Abstract
During acute Trypanosoma cruzi infection in mice, many leucocytes undergo apoptosis. Although apoptosis has been ascribed to increased levels of nitric oxide (NO) and Fas–FasL interaction, the importance of this phenomenon in modulating the host response against T. cruzi is unknown. Herein, the role of NO- and Fas–FasL-induced apoptosis in modulating the immune response to T. cruzi was evaluated using mice deficient in Fas expression (MRL/MpJ-Fas lpr) and inducible nitric oxide synthase (iNOS) knockout mice (iNOS–/–). The results showed that besides decreasing apoptosis induction after infection, impairment of the Fas–FasL interaction resulted in decreased NO production, as a consequence of enhanced T helper 2 (Th2) cytokine production. Differently, blockage of NO-induced apoptosis resulted in uncontrolled cytokine production, rather than a biased Th2 cytokine pattern. Together, these results suggested that Fas and FasL-induced apoptosis could be implied in modulation of the immune response against T. cruzi by interfering with cytokine and NO production during the acute phase of the infection.
Introduction
The obligate intracellular protozoan parasite Trypanosoma cruzi is the causative agent of South American trypanosomiasis or Chagas' disease, a chronic and debilitating syndrome that affects millions of people in Latin America.1 Murine T. cruzi infection recapitulates many of the major pathological and immunological alterations reported in human Chagas' disease. In addition, lymphoproliferative responses are suppressed in acutely infected mice,2 and this is probably caused by infection-associated increases in nitric oxide (NO).3,4 NO is produced as a result of the induction of inducible nitric oxide synthase (iNOS)5,6 by cytokines such as interferon-γ (IFN-γ) and tumour necrosis factor-α (TNF-α), known to be increased as a result of infection.7 The resultant increase in NO production contributes to parasite killing and host survival6,8 but may also lead to myocardial dysfunction.9 Additionally, NO has been implicated in the apoptosis of lymphocytes, macrophages (Mφ) and many other cells (reviewed in refs 10 and 11) in a variety of clinical and experimental settings and with several micro-organisms,12–15 including T. cruzi.4
Apoptosis is a cell-intrinsic process that is essential for animal development and tissue homeostasis. Malfunction of this tightly controlled mechanism of cell suicide may result in cancer, neurodegenerative diseases, or other pathological conditions (reviewed in ref. 16). Both extracellular and intracellular signals can trigger apoptosis, including growth factor deprivation16 and overexpression of certain oncogenes or tumour-suppressor genes.17 Moreover, cross-linking of the so-called ‘death receptors’, including Fas (CD95) and TNF receptor 1 by their respective ligands, FasL (CD95L) and TNF-α,18 might lead to apoptosis. Spleen cells from mice acutely infected with T. cruzi exhibit increased expression of Fas and FasL as a consequence of the IFN-γ produced, as in IFN-γ–/– mice the expression of these molecules is unchanged as a result of infection.19 Moreover, in vitro, Fas and FasL have been implicated in activation-induced apoptosis in CD4+ T cells from infected mice.20
Mice genetically deficient in Fas (lpr)21 or FasL (gld)22 have been shown to be more susceptible to T. cruzi infection than their wild-type (WT) infected control. However, the mechanisms that mediate this enhanced susceptibility are not understood. Moreover, less is known about the importance of apoptosis induction in the establishment of an efficient immune response and in the control of T. cruzi infection, or in the resulting autoimmune mechanisms that are reported to occur in infected mice.23
Herein, we addressed these questions by infecting the MRL/MpJ-Fas (lpr) and iNOS–/– mice with T. cruzi and assaying apoptosis rates and the production of cytokines and NO. The results showed that while blockage of NO-induced apoptosis results in dysregulated cytokine production, inhibition of Fas–FasL-induced apoptosis led to a marked inhibition of NO production, as a consequence of an increased T helper 2 (Th2) cytokine production. Our data support the hypothesis that the Fas pathway of apoptosis induction is important for modulating the immune response against T. cruzi infection.
