 264·7 was cultured in Petri dishes (Falcon, Becton Dickinson, Heidelberg, Germany) in cRPMI-1640 media (RPMI-1640 containing 10% heat inactivated fetal calf serum (FCS), 1% non-essential amino acids (NEAA), 100 U/ml penicillin, 100 µg/ml streptomycin, and 2·5 g/l glucose) at 37° and 5% CO2.
Bone-marrow-derived macrophages (BMDM)
BMDM were differentiated in vitro from bone marrow precursor cells as described by Hoffmann et al.30 Briefly, bone marrow cells were flushed from femurs and tibia of 6–10-week-old mice, washed twice in RPMI-1640 and grown for 17 days in liquid cultures in Teflon film bags (SLG, Gauting, Germany) at 37° and 5% CO2. The culture medium consisted of RPMI-1640 supplemented with 15% L-cell-conditioned medium as a source of macrophage colony-stimulating factor (M-CSF), 10% heat inactivated FCS, 5% heat inactivated horse serum, 1 mm sodium pyruvate (both from Seromed Biochrom KG, Germany), 50 U/ml penicillin, 50 µg/ml streptomycin, and 5 × 10−5 m mercaptoethanol. Cultures were set up with 6 × 106 cells/50 ml. After harvesting, the macrophages were washed once, counted and resuspended at 2 × 106 cells/ml. For the preparation of L-cell-conditioned medium, 1 × 105 L929 cells/ml were cultured in 100 ml-batches in cell culture flasks (Falcon, Becton Dickinson) in cRPMI-1640 at 37° and 5% CO2. After 7 days, the culture supernatants were harvested, cleared from cell debris by centrifugation (1500 g, 4°, 15 min) and stored at −20°.
Induction and determination of NO release in BMDM and the macrophage cell line RAW 264·7
BMDM and RAW 264·7 were harvested, washed once and resuspended in RPMI-1640 medium. 1 × 105 cells/well were seeded into 96-well flat bottom microtitre plates (Falcon, Becton Dickinson) and stimulated with different concentrations of P3CSK4 or LPS in a total volume of 150 µl. Culture supernatants were harvested after 48 hr (BMDM) or 24 hr (RAW 264·7). All assays were performed in triplicate. Production of NO was determined by measuring nitrite, a stable metabolite of NO, in the culture supernatants using the Griess reaction.31 Accordingly, 100 µl culture supernatant were mixed with 100 µl Griess reagent (1% sulphanilamide and 0,1% N-(1-naphtyl)ethylendiamine in 2,5% phosphoric acid), and the absorbance at 550 nm was monitored with a Dynatec MRX enzyme-linked immunosorbent assay (ELISA) plate reader (Dynatec, Denkendorf, Germany). Nitrite concentrations were calculated by using sodium nitrite as a standard.
Stimulation of BMDM and macrophage cell lines and preparation of cellular extracts
Seventeen-day-old BMDM, or RAW 264·7 cells (1 × 106), were seeded into the wells of 24-well-plates in RPMI-1640 with NEAA and antibiotics, incubated overnight and then stimulated with various concentrations of P3CSK4 or LPS for different periods of time. The plates were placed on ice, the medium was replaced by 100 µl/well lysis buffer (62·5 mm tris(hydroxymethyl)aminomethane/HCl, pH 6·8, containing 10 mm dithiothreitol, 2·5% sodiumdodecyl sulphate, 1 mm Na3VO4, 50 mm NaF, 0·05% bromophenol blue, one protease-inhibitor-cocktail pill/10 ml (Boehringer Mannheim, Germany), 7·4 mm tris(hydroxymethyl)aminomethane, 0·06 mm glycine, and 15·5 mm glycerol). The cell lysate was removed with a syringe, placed in a reaction vial, incubated at 95° for 5 min, centrifuged (30 s, 13 250 g.) and stored frozen at −70°.
Polyacrylamide gel electrophoresis and Western blotting
Polyacrylamide gel electrophoresis was performed according to Laemmli32 using 10% resolution gels with 5% stacking gels. Transfer of the separated samples onto a polyvinylidene fluoride (PVDF) transfer membrane (Hybond-P, RPN303F, Amersham, Germany) was performed with a Mini-Protean II system (BioRad, München, Germany). PVDF membranes were blocked overnight at 4° in blocking buffer (20 mm tris(hydroxymethyl)aminomethane/HCl, pH 7·6, containing 0·5 m NaCl, 0·05% Tween-20, and 5% skimmed milk powder) and then incubated for 1 hr at room temperature in blocking buffer containing antiphospho-p44/42 MAP kinase E10 monoclonal antibody (New England BioLabs, Beverly, MA) or antiphospho MEK1/2 kinase antibody (New England BioLabs). After washing three times with TTBS (20 mm tris(hydroxymethyl)aminomethane/HCl, pH 7·6, containing 0·5 m NaCl and 0·05% Tween-20), the membranes were incubated in blocking buffer with horseradish peroxidase (HRP)-conjugated second antibodies for 1·5 hr at room temperature (rabbit anti-mouse immunoglobulin G (IgG) peroxidase conjugated, Tago, CA/goat anti-rabbit immunoglobulin peroxidase conjugated; Dako, Denmark). After washing three times with TTBS and once with Tris-buffered saline (TBS; 20 mm tris(hydroxymethyl)aminomethane/HCl, pH 7·6, containing 0·5 m NaCl and 0·05% Tween-20), bound antibodies were detected using the ECL+ Plus Western Blotting Detection System (Amersham Pharmacia Biotech, Germany).
Stimulation of cells and analysis of NFκB translocation
Indirect immunofluorescence analyses were performed as previously described.33 BMDM of BALB/c mice were seeded into an eight-well glass slide (Nunc, Denmark) at a concentration of 2·5 × 104 cells/well and allowed to grow to about 70% confluency. Cells were washed with phosphate-buffered saline (PBS) and fixed in 3·5% paraformaldehyde/PBS for 15 min at room temperature. Cells were permeabilized in PBS containing 0·5% saponin, 0·2% BSA, 2% FCS, and 5 mm MgCl2 for 10 min. All further washes and incubations were carried out in the same solution containing 0·05% saponin. After fixation and permeabilization, cells were washed three times for 3 min with constant gentle agitation. For detection of NFκB, cells were incubated for 2 hr with an anti-NFκB antibody (rabbit polyclonal IgG, NFκB p65, SC-109, Santa Cruz Biotechnology, Santa Cruz, CA) at a 1:40 dilution. Subsequently, cells were washed 5 × 3 min, followed by addition of the secondary antibody (goat anti-rabbit IgG, biotin-conjugated, Sigma, Deisenhofen, Germany), diluted 1:50 and incubated for 2 hr at room temperature. Fluoroscein isothiocyanate (FITC)-labelled avidin (Sigma), diluted 1:50, was added and the cells were further incubated for 2 hr in the dark. Cells were washed 6 × 3 min with occasional agitation at room temperature and then examined under a fluorescence microscope (Polyvar, Reichert-Jung, Jena, Germany).
Results
Induction of MAP kinase phosphorylation by P3CSK4
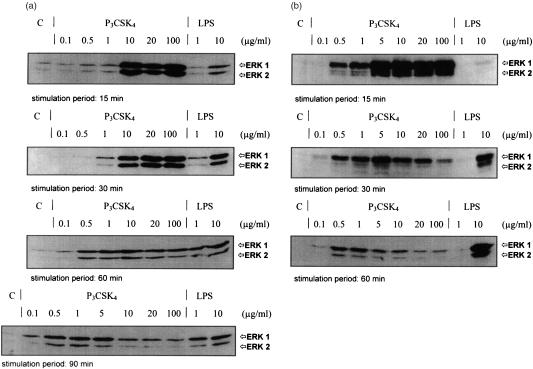
The activation of MAP kinases (p42/44) requiring phosphorylation at threonine and tyrosine was monitored by immunoblot analysis using anti-phospho-specific MAP kinase antibodies. Stimulation with P3CSK4 of the macrophage cell line RAW 264·7 or of BMDM from BALB/c mice resulted in a dose-dependent modulation of phosphorylation of the MAP kinases ERK1 and ERK2. As shown in Fig. 1(a), short stimulation periods (15 min) of RAW 264·7 cells with P3CSK4 led to an increased phosphorylation of MAP kinases ERK1/2, when using concentrations in the range of 10–100 µg/ml. Similar results were obtained with BMDM from BALB/c mice when stimulated with P3CSK4 in the concentration range of 5–100 µg/ml (Fig. 1b). Lower P3CSK4 concentrations (0·1–1·0 µg/ml) showed no increase in phosphorylation after 15 min, followed by a slight increase after 30, 60–90 min only for RAW 264·7 cells (Fig. 1a). In BMDM from BALB/c mice low P3CSK4 concentrations (0·1–1·0 µg/ml) had a similar effect (Fig. 1b). When stimulating cells with high P3CSK4 concentrations (5–100 µg/ml) we observed a rapid decrease of the high phosphorylation level found after 15 min with time with respect to both cell lines (Fig. 1a, b). In contrast, stimulation with LPS, which is known to activate both MAP kinases, led to a dose- and stimulation time-dependent steady increase in phosphorylation: from 1 to 10 µg/ml LPS and/or from short (15 min) to longer (90 and 60 min) stimulation time intervals an increased phosphorylation of ERK1 and ERK2 was detected in RAW 264·7 cells and BMDM (Fig. 1a, b).
Figure 1.
Induction of MAP kinase (ERK1/2) phosphorylation of P3CSK4- and LPS-stimulated RAW 264·7 cells (a) and BMDM from BALB/c mice (b). (a) RAW 264·7 cells were stimulated for 15, 30, 60 and 90 min with the indicated amounts of P3CSK4 and LPS and the induced phosphorylation of MAP kinases was monitored by Western blot using phospho-p44/42 MAP kinase E10 monoclonal antibodies. Bands were visualized by the enhanced chemiluminescence method as described in Materials and Methods. (b) BMDM from BALB/c mice were stimulated for 15, 30, and 60 min with the indicated amounts of P3CSK4 and LPS. The resulting MAP kinase phosphorylation was detected as described under (a). C represents resting, unstimulated cells (control).
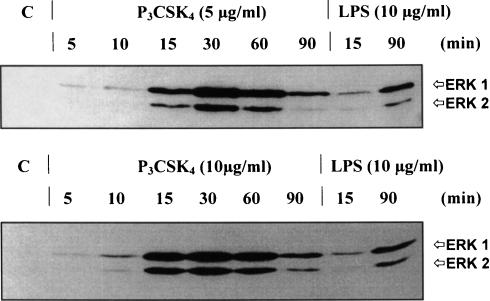
Figure 2 shows the kinetics of the P3CSK4 induced phosphorylation of the MAP kinases using two lipopeptide concentrations (5 and 10 µg/ml). P3CSK4-induced MAP kinase phosphorylation was already detected after 5 min, increased to a maximum at 30 min (5 µg/ml P3CSK4) or at 15–60 min (10 µg/ml P3CSK4), before decreasing to lower levels by 90 min. In contrast, LPS (10 µg/ml) induced a low signal at 15 min, and a more pronounced signal at 90 min indicating different activation processes of ERK1/2 with respect to LPS and P3CSK4.
Figure 2.
Kinetics of P3CSK4- and LPS-induced MAP kinase (ERK1/2) phosphorylation in RAW 264·7 cells. RAW 264·7 cells were stimulated for 5, 10 15, 30, 60 and 90 min with either 5 (upper panel) or 10 µg (lower panel) P3CSK4. Phosphorylation of MAP kinases (ERK1/2) was monitored by immunoblot using phospho-p44/42 MAP kinase E10 monoclonal antibodies. Bands were visualized by the enhanced chemiluminescence method. LPS (10 µg/ml) was used as a positive control (15 and 90 min stimulation periods). C represents resting, unstimulated cells (control).
Induction of MAPK kinase phosphorylation by P3CSK4
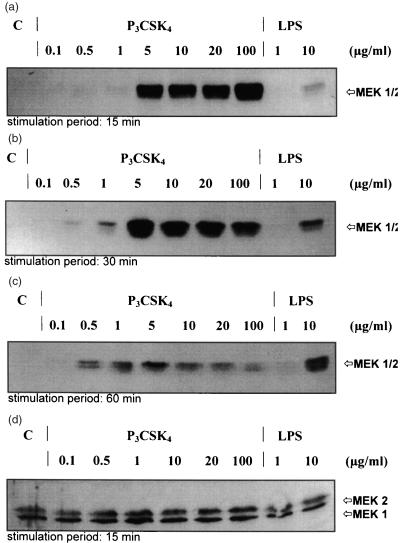
Activation of the upstream MAP kinase regulator proteins MEK1 and MEK2 (MAPK kinases) occurs through phosphorylation of two serine residues at position 217 and 221. Therefore, an anti-phospho-MEK antibody was used for monitoring by immunoblot analysis the MEK1/2 activation stage; this antibody selectively recognizes activated, phosphorylated MEK1/2. As shown in Fig. 3, incubation of RAW 264·7 cells with P3CSK4 for 15, 30, and 60 min resulted in a dose-dependent increase of phosphorylation of MEK1 and MEK2. Again, as observed for ERK1/2, high amounts of P3CSK4 (5–100 µg/ml) resulted already after short stimulation periods (15 min) in high phosphorylation levels; longer stimulation intervals (30–60 min) induced an increase of phosphorylation only at lower P3CSK4 concentrations (0·5–1 µg/ml) but lead to a decrease of phosphorylation at higher concentrations. In contrast, stimulation with LPS showed a dose- and time-dependent steady increase in activation of both MEK1 and MEK2 (Fig. 3a–c, lanes 9 and 10). All samples used to detect phosphorylation contained a comparable amount of MEK1 and MEK2 (Fig. 3d), indicating that the detected differences in phosphorylation are not caused by different protein levels of MEK1 and MEK2, and suggesting that lipopeptide and LPS are not inducing de novo MEK1 and MEK2 protein expression. P3CSK4 and LPS are thus inducing different levels of phosphorylation suggesting different signal transduction mechanisms for both compounds with respect to macrophages.
Figure 3.
Induction of MAPK kinase (MEK1/2) phosphorylation of P3CSK4- and LPS-stimulated RAW 264·7 cells. RAW 264·7 cells were stimulated for 15 (a), 30 (b), and 60 (c) minutes with the indicated amounts of P3CSK4 and LPS and the induced phosphorylation of MAPK kinases (MEK1/2) was monitored by Western blot using phospho-MEK1/2 kinase monoclonal antibodies. Bands were visualized by the enhanced chemiluminescence method as described in Materials and Methods. (d) represents an immunoblot, which was performed with anti MEK1 (12-B) polyclonal antibodies after a 15-min stimulation period. Anti-MEK1(12-B) polyclonal antibodies recognize the phosphorylated and non-phosphorylated forms of MEK1/2. C represents resting, unstimulated cells (control).
Induction of MAP kinase phosphorylation in BMDM from LPS responder/LPS non-responder mice
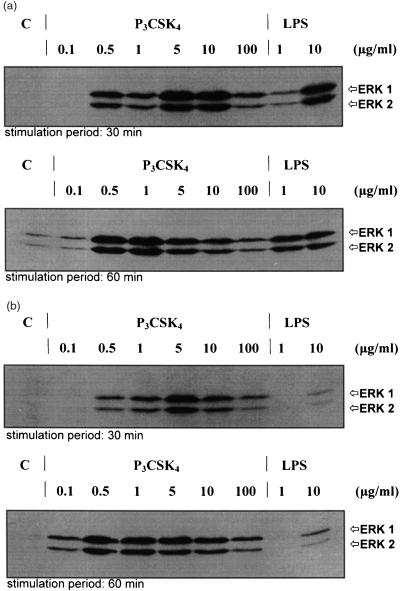
Mutation of TLR4 in mice of the strain C57BL/10ScCr impedes LPS-induced signal transduction preventing the response to LPS. Animals of the control strain C57BL/10ScSn are normally responsive.34 In order to further elucidate the signal transduction mechanisms induced by P3CSK4 and to detect possible differences to LPS stimulation, BMDM derived from LPS responder and non-responder mice were stimulated with both compounds and monitored for ERK1 and ERK2 activation. As can be seen in Fig. 4(a), cells of the LPS responder strain C57BL/10ScSn showed a strong, dose-dependent activation of the MAP kinases ERK1 and ERK2 following P3CSK4 and LPS stimulation. Figure 4 (a, upper panel) represents the MAP kinase phosphorylation patterns obtained after a 30-min stimulation with P3CSK4 (0·1, 0·5, 1, 5, 10, and 100 µg/ml) or LPS (1 and 10 µg/ml). MAP kinase activation started when stimulated for 30 min at 0·5 µg/ml P3CSK4, reached an optimum at 5 and 10 µg/ml P3CSK4 and decreased to lower phosphorylation levels at 100 µg/ml P3CSK4; no activation was observed following stimulation with 0·1 µg/ml P3CSK4 and in control cells. Sixty-minute stimulation periods (Fig. 4a, lower panel) resulted in a shift of the above observed phosphorylation pattern to lower P3CSK4 concentrations: MAP kinase activation started at 0·1 µg/ml P3CSK4, reached an optimum at 0·5 and 1 µg/ml P3CSK4 and decreased to lower phosphorylation levels over 5, 10–100 µg/ml P3CSK4. With respect to LPS, a strong phosphorylation was detected at 10 µg/ml (30 min stimulation), which was comparable to that obtained after stimulation (30 min) with 10 µg/ml P3CSK4. Stimulation of C57BL/10ScSn cells with 1 µg/ml LPS for 30 min resulted in an activation of MAP kinase which reached about 1/10 of that observed after stimulation using 10 µg/ml LPS. MAP kinase phosphorylation levels increased from 30 min to 60 min stimulation periods when using 1 µg/ml LPS but decreased for about 50% when stimulating the cells for 60 min with 10 µg/ml LPS (compared to a stimulation period of 30 min). Incubation of BMDM prepared from the LPS non-responder strain C57BL/10 ScCr with P3CSK4 resulted in activation patterns comparable to the responder strain (Fig. 4b). In contrast, incubation with LPS induced no phosphorylation of ERK1 and ERK2 at concentrations of 1 µg/ml, and the phosphorylation levels after 30 and 60 min stimulation periods were drastically reduced when using 10 µg/ml LPS (Fig. 4b, upper and lower panel). Our results clearly indicate different signal transduction mechanisms for P3CSK4 and LPS.
Figure 4.
Induction of MAP kinase (ERK1/2) phosphorylation of P3CSK4-and LPS-stimulated BMDM from C57 BL/10 ScSn (a) and C57 BL/10 ScCr mice (b). (a) BMDM from C57 BL/10 ScSn (LPS responder) mice were stimulated for 30 and 60 min with the indicated amounts of P3CSK4 and LPS and the induced phosphorylation of the MAP kinases ERK1 and ERK2 was monitored by Western blot using phospho-p44/42 MAP kinase E10 monoclonal antibodies. Bands were visualized by the enhanced chemiluminescence method as described in Materials and Methods. (b) BMDM from C57 BL/10 ScCr (LPS non-responder) mice were stimulated for 30 and 60 min with the indicated amounts of P3CSK4 and LPS. The resulting MAP kinase phosphorylation was detected as described under (a). C represents resting, unstimulated cells (control).
Induction of NFκB translocation in BMDM derived from LPS responder/LPS non-responder and BALB/c mice and in RAW 264·7 cells
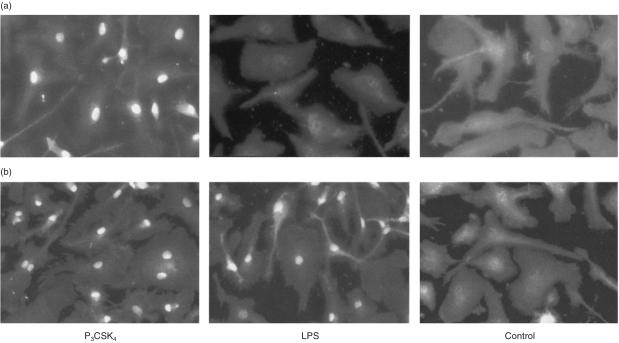
Stimulation of BMDM from LPS non-responder mice (C57BL/10ScCr) with P3CSK4 resulted in translocation of NFκB into the cell nucleus (Fig. 5a, left panel), which corresponds to the observed ERK1/2 and MEK1/2 phosphorylation. In contrast, LPS-stimulation of BMDM from LPS non-responder mice did not induce translocation of p65 NFκB into the cell nucleus (Fig. 5a, middle panel) corresponding again to the observed lack of phosphorylation of ERK1/2 and MEK1/2. BMDM from LPS responder mice, however, after P3CSK4 or LPS stimulation showed a translocation of p65 NFκB into the nucleus (Fig. 5b, left and middle panels). These different effects of P3CSK4 and LPS in LPS responder and non-responder mice indicate that the P3CSK4-induced signalling pathway is, in contrast to the LPS-activation, TLR4 independent.
Figure 5.
P3CSK4- and LPS-dependent induction of NFκB translocation in BMDM from C57 BL/10 ScCr (a) and C57 BL/10 ScSn (b) mice. (a) BMDM from C57 BL/10 ScCr (LPS non-responder) mice were stimulated for 1 hr with either P3CSK4 (1 µg/ml) or LPS (1 µg/ml). Subsequently, the cells were immunostained with anti-NFκB p65, biotinylated goat anti-rabbit IgG and FITC-conjugated avidin, as described in Materials and Methods. The control represents resting, unstimulated macrophages derived from the bone marrow of C57 BL/10 ScCr mice. (b): BMDM from C57 BL/10 ScSn (LPS responder) mice were stimulated for 1 hr with either P3CSK4 (1 µg/ml) or LPS (1 µg/ml). The resulting NFκB translocation was monitored as described under (a). The control represents resting, unstimulated BMDM from the mice strain C57 BL/10 ScSn.
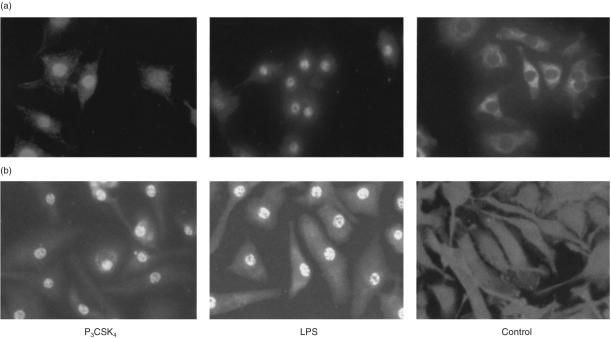
Analysis of the subcellular distribution of the p65 NFκB in RAW 264·7 cells after stimulation of the cells with P3CSK4 (1 µg/ml) for 2 hr resulted in NFκB translocation into the nucleus (Fig. 6a, left panel); similar results were obtained after treatment with LPS (1 µg/ml) which was used as a positive control (Fig. 6a, middle panel). No NFκB translocation was observed in non-treated control cells (Fig. 6a, right panel). Analysis of the subcellular distribution of the p65 NFκB in BMDM derived from the LPS responder BALB/c mice resulted after treatment of the cells with P3CSK4 (1 µg/ml) for 2 hr in NFκB translocation into the nucleus (Fig. 6b, left panel); similar results were obtained after stimulation with LPS (1 µg/ml), which was used as a positive control (Fig. 6b, middle panel). No NFκB translocation was observed in control cells (Fig. 6b, right panel).
Figure 6.
P3CSK4-and LPS-dependent induction of NFκB translocation in RAW 264·7 cells (a) and BMDM from BALB/c mice (b). (a) RAW 264·7 cells were stimulated for 2 hr with either P3CSK4 (1 µg/ml) or LPS (1 µg/ml). Subsequently, the cells were immunostained with anti-NFκB p65 antibodies. Bound antibodies were visualized by biotinylated goat anti-rabbit IgG and FITC-conjugated avidin, as described in Materials and Methods. The control represents resting, unstimulated RAW 264·7 cells. (b) BMDM from BALB/c mice were stimulated for 2 hr with either P3CSK4 (1 µg/ml) or LPS (1 µg/ml). The resulting NFκB translocation was monitored as described under (a). The control represents resting, unstimulated macrophages derived from the bone marrow of BALB/c mice.
Induction of NO release by P3CSK4 and LPS in BMDM derived from LPS responder/LPS non-responder and BALB/c mice, and in RAW 264·7 cells
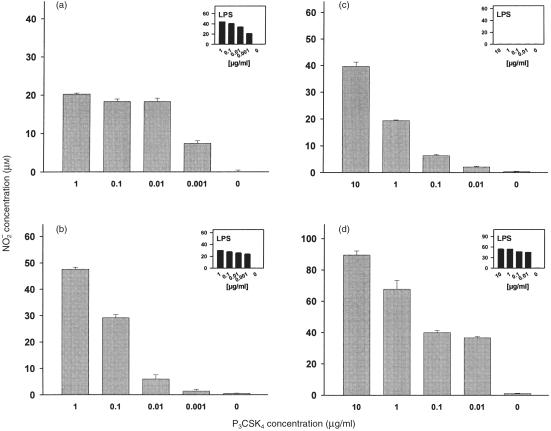
We examined the release of NO from the macrophage cell line RAW 264·7 and BMDM from BALB/c mice after stimulation with P3CSK4. Cells were stimulated for 24 hr (RAW 264·7) or 48 hr (BMDM), subsequently the culture supernatants were monitored for nitrite contents. As shown in Fig. 7, P3CSK4 induced the release of NO in a dose-dependent manner in both, RAW 264·7 cells (Fig. 7a) and BMDM from BALB/c mice (Fig. 7b). A similar dose–response-dependent induction of NO release was observed for both cell lines after stimulation with LPS (Fig. 7a, b, inserts). No induction of NO release was observed when stimuli were omitted.
Figure 7.
Dose-dependent induction of NO release in RAW 264·2 cells (a), BMDM derived from BALB/c mice (b), and BMDM derived from C57 BL/10 ScCr (c) and C57 BL/10 ScSn mice (d) by P3CSK4 and LPS. (a) RAW 264·2 cells were seeded into microtitre plates (1 × 105 cells/well). After 24 hr medium was removed and the cells were stimulated with P3CSK4 or LPS for 24 hr at the indicated concentrations. Total volume/well was 150 µl. NO release was determined by measuring the nitrite concentrations in the culture supernatants. Values represent means ±SD of triplicate cultures. (b) BMDM derived from BALB/c mice. (c) BMDM derived from LPS non-responder mice (C57BL/10ScCr). (d) BMDM prepared from LPS-responder mice (C57BL/10ScSn). Primary macrophages (b, c, d) were seeded into microtitre plates (1 × 105 cells/well) and the cells were stimulated with P3CSK4 and LPS for 48 hr at the indicated concentrations. NO induction was monitored as described under (a). Values represent means ±SD of triplicate cultures.
BMDM prepared from both, LPS responder (C57BL/10ScSn) and LPS non-responder (C57BL/10ScCr) mice released comparable quantities of NO after stimulation with P3CSK4 (Fig. 7c, d). In addition, the kinetics of P3CSK4-induced NO production were comparable in BMDM prepared from C57BL/10ScSn and C57BL/10ScCr animals (data not shown). Corresponding to the lacking ERK1/2/MEK1/2 phosphorylation and NFκB translocation, BMDM prepared from the C57BL/10ScCr mice (LPS non-responder) were not able to respond to LPS-stimulation. No induction of NO release was detected after a 48-hr stimulation period with LPS (Fig. 7c, insert). In contrast, BMDM derived from closely related C57BL/10ScSn mice (LPS responder) showed a strong enhancement of NO release after LPS stimulation for 48 hr (Fig. 7d, insert), which was similar to that observed with BMDM derived from BALB/c mice also containing an intact TLR4 receptor (Fig. 7b, insert).
Inhibition of P3CSK4-and LPS-dependent induction of NO release in BMDM by anti-CD14-directed antibodies
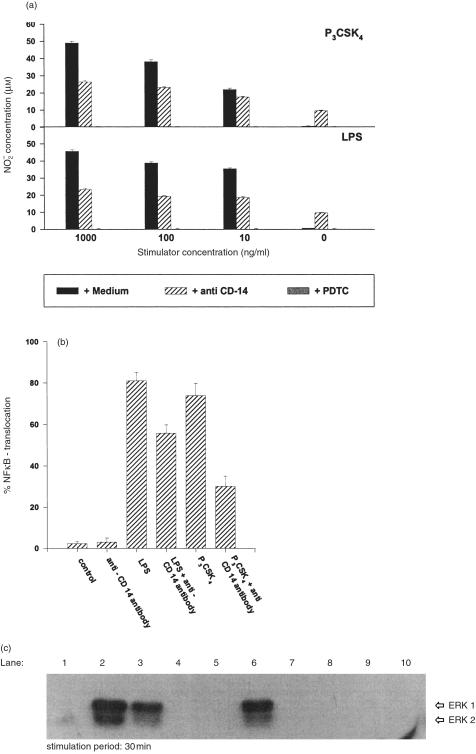
LPS-induced NO release was inhibited by about 50% when stimulation was performed in the presence of anti-CD14-directed antibodies (Fig. 8a, lower panel). This 50% inhibition was observed for all tested LPS concentrations (1, 0·1, and 0·01 µg/ml). Later, at all used LPS concentrations, no induction of NO release was monitored when the stimulation was performed in the presence of the NFκB inhibitor PDTC. No induction was detected in the presence of media (control); an about 10 µm NO release was observed in the presence of anti-CD14 antibodies alone. For P3CSK4, a similar inhibitory effect (≈ 50%) – as seen with LPS – was obtained when stimulation was performed in the presence of anti-CD14-directed antibodies. Again, as also seen with LPS, complete inhibition was detected when P3CSK4 stimulation was performed in the presence of PDTC (Fig. 8a, upper panel). CD14-non-related antibodies showed no inhibitory effect on LPS- and P3CSK4-dependent NO release (data not shown).
Figure 8.
Inhibition of P3CSK4-dependent ERK1/2 phosphorylation and P3CSK4-/LPS-dependent induction of NO release/NFκB translocation by anti-CD14- directed antibodies in BMDM. (a) Inhibition of NO release. BMDM derived from BALB/c mice were seeded into microtitre plates (1×105 cells/well) and the cells were stimulated with P3CSK4 or LPS for 48 hr at the indicated concentrations. PDTC (10 µg/ml/50 µl), anti-CD14- directed antibodies (10 µg/ml, 50 µl) or media as control (50 µl) were added 1 hr prior to stimulation. NO release was determined by measuring the nitrite concentrations in the culture supernatants as described in Materials and Methods. Values represent means ±SD of triplicate cultures. (b) Inhibition of NFκB translocation. BMDM from BALB/c mice were stimulated for 2 hr with either P3CSK4 (1 µg/ml) or LPS (1 µg/ml) in the presence or absence of anti-CD14 antibodies. The resulting NFκB translocation was monitored as described in Materials and Methods. The control represents resting, unstimulated macrophages derived from the bone marrow of BALB/c mice. Values represent means ±SD of triplicate cultures. (c) Inhibition of P3CSK4-dependent ERK1/2 phosphorylation by anti-CD14- directed antibodies and PD98059. BMDM from BALB/c mice (1×106 cells in 1 ml media) were stimulated for 30 min with 1 µg P3CSK4 (lane 2). Anti-CD14 antibodies (10 µg, lane 3), MEK inhibitor PD98059 (25 µg, lane 4 and 50 µg, lane 5) or PDTC (10 µg, lane 6) were added 1 hr prior to stimulation. The resulting MAP kinase phosphorylation was detected as described in the legend of Fig. 1(a). Lane 1 represents resting, unstimulated cells. Lane 7 represents unstimulated cells treated with anti-CD14 (10 µg) antibodies; lanes 8 and 9 represent unstimulated cells incubated with PD98059 (25 and 50 µg, respectively) and lane 10 represents unstimulated cells in the presence of PDTC (10 µg).
Inhibition of P3CSK4- and LPS-dependent NFκB tranlocation in BMDM from BALB/c mice by anti-CD14-directed antibodies
Inhibition experiments using anti-CD14-directed antibodies resulted in an about 70% reduction of NFκB translocation when cells were stimulated with 1 µg/ml P3CSK4 (Fig. 8b). With LPS an approximately 30% inhibition of translocation was detected in the presence of anti-CD14 antibodies. No translocation was found in control cultures containing media or in the presence of antibodies.
Inhibition of P3CSK4-dependent ERK1/2 phosphorylation in BMDM from BALB/c mice by anti-CD14-directed antibodies and PD98059
Lipopeptide-induced ERK1/2 phosphorylation was inhibited by about 40% when cells were stimulated with lipopeptide for 30 min in the presence of anti-CD14-directed antibodies (Fig. 8c, lanes 2 and 3). With respect to LPS-induced ERK1/2 phosphorylation, incubation of the cells in the presence of anti-CD14-directed antibodies resulted in a significant weaker inhibition (data not shown). Lipopeptide-induced ERK1/2 phosphorylation was completely reduced in the presence of PD98059, a specific inhibitor of the upstream located kinases MEK1/2 (Fig. 8c, lanes 4 and 5). In contrast, lipopeptide stimulation was not inhibited by PDTC, a NFκB inhibitor (Fig. 8c, lane 6). No phosphorylation was observed in the control containing media (Fig. 8c, lane 1) and in the presence of PDTC (Fig. 8c, lane 10).
Discussion
P3CSK4, a synthetic lipopeptide derived from bacterial lipoprotein, has shown to be an effective immunoadjuvant in parenteral, nasal and oral immunization.4–8 In vitro P3CSK4 constitutes a potent macrophage/monocyte activator resulting in the induction of lymphokine production, phagocytosis, activation for tumour cytotoxicity, tumour necrosis factor-α (TNF-α) production, and release of reactive oxygen and nitrogen intermediates.1,3,35 The focus of this study was to further elucidate the signalling mechanisms of P3CSK4-induced activation of macrophages. Because bacterial compounds such as LPS, lipoproteins of different sources and other bacterial cell-wall components are known to stimulate macrophages via activation of MAP kinases,36–38 which are linked to the regulation and expression of cytokine genes as well as to other important cellular functions, we concentrated our investigations on the ability of P3CSK4 to induce the ERK1/2 signal transduction pathway. We also monitored differences between P3CSK4- and LPS-induced activation. We checked the MAP kinase signalling pathway at different levels after LPS and P3CSK4 stimulation measuring kinase phosphorylation (ERK1/2), kinase kinase phosphorylation (MEK1/2), NFκB translocation, and the induction of NO release. Determinations were performed in RAW 264·7 cells and BMDM from BALB/c, C57BL/10ScSn (LPS responder) and C57BL/10ScCr (LPS non-responder) mice.
First, we observed differences in the kinetics of LPS- and lipopeptide-induced ERK1/2 and MEK1/2 phosphorylation. P3CSK4 stimulation of RAW 264·7 cells or BMDM from BALB/c mice resulted in a strong dose- and time-dependent increase of ERK1/2 and MEK1/2 phosphorylation. In contrast, LPS, which is known to signal via CD14 and TLR4, resulted in a comparably weak increase of ERK1/2 and MEK1/2 phosphorylation in RAW 264·7, or in BMDM from BALB/c mice. The differences in the kinetics of P3CSK4- and LPS-induced ERK1/2 phosphorylation indicate the involvement of different signal transduction mechanisms and receptors for LPS and the synthetic lipopeptide P3CSK4. These results are in accordance with earlier investigations indicating that TLR2 makes no contribution to LPS signal transduction.39
Experiments monitoring the P3CSK4- and LPS-induced activation of the upstream MAP kinase regulator proteins (MAPK kinases) MEK1 and MEK2 revealed phosphorylation patterns corresponding to the ones observed for ERK1/2: Again, P3CSK4 induced a strong phosphorylation after short-time stimulation at high doses. In contrast, LPS-induced phosphorylation of MEK1/2 reached a late maximum at 90 min at high LPS concentrations. Control experiments showed that all samples taken after stimulation using different LPS or P3CSK4 concentrations contained comparable amounts of 1EK1/2 suggesting no LPS- or P3CSK4-induced de novo synthesis. Thus, mainly the upstream MAPkinase kinases MEK1/2 are responsible for the phosphorylation of the kinases ERK1/2 after LPS and P3CSK4 stimulation. Further, the differences in kinetics of LPS- and P3CSK4-induced phosphorylation of MEK1/2 and ERK1/2 strongly suggest different signalling transduction pathways for both bacterial compounds. These data are in accordance with earlier investigations reporting distinct pathways for LPS and lipoproteins from Treponema pallidum and Borrelia burgdorferi.40
In order to monitor differences in the LPS and P3CSK4 signalling pathway, we investigated BMDM derived from LPS responder and non-responder mice. A null mutation of TLR4 in mice of the strain C57BL/10ScCr impedes LPS-induced signal transduction, and therefore prevents a response to LPS. Animals of the control strain C57BL/10ScSn are normally responsive.34 Stimulation of BMDM prepared from LPS responder mice (C57BL/10ScSn) showed an almost identical time- and dose-dependent pattern of ERK1/2 phosphorylation as observed for RAW 264·7 cells and BMDM derived from BALB/c mice, which all contain the intact TLR4. In contrast, BMDM prepared from LPS non-responder mice were not able to phosphorylate ERK1/2 after LPS stimulation, but still showed a dose- and time-dependent P3CSK4-induced phosphorylation pattern almost identical to that detected in BMDM obtained from LPS responder mice, BALB/c mice or RAW 264·7 cells. Our results clearly indicate that TLR4 plays no major role in the activation of macrophages by P3CSK4.
LPS and bacterial lipoproteins have been shown to stimulate the nuclear translocation factor NFκB,15,40–42 which in turn upregulates the transcription of a number of genes whose products are involved in inflammation.43 Because earlier investigations also suggested distinct signalling pathways for LPS and bacterial lipoproteins we investigated the LPS- and P3CSK4-induced translocation of NFκB in BMDM from LPS responder and non-responder mice. Analysing the subcellular distribution of p65 NFκB we could demonstrate that BMDM from both strains showed NFκB translocation when stimulated with P3CSK4. When stimulated with LPS, only the LPS responder cells showed NFκB translocation, whereas no NFκB-translocation was observed in BMDM derived from LPS non-responder mice. Our findings clearly demonstrate that P3CSK4 induces phosphorylation of ERK1/2 and MEK1/2 and the translocation of NFκB. Further on, the P3CSK4 induced signal transduction is TLR4 independent. This is in agreement with prior studies reporting that murine TLR4 does not function analogously in LPS and bacterial lipoprotein signalling because a TLR4 mutation, which renders cells insensitive to LPS, does not abrogate bacterial lipoprotein-induced responses.34,44,45
LPS and P3CSK4 activate nitric oxide synthase (NOS) in macrophages.46–49 Accordingly, LPS and P3CSK4 stimulation resulted in the induction of NO release in the macrophage cell line RAW 264·7, and in BMDM from BALB/c mice. Experiments with BMDM derived from LPS responder and non-responder strains showed a comparable dose-dependent P3CSK4-dependent NO release. In contrast, no release was detected in LPS non-responder cells after stimulation with LPS. The presence of PDTC, an inhibitor of NFκB-activation, almost completely reduced P3CSK4-induced NO release indicating that NFκB is the critical transcription factor for the upregulation of P3CSK4-induced inducible nitric oxide synthase (iNOS) expression; activation of the transcription factors AP-1, octamer binding protein-1, the cAMP response element-binding protein, and the promoter-selective transcription factor (Sp1) is not influenced by PDTC.50,51 These results are in agreement with earlier investigations showing that the critical transcriptional factor in LPS-induced NO release is NFκB.52 However, our investigations clearly indicate that LPS- and P3CSK4-induction of NFκB translocation are caused by different signalling transduction mechanisms. Accordingly, P3CSK4 induced (in contrast to LPS) no ERK1/2 signal transduction, no NFκB translocation, and no induction of NO release in TLR2-deficient BMDM prepared from TLR2 knockout mice (manuscript in preparation).
We also investigated the involvement of CD14 in lipopeptide-dependent activation of macrophages. We found that both NFκB translocation and induction of NO release was significantly reduced when P3CSK4 stimulation was performed in the presence of anti-CD14 antibodies. Accordingly, in the presence of anti-CD14 antibodies the NFκB translocation was inhibited by about 70%, and NO release was reduced by about 50%. These unexpected data strongly suggest the involvement of CD14 in the P3CSK4-induced dependent signal transduction mechanism. Finally, we observed an about 40% inhibition of the P3CSK4-dependent ERK1/2 phosphorylation by anti-CD14-directed antibodies, which clearly indicates that P3CSK4-dependent NFκB translocation and NO release is regulated via CD14.
In summary, the present studies indicate that the P3CSK4-dependent activation of macrophages is induced via phosphorylation of the MAP kinases ERK1/2 and MEK1/2 resulting in NFκB translocation and the induction of NO release. Further on, the P3CSK4-dependent signalling process is likely to be CD14/TLR2 dependent and TLR4 independent. Presently we are investigating if further genes are activated or otherwise modified in their transcription efficiency in the P3CSK4-induced signal transduction pathway.
Acknowledgments
The authors would like to thank C. Galanos (MPI, Freiburg, Germany) for supplying LPS responder/LPS non-responder mice and LPS. Special thanks to A. Haber, M. Eckert, and N. Kegel for their excellent technical assistance. This study was supported by the Deutsche Forschungsgemeinschaft.
Abbreviations
- BMDM
Bone marrow-derived macrophages
- BSA
bovine serum albumin
- ELISA
enzyme-linked immunosorbent assay
- ERK
extracellular signal-regulated protein kinase
- FCS
fetal calf serum
- FITC
fluorescein isothiocyanate
- HRP
horseradish peroxidase
- IL
interleukin
- JNK
c-Jun NH2-terminal kinase
- LPS
lipopolysaccharide
- MAPK
mitogen-activated protein kinase
- M-CSF
macrophage colony-stimulating factor
- MEK
mitogen-activated protein kinase kinase
- MHC
major histocompatibility complex
- NEAA
non-essential amino acids
- NO
nitric oxide
- PBS
phosphate-buffered saline
- P3CSK4
N-palmitoyl-S-[2,3-bis(palmitoyloxy)-(2R,S)-propyl]-(R)-cysteinyl-seryl-(lysyl)3-lysine
- PDTC
pyrrolidine dithiocarbamate
- PVDF
polyvinylidene difluoride
- RAW 264·7
murine macrophage cell line
- TBS
Tris-buffered saline, pH 7·6
- TLR
Toll-like receptor
- TTBS
Tris-buffered saline, pH 7·6, containing 0·1% Tween 20 (v/v)
- TFA
trifluoroacetic acid
- Tris
tris(hydroxymethyl)aminomethane.
References
- 1.Hoffmann P, Jiménez-Dias M, Loleit M, et al. Preparation of human and murine monoclonal antibodies: antigens combined with or conjugated to lipopeptides constitute potent immunogens for in vitro and in vivo immunizations. Hum Antibodies Hybridomas. 1990;1(3):137–44. [PubMed] [Google Scholar]
- 2.Hoffmann P, Semmler K, Müller B, Wiesmüller K-H, Jung G, Bessler WG. Bacterial lipopeptides as activators of human monocytes and monocyte-derived macrophages for tumor cytostasis. Immunobiology. 1997;197:344. [Google Scholar]
- 3.Kreutz M, Ackermann U, Hauschildt S, Krause SW, Riedel D, Bessler WG, Andreesen R. A comparative analysis of cytokine production and tolerance induction by bacterial lipopeptides, lipopolysaccharides, and Staphyloccocus aureus in human monocytes. Immunology. 1997;92:396–401. doi: 10.1046/j.1365-2567.1997.00365.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baier W, Heinevetter L, Wiesmüller K-H, Jung G, Huber M, Bessler WG. The lipopeptide P3CSK4 constitutes an adjuvant in parenteral and oral immunization. Vaccine Res. 1997;6(3):127–40. [Google Scholar]
- 5.Baier W, Masihi N, Huber M, Hoffmann P, Bessler WG. Lipopeptides as immunoadjuvants and immunostimulants in mucosal immunization. Immunobiol. 1999;201:391–405. doi: 10.1016/s0171-2985(00)80093-5. [DOI] [PubMed] [Google Scholar]
- 6.Baier W, Loleit M, Fischer B, et al. Generation of antibodies directed against the low-immunogenic peptide-toxins microcystin-LR/RR and nodularin. Int J Immunopharmacol. 2000;22:339–53. doi: 10.1016/s0192-0561(99)00086-7. 10.1016/s0192-0561(99)00086-7. [DOI] [PubMed] [Google Scholar]
- 7.Mittenbühler K, Baier W, vd Esche U, et al. Lipopeptides are efficient novel immunogens and adjuvants in parenteral and oral immunization. Curr Top Peptide Protein Res. 1997;2:125–35. [Google Scholar]
- 8.Mittenbühler K, Loleit M, Baier W, et al. Drug specific antibodies: T-cell epitope-lipopeptide conjugates are potent adjuvants for small antigens in vivo and in vitro. Int J Immunopharmacol. 1997;19:277–8. doi: 10.1016/s0192-0561(97)00069-6. 10.1016/s0192-0561(97)00069-6. [DOI] [PubMed] [Google Scholar]
- 9.Schnorrenberg G, Gerhardt H. Fully automatic simultaneous multiple peptide synthesis in micromolar scale-rapid synthesis of series of peptides for screening in biological assays. Tetrahedron. 1989;45:7759–64. 10.1016/s0040-4020(01)85791-4. [Google Scholar]
- 10.Metzger J, Jung G, Bessler WG, Hoffmann P, Strecker M, Lieberknecht A, Schmidt U. Lipopeptides containing 2-palmitoylamino-6,7-bis (palmitoyloxy)-heptanoic acid: synthesis, stereospecific stimulation of B-lymphocytes and adjuvanticity. J Med Chem. 1991;34:1969–74. doi: 10.1021/jm00111a008. [DOI] [PubMed] [Google Scholar]
- 11.Jung G, Beck-Sickinger AG. Multiple peptide synthesis methods and their applications. Angew Chem Int Ed Engl. 1992;31:367–83. [Google Scholar]
- 12.Erhard MH, Schmidt P, Hofmann A, et al. The lipopeptide Pam3Cys-Ser-(Lys)4. An alternative adjuvant to Freund's adjuvant for the immunization of chicken to produce egg yolk antibodies. ATLA. 1997;25:173–81. [Google Scholar]
- 13.Wiedemann F, Link R, Pumpe K, et al. Histopathological studies on the local reactions induced by complete Freund's adjuvant (CFA), bacterial lipopolysaccharide (LPS), and synthetic lipopeptide (P3C) conjugates. J Pathol. 1991;164:265–71. doi: 10.1002/path.1711640313. [DOI] [PubMed] [Google Scholar]
- 14.Hirschfeld M, Kirschning CJ, Schwandner R, Wesche H, Weis JH, Wooten RM, Weis JJ. Inflammatory signaling by Borrelia burgdorferi lipoproteins is mediated by toll-like receptor 2. J Immunol. 1999;163:2382–6. [PubMed] [Google Scholar]
- 15.Giambartolomei GH, Dennis VA, Lasater BL, Philipp MT. Induction of pro- and anti-inflammatory cytokines by Borrelia burgdorferi lipoproteins in monocytes is mediated by CD14. Infect Immun. 1999;67:140–7. doi: 10.1128/iai.67.1.140-147.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Uhl B, Wolf B, Schwinde A, Metzger J, Jung G, Bessler WG, Hauschildt S. Intracellular localization of a lipopeptide macrophage activator: immunocytochemical investigations and EELS analysis on ultrathin cryosections of bone marrow-derived macrophages. J Leukocyte Biol. 1991;50:10–8. doi: 10.1002/jlb.50.1.10. [DOI] [PubMed] [Google Scholar]
- 17.Cobb MH, Goldsmith EJ. How MAP kinases are regulated. J Biol Chem. 1995;270:14843–6. doi: 10.1074/jbc.270.25.14843. [DOI] [PubMed] [Google Scholar]
- 18.Paul A, Wilson S, Belham CM, Robinson CJ, Scott PH, Gould GW, Plevin R. Stress-activated protein kinases: activation, regulation and function. Cell Signal. 1997;9:403–10. doi: 10.1016/s0898-6568(97)00042-9. 10.1016/s0898-6568(97)00042-9. [DOI] [PubMed] [Google Scholar]
- 19.Widmann C, Gibson S, Jarpe MB, Johnson GL. Mitogen-activated protein kinase: conservation of a three-kinase module from yeast to human. Physiol Rev. 1999;79:143–80. doi: 10.1152/physrev.1999.79.1.143. [DOI] [PubMed] [Google Scholar]
- 20.Cowley S, Paterson H, Kemp P, Marshall CJ. Activation of MAP kinase kinase is necessary and sufficient for PC12 differentiation and for transformation of NIH 3T3 cells. Cell. 1994;77:841–52. doi: 10.1016/0092-8674(94)90133-3. [DOI] [PubMed] [Google Scholar]
- 21.Mansour SJ, Matten WT, Hermann AS, Candia JM, Rong S, Fukasawa K, Vande Woude GF, Ahn NG. Transformation of mammalian cells by constitutively active MAP kinase kinase. Science. 1994;265:966–70. doi: 10.1126/science.8052857. [DOI] [PubMed] [Google Scholar]
- 22.Alessi DR, Cuenda A, Cohen P, Dudley DT, Saltiel AR. PD 098059 is a specific inhibitor of the activation of mitogen-activated protein kinase kinase in vitro and in vivo. J Biol Chem. 1995;270:27489–94. doi: 10.1074/jbc.270.46.27489. [DOI] [PubMed] [Google Scholar]
- 23.Sale EM, Atkinson PG, Sale GJ. Requirement of MAP kinase for differentiation of fibroblasts to adipocytes, for insulin activation of p90, S6 kinase and for insulin or serum stimulation of DNA synthesis. EMBO J. 1995;14::674–684. doi: 10.1002/j.1460-2075.1995.tb07046.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cox ME, Parsons SJ. Roles for protein kinase C and mitogen-activated protein kinase in nicotine-induced secretion from bovine adrenal chromaffin cells. J Neurochem. 1997;69:1119–30. doi: 10.1046/j.1471-4159.1997.69031119.x. [DOI] [PubMed] [Google Scholar]
- 25.Klemke RL, Cai S, Giannini AL, de Gallagher PJ, Lanerolle P, Cheresh DA. Regulation of cell motility by mitogen-activated protein kinase. J Cell Biol. 1997;137:481–92. doi: 10.1083/jcb.137.2.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kornhauser JM, Greenberg ME. A kinase to remember: dual roles for MAP kinase in long-term memory. Neuron. 1997;18:839–42. doi: 10.1016/s0896-6273(00)80322-0. [DOI] [PubMed] [Google Scholar]
- 27.Cheresh DA, Leng J, Klemke RL. Regulation of cell contraction and membrane ruffling by distinct signals in migratory cells. J Cell Biol. 1999;146:1107–16. doi: 10.1083/jcb.146.5.1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Whitmarsh AJ, Davis RJ. A central control for cell growth. Nature. 2000;403:255–6. doi: 10.1038/35002220. 10.1038/35002220. [DOI] [PubMed] [Google Scholar]
- 29.Graves LM, Guy HI, Kozlowski P, et al. Regulation of carbamoyl phosphate synthetase by MAP kinase. Nature. 2000;403:328–32. doi: 10.1038/35002111. 10.1038/35002111. [DOI] [PubMed] [Google Scholar]
- 30.Hoffmann P, Jiménez-Dias M, Weckesser J, Bessler WG. Murine bone marrow-derived macrophages constitute feeder cells for human B cell hybridomas. J Immunol Methods. 1996;196:85–91. doi: 10.1016/0022-1759(96)00121-4. 10.1016/0022-1759(96)00121-4. [DOI] [PubMed] [Google Scholar]
- 31.Ding AH, Nathan CF, Stuehr DJ. Release of reactive nitrogen intermediates and reactive oxygen intermediates from mouse peritoneal macrophages. Comparison of activating cytokines and evidence for independent production. J Immunol. 1988;141:2407–12. [PubMed] [Google Scholar]
- 32.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–5. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 33.Beranger F, Paterson H, Powers S, de Gunzburg J, Hancock JF. The effector domain of Rab6, plus highly hydrophobic C terminus, is required for Golgi apparatus localization. Mol Cell Biol. 1994;14:744–58. doi: 10.1128/mcb.14.1.744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Poltorak A, He X, Smirnova I, et al. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice. Mutations in Tlr4 gene. Science. 1998;282:2085–8. doi: 10.1126/science.282.5396.2085. [DOI] [PubMed] [Google Scholar]
- 35.Terenzi F, Diáz-Guerra MJM, Casado M, Hortelano S, Leoni S, Boscá L. Bacterial lipopeptides induce nitric oxide synthase and promote apoptosis through nitric oxide-independent pathways in rat macrophages. J Biol Chem. 1995;270:6017–21. doi: 10.1074/jbc.270.11.6017. [DOI] [PubMed] [Google Scholar]
- 36.Rawadi G, Ramez V, Lemercier B, Roman-Roman S. Activation of mitogen-activated protein kinase pathways by Mycoplasma fermentans membrane lipoproteins in murine macrophages: involvement in cytokine synthesis. J Immunol. 1998;160:1330–9. [PubMed] [Google Scholar]
- 37.Schumann RR, Pfeil D, Freyer D, Buerger W, Lamping N, Kirschning CJ, Goebel UB, Weber JR. Lipopolysaccharide and pneumococcal cell wall components activate the mitogen activated protein kinases (MAPK) erk-1, erk-2, and p38 in astrocytes. Glia. 1998;22:295–305. doi: 10.1002/(sici)1098-1136(199803)22:3<295::aid-glia8>3.0.co;2-4. 10.1002/(sici)1098-1136(199803)22:3<295::aid-glia8>3.3.co;2-e. [DOI] [PubMed] [Google Scholar]
- 38.Marie C, Roman-Roman S, Rawadi G. Involvement of mitogen-activated protein kinase pathways in interleukin-8 production by human monocytes and polymorphonuclear cells stimulated with lipopolysaccharide or Mycoplasma fermentans membrane. Infect Immun. 1999;67:688–93. doi: 10.1128/iai.67.2.688-693.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Beutler B. Tlr4: central component of the sole mammalian LPS sensor. Curr Opin Immunol. 2000;12:20–6. doi: 10.1016/s0952-7915(99)00046-1. 10.1016/s0952-7915(99)00046-1. [DOI] [PubMed] [Google Scholar]
- 40.Norgard MV, Arndt LL, Akins DR, Curetty LL, Harrich DA, Radolf JD. Activation of human monocytic cells by Treponema pallidum and Borrelia burgdorferi lipoproteins and synthetic lipoproteins proceeds via a pathway distinct from that of lipopolysaccharide but invovlves the transcriptional activator NF-κB. Infect Immun. 1996;64:3845–52. doi: 10.1128/iai.64.9.3845-3852.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rescigno M, Granucci F, Citterio S, Foti M, Ricciardi-Castagnoli P. Coordinated events during bacteria-induced DC maturation. Immunol Today. 1999;20:200–3. doi: 10.1016/s0167-5699(98)01427-3. 10.1016/s0167-5699(98)01427-3. [DOI] [PubMed] [Google Scholar]
- 42.Wang P, Wu P, Siegel MI, Egan RW, Billah MM. Interleukin (IL) -10 inhibits nuclear factor kappa B (NF kappa B) activation in human monocytes. IL-10 and IL-4 suppress cytokine synthesis by different mechanisms. J Biol Chem. 1995;270:9558–63. doi: 10.1074/jbc.270.16.9558. [DOI] [PubMed] [Google Scholar]
- 43.Baeuerle PA, Henkel T. Function and activation of NF-kappa B in the immune system. Annu Rev Immunol. 1994;12:141–79. doi: 10.1146/annurev.iy.12.040194.001041. [DOI] [PubMed] [Google Scholar]
- 44.Zhang H, Peterson JW, Niesel DW, Klimpel GR. Bacterial lipoprotein and lipopolysaccharide act synergistically to induce lethal shock and proinflammatory cytokine production. J Immunol. 1997;159:4868–78. [PubMed] [Google Scholar]
- 45.Aliprantis AO, Yang R-B, Mark MR, et al. Cell activation and apoptosis by bacterial lipoproteins through toll-like receptor-2. Science. 1999;285:736–9. doi: 10.1126/science.285.5428.736. 10.1126/science.285.5428.736. [DOI] [PubMed] [Google Scholar]
- 46.Hauschildt S, Scheipers P, Bessler WG. Inhibitors of poly (ADP-ribose) polymerase suppress lipopolysaccharide-induced nitrite formation in macrophages. Biochem Biophys Res Commun. 1991;179:865–71. doi: 10.1016/0006-291x(91)91898-m. [DOI] [PubMed] [Google Scholar]
- 47.Lyons CR, Orloff GJ, Cunningham JM. Molecular cloning and functional expression of an inducible nitric oxide synthase from a murine macrophage cell line. J Biol Chem. 1992;267:6370–4. [PubMed] [Google Scholar]
- 48.May MJ, Ghosh S. Signal transduction through NF-κB. Immunol Today. 1998;19:80–8. doi: 10.1016/s0167-5699(97)01197-3. 10.1016/s0167-5699(97)01197-3. [DOI] [PubMed] [Google Scholar]
- 49.Chen C-C, Wang J-K, Lin S-B. Antisense oligonucleotides targeting protein kinase C-α, -βI, or -δ but not -η inhibit lipopolysaccharide-induced nitric oxide synthase expression in RAW 264.7 macrophages: involvement of a nuclear factor κB-dependent mechanism. J Immunobiol. 1998;161:6206–14. [PubMed] [Google Scholar]
- 50.Schreck R, Meier B, Mannel DN, Droge W, Baeuerle PA. Dithiocarbamates as potent inhibitors of nuclear transcription factor κB activation in intect cells. J Exp Med. 1992;175:1181–94. doi: 10.1084/jem.175.5.1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ziegler-Heitbrock HW, Sternsdorf T, Liese J, et al. Pyrrolidine dithiocarbamate inhibits NF-kappa B mobilization and TNF production in human monocytes. J Immunol. 1993;151:6986–93. [PubMed] [Google Scholar]
- 52.Liu SF, Ye X, Malik AB. In vivo inhibition of nuclear factor-κB activation prevents inducible nitric oxide synthase expression and systemic hypotension in a rat model of septic shock. J Immunol. 1997;159:3976–83. [PubMed] [Google Scholar]