Abstract
Oestrogens affect the development and regulation of the immune system. To determine the role of oestrogen receptors α (ER-α) and β (ER-β) on the development of the immune system, male ER-α (ERKO) and ER-β (BERKO) mice, as well as αβ-double knockout (DERKO) mice, were studied. Deletion of ER-α led to hypoplasia of both thymus and spleen. Interestingly, a higher frequency of immature double CD4+ CD8+ thymocytes was found in ER-α− mice compared with ER-α+ mice. Female oophorectomized BERKO mice given oestradiol (E2) displayed a similar degree of thymic atrophy compared with the wild-type strain but showed only limited involution of thymus cortex and no alteration of thymic CD4/CD8 phenotype expression. Our data demonstrate that expression of ER-α, but not ER-β, is mandatory in males for development of full-size thymus and spleen, whereas expression of ER-β is required for E2-mediated thymic cortex atrophy and thymocyte phenotype shift in females. A potential background for the above findings may be down-regulated activity in the growth hormone/insulin-like growth factor-1 (GH/IGF-1) axis in males lacking ER-α and suppressed sensitivity of females lacking ER-β to E2-mediated suppression of IGF-1.
Introduction
Oestrogens are known to exert multiple effects on the development and regulation of the immune system. The cellular and molecular mechanisms through which oestrogens mediate these effects are, to a large extent, still unknown. It is well established that oestrogens are important both for the development of thymus and for thymic atrophy during pregnancy.1,2 Oestrogens have been suggested to be responsible for the heightened immune responsiveness and increased incidence of autoimmune diseases in females compared to males.3,4 In experimental systems, oestrogens have been shown to decrease T-lymphocyte-dependent delayed-type hypersensitivity (DTH),5 natural killer (NK)-cell mediated cytotoxicity6–8 and granulocyte-mediated inflammation,9 and to enhance immunoglobulin and antibody production.5
Oestrogens exert their different biological effects by binding to specific nuclear oestrogen receptors (ER). Oestradiol (E2) was formerly believed to accomplish its effects through only one receptor. Hence, it was puzzling that oestrogens could have such diverse effects in different tissues and cells. In 1996 a novel oestrogen receptor, named oestrogen receptor β (ER-β), was cloned from rat prostate.10 mRNA and protein expression of the ER-β gene have been detected in different tissues in various species.11–13 Furthermore, cells involved in the immune system express ER-β, for instance thymus and spleen of human mid-gestational fetus14 and lymphocytes in human lymph nodes.11 With the existence of two different ERs, the diverse action of oestrogen might be more easily explained and the variability possibly even greater, as it has been shown in vitro that ER-α and ER-β can form heterodimers on DNA.15,16
ER knockout mice, both the ER-α knockout, ERKO,17 and the novel ER-β knockout, BERKO,18 are valuable tools for using to understand through which of the two receptors oestrogens act (reviewed in ref. 19).
Sex hormones influence the fetal development of thymus and regulate the size of the thymus gland in adult mice. Typically, castration of both sexes leads to an increase of thymus weight and cellularity.20,21 Exposure to endogenous oestrogen during pregnancy,22 or treatment of castrated animals with exogenous sex hormones, causes massive atrophy of the thymus with typically increased frequencies of single CD4+ and CD8+ T lymphocytes.20,21,23 In one study published recently it was shown that male ERKO mice had smaller thymi than wild-type (WT) littermates and that they displayed less thymic atrophy and lacked the typical phenotypic alterations of T cells upon exposure to E2.24 These results indicate that ER-α is mandatory for full development of the thymus and for E2-induced thymic alterations.
In order to investigate the role of ER-α and -β in development of the immune system of male mice, we used ERKO, BERKO, double ER knockout (DERKO) and WT mice, and analysed lymphocyte phenotypes and thymus and spleen size. Furthermore, we exposed oophorectomized female BERKO mice to E2 to investigate the role of ER-β on oestrogen-mediated effects on thymus gland involution. Serum levels of insulin-like growth factor-1 (IGF-1) were analysed, and the possible role is discussed of the growth hormone (GH)/IGF-1 axis on oestrogen-mediated effects on the development and regulation of the immune system.
Materials and methods
Mice
Generation of DERKO mice.
Male double-heterozygous (ER-α+/− β+/−) mice were mated with female double-heterozygous (ER-α+/− β+/−) mice on a mixed C57Bl/6 J/129 background resulting in WT, ERKO, BERKO and DERKO offspring. Genotyping of tail DNA was performed by using the polymerase chain reaction (PCR). The ER-α gene was analysed with the primer pairs 5′-AACTCGCCGGCTGCCACTTACCAT-3′ and 5′-CATCAGCGGGCTAGGCGACACG-3′, resulting in a 320-bp fragment for the WT gene. Primers 5′-TGTGGCCGGCTGGGTGTG-3′ and 5′-GGCGCTGGGCTCGTTCTC-3′ resulted in a 700-bp fragment for the mutant gene. The ER-β gene was analysed with the following primers: βNHD4-25 (5′-AGAATGTTGCACTGCCCCTGCTGCT-3′), C1wt-27 (5′-GGAGTAGAAACAAGCAATCCAGACATC-3′) and Neo-25 (5′-GCAGCCTCTGTTCCACATACACTTC-3′). A 650-bp product (βNHD4-25 and C1wt-27) was amplified for the WT gene and a 450-bp product (βNHD4-25 and Neo-25) was amplified for the mutant gene.
Breeding of BERKO mice.