Materials and methods
Infection of mice
The Y strain of T. cruzi was used in all experiments and maintained in BALB/c mice (Jackson Laboratories, Bar Harbor, ME). The iNOS–/–24 and syngeneic 129sv × C57BL/6 WT mice (kindly provided Drs Carl Nathan and John Mudgett, respectively) were maintained under pathogen-free conditions. MRL/MpJ-Fas lpr (lpr) and C57BL/6J mice (WT) were obtained from Jackson Laboratories. Six-week-old mice were infected with 103 trypomastigotes, and parasitaemia was assessed as previously described.4
Spleen cell cultures
Suspensions of splenocytes from uninfected and infected WT, iNOS–/– or lpr mice were washed in Hank's balanced salt solution (HBSS) and treated with lysing buffer (9:1) (0·16 m NH4Cl:0·17 m Tris-HCl, pH 7·5, respectively) for 4 min. The erythrocyte-free cells were then washed three times in HBSS and adjusted to 3 × 106 cells/ml in RPMI-1640 (Flow Laboratories, Inc., McLean, VA) supplemented with 10% fetal calf serum (Hyclone, Logan, UT), 2-mercaptoethanol (5 × 10−5 m), l-glutamine (2 mm) and antibiotics (all purchased from Sigma Chemicals Co., St. Louis, MO). The cell suspension was distributed (1 ml/well) into 24-well tissue culture plates (Corning Inc. Life Sciences, Acton, MA) and cultured for 48 hr at 37° in a humidified 5% CO2 atmosphere, in the presence or absence of parasite lysate (10 µg/ml), concanavalin A (Con A) (2 µg/ml), neutralizing monoclonal antibody (mAb) anti-murine FasL (5 µg/ml) (clone Kay-10; Pharmingen, San Diego, CA), or control immunoglobulin G (IgG) (5 µg/ml). The cells were used to assay apoptosis, or expression of CD95 (Fas) or CD95L (FasL), and the supernatant was collected to evaluate cytokine and NO production.
Quantification of nitrite and nitrate
Blood was obtained from the retro-orbital plexus of uninfected or infected mice at various time-points postinfection. The nitrate was reduced to nitrite with nitrate reductase, as described previously,6 and the nitrite concentration was determined according to the Griess method.25 For this assay, 0·1 ml of cell-free culture medium or serum was mixed with 0·1 ml of Griess reagent in a multiwell plate and the absorbance at 550 nm was then read 10 min later. The NO2 concentration was determined by reference to a standard curve of NaNO2 (1–200 µm).
DNA labelling technique and flow cytometry (FCM) analysis
The percentage of apoptotic cells in the samples was estimated by labelling cells with 7-amino-actinomycin D (7-AAD) (Calbiochem-Novabiochem Corp., La Jolla, CA), as previously described,26 with a few modifications. Briefly, cells (3 × 106) were washed twice in phosphate-buffered saline (PBS) and resuspended in 500 µl of 7-AAD (10 µg/ml) in PBS and incubated for 20 min at 4° in 12 × 75 mm polypropylene tubes (Becton-Dickinson, Mountain View, CA) protected from light. The 7-AAD fluorescence (FL-3) from at least 104 cells in each sample was analysed in a fluorescence-activated cell sorter (FACScan flow cytometer; Becton-Dickinson, San Jose, CA). Debris and clumps were excluded from the analysis by setting the appropriate gate on a side scatter (SSC) versus forward scatter (FSC) dot-plot. All measurements were made using the same instrumental settings.
To evaluate the expression of Fas and FasL, spleen cells from uninfected or infected WT, iNOS–/– or lpr mice were incubated for 30 min at 4° with 0·5 µg of anti-CD16/CD32 mAb (Fc block), followed by the addition of 0·5 µg of fluorescein isothiocyanate (FITC)-labelled anti-Fas or 0·5 µg of phycoerythrin (PE) -labelled anti FasL (all purchased from Pharmingen). To determine the level of background staining, cells were incubated with 0·5 µg each of hamster IgG1 anti-trinitrophenyl (TNP) FITC (Pharmingen), for 30 min at 4°, in the dark in 100 µl of PBS containing 1% bovine serum albumin (PBS-BSA). Subsequently, cells were washed twice and resuspended in 300 µl of PBS-BSA. Multivariate data analysis was performed using cellquest software (Becton-Dickson), by setting a gate on the lymphocytes on a SSC versus FSC scatter dot-plot and determining the expression of Fas or FasL FL-1 histograms.
Cytokine assays
Total spleen cells were cultured in the presence or absence of T. cruzi lysate, as described above. Supernatants were harvested after 48 hr, and stored at −20° until use. Cytokine (IFN-γ, interleukin [IL]-4 and IL-10) concentrations in the supernatants were evaluated by using a sandwich enzyme-linked immunosorbent assay (ELISA), as previously described.27
Statistical analysis
The results are expressed as the mean±standard error of the mean (SEM) of the indicated number of animals or experiments. Statistical analysis was performed using analysis of variance (anova) followed by the Student Newman–Keuls test (instat software,;GraphPad, San Diego, CA). A P-value of < 0·05 was considered to indicate significance.
Results
Susceptibility of lpr and iNOS–/– mice to infection with the Y strain of T. cruzi
On day 5 postinfection, the parasitaemia in iNOS–/– and lpr mice was similar to that found in infected WT mice (Fig. 1a, 1c). However, on day 9 postinfection, lpr (Fig. 1a) and iNOS–/– (Fig. 1c) mice presented ≈threefold higher parasitaemia than WT mice. By day 10 postinfection there was no significant difference between any of the groups. Importantly, infected lpr and iNOS–/– mice had increased mortality as compared to the WT mice. All of the infected WT mice survived acute infection. The mortality rate of infected lpr mice was 90% by day 21 postinfection (Fig. 1b) and 100% of infected iNOS–/– mice had died by day 18 postinfection (Fig. 1d).
Figure 1.
Impairment of Fas–FasL interaction or absence of inducible nitric oxide synthase (iNOS) leads to increased parasitaemia and mortality. Wild-type (WT) control (squares), lpr (circles) or iNOS–/– (triangles) mice were each infected intraperitoneally (i.p.) with 103 blood trypomastigotes (Y strain), and the parasitaemia (n = 10) (a) and (c) and mortality (b) and (d) were evaluated. The data in panels (a) and (c) are expressed as median ±SEM. Data from one of two experiments shown. Asterisks indicate statistical significance, P ≤ 0·01 (Student Newman–Keuls test), compared with infected WT mice.
Apoptosis in the absence of Fas–FasL interaction in T. cruzi-infected mice
The percentage of apoptosis was determined in spleen cells obtained from lpr or WT mice on day 11 postinfection, as at this time-point the number of apoptotic cells is significantly enhanced in infected compared to non-infected mice, as previously reported.4 The phenotype of apoptotic cells was similar to that previously published,4 being ≈65% CD4+ T cells, 23% CD3+ CD8+ T cells and 11% CD19+ CD3− B cells.
Figure 2 shows the percentage enhancement of apoptosis in spleen cells after infection of WT and lpr mice, before (Fig. 2a) and after (Fig. 2b) culture. Although infection with T. cruzi led to a significant increase of apoptosis in spleen cells from both WT and lpr mice, enhancement of apoptosis was considerably increased in cells from WT mice compared with cells from lpr mice (Fig. 2a). Moreover, after culture for 48 hr, the cells obtained from the infected lpr mice showed lower levels of apoptosis than those from infected WT mice (Fig. 2b).
Figure 2.
Apoptosis induced by Trypanosoma cruzi infection is decreased in the lpr mice. The percentage of apoptotic cells was evaluated in spleen from T. cruzi-infected (11 days postinfection) wild-type (WT) and lpr mice (n = 4 mice per group) before (a) or after (b) culture in medium for 48 hr. The apoptotic cells were detected by flow cytometry, as described in the Materials and methods. Each column (mean ±SEM) represents the percentage of enhancement of apoptotic cells after infection in relation to the respective uninfected controls (100%). The results shown are representative of three separate experiments. Asterisks indicate statistical significance, P ≤ 0·01 (Student Newman–Keuls test), compared with spleen cells from the respective control infected WT mice.
NO production after T. cruzi infection in the absence of Fas–FasL interaction
In an effort to understand the mechanism by which the absence of Fas and FasL interaction rendered mice more susceptible to infection with the Y strain of T. cruzi, we evaluated NO production in infected lpr mice. The results showed that the levels of nitrite and nitrate in the sera of WT mice were significantly increased as compared with the levels found in lpr mice (Fig. 3a). In addition, the level of nitrite in supernatants of spleen cells cultured in the presence of parasite antigens was significantly reduced in the infected lpr mice as compared to that of WT mice (Fig. 3b). Moreover, addition of the blocking mAb, anti-FasL, to cultures of spleen cells from infected WT mice resulted in significant inhibition of NO production (Fig. 3c). Interestingly, the concentration of IFN-γ in these supernatants was reduced by 50% as compared with controls (data not shown). Together, these results indicate that the interaction of Fas and FasL are implied in the modulation of NO production during T. cruzi infection.
Figure 3.
Nitric oxide (NO) production by spleen cells, after Trypanosoma cruzi infection, in the absence of Fas and FasL interaction. NO production by normal or T. cruzi-infected (11 days postinfection) wild-type (WT) and lpr mice was evaluated by measuring nitrite and nitrate levels in serum (a) or in the supernatants of spleen cells cultured for 48 hr (b) and (c) in the presence or absence of parasite lysate (Tc lysate) (10 µg/ml) (b), or of neutralizing anti-FasL monoclonal antibody (mAb) (5 µg/ml) (c) or control immunoglobulin G (IgG) (5 µg/ml) (c). Each column (mean±SEM) represents the results for three mice in one experiment representative of two performed separately. Asterisks indicate statistical significance, P ≤ 0·01 (Student Newman–Keuls test), compared with the value from the control uninfected (*) or infected WT (**) mice, or cells from infected WT mice cultured in medium only (***) or in the presence of parasite lysate (****).
Cytokine production by T. cruzi-infected lpr mice
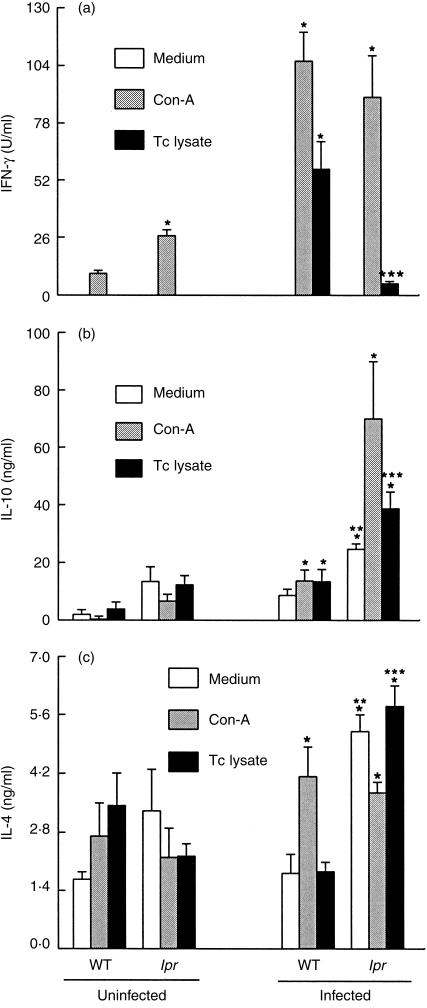
To investigate whether the inhibition of NO production in infected lpr mice could be related to a unbalanced T helper 1 (Th1)/Th2 activation, we assayed the production of Th1 and Th2 cytokines in spleen cells harvested from infected lpr and WT mice and cultured for 48 hr in the presence or absence of Con A or parasite antigens. The results showed that infection with T. cruzi led to a significant increase in the production of IFN-γ, but not of IL-4 and IL-10, as detected in the supernatants of splenic cells cultured in the presence of parasite lysate (Fig. 4). In comparison to the cells from WT mice, cells from lpr mice produced significantly less IFN-γ (Fig. 4a) and significantly more IL-10 (Fig. 4b) and IL-4 (Fig. 4c). When cultured in the presence of Con A, there was an increase in IFN-γ and IL-4 production by cells from both WT and lpr mice (Fig. 4c) and an increased IL-10 production in cells from lpr, but not from WT, mice (Fig. 4b). These data indicate that although spleen cells of infected lpr mice are able to secrete normal levels of IFN-γ when cultured with Con A, in the presence of parasite antigens they produce preferentially Th2 cytokines.
Figure 4.
Production of cytokines in cells from infected lpr mice. Spleen cells were harvested from normal and infected (day 11) wild-type (WT) and lpr mice (n = 4 mice/group) and were cultured in medium only or in the presence of concanavalin A (Con A) (2 µg/ml) or parasite lysate (Tc lysate, 10 µg/ml). Supernatants were harvested after 48 hr of incubation and the concentration of interferon-γ (IFN-γ) (a), interleukin-10 (IL-10) (b) and interleukin-4 (IL-4) (c) was determined by enzyme-linked immunosorbent assay (ELISA). Bars (mean±SD) represent the results (in triplicate) from one experiment representative of two performed separately. Asterisks indicate statistical significance, P ≤ 0·01 (Student Newman–Keuls test), compared with the values for cells from the respective controls: WT uninfected mice (*) or cells from infected mice cultured in medium only (**) or in the presence of Tc lysate (***).
Apoptosis and cytokine production in the absence of iNOS in T. cruzi-infected mice
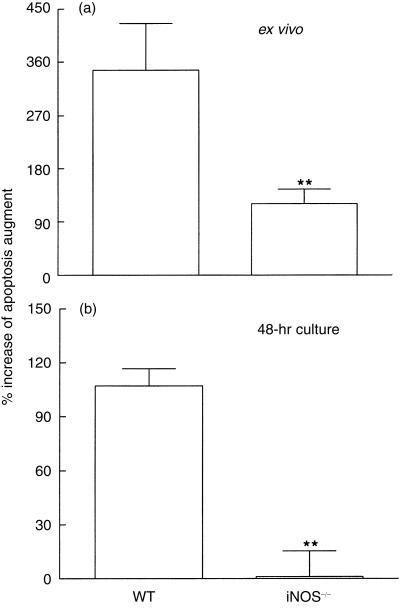
We next considered whether the blockage of NO-induced apoptosis could interfere with cytokine production after infection, as occurred when Fas- and FasL-induced apoptosis was blocked. To answer this question we evaluated the number of apoptotic spleen cells and determined cytokine levels in the iNOS knockout mice. As shown in Fig. 5, infection of WT mice with T. cruzi led to a significant enhancement in the percentage of apoptosis in spleen cells. In iNOS–/– mice, the infection also led to an increase in apoptosis levels; however, this was significantly less pronounced than that observed in cells from WT mice (Fig. 5a). Moreover, after culture for 48 hr, spleen cells obtained from the infected iNOS–/– mice showed lower levels of apoptosis than those from infected WT mice (Fig. 5b).
Figure 5.
Apoptosis induced by Trypanosoma cruzi infection is decreased in the inducible nitric oxide synthase-deficient (iNOS–/–) mice. The percentage of apoptotic cells was evaluated in spleen from T. cruzi-infected (11 days postinfection) wild-type (WT) and iNOS –/– mice (n = 4 mice/group) before (a) or after (b) culture in medium alone for 48 hr. The apoptotic cells were detected by flow cytometry, as described in the Materials and methods. Each column (mean±SEM) represents the percentage of enhancement of apoptotic cells after infection in relation to the respective uninfected controls (100%). The results shown are representative of two experiments performed separately. Asterisks indicate statistical significance, P ≤ 0·01 (Student Newman–Keuls test), compared with the value for cells from the respective infected control WT mice.
Analysis of IFN-γ, IL-4 and IL-10 production was performed in supernatants of spleen cells from normal or infected WT or iNOS–/– mice, cultured for 48 hr in medium only or in the presence of parasite antigens. The results are presented in Fig. 6 and show that, in contrast to the results obtained from cells of infected lpr mice, cells from infected iNOS–/– mice showed an increase in IFN-γ (Fig. 6a) and IL-10 (Fig. 6b) production when cultured in the presence of parasite antigens, as compared to infected WT mice. There was no significant enhancement in IL-4 production by cells from WT or iNOS–/– mice after infection (Fig. 6c).
Figure 6.
Production of cytokines in cells from T. cruzi-infected inducible nitric oxide synthase-deficient (iNOS–/–) mice. Spleen cells were harvested from normal and infected (day 11) wild-type (WT) or iNOS–/– mice (n = 4 mice/group) and were cultured in medium alone or in the presence of parasite lysate (Tc) (10 µg/ml). Supernatants were harvested after 48 hr of incubation, and the concentrations of interferon-γ (IFN-γ) (a), interleukin-10 (IL-10) (b) and interleukin-4 (IL-4) (c) were determined by enzyme-linked immunosorbent assay (ELISA). Bars (mean ±SD) represent the results (in triplicate) from one experiment representative of two performed separately. Asterisks indicate statistical significance, P ≤ 0·01 (Student Newman–Keuls test), compared with the values for cells from the respective uninfected control WT mice (*) or cells from infected mice cultured in the presence of parasite lysate (***).
Discussion
In this present study we analysed the roles of NO and Fas–FasL-induced apoptosis in modulating the immune response against T. cruzi in mice. iNOS–/– and lpr mice were utilized in order to ensure that high levels of NO6 and Fas expression19 would not be increased as a result of infection. Induction of apoptosis in leucocytes has been observed during infection with many different parasites,13–15,28–30 including T. cruzi.4,31 We have previously observed spontaneous apoptosis in vivo and in vitro in CD4+ and CD8+ T lymphocytes from T. cruzi Y strain-infected mice.4 In addition, activation-induced cell death (AICD) was reported to occur in CD4+, but not in CD8+, T lymphocytes during this infection.31 While spontaneous apoptosis was demonstrated to be modulated by NO,4 AICD was ascribed to the Fas–FasL pathway.22
Infection of lpr mice with the Y strain of T. cruzi resulted in high parasitaemia and mortality, associated with decreased NO production (Fig. 3). This is consistent with previous work demonstrating that NO is critical for host survival during T. cruzi infection.6,8 The finding that blockage of Fas–FasL interaction in infected WT mice (Fig. 4) led to a significant inhibition of NO production in vitro, additionally confirmed the implication of Fas and FasL in modulating NO production during the acute phase of T. cruzi infection.
One probable mechanism by which the Fas–FasL interaction could participate in the modulation of NO production after infection with T. cruzi could be through interference with cytokine production. As demonstrated by our results (Fig. 4b, 4c), the infected lpr mice produced significantly higher amounts of IL-10 and IL-4 than infected WT mice. Also, the production of IFN-γ was reduced in infected lpr mice when compared to the infected WT mice (Fig. 4a). Moreover, blockage of FasL in cultured cells from infected WT mice resulted in decreased production of IFN-γ (data not shown). Strikingly, data reported recently from separate work showed that gld mice (which have a defect in FasL) are also highly susceptible to T. cruzi infection and produce higher levels of Th2 cytokines than WT mice when infected with T. cruzi.22 Our data confirm this observation and additionally extend it, demonstrating that after infection with a more virulent strain of T. cruzi, impairment of the Fas–FasL interaction led to a decrease in NO production and enhanced susceptibility to infection. The decreased NO synthesis could be related to the reduced production of IFN-γ observed in the infected lpr mice as compared to WT mice.
The mechanism(s) underlying this enhanced production of IL-4 and IL-10 in the absence of Fas is (are) not clear. Some authors have reported that Th1 cells are more susceptible to Fas-induced apoptosis than Th2 cells,32 while others have demonstrated that Th1 and Th2 cells are similarly susceptible to Fas–FasL-induced apoptosis.33 However, the possibility that preferential apoptosis of Th1 or Th2 cells is occurring in T. cruzi-infected mice cannot be ruled out because, as shown here in the infected lpr mice, there is an enhanced production of Th2 cytokines. In this context, it has been recently reported that apoptosis induction is associated with a decreased production of Th1 cytokines in susceptible, but not in resistant, mice infected with Leishmania donovani.34 In addition, in mice infected with Mycobacterium tuberculosis, the selective loss of CD4+ Th1 cells was associated with enhanced expression of Fas.35
In contrast to that observed with the inhibition of Fas–FasL-induced apoptosis, blockage of NO-induced apoptosis rendered an unbalanced cytokine production rather than a biased Th2 pattern, suggesting that Fas, more than NO-induced apoptosis, could participate in the control of the immune response through modulating the production of cytokines.
Our results showed that infected iNOS–/– mice produced significantly more IFN-γ than the infected WT mice. In agreement with this finding, it has been reported that IFN-γ production is increased in iNOS–/– mice infected with other parasites, such as mycobacterium24 and influenza A virus.36 The explanation for this is not completely understood. However, it has been demonstrated that NO can inhibit activation of IL-1β-converting enzyme (ICE),37 which is important in the production of active IL-18, the IFN-γ-inducing factor.38 If production of IFN-γ during T. cruzi infection depends on IL-18, then the inhibition of NO production in iNOS–/– mice could result in higher levels of IL-18 and, as a consequence, increased production of