Abstract
The secretory lysosomes found in haemopoietic cells provide a very efficient mechanism for delivering the effector proteins of many immune cells in response to antigen recognition. Although secretion shows some similarities to the secretion of specialized granules in other secretory cell types, some aspects of secretory lysosome release appear to be unique to melanocytes and cells of the haemopoietic lineage. Mast cells and platelets have provided excellent models for studying secretion, but recent advances in characterizing the immunological synapse allow a very fine dissection of the secretory process in T lymphocytes. These studies show that secretory lysosomes are secreted from the centre of the talin ring at the synapse. Proper secretion requires a series of Rab and cytoskeletal elements which play critical roles in the specialized secretion of lysosomes in haemopoietic cells.
Introduction
The function of many of the cells from the haemopoietic lineage is mediated by the release of specific effector molecules in response to an external stimulus. This process is termed regulated secretion. These molecules are stored intracellularly within specialized secretory storage compartments which are mobilized to, and fuse with, the cell surface upon cell stimulation. Cytotoxic T lymphocytes (CTL), natural killer (NK) cells, mast cells, platelets and neutrophils are amongst those which use specialized secretory granules in their effector functions (reviewed in ref. 1). The importance of regulated secretion is that it provides a very tight control over the release of proteins. First, they are only released upon external stimulation of the cells; and secondly, release can be restricted to specific locations, for example, the site of membrane contact, by recruiting the granules to specific sites on the plasma membrane. For example, CTL are able to secrete cytotoxic molecules into the target cell by polarizing their granules to the site of contact with the target cell on activation by contact with a target cell. This provides several different levels at which the secretory process and effector functions of the cells can be controlled: receptor signalling; granule polarization and membrane fusion. As such, regulated secretion provides an important mechanism for controlling immune responses.
All cells possess lysosomes in which proteins are degraded intracellularly. The cells of the haemopoietic lineage are unusual in that they use their lysosomes as their regulated secretory storage organelles. These compartments are known as ‘secretory lysosomes’. Although all cells possess lysosomes in which proteins are degraded intracellularly, in certain cell types of the haemopoieitic lineage these organelles are also regulated secretory granules.2 This differs from conventional secretory cells (e.g. neuroendocrine) which possess separate secretory granules and lysosomes (reviewed in ref. 3).
Current data support the idea that secretory lysosomes have acquired specialized machinery enabling them to function as regulated secretory organelles. Some of the best evidence for this comes from observations on genetic diseases in which secretory lysosome release is affected, but conventional secretory granule release and lysosomal functions are normal. The selective defects observed in these diseases suggest that the affected gene products are essential components in the secretory machinery. Recent studies have now identified some of these gene products.
Secretion in normal haemopoietic cells
Like other calcium-mediated exocytosis events, secretion from lysosomes involves several distinct steps following cell activation; transport of secretory organelles to sites of exocytosis, detachment from the cytoskeleton and docking with the plasma membrane. Subsequent fusion of the secretory granules with the plasma membrane releases the secretory lysosome contents.
The T-cell immunological synapse
CD4 and CD8 T-cell function is dependent on secretion of cytokines and cytolytic proteins, respectively, in response to recognition and interaction with a target cell. What happens when a T-cell recognizes its target? It has long been known that activation of the T cell upon target cell recognition results in dramatic polarization of both plasma membrane-associated proteins and the cytoplasmic organelles towards the site of cell–cell contact.4 Initial recognition involves interaction between the adhesion molecules lymphocyte function-associated antigen-1 (LFA-1) on the T cell and intracellular adhesion molecule (ICAM) on the target cell. Antibodies to LFA-1 prevent both conjugate formation and cytotoxicity.5,6 This facilitates the recognition of target-cell-associated major histocompatibility complex (MHC) I or II and peptide by T-cell receptors (TCR) which signals a cascade of effector events. The topological distribution of these proteins at the site of membrane contact is highly organized. Shortly after cell–cell contact, LFA-1 and ICAM segregate to an outer ring which surrounds a central core containing the TCR and MHC. Activation of protein kinase C (PKC) molecules associated with the synapse leads to association of the cytoskeletal protein, talin, with LFA-17 so that talin also forms a ring at the synapse. This contact site has recently been termed the ‘immunological synapse’8 or ‘supramolecular activation cluster (SMAC)’9 reflecting the dynamic and highly organized nature of this interaction site. In CD4 T cells the TCR has been found to segregate to the inner core of the immunological synapse, surrounded by LFA-1 with talin associated inside the plasma membrane (reviewed in ref. 8).
How do secretory events fit into the synapse? Following TCR, triggering there is a Vav-dependent phosphorylation of Rac-110 which leads to dramatic changes in the cytoskeleton of the T cell. The recruitment of talin to the outer ring of the synapse may contribute to the polarization of the actin at the synapse.8 The microtubule organizing centre (MTOC) also polarizes towards the site of membrane contact.11 This rearrangement of the cytoskeleton in turn results in a dramatic polarization of the secretory apparatus of the T cell towards the contact site, illustrated in Fig. 1. The secretory granules move along the microtubules, driven by kinesin-based motors,12 and rapidly accumulate at the site of membrane contact between the two cells. Following polarization, the granules fuse with the plasma membrane13 and their electron-dense contents can be seen secreted into a cleft that is often observed within the contact area (Fig. 1, lower panel). This polarization of the granules to the site of contact before fusion therefore directs release of granule contents towards the target cell. We recently showed in murine CTL that release is restricted to the inner area of the synapse formed by the talin cleft.14 Many endosomes, and the Golgi apparatus, also polarize towards the site of membrane contact11 (see Fig. 1). The fact that the Golgi apparatus polarizes very close to the membrane means that even constitutively secreted proteins will be secreted in a highly focused manner. The mitochondria also become polarized and are often seen very closely associated with the secretory granules.
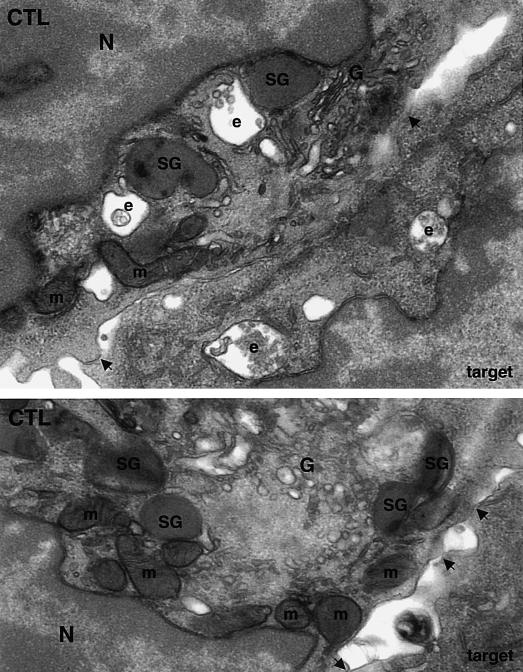
Figure 1.
Polarization of the CTL secretory apparatus to the site of membrane contact on target cell recognition. Thin (50–100 nm, top panel) and semi-thin (150–200 nm, bottom panel) lead citrate-stained sections of epon-embedded mouse CD8+ CTL (CTL) conjugated to P815 target cells (target) showing the polarization of the CTL secretory apparatus on target cell recognition and visualized by electron microscopy. The area of cell–cell contact (e.g. arrowheads) corresponding to the immunological synapse is shown; N = nucleus. At early time-points of recognition (top panel) the two cellular membranes form a tight contact. The Golgi complex (G) polarizes to the centre of the contact site and the secretory granules (SG), endosomes (e) and mitochondria accumulate around the Golgi complex. Interestingly, endosomes (e) in the target cell cytoplasm are also found in the area of the contact site. At later time-points (bottom panel) the secretory granules are transported to the plasma membrane at the contact site where they release their contents. Note the appearance of ‘gaps’ between the two cells which contain electron-dense material characteristic of that present within the secretory granules. This most probably represents material already secreted from the CTL. Note also the close association of mitochondria with the secretory organelles.
The molecular mechanisms of lysosome secretion
What controls the secretion of the granules once they have polarized to the MTOC? Two approaches have been taken to identify the proteins regulating secretion in haemopoietic cells. The first approach involves looking in haemopoietic tissues for members of protein families which are known to be involved in regulated secretion in other, better characterized, secretory cells, such as neurosecretory cells (reviewed in ref. 3). The second involves identifying those proteins that are defective in human genetic diseases and their mouse models in which haemopoietic cell secretion is selectively impaired.
SNARES, Rabs and THE EXOCYST
Current views suggest that membrane–membrane fusion events in the cell are mediated by a core complex of three proteins, known as the SNARE [soluble N-ethylmaleimide-sensitive fusion factor (NSF) attachment receptor] complex, which bring vesicle and target membranes together and drive fusion. This has been most thoroughly characterized for synaptic vesicle exocytosis in the nerve terminal (reviewed in ref. 3). This complex consists of a protein on the vesicle cell membrane that belongs to the v-SNARE or VAMP family of proteins (VAMP-1/synaptobrevin-1 on the synaptic vesicle). This v-SNARE or VAMP interacts with two proteins on the target membrane; a partner t-SNARE (or syntaxin) and a SNAP protein (syntaxin-1 and SNAP-25, respectively, in the nerve terminus). Distinct members of the VAMP, syntaxin and SNAP families of proteins appear to operate at the different membrane fusion events which occur throughout the cell and in different cells. This provides some of the specificity needed to direct each distinct vesicle or organelle to fuse with its correct target membrane.
Syntaxins 2, 3 and 4, VAMPs 2 and 7, and SNAP-23 (the non-neuronal form of SNAP-25) have all been found in the mast cell line, RBL.15,16 VAMP-2 is involved in exocytosis in neuronal cells17,18 and is also found on mast cell granules. However, it does not appear to be involved in mast cell lysosome exocytosis since VAMP-2 is cleaved by tetanus toxin and mast cell degranulation is insensitive to tetanus toxin treatment.19 This highlights the problem of the approach of simply identifying homologues of proteins identified in conventional secretory cells.
Another complicating factor in the approach of simply identifying homologues of proteins in conventional secretory cells is that some haemopoietic cells contain several distinct secretory organelles which are mobilized to secrete by different stimuli. Syntaxin 2, syntaxin 4, a VAMP homologue and SNAP-23 have also been found in platelets.20–22 Interestingly, both syntaxins 2 and 4 are involved in exocytosis but play distinct roles in these cells. Densegranule secretion appears to involve syntaxin 2 but not syntaxin 4, whilst alpha-granule and lysosomal secretion appear to require both syntaxin 2 and 4.23,24 All three events require SNAP-23. Interestingly, the rate of exocytosis from the granules and lysosomes differs with both dense and alpha-granule contents being secreted more rapidly than the lysosomal enzymes, suggesting that although alpha-granules and lysosomes appear to use the same fusion machinery, regulation of their exocytosis is distinct.
Syntaxins 3 and 4, VAMP-2 and SNAP-23 have also been detected in neutrophils syntaxins 1A, 5, 6, 7, 9, 11 and 16.25 Syntaxin 1, VAMP-2 and SNAP-23 have all been localized to subpopulations of granular structures, while syntaxins 4 and 6 have been found on the neutrophil plasma membrane. One report has suggested that neutrophil exocytosis involves syntaxin 6, (which is found on the neutrophil plasma membrane) and SNAP-23, (which is in the membranes of both tertiary and specific granules).26 SNAP-23 and syntaxin 6 were found to be co-localized after mobilization of these granules to the surface upon cell stimulation, and to interact both in vitro and in vivo, and this in vivo interaction was increased upon activation. Tertiary and specific granules are rapidly mobilized to the cell surface upon activation, whereas auzophilic granules are hardly mobilized. Interestingly, antibodies against syntaxin 6 inhibit exocytosis of both specific and auzophilic granules, whereas those to SNAP-23 inhibited specific but not auzophilic granule exocytosis. This again indicates that different secretory granules in the same cell with different secretory properties use distinct but overlapping proteins for secretion. To date, no SNARE proteins have been identified which are unique to haemopoietic cells. This is consistent with the large number of syntaxin isoforms found in these cells.
The membrane–membrane fusion events mediated by SNARE proteins are thought to be regulated by a number of different proteins, including synaptotagmins (see below) and Rab proteins.27 The Rab proteins are a family of small GTPases present in the cytosol which can confer specificity on the SNARE interactions.28 When modified by attachment of a lipid group (termed prenylation) these proteins can attach to specific membranes and ‘catalyse’ interactions between t-SNARE and v-SNARE proteins present on the transport and vesicle docking membranes. In this way it is thought that Rab proteins regulate fusion and bestow specificity upon SNARE interactions. Recent data also suggest that the Rab proteins might also be involved in regulating the movement of organelles to their target membrane sites.29 The specificity of the Rab proteins is determined by the membranes with which individual Rabs associate.30 There are more than 52 different Rabs identified to date28 and several have now been identified in haemopoietic cells.
One of the best studied secretory cell types in the haemopoietic lineage is the mast cell. Rab3d has been demonstrated to be present on mast cell secretory granules and is essential for secretion in these cells.31,32 Mast cells also contain Rab37, a novel Rab GTPase which is specifically expressed in mast cells, where it co-localizes with secretory lysosomal markers.33 The function of Rab37 is not known, although it is likely that it is involved in interacting with mast-cell-specific effector proteins. Rab37 has 47% homology with Rab8, a Rab involved in transport from Golgi apparataus to plasma membrane, and might therefore be involved in regulating mobilization of the mast cell granules to the plasma membrane. Interestingly, in this respect, Rab8 and Rab6 have been shown to be phosphorylated by PKC pathways on stimulation of the cells by thrombin in platelets and may be involved in degranulation in these cells.34 Platelets also express Rab3b, Rab4 and Rab27a.34,35 Rab4 fractionates with the alpha-granule markers.36 Expression of a dominant-negative mutant of Rab4 inhibited alpha-granule, but not dense-granule secretion, supporting the idea that the exocytosis of distinct secretory organelles in the same cell may be regulated by different proteins. Other Rab proteins have been found to be selectively expressed in cells with secretory lysosomes, perhaps the best example being Rab27a,37 which is discussed in more detail below.
Synaptotagmins (Syt) are a family of calcium and phospholipid-binding membrane proteins which have been found on regulated secretory vesicles in neurons and other conventional secretory cell tissues. Binding of calcium results in a conformational or electrostatic change in the synaptotagmin molecule and so these proteins are thought to act as the calcium sensors which activate exocytosis of the secretory organelles upon calcium influx. The neuronal synapse contains abundant amounts of SytI and II. A number of synaptotagmins have been identified in haemopoietic cells. A role for synaptotagmins in mast cells has been suggested since calcium-dependant exocytosis was dramatically increased upon expression of neuronal synaptotagmin I, in mast cells. Mast cells do not express endogenous synaptotagmin I but do express synaptotagmins II, III and V.38 SytII has been found on the lysosomal membrane of these cells and recent results suggest that it can act as a negative regulator of exocytosis from lysosomes from mast cells.38
In the nerve terminus, synaptic vesicles attach to the plasma membrane at specific exocytosis sites defined by a complex of proteins called the ‘exocyst’.39 An analogous complex of proteins is found in the budding tip of yeast undergoing division. It is thought that this complex is involved in defining the sites at which exocytosis occurs. It is likely that such complexes might also be involved in defining the sites of exocytosis in haemopoietic cells, especially in cells such as lymphocytes in which exocytosis is highly polarized. No evidence is available to date as to whether or not the exocyst does play a role in these cells and if so whether haemopoietic-specific components might be involved.
Secretion in mutant ctl
Immunodeficiency and albinism
For some time, the best evidence that unique components are involved in secretory lysosome exocytosis has come from the autosomal recessive disease Chediak–Higashi syndrome (CHS).40 The beige mouse model of this disease is perhaps best known to immunologists as a mouse model lacking functional T- and NK-cell activity. The defect in these mice turns out to be neither an absence of T and NK cells, nor their ability to produce lytic proteins, but rather their inability to secrete their lytic granules.41 This defect manifests itself in most of the cells of the haemopoietic lineage which function by secretory lysosome release (reviewed in ref. 2). But in addition, as suggested by the name of the mouse, coat colour is also affected. This turns out to reflect an inability of the pigment producing cells, the melanocytes, to secrete their melanosomes and give rise to proper hair pigmentation. This, in turn, is due to the fact that melanosomes are modified lysosomes that undergo regulated secretion.42 The importance of this observation is that the defective gene in CHS is presumably involved in control of secretory lysosome release in both haemopoietic cells and melanocytes. Furthermore, this highlights a means for identifying other proteins involved in this specialized secretory pathway, since other mutations giving rise to immunodeficiencies with associated albinism may well arise from defects in proteins affecting secretory lysosome release.
Chediak–Higashi syndrome
Although the CHS gene has now been identified, it is not clear what role it plays in secretory lysosome release. The CHS gene, termed Lyst, encodes a cytosolic protein with a predicted MW of 400 kD.43,44 In CHS CTL the granules can polarize at the point of membrane contact between the two cells, but cannot fuse with the membrane.41 Curiously lysosomes in all cells, including those with secretory lysosomes, are abnormally enlarged in CHS and beige mice, suggesting that the Lyst gene product may be involved in regulation of membrane fusion events. However, since attempts to raise antibodies have failed, it is unclear where and how this relates to secretory lysosome release.40
Griscelli syndrome
Patients with Griscelli syndrome also have severely impaired CTL responses, as well as a much more marked albinism.45 Recently, two defective genes have been identified in different Griscelli syndrome patients. Both map very close on chromosome 15 and arise from mutations in the genes encoding either myosin V or Rab27a.46,47 Although both sets of patients possess silvery grey hair, only those with a defect in Rab27a show immunological problems. Studies on the mouse models of these defects (ashen and dilute for mutations in Rab27a and myosin V, respectively) have revealed the molecular basis of these differences and point to a critical role for Rab27a in secretory lysosome secretion in CTL.
The ashen mouse lacks Rab27a48 and, as suggested by its name, shows a diluted pigmentation in its coat. CTL derived from these mice are unable to lyse targets due to an inability to secrete their granules.14 Intriguingly, although the granules of ashen CTL are able to polarize towards the immunological synapse, they are unable to squeeze in to the centre of the talin ring as granules from normal cells do (Fig. 2). Electron microscopy revealed that the granules of ashen mice are unable to dock at the membrane, suggesting that although they can move along the microtubules, they are then unable to reach the membrane in order to dock and fuse.14 What exactly is Rab27a doing? Although Rab proteins are primarily known for facilitating membrane–membrane interactions, some such as Rab6, have been shown to mediate interactions with the cytoskeleton.29 Some clues as to the potential role of Rab27a come from studies on the other model of Griscelli syndrome in which myosin V is missing – the dilute mouse.
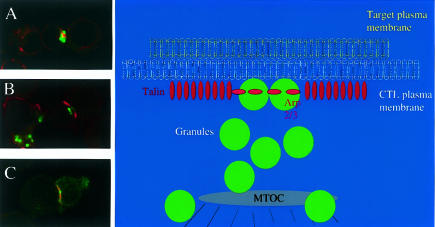
Figure 2.
Polarization of CTL microtubule organizing centre and secretory granules and formation of the immunological synapse upon target cell recognition by CTL. (a–c) Confocal images of methanol-fixed mouse CD8+ CTL (left hand side) conjugated to P815 target cells (right hand side) and labelled with antibodies to the lysosomal protein, cathepsin D (green, a and b), tubulin (red, a), talin (red, b and c) and arp2/3 (green, c).On initial recognition of the target cell, the CTL microtubule organizing centre (a; tubulin, red) polarizes towards the target cell and the secretory granules (a; cathepsin D, green) accumulate around it before moving to the plasma membrane for secretion (green; b). Talin (b and c; red) and arp2/3 (c; green) associate with membrane-associated proteins at the plasma membrane at the contact site. Note the clear topological distribution of these two proteins at the membrane, characteristic of the immunological synapse, with talin forming a ring surrounding a central hole which contains arp2/3. Note in (b), that the secretory granules which are tightly associated with the plasma membrane are confined to the central core of the immunological synapse. (d) Schematic diagram showing the distribution of proteins and secretory granules at the immunological synapse which forms at the site of contact between the target cell plasma membrane (yellow, top) and CTL plasma membrane (white, bottom), and shown in (a)–(c). CTL LFA-1 binds to ICAM-1 on the target and recruits talin (red vertical ovals) from the cytoplasm. These proteins are rapidly distributed into an outer ring which surrounds a central core which is likely to contain the TCR and recruits arp3/2 (red horizontal ovals). The secretory granules (green) polarized around the MTOC (grey) are secreted through the central core of the synapse.
Unlike CTL from ashen mice, CTL from dilute mice can kill normally, suggesting that the secretory granules of CTL can be secreted normally49. However, in both mice the coat colour is affected, indicating that the secretory lysosomes of these cells require both Rab27a and myosin V for secretion. Studies in melanosomes from dilute mice have demonstrated that the melanosomes need myosin V to remain ‘tethered’ at the cell periphery once they have migrated along the microtubules.50,51 This is consistent with biochemical data demonstrating a direct interaction between Rab27a and myosin V.52 Why then should secretion be affected in melanocytes from dilute mice, but not in CTL? Myosin V is one of a number of unconventional myosins and it is possible that in T cells Rab27a can interact with another myosin, or that the requirement for this interaction is less stringent. Either way, it is consistent with a role for Rab27a in mediating a final movement of the granules into the talin ‘hole’ so that the granules can fuse at the plasma membrane. Interestingly, the Arp2/3 complex of proteins involved in actin polymerization, localizes at this talin hole in the immunological synapse and may also be important in secretion.14
Hermansky–Pudlak syndrome
Hermansky–Pudlak syndrome (HPS) is another autosomal recessive disease that gives rise to immune defects, most notably affecting platelet secretion, and varying levels of albinism. Recent advances in genetic mapping have identified several of the defective genes which can give rise to this disorder.53 In many patients mutations have been identified in a novel gene, termed HPS-1.54 In other patients mutations have been identified in subunits of the adaptor protein, AP3, which is involved in sorting of proteins from the Golgi to the lysosome. Both HPS-1 and AP3 are ubiquitously expressed, but mutations in these proteins seem to have a devastating affect on cells using secretory lysosomes. The list of potential genes giving rise to HPS has been greatly extended by a long-term study by Swank and colleagues on a series of mice with coat colour and platelet secretion disorders.55 This study has identified at least 15 mouse strains, with mutations mapping to different loci on different chromosomes, indicating that the true number of genes which can give rise to the HPS phenotype will be much greater.
One of the strains identified as an HPS model is the gunmetal mouse. Gunmetal is defective in a rab geranylgeranyl transferse-II (RGGT) that is required to prenylate, and therefore regulate the membrane association and activity of a subset of Rab proteins.56 Amongst these is Rab27a, which is affected in the ashen mouse. It is important to point out that the gunmetal mutation reduces the level of prenylation by about 80%, but there is still 20% activity which gives rise to a reduced level of active Rab27a in the cells. Remarkably, CTL from gunmetal mice are able to kill targets, although at a slightly reduced level compared with wild-type cells, indicating that 20% of normal Rab27a levels are sufficient for secretion.14 What is striking about gunmetal CTL is that they only show partial polarization of their secretory granules at the immunological synapse. Many granules remain scattered around the periphery of the cell away from the sites of exocytosis in these cells, suggesting that a Rab protein prenylated by RGGT is involved in regulating transfer onto or along polarized microtubules in these cells upon activation. Interestingly, a yeast mutant, mrs6-2, shows a defect in a Rab escort protein and reduced prenylation of yeast Rab homologues and fails to polarize vesicles towards the bud site, resulting in the accumulation of small-budded binucleate cells.57 This defect can be suppressed by the over-expression of a number of different proteins involved in cell wall maintenance, or MLC1, a myosin light chain able to bind myo2, the yeast myosin V homologue.
Acknowledgments
G.M.G. is a Wellcome Trust Senior Fellow. The laboratory is supported entirely by grants from the Wellcome Trust.
References
- 1.Stinchcombe JC, Griffiths GM. Regulated secretion from hemopoietic cells. J Cell Biol. 1999;147:1–6. doi: 10.1083/jcb.147.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Griffiths GM. Secretory lysosomes – a special mechanism of regulated secretion in haemopoieitic cells. Trends Cell Biology. 1996;6:329–32. doi: 10.1016/0962-8924(96)20031-5. [DOI] [PubMed] [Google Scholar]
- 3.Lin RC, Scheller RH. Structural organization of the synaptic exocytosis core complex. Neuron. 1997;19:1087–94. doi: 10.1016/s0896-6273(00)80399-2. [DOI] [PubMed] [Google Scholar]
- 4.Podack ER, Kupfer A. T-cell effector functions: mechanisms for delivery of cytotoxicity and help. Annu Rev Cell Biol. 1991;7:479–504. doi: 10.1146/annurev.cb.07.110191.002403. [DOI] [PubMed] [Google Scholar]
- 5.Springer TA, Davignon D, Ho MK, Kurzinger K, Martz E, Sanchez-Madrid F. LFA-1 and Lyt-2,3 molecules associated with T lymphocyte-mediated killing; and Mac-1, an LFA-1 homologue associated with complement receptor function. Immunol Rev. 1982;68:171–95. doi: 10.1111/j.1600-065x.1982.tb01064.x. [DOI] [PubMed] [Google Scholar]
- 6.Krensky AM, Robbins E, Springer TA, Burakoff SJ. LFA-1, LFA-2, and LFA-3 antigens are involved in CTL-target conjugation. J Immunol. 1984;132:2180–2. [PubMed] [Google Scholar]
- 7.Burn P, Kupfer A, Singer SJ. Dynamic membrane–cytoskeletal interactions: specific association of integrin and talin arises in vivo after phorbol ester treatment of peripheral blood lymphocytes. Proc Natl Acad Sci USA. 1988;85:497–501. doi: 10.1073/pnas.85.2.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grakoui A, Bromley SK, Sumen C, Davis MM, Shaw AS, Allen PM, Dustin ML. The immunological synapse: a molecular machine controlling T cell activation [see comments] Science. 1999;285:221–7. doi: 10.1126/science.285.5425.221. 10.1126/science.285.5425.221. [DOI] [PubMed] [Google Scholar]
- 9.Monks CR, Freiberg BA, Kupfer H, Sciaky N, Kupfer A. Three-dimensional segregation of supramolecular activation clusters in T cells. Nature. 1998;395:82–6. doi: 10.1038/25764. 10.1038/25764. [DOI] [PubMed] [Google Scholar]
- 10.Kuhne MR, Ku G, Weiss A. A guanine nucleotide exchange factor-independent function of Vav1 in transcriptional activation. J Biol Chem. 2000;275:2185–90. doi: 10.1074/jbc.275.3.2185. [DOI] [PubMed] [Google Scholar]
- 11.Kupfer A, Singer SJ. Cell biology of cytotoxic and helper T cell functions: immunofluorescence microscopic studies of single cells and cell couples. Annu Rev Immunol. 1989;7:309–37. doi: 10.1146/annurev.iy.07.040189.001521. [DOI] [PubMed] [Google Scholar]
- 12.Burkhardt JK, McIlvain Jm, Jr, Sheetz MP, Argon Y. Lytic granules from cytotoxic T cells exhibit kinesin-dependent motility on microtubules in vitro. J Cell Sci. 1993;104:151–62. doi: 10.1242/jcs.104.1.151. [DOI] [PubMed] [Google Scholar]
- 13.Yannelli JR, Sullivan JA, Mandell GL, Engelhard VH. Reorientation and fusion of cytotoxic T lymphocyte granules after interaction with target cells as determined by high resolution cinemicrography. J Immunol. 1986;136:377–82. [PubMed] [Google Scholar]
- 14.Stinchcombe JC, Mules E, Booth S, Barral D, Hume A, Seabra M, Griffiths GM. Rab27a is required for regulated secretion in cytotoxic T lymphocytes. J Cell Biol. 2001;152:825–33. doi: 10.1083/jcb.152.4.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hibi T, Hirashima N, Nakanishi M. Rat basophilic leukemia cells express syntaxin-3 and VAMP-7 in granule membranes. Biochem Biophys Res Commun. 2000;271:36–41. doi: 10.1006/bbrc.2000.2591. [DOI] [PubMed] [Google Scholar]
- 16.Paumet F, Le Mao J, Martin S, Galli T, David B, Blank U, Roa M. Soluble NSF attachment protein receptors (SNAREs) in RBL-2H3 mast cells: functional role of syntaxin 4 in exocytosis and identification of a vesicle-associated membrane protein 8-containing secretory compartment. J Immunol. 2000;164:5850–7. doi: 10.4049/jimmunol.164.11.5850. [DOI] [PubMed] [Google Scholar]
- 17.Sollner T, Bennett MK, Whiteheart SW, Scheller RH, Rothman JE. A protein assembly-disassembly pathway in vitro that may correspond to sequential steps of synaptic vesicle docking, activation, and fusion. Cell. 1993;75:409–18. doi: 10.1016/0092-8674(93)90376-2. [DOI] [PubMed] [Google Scholar]
- 18.Schiavo G, Benfenati F, Poulain B, Rossetto O, de Polverino Laureto P, DasGupta BR, Montecucco C. Tetanus and botulinum-B neurotoxins block neurotransmitter release by proteolytic cleavage of synaptobrevin [see comments] Nature. 1992;359:832–5. doi: 10.1038/359832a0. [DOI] [PubMed] [Google Scholar]
- 19.Arora N, Williamson LC, Leppla SH, Halpern JL. Cytotoxic effects of a chimeric protein consisting of tetanus toxin light chain and anthrax toxin lethal factor in non-neuronal cells. J Biol Chem. 1994;269:26165–71. [PubMed] [Google Scholar]
- 20.Lemons PP, Chen D, Bernstein AM, Bennett MK, Whiteheart SW. Regulated secretion in platelets: identification of elements of the platelet exocytosis machinery [see comments] Blood. 1997;90:1490–500. [PubMed] [Google Scholar]
- 21.Chen D, Lemons PP, Schraw T, Whiteheart SW. Molecular mechanisms of platelet exocytosis: role of SNAP-23 and syntaxin 2 and 4 in lysosome release. Blood. 2000;96:1782–8. [PubMed] [Google Scholar]
- 22.Chen D, Bernstein AM, Lemons PP, Whiteheart SW. Molecular mechanisms of platelet exocytosis: role of SNAP-23 and syntaxin 2 in dense core granule release. Blood. 2000;95:921–9. [PubMed] [Google Scholar]
- 23.Lemons PP, Chen D, Whiteheart SW. Molecular mechanisms of platelet exocytosis: requirements for alpha-granule release. Biochem Biophys Res Commun. 2000;267:875–80. doi: 10.1006/bbrc.1999.2039. 10.1006/bbrc.1999.2039. [DOI] [PubMed] [Google Scholar]
- 24.Flaumenhaft R, Croce K, Chen E, Furie B, Furie BC. Proteins of the exocytotic core complex mediate platelet alpha-granule secretion. Roles of vesicle-associated membrane protein, SNAP-23, and syntaxin 4. J Biol Chem. 1999;274:2492–501. doi: 10.1074/jbc.274.4.2492. [DOI] [PubMed] [Google Scholar]
- 25.Brumell JH, Volchuk A, Sengelov H, Borregaard N, Cieutat AM, Bainton DF, Grinstein S, Klip A. Subcellular distribution of docking/fusion proteins in neutrophils, secretory cells with multiple exocytic compartments. J Immunol. 1995;155:5750–9. [PubMed] [Google Scholar]
- 26.Martin-Martin B, Nabokina SM, Blasi J, Lazo PA, Mollinedo F. Involvement of SNAP-23 and syntaxin 6 in human neutrophil exocytosis [In Process Citation] Blood. 2000;96:2574–83. [PubMed] [Google Scholar]
- 27.Schimmoller F, Simon I, Pfeffer SR. Rab GTPases, directors of vesicle docking. J Biol Chem. 1998;273:22161–4. doi: 10.1074/jbc.273.35.22161. [DOI] [PubMed] [Google Scholar]
- 28.Pereira-Leal JB, Seabra MC. The mammalian Rab family of small GTPases: definition of family and subfamily sequence motifs suggests a mechanism for functional specificity in the Ras superfamily. J Mol Biol. 2000;301:1077–87. doi: 10.1006/jmbi.2000.4010. 10.1006/jmbi.2000.4010. [DOI] [PubMed] [Google Scholar]
- 29.Echard A, Jollivet F, Martinez O, Lacapere JJ, Rousselet A, Janoueix-Lerosey I, Goud B. Interaction of a Golgi-associated kinesin-like protein with Rab6. Science. 1998;279:580–5. doi: 10.1126/science.279.5350.580. 10.1126/science.279.5350.580. [DOI] [PubMed] [Google Scholar]
- 30.Lutcke A, Parton RG, Murphy C, Olkkonen VM, Dupree P, Valencia A, Simons K, Zerial M. Cloning and subcellular localization of novel rab proteins reveals polarized and cell type-specific expression. J Cell Sci. 1994;107:3437–48. doi: 10.1242/jcs.107.12.3437. [DOI] [PubMed] [Google Scholar]
- 31.Roa M, Paumet F, Le Mao J, David B, Blank U. Involvement of the ras-like GTPase rab3d in RBL-2H3 mast cell exocytosis following stimulation via high affinity IgE receptors (Fc epsilonRI) J Immunol. 1997;159:2815–23. [PubMed] [Google Scholar]
- 32.Tuvim MJ, Adachi R, Chocano JF, et al. Rab3D, a small GTPase, is localized on mast cell secretory granules and translocates to the plasma membrane upon exocytosis. Am J Respir Cell Mol Biol. 1999;20:79–89. doi: 10.1165/ajrcmb.20.1.3279. [DOI] [PubMed] [Google Scholar]
- 33.Masuda ES, Luo Y, Young C, et al. Rab37 is a novel mast cell specific GTPase localized to secretory granules. FEBS Lett. 2000;470:61–4. doi: 10.1016/s0014-5793(00)01288-6. [DOI] [PubMed] [Google Scholar]
- 34.Karniguian A, Zahraoui A, Tavitian A. Identification of small GTP-binding rab proteins in human platelets: thrombin-induced phosphorylation of rab3B, rab6, and rab8 proteins. Proc Natl Acad Sci USA. 1993;90:7647–51. doi: 10.1073/pnas.90.16.7647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nagata K, Nozawa Y. [Role of GTP-binding proteins in phospholipid metabolism in human platelets] Nippon Rinsho. 1992;50:223–9. [PubMed] [Google Scholar]
- 36.Shirakawa R, Yoshioka A, Horiuchi H, Nishioka H, Tabuchi A, Kita T. Small GTPase rab4 regulates Ca2+-induced alpha-granule secretion in platelets [In Process Citation] J Biol Chem. 2000;275:33844–9. doi: 10.1074/jbc.M002834200. 10.1074/jbc.m002834200. [DOI] [PubMed] [Google Scholar]
- 37.Chen D, Guo J, Miki T, Tachibana M, Gahl WA. Molecular cloning and characterisation of rab27a and rab27b, novel human rab proteins shared by melanocytes and platelets. Biochem Mol Med. 1997;60:27–37. doi: 10.1006/bmme.1996.2559. 10.1006/bmme.1996.2559. [DOI] [PubMed] [Google Scholar]
- 38.Baram D, Adachi R, Medalia O, Tuvim M, Dickey BF, Mekori YA, Sagi-Eisenberg R. Synaptotagmin II negatively regulates Ca2+-triggered exocytosis of lysosomes in mast cells. J Exp Med. 1999;189:1649–58. doi: 10.1084/jem.189.10.1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kee Y, Yoo JS, Hazuka CD, Peterson KE, Hsu SC, Scheller RH. Subunit structure of the mammalian exocyst complex. Proc Natl Acad Sci USA. 1997;94:14438–43. doi: 10.1073/pnas.94.26.14438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McVey Ward D, Griffiths GM, Stinchcombe JC, Kaplan J. Analysis of the lysosomal disease Chediak–Higashi Syndrome. Traffic. 2000;1:816–22. doi: 10.1034/j.1600-0854.2000.011102.x. 10.1034/j.1600-0854.2000.011102.x. [DOI] [PubMed] [Google Scholar]
- 41.Baetz K, Isaaz S, Griffiths GM. Loss of cytotoxic T lymphocyte function in Chediak–Higashi syndrome arises from a secretory defect that prevents lytic granule exocytosis. J Immunol. 1995;154:6122–31. [PubMed] [Google Scholar]
- 42.Orlow SJ. Melanosomes are specialized members of the lysosomal lineage of organelles. J Invest Dermatol. 1995;105:3–7. doi: 10.1111/1523-1747.ep12312291. [DOI] [PubMed] [Google Scholar]
- 43.Barbosa MD, Nguyen QA, Tchernev VT, et al. Identification of the homologous beige and Chediak–Higashi syndrome genes [published erratum appears in Nature 1997; 385:97] Nature. 1996;382:262–5. doi: 10.1038/382262a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nagle DL, Karim MA, Woolf EA, et al. Identification and mutation analysis of the complete gene for Chediak–Higashi syndrome [see comments] Nat Genet. 1996;14:307–11. doi: 10.1038/ng1196-307. [DOI] [PubMed] [Google Scholar]
- 45.Mancini AJ, Chan LS, Paller AS. Partial albinism with immunodeficiency: Griscelli syndrome: report of a case and review of the literature. J Am Acad Dermatol. 1998;38:295–300. doi: 10.1016/s0190-9622(98)70568-7. [DOI] [PubMed] [Google Scholar]
- 46.Pastural E, Ersoy F, Yalman N, et al. Two genes are responsible for Griscelli syndrome at the same 15q21 locus. Genomics. 2000;63:299–306. doi: 10.1006/geno.1999.6081. 10.1006/geno.1999.6081. [DOI] [PubMed] [Google Scholar]
- 47.Menasche G, Pastural E, Feldmann J, et al. Mutations in RAB27A cause Griscelli syndrome associated with haemophagocytic syndrome. Nat Genet. 2000;25:173–6. doi: 10.1038/76024. 10.1038/76024. [DOI] [PubMed] [Google Scholar]
- 48.Wilson SM, Yip R, Swing DA, et al. A mutation in Rab27a causes the vesicle transport defects observed in ashen mice. Proc Natl Acad Sci USA. 2000;97:7933–8. doi: 10.1073/pnas.140212797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Haddad EK, Wu X, Hammer JA, Henkart PA. Defective granule exocytosis in Rab27a-deficient lymphocytes. J Cell Biol. 2001;152:835–41. doi: 10.1083/jcb.152.4.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wu X, Bowers B, Wei Q, Kocher B, Hammer JA. Myosin V associates with melanosomes in mouse melanocytes: evidence that myosin V is an organelle motor. J Cell Sci. 1997;110:847–59. doi: 10.1242/jcs.110.7.847. 3rd. [DOI] [PubMed] [Google Scholar]
- 51.Wu X, Jung G, Hammer JA. Functions of unconventional myosins. Curr Opin Cell Biol. 2000;12:42–51. doi: 10.1016/s0955-0674(99)00055-1. 10.1016/s0955-0674(99)00055-1 3rd. [DOI] [PubMed] [Google Scholar]
- 52.Hume AN, Collinson L, Rapak A, Gomes AQ, Hopkins CR, Seabra MC. Rab27a regulates the peripheral distribution of melanosomes in melanocytes. J Cell Biol. 2001;152:795–808. doi: 10.1083/jcb.152.4.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Huizing M, Anikster Y, Gahl WA. Hermansky–Pudlak Syndrome and related disorders of organelle formation. Traffic. 2000;1:823–35. doi: 10.1034/j.1600-0854.2000.011103.x. 10.1034/j.1600-0854.2000.011103.x. [DOI] [PubMed] [Google Scholar]
- 54.Oh J, Bailin T, Fukai K, et al. Positional cloning of a gene for Hermansky–Pudlak syndrome, a disorder of cytoplasmic organelles [see comments] Nat Genet. 1996;14:300–6. doi: 10.1038/ng1196-300. [DOI] [PubMed] [Google Scholar]
- 55.Swank RT, Novak EK, McGarry MP, Rusiniak ME, Feng L. Mouse models of Hermansky Pudlak syndrome: a review. Pigment Cell Res. 1998;11:60–80. doi: 10.1111/j.1600-0749.1998.tb00713.x. [DOI] [PubMed] [Google Scholar]
- 56.Detter JC, Zhang Q, Mules EH, et al. Rab geranylgeranyl transferase alpha mutation in the gunmetal mouse reduces Rab prenylation and platelet synthesis. Proc Natl Acad Sci USA. 2000;97:4144–9. doi: 10.1073/pnas.080517697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bialek-Wyrzykowska U, Bauer BE, Wagner W, Kohlwein SD, Schweyen RJ, Ragnini A. Low levels of Ypt protein prenylation cause vesicle polarization defects and thermosensitive growth that can be suppressed by genes involved in cell wall maintenance. Mol Microbiol. 2000;35:1295–311. doi: 10.1046/j.1365-2958.2000.01782.x. 10.1046/j.1365-2958.2000.01782.x. [DOI] [PubMed] [Google Scholar]