ER-β−/− (BERKO) and WT (ER-β+/+) littermates were bred from heterozygous ER-β+/− mice, and the ER-β gene was analysed as described above.
Housing conditions.
Mice were housed with five to 10 animals in each cage under standard conditions of temperature and light, and fed standard laboratory chow ad libitum.
Castration and hormone treatment
In the oestrogen exposure experiments, all mice were oophorectomized. Ovaries were removed after a flank incision and the incisions were kept closed with metallic clips. The oophorectomy was carried out under Hypnorm®/Dormicum® anaesthesia. Mice were rested for 1 week after castration before the experiments were started, when mice were injected subcutaneously (s.c.) with 0·1 mg/kg/day of 17β-estradiol benzoate (E2) (Sigma, St Louis, MO). Control mice received injections of vehicle oil (Apoteksbolaget, Göteborg, Sweden).
Cellular parameters
Tissue collection and single-cell preparation.
The mice were killed by cervical dislocation 24 hr after the last hormone injection. Spleens and thymus glands were removed and weighed. Single-cell suspensions were obtained after tissue was mashed and passed through a nylon wool sieve. The cells were centrifuged at 515 g for 5 min, and pelleted spleen cells were resuspended in Tris-buffered 0·83% NH4Cl solution (pH 7·29) to lyse erythrocytes. After washing in phosphate-buffered saline (PBS) the total number of leucocytes was calculated using an automated cell counter (Sysmex, Kobe, Japan), and the cells were subjected to fluorescence-activated cell sorter (FACS) analysis. Cell numbers were not adjusted for viability as in earlier studies no changes in cell viability were observed after repeated exposure of mice to 0·1 mg/kg/day of oestradiol.25
Flow cytometry for analysis of cell phenotypes.
Isolated thymocytes were stained with fluorescein isothiocyanate (FITC)-labelled antibodies to CD8a (clone 53-6·7) (Becton-Dickinson, Franklin Lakes, NJ) and phycoerythrin (PE)-conjugated antibodies to CD4 (clone H129·19) (Becton-Dickinson). Spleen cells were labelled with αCD8-FITC, αCD4-PE, αCD3ε-PE (clone 145-2C11) (Becton-Dickinson) and αCD45R/B220-FITC (clone RA3-6B2) (Becton-Dickinson). The cells were analysed in a fluorescence-activated cell sorter (FACSCalibur; Becton-Dickinson). Stained cells were expressed as percentage of gated mononuclear cells.
Histological parameters
Thymus glands taken at autopsy were placed in 4% formaldehyde, dehydrated, sectioned and stained with haematoxylin and eosin. The sections were coded and examined in a Leica microscope (Leica, Wetzlar, Germany) with the computer application leica qwin (Leica). Whole thymus, cortex and medulla areas were measured, and the ratio between the cortex and total area was calculated (cortex area fraction).
Serological assays
Serum IGF-1 levels were measured by double-antibody IGF-binding protein-blocked radioimmunoassay, according to Blum & Breier.26
Statistical analysis
In the experiments comparing male ERKO, BERKO, DERKO and WT mice we first analysed each knockout group against WT mice by use of the Mann–Whitney U-test. Next we used a two-way analysis of variance (anova) to find statistical differences with respect to the expression of ER-α and ER-β. These analyses clearly demonstrated that all statistically significant differences were linked to the presence or absence of ER-α. Therefore, we pooled data from WT and BERKO (ER-α+), and ERKO and DERKO (ER-α−) mice and subsequently analysed the pools by using the Mann–Whitney U-test.
In the experiments analysing effects of E2 treatment in female mice, the non-parametric Mann–Whitney U-test was used. Correlation analyses were performed by use of Fisher's r to z-test. Results are presented as mean ± standard error of mean (SEM). A P-value of < 0·05 was considered statistically significant.
Results
The role of oestrogen in the development of thymus and spleen in male mice
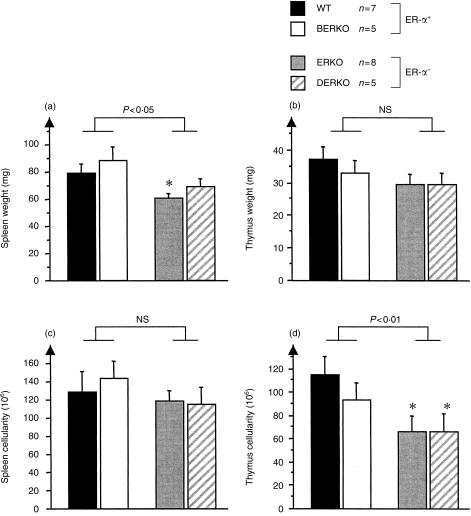
To determine the role of oestrogen on body weight and the development of thymus and spleen, 4-month-old male ERKO, BERKO, DERKO and WT littermates were studied. When the mice were killed, body weight did not differ among any of the four groups (data not shown). After killing, the weight and cell count of spleens and thymus glands were determined. ER-α− mice (ERKO and DERKO) displayed significantly lower thymic cellularity compared with ER-α+ (WT and BERKO) mice (Fig. 1d). The spleen weights of ER-α− mice were significantly lower than those of ER-α+ mice (Fig. 1a). The spleen cellularity was, however, not significantly reduced in ER-α− mice (Fig. 1c). These results clearly indicate that oestrogen, through action on ER-α, is an important growth factor for thymus and spleen of male mice and that ER-β has no such role.
Figure 1.
Mice lacking oestrogen receptor-α (ER-α) have smaller spleens and thymus glands than ER-α+ mice. Male 4-month-old ER-α knockout (ERKO) mice, ER-β knockout (BERKO) mice and αβ-double knockout (DERKO) mice were compared with littermate wild-type (WT) mice with respect to splenic (a) and thymic (b) weight and spleen (c) and thymus (d) cellularity. The non-parametric Mann–Whitney U-test was used to detect statistical significance between WT mice and the different knock-out groups. *P < 0·05 versus WT mice. A two-way analysis of variance (anova) was used to detect statistical differences with respect to the expression of ER-α and ER-β. As all statistically significant effects were found relative to the presence or absence of ER-α, two groups were obtained by pooling ER-α+ mice (WT and BERKO) and ER-α− mice (ERKO and DERKO). The pools were then analysed by using the Mann–Whitney U-test to obtain the P-values given in the figure. Mean±SEM values are shown. NS, not significant.
Histological examination of sections of thymus glands revealed that, irrespective of ER genotype, all groups of mice displayed similar cortex area fraction (data not shown).
Lymphocyte phenotypes in thymus were analysed by use of flow cytometry. ER-α− mice had a significantly lower frequency of single CD4+ and CD8+ thymocytes and a higher frequency of double CD4+ CD8+ thymocytes compared with ER-α+ mice (Table 1). In contrast, BERKO mice displayed CD4+ and CD8+ frequencies similar to those of WT mice. These data indicate a role of ER-α in the maturation process of T lymphocytes in the thymus of male mice.
Table 1. Phenotypes of thymic T cells in wild-type (WT), oestrogen receptor (ER)-α knockout (ERKO), ER-β knockout (BERKO) and αβ-double knockout (DERKO) mice.
| CD4+ thymocytes (%) | CD8+ thymocytes (%) | CD4+/CD8+ thymocytes (%) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| ER-α+ (n = 12) | WT (n = 7) | 11·3 ± 0·7 | WT (n = 7) | 2·1 ± 0·3 | WT (n = 7) | 82·7 ± 0·9 | |||
| 11·9 ± 0·5 | 1·9 ± 0·2 | 81·7 ± 0·7 | |||||||
| BERKO (n = 5) | 12·7 ± 0·4 | BERKO (n = 5) | 1·7 ± 0·2 | BERKO (n = 5) | 80·1 ± 0·6 | ||||
| P < 0·05* | P < 0·05* | P < 0·05* | |||||||
| ER-α− (n = 11) | ERKO (n = 8) | 9·9 ± 0·8 | ERKO (n = 8) | 1·5 ± 0·3 | ERKO (n = 8) | 84·1 ± 1·5 | |||
| 9·8 ± 0·7 | 1·5 ± 0·2 | 84·2 ± 1·1 | |||||||
| DERKO (n = 3) | 9·3 ± 1·2 | DERKO (n = 3) | 1·4 ± 0·3 | DERKO (n = 3) | 84·4 ± 1·7 |
Four-month-old male ERKO, BERKO, DERKO and WT mice were generated from breeding of ER-α+/−β+/− mice. Thymocytes were stained with α-CD4 phycoerythrin (PE) and α-CD8 fluorescein isothiocyanate (FITC) and analysed by flow cytometry. The non-parametric Mann–Whitney U-test was used to find statistical significance between WT and the different knockout groups. (No statistical differences were found.) A two-way analysis of variance (anova) was used to find statistical differences with respect to the expression of ER-α and ER-β. As all statistically significant effects found were linked to the presence or absence of ER-α, two groups were obtained by pooling ER-α+ mice (WT and BERKO) and ER-α− mice (ERKO and DERKO). The pools were than analysed using the Mann–Whitney test to obtain the P-values
given in the table. Data is shown as mean ± SEM.
The frequency of B- and T-cell phenotypes in spleen did not differ between any of the four groups of mice (data not shown).
Role of ER-β in E2-mediated thymic atrophy
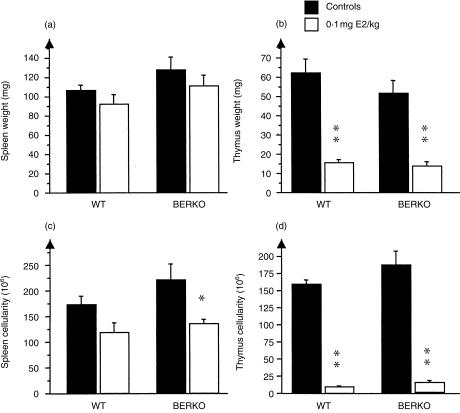
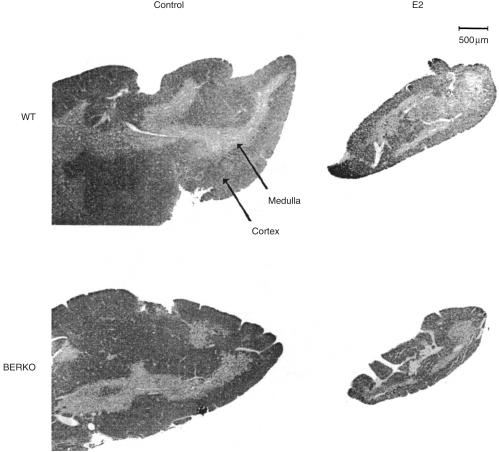
Four-month-old oophorectomized female BERKO and WT littermates were treated for 2 weeks with s.c. injections of E2 (0·1 mg/kg) for 5 days/week or with olive oil as a control. Both BERKO and WT mice displayed a similar degree of thymic atrophy after E2 treatment, with a massive reduction of both thymic weight and cellularity (Fig. 2b, 2d). In histological sections of thymus glands (representative pictures are shown in Fig. 3) the cortex area fraction was calculated. Upon exposure to E2, WT mice displayed a significant reduction of the cortex area fraction, while thymuses of BERKO mice exposed to E2 showed no such significant reduction (Table 2).
Figure 2.
Oestrogen receptor-β knockout (BERKO) and wild-type (WT) mice react similarly to oestradiol (E2) treatment with respect to splenic (a) and thymic (b) weight and spleen (c) and thymus (d) cellularity. Female 4-month-old BERKO and WT mice were treated with 0·1 mg/kg of E2 for 5 days/week or with olive oil as a control. After 2 weeks of treatment, the mice were killed and body weight, thymic and splenic weight, and cellularity were measured. Black bars represent vehicle controls and open bars represent E2-treated mice. Mean±SEM values are shown. The statistical method used was the non-parametric Mann–Whitney U-test. *P < 0·05, **P < 0·01.
Figure 3.
Lack of oestradiol (E2)-induced reduction of cortex area fraction in female oestrogen receptor-β knockout (BERKO) mice. Representative microphotographs are shown of haematoxylin and eosin-stained sections of thymi from female BERKO and wild-type (WT) mice treated for 2 weeks with 0·1 mg/kg of E2 for 5 days/week and from control mice (treated with olive oil). The sections of thymi shown from oil-treated control mice are approximately one-third of the total section area. Original data are shown in Table 2.
Table 2. Cortex area fraction in thymi of oestradiol (E2)-treated oestrogen receptor (ER)-β knockout (BERKO) and wild-type (WT) mice.
| Genotype | Treatment | n | Cortex area fraction (%) |
|---|---|---|---|
| Wild type | Controls | 6 | 79·5 ± 3·7* |
| E2 | 5 | 61·3 ± 2·7 | |
| BERKO | Controls | 4 | 78·9 ± 4·3 |
| E2 | 4 | 69·4 ± 6·4 |
Four-month-old female BERKO and WT mice were treated for 2 weeks with 0·1 mg/kg of E2 for 5 days/week or with olive oil as a control. The thymus cortex area fraction was calculated as: (cortex area) ÷ (total area) of thymus sections stained with haematoxylin and eosin (seeFig. 3). The statistical method used was the non-parametric Mann–Whitney U-test. Data is presented as mean±SEM.
P < 0·05, control versus E2-treated WT mice.
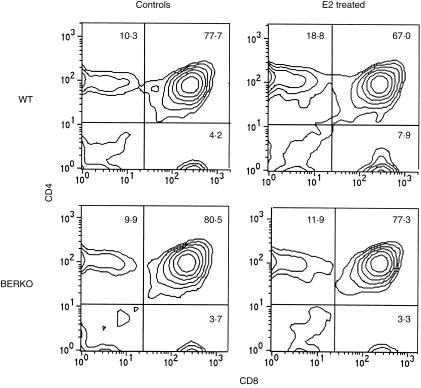
Thymocytes were subjected to flow cytometry for analysis of T-cell phenotypes. WT mice exposed to E2 displayed significantly increased frequencies of single CD4+ and CD8+ thymocytes and a reduced frequency of double CD4+ CD8+ thymocytes. In BERKO mice, the phenotypic alteration of T cells upon exposure to E2 was not seen (Table 3). FACS plots from representative mice are depicted in Fig. 4. Together these results demonstrate that ER-β is important for oestrogen-mediated changes in thymus gland gross morphology and for alteration of T-cell phenotypes, but is not required for reduction of thymic weight and cellularity.
Table 3. Cellularity and phenotypes of thymic T cells in wild-type (WT) and oestrogen receptor (ER)-β knockout (BERKO) mice after treatment with oestradiol (E2).
| Genotype | Treatment | n | Cellularity (106 cells) | CD4+ thymocytes (%) | CD8+ thymocytes (%) | CD4+ CD8+ thymocytes (%) |
|---|---|---|---|---|---|---|
| WT | Controls | 6 | 159 ± 6** | 10·1 ± 0·4** | 3·1 ± 0·4* | 76·3 ± 0·6* |
| E2 | 5 | 10 ± 2 | 20·0 ± 2·9 | 6·6 ± 1·0 | 58·3 ± 6·0 | |
| BERKO | Controls | 4 | 185 ± 21* | 10·2 ± 0·3 | 3·6 ± 0·3 | 75·4 ± 1·2 |
| E2 | 4 | 15 ± 2 | 13·0 ± 1·7 | 3·4 ± 0·6 | 71·8 ± 2·5 |
Four-month-old female BERKO and WT mice were treated for 2 weeks with 0·1 mg/kg of E2 for 5 days/week or with olive oil as a control. Thymocytes were stained with α-CD4 phycoerythrin (PE) and α-CD8 fluorescein isothiocyanate (FITC) and analysed by flow cytometry. Data shown represent mean±SEM values. The statistical method used was the non-parametric Mann–Whitney U-test. Representative plots are shown in Fig. 3.
P < 0·05,
P < 0·01, controls versus E2-treated mice.
Figure 4.
Upon exposure to oestradiol (E2), oestrogen receptor-β knockout (BERKO) mice do not display the typical T-lymphocyte phenotypic alterations seen in wild-type (WT) mice. The plots show representative results from flow cytometry of thymocytes from female BERKO and WT mice treated for 2 weeks with 0·1 mg/kg of E2 for 5 days/week and olive oil control mice. (See Table 3.)
Role of ER-β in oestrogen-mediated effects on spleen
Female 4-month-old WT and BERKO mice were treated with 0·1 mg/kg of E2 for 2 weeks. In both groups of mice, E2 treatment slightly reduced spleen weight (Fig. 2a) and there was a reduction of the spleen cellularity, statistically significant for BERKO mice (Fig. 2c).
Role of ER-β in E2-mediated effects on IGF-1 production
Serum levels of IGF-1 were measured in female oophorectomized WT and BERKO mice treated with E2 for 2 weeks. In Table 4 it is shown that upon exposure to E2, serum levels of IGF-1 were significantly decreased in WT, but not in BERKO, mice.
Table 4. Serum levels of insulin-like growth factor-1 (IGF-1) in oestradiol (E2)-treated female oestrogen receptor (ER)-β knockout (BERKO) and wild-type (WT) mice.
| Genotype | Treatment | n | IGF-1 (ng/ml) |
|---|---|---|---|
| WT | Controls | 6 | 365 ± 23* |
| E2 | 5 | 307 ± 22 | |
| BERKO | Controls | 4 | 350 ± 9 |
| E2 | 4 | 333 ± 37 |
Four-month-old female BERKO and WT mice were treated for 2 weeks with 0·1 mg/kg of E2 for 5 days/week or with olive oil as a control. Serum levels of IGF-1 were measured by use of a radioimmunoassay. Data is presented as mean±SEM. The statistical method used was the non-parametric Mann–Whitney U-test.
P < 0·05, controls versus E2-treated mice.
In both WT and BERKO mice, and irrespective of E2 treatment, there was a significant, negative correlation between serum levels of IGF-1 and the frequency of single CD4+, CD8+ thymocytes and a positive correlation with double CD4+ CD8+ thymocytes, r = − 0·524 (P < 0·05), r = − 0·481 (P < 0·05) and r = 0·460 (P < 0·05), respectively.
Discussion
A breakthrough in the understanding of the biological effects of oestrogen was achieved when the second receptor, ER-β, was discovered. By use of ER-α and -β gene-targeted mice we are now able to elucidate some of the different roles of the two receptors in the development of immune organs. We chose to perform these experiments in male mice as it is known that female ERKO mice display increased production of oestrogens,27 a trait not found in BERKO females (K. Korach, J. Å. Gustafsson, personal communication). Therefore it would be difficult to interpret data from intact female ER-α and -β gene-targeted mice, as oestrogen levels vary. Our data clearly show that ER-α is mandatory for achieving full-size thymus in male mice, whereas ER-β has no such role (Fig. 1b, 1d). In a recently published study24 it was demonstrated that male ERKO mice have significantly smaller thymi than their WT littermates and that this effect was the result of a lack of ER-α in stromal elements in thymus rather than in haemopoetic elements. In addition, we showed that mice not expressing ER-α display a significantly lower percentage of single CD4+ and CD8+ cells and higher frequency of double CD4+ CD8+ cells compared with ER-α+ mice and that ER-β has no influence on the phenotypic expression of thymocytes (Table 1).
Thymic growth and T-cell differentiation are dependent on the secretion of thymic hormones, particularly thymulin. GH, via stimulation of IGF-1, is known to stimulate thymic growth28,29 (reviewed in ref. 30), possibly via the known direct stimulatory effect of IGF-1 on thymulin production.31 A direct association between the GH/IGF-1 axis and the cellularity of thymus has been demonstrated in several conditions, including GH-deficient dwarf mice28 and cyclosporin A-induced thymic atrophy.32 Interestingly, we recently demonstrated that male ER-α− mice have lower serum levels of IGF-1 compared with ER-α+ littermates.33,34 Our novel finding that phenotypic thymocyte changes occur in ER-α− mice (Table 1) could also be explained by reduced IGF-1 production in these mice, as a recent study demonstrated that blocking of IGF-1 in fetal thymic organ cultures increased the frequency of double CD4+ CD8+ cells.35 Thus, one attractive explanation for the reduced thymic growth and cellularity, as well as a changed phenotypic T-lymphocyte pattern of ER-α− mice, is that these effects are mediated by lowered production of IGF-1.
The spleen weight in ER-α− mice was reduced but there was no reduction in the number of mononuclear cells. We can only speculate on the reason for this finding. It might be the result of ER-α-dependent effects in male mice on spleen stromal elements, number of erythrocytes or extracellular fluid content. The activity of the GH/IGF-1 axis is known to be important for splenic growth (reviewed in ref. 36), and it has been demonstrated that serum levels of IGF-1 are positively correlated with the spleen weight in mice.37 As male ER-α− mice are known to have lower IGF-1 levels in serum,33,34 we propose that their smaller spleens are caused by suppressed activity in the GH/IGF-1 axis. In order to test directly the hypothetical importance of IGF-1 in oestrogen-mediated effects on male immune organ development, replacement studies with IGF-1 in ERKO mice are currently underway in our laboratory.
Pregnancy and treatment of mice with higher doses of oestrogen is well known to induce thymic atrophy. This involution is characterized by atrophy of the thymic cortex with relatively preserved medulla, massive loss of cellularity and phenotypic alteration of T lymphocytes, i.e. increased frequencies of single CD4+ and CD8+ cells and decreased frequency of double CD4+ CD8+ cells. We chose to study oestrogen-induced thymic atrophy in female mice because this is a phenomenon that occurs during pregnancy. Endogenous production of oestrogen was removed by oophorectomy in order to obtain a clear experimental situation during subsequent supplementation with known doses of oestrogen. We showed that in E2-triggered thymic involution, expression of ER-β is not required, whereas it is needed both for the extensive atrophy of thymic cortex (Table 2 and Fig. 3) and for the phenotypic alteration of thymic T lymphocytes (Table 3 and Fig. 4) in mice exposed to oestrogen. The recent finding that blocking of IGF-1 decreases the frequency of double CD4+ CD8+ cells in fetal thymic organ cultures,35 is in accordance with our present results demonstrating reduction of this cell population in parallel with lowered serum levels of IGF-1 in E2-exposed female WT mice. The correlation between serum levels of IGF-1 and the frequencies of single CD4+, CD8+ and double CD4+ CD8+ could indicate a direct role of this hormone in the maturation process of T cells within the thymus. The less extensive cortical atrophy and lack of thymocyte phenotype shift in BERKO mice upon exposure to E2 may reflect a lower sensitivity to oestrogen in thymic cortical epithelial cells of mice lacking ER-β. In WT mice, treatment with E2 reduces the number of cortical epithelial cells and thus limits the capacity of positive selection of early T cells in these mice, resulting in a relative accumulation of more mature single CD4+ and CD8+ cells in the preserved thymic medulla.
It was recently demonstrated that ERKO mice were susceptible to E2-induced thymic atrophy, but the extent of their atrophy was less than that seen in WT mice.24 In accordance with our finding in E2-treated BERKO mice it was shown that, compared with WT littermates, ERKO mice failed to alter their CD4/CD8 phenotypes upon E2 exposure. In addition, it was clearly demonstrated that ER-α expression was needed in either haemopoetic or thymic stromal cells to obtain an E2-mediated T-lymphocyte phenotype shift. Taken together, these data demonstrate that both ER-α and ER-β are required for oestrogen-induced phenotypic shifts of T lymphocytes in the thymus of female mice. At this point we can only speculate whether the two receptors work together as separate homodimers or if they form functional heterodimers in the thymus gland or bone marrow stroma cells to mediate this alteration.
In human and rat thymus, both ER-α and ER-β have been detected at the mRNA and protein levels.14,38 ER-α is expressed in thymus stromal cells,39 haemopoetic cells and bone marrow40 of mice. ER-β expression has been detected in murine bone marrow41,42 but no clear-cut data on ER-β expression in thymus of mice are yet available. If ER-β is not expressed in the murine thymus it is possible that the role of this receptor in E2-mediated alteration of thymic T-lymphocyte phenotypes is mediated through action at the prethymic haemopoetic level.
In summary, the present report demonstrates that ER-α is important for effects mediated by a low concentration of oestrogen during thymus gland and spleen development in male mice, whereas both ER-α and -β are required for full thymus involution induced by higher concentrations of oestrogen in female mice.
Further studies of ER-targeted mice are important for elucidating the specific role and the interplay between these two receptors in the regulatory role of oestrogen on immune responsiveness. Such knowledge is of great importance for appropriate use of future ER-specific ligands in postmenopausal women and in females suffering from sex steroid-influenced immune-mediated diseases, such as rheumatoid arthritis and systemic lupus erythematosus.
Acknowledgments
We thank Mrs Lena Svensson and Mrs Zai-Qing Liu for excellent technical assistance. This study was supported by grants from the Swedish cancer foundation, the Börje Dahlin foundation, the Göteborg Medical Society, Association against Rheumatism, the King Gustav V's 80 years foundation, Reuma forskningsfond margareta, the Medical Faculty of University of Göteborg (LUA) and the Swedish Medical Research Council.
References
- 1.Marotti T, Sirotkovic M, Pavelic J, Gabrilovac J, Pavelic K. In vivo effect of progesteron and estrogen on thymus mass and T-cell functions in female mice. Horm Metab Res. 1984;16:201–3. doi: 10.1055/s-2007-1014742. [DOI] [PubMed] [Google Scholar]
- 2.Bodey B, Bodey B, Siegel SE, Kaiser HE. Involution of the mammalian thymus, one of the leading regulators of aging. In Vivo. 1997;11:421–40. [PubMed] [Google Scholar]
- 3.Beeson PB. Age and sex associations of 40 autoimmune diseases. Am J Med. 1994;96:457–62. doi: 10.1016/0002-9343(94)90173-2. [DOI] [PubMed] [Google Scholar]
- 4.Grossman CJ, Roselle GA, Mendenhall CL. Sex steroid regulation of autoimmunity. J Steroid Biochem Mol Biol. 1991;40:649–59. doi: 10.1016/0960-0760(91)90287-f. [DOI] [PubMed] [Google Scholar]
- 5.Carlsten H, Holmdahl R, Tarkowski A, Nilsson LA. Oestradiol- and testosterone-mediated effects on the immune system in normal and autoimmune mice are genetically linked and inherited as dominant traits. Immunology. 1989;68:209–14. [PMC free article] [PubMed] [Google Scholar]
- 6.Hanna N, Schneider M. Enhancement of tumor metastasis and suppression of natural killer cell activity by beta-estradiol treatment. J Immunol. 1983;130:974–80. [PubMed] [Google Scholar]
- 7.Screpanti I, Santoni A, Gulino A, Herberman RB, Frati L. Estrogen and antiestrogen modulation of the levels of mouse natural killer activity and large granular lymphocytes. Cell Immunol. 1987;106:191–202. doi: 10.1016/0008-8749(87)90163-8. [DOI] [PubMed] [Google Scholar]
- 8.Nilsson N, Carlsten H. Estrogen induces suppression of natural killer cell cytotoxicity and augmentation of polyclonal B cell activation. Cell Immunol. 1994;158:131–9. doi: 10.1006/cimm.1994.1262. 10.1006/cimm.1994.1262. [DOI] [PubMed] [Google Scholar]
- 9.Josefsson E, Tarkowski A, Carlsten H. Anti-inflammatory properties of estrogen. I. In vivo suppression of leukocyte production in bone marrow and redistribution of peripheral blood neutrophils. Cell Immunol. 1992;142:67–78. doi: 10.1016/0008-8749(92)90269-u. [DOI] [PubMed] [Google Scholar]
- 10.Kuiper GG, Enmark E, Pelto-Huikko M, Nilsson S, Gustafsson JA. Cloning of a novel receptor expressed in rat prostate and ovary. Proc Natl Acad Sci USA. 1996;93:5925–30. doi: 10.1073/pnas.93.12.5925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Enmark E, Pelto-Huikko M, Grandien K, et al. Human estrogen receptor beta-gene structure, chromosomal localization, and expression pattern. J Clin Endocrinol Metab. 1997;82:4258–65. doi: 10.1210/jcem.82.12.4470. [DOI] [PubMed] [Google Scholar]
- 12.Couse JF, Lindzey J, Grandien K, Gustafsson JA, Korach KS. Tissue distribution and quantitative analysis of estrogen receptor-alpha (ERalpha) and estrogen receptor-beta (ERbeta) messenger ribonucleic acid in the wild-type and ERalpha-knockout mouse. Endocrinology. 1997;138:4613–21. doi: 10.1210/endo.138.11.5496. [DOI] [PubMed] [Google Scholar]
- 13.Wilson ME, Price Rh, Jr, Handa RJ. Estrogen receptor-beta messenger ribonucleic acid expression in the pituitary gland. Endocrinology. 1998;139:5151–6. doi: 10.1210/endo.139.12.6354. [DOI] [PubMed] [Google Scholar]
- 14.Brandenberger AW, Tee MK, Lee JY, Chao V, Jaffe RB. Tissue distribution of estrogen receptors alpha (ER-alpha) and beta (ER-beta) mRNA in the midgestational human fetus. J Clin Endocrinol Metab. 1997;82:3509–12. doi: 10.1210/jcem.82.10.4400. [DOI] [PubMed] [Google Scholar]
- 15.Cowley SM, Hoare S, Mosselman S, Parker MG. Estrogen receptors alpha and beta form heterodimers on DNA. J Biol Chem. 1997;272:19858–62. doi: 10.1074/jbc.272.32.19858. [DOI] [PubMed] [Google Scholar]
- 16.Pettersson K, Grandien K, Kuiper GG, Gustafsson JA. Mouse estrogen receptor beta forms estrogen response element-binding heterodimers with estrogen receptor alpha. Mol Endocrinol. 1997;11:1486–96. doi: 10.1210/mend.11.10.9989. [DOI] [PubMed] [Google Scholar]
- 17.Korach KS, Couse JF, Curtis SW, et al. Estrogen receptor gene disruption: molecular characterization and experimental and clinical phenotypes. Recent Prog Horm Res. 1996;51:159–86. [Discussion 86–8.] [PubMed] [Google Scholar]
- 18.Krege JH, Hodgin JB, Couse JF, et al. Generation and reproductive phenotypes of mice lacking estrogen receptor beta. Proc Natl Acad Sci USA. 1998;95:15677–82. doi: 10.1073/pnas.95.26.15677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Couse JF, Korach KS. Estrogen receptor null mice: what have we learned and where will they lead us? Endocr Rev. 1999;20:358–417. doi: 10.1210/edrv.20.3.0370. [DOI] [PubMed] [Google Scholar]
- 20.Rijhsinghani AG, Thompson K, Bhatia SK, Waldschmidt TJ. Estrogen blocks early T cell development in the thymus. Am J Reprod Immunol. 1996;36:269–77. doi: 10.1111/j.1600-0897.1996.tb00176.x. [DOI] [PubMed] [Google Scholar]
- 21.Aboudkhil S, Bureau JP, Garrelly L, Vago P. Effects of castration, depo-testosterone and cyproterone acetate on lymphocyte T subsets in mouse thymus and spleen. Scand J Immunol. 1991;34:647–53. doi: 10.1111/j.1365-3083.1991.tb01588.x. [DOI] [PubMed] [Google Scholar]
- 22.Clarke AG, Kendall MD. The thymus in pregnancy: the interplay of neural, endocrine and immune influences. Immunol Today. 1994;15:545–51. doi: 10.1016/0167-5699(94)90212-7. [DOI] [PubMed] [Google Scholar]
- 23.Guevara Patino JA, Marino MW, Ivanov VN, Nikolich-Zugich J. Sex steroids induce apoptosis of CD8+ CD4+ double-positive thymocytes via TNF-alpha. Eur J Immunol. 2000;30:2586–92. doi: 10.1002/1521-4141(200009)30:9<2586::AID-IMMU2586>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 24.Staples JE, Gasiewicz TA, Fiore NC, Lubahn DB, Korach KS, Silverstone AE. Estrogen receptor alpha is necessary in thymic development and estradiol-induced thymic alterations. J Immunol. 1999;163:4168–74. [PubMed] [Google Scholar]
- 25.Carlsten H, Tarkowski A, Holmdahl R, Nilsson LA. Oestrogen is a potent disease accelerator in SLE-prone MRL lpr/lpr mice. Clin Exp Immunol. 1990;80:467–73. doi: 10.1111/j.1365-2249.1990.tb03311.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Blum WF, Breier BH. Radioimmunoassays for IGFs and IGFBPs. Growth Regul. 1994;4(Suppl. 1):11–9. [PubMed] [Google Scholar]
- 27.Couse JF, Curtis SW, Washburn TF, et al. Analysis of transcription and estrogen insensitivity in the female mouse after targeted disruption of the estrogen receptor gene. Mol Endocrinol. 1995;9:1441–54. doi: 10.1210/mend.9.11.8584021. [DOI] [PubMed] [Google Scholar]
- 28.Murphy WJ, Durum SK, Longo DL. Differential effects of growth hormone and prolactin on murine T cell development and function. J Exp Med. 1993;178:231–6. doi: 10.1084/jem.178.1.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Goya RG, Gagnerault MC, De Moraes MC, Savino W, Dardenne M. In vivo effects of growth hormone on thymus function in aging mice. Brain Behav Immun. 1992;6:341–54. doi: 10.1016/0889-1591(92)90033-k. [DOI] [PubMed] [Google Scholar]
- 30.Clark R. The somatogenic hormones and insulin-like growth factor-1: stimulators of lymphopoiesis and immune function. Endocr Rev. 1997;18:157–79. doi: 10.1210/edrv.18.2.0296. [DOI] [PubMed] [Google Scholar]
- 31.Timsit J, Savino W, Safieh B, Chanson P, Gagnerault MC, Bach JF, Dardenne M. Growth hormone and insulin-like growth factor-I stimulate hormonal function and proliferation of thymic epithelial cells. J Clin Endocrinol Metab. 1992;75:183–8. doi: 10.1210/jcem.75.1.1619008. [DOI] [PubMed] [Google Scholar]
- 32.Dorup I, Flyvbjerg A, Everts ME, Orskov H. Effects of growth hormone on growth and muscle Na+-K+ pump concentration in K+-deficient rats. Am J Physiol. 1992;262:E511–7. doi: 10.1152/ajpendo.1992.262.4.E511. [DOI] [PubMed] [Google Scholar]
- 33.Vidal O, Lindberg M, Sv L, Lubahn DB, Ritzen EM, Gustafsson J, Ohlsson C. Disproportional body growth in female estrogen receptor-alpha-inactivated mice. Biochem Biophys Res Commun. 1999;265:569–71. doi: 10.1006/bbrc.1999.1711. 10.1006/bbrc.1999.1711. [DOI] [PubMed] [Google Scholar]
- 34.Vidal O, Lindberg M, Hollberg K, et al. Estrogen receptor specificity in the regulation of skeletal changes in male mice. Proc Natl Acad Sci USA. 2000;97:5474–9. doi: 10.1073/pnas.97.10.5474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kecha O, Brilot F, Martens H, et al. Involvement of insulin-like growth factors in early T cell development: a study using fetal thymic organ cultures. Endocrinology. 2000;141:1209–17. doi: 10.1210/endo.141.3.7360. [DOI] [PubMed] [Google Scholar]
- 36.van Buul-Offers SC, Kooijman R. The role of growth hormone and insulin-like growth factors in the immune system. Cell Mol Life Sci. 1998;54:1083–94. doi: 10.1007/s000180050237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Siddiqui RA, McCutcheon SN, Blair HT, Mackenzie DD, Morel PC, Breier BH, Gluckman PD. Growth allometry of organs, muscles and bones in mice from lines divergently selected on the basis of plasma insulin-like growth factor-I. Growth Dev Aging. 1992;56:53–60. [PubMed] [Google Scholar]
- 38.Kuiper GG, Carlsson B, Grandien K, Enmark E, Haggblad J, Nilsson S, Gustafsson JA. Comparison of the ligand binding specificity and transcript tissue distribution of estrogen receptors alpha and beta. Endocrinology. 1997;138:863–70. doi: 10.1210/endo.138.3.4979. [DOI] [PubMed] [Google Scholar]
- 39.Seiki K, Sakabe K. Sex hormones and the thymus in relation to thymocyte proliferation and maturation. Arch Histol Cytol. 1997;60:29–38. doi: 10.1679/aohc.60.29. [DOI] [PubMed] [Google Scholar]
- 40.Smithson G, Medina K, Ponting I, Kincade PW. Estrogen suppresses stromal cell-dependent lymphopoiesis in culture. J Immunol. 1995;155:3409–17. [PubMed] [Google Scholar]
- 41.Vidal O, Kindblom LG, Ohlsson C. Expression and localization of estrogen receptor-beta in murine and human bone. J Bone Miner Res. 1999;14:923–9. doi: 10.1359/jbmr.1999.14.6.923. [DOI] [PubMed] [Google Scholar]
- 42.Smithson G, Couse JF, Lubahn DB, Korach KS, Kincade PW. The role of estrogen receptors and androgen receptors in sex steroid regulation of B lymphopoiesis. J Immunol. 1998;161:27–34. [PubMed] [Google Scholar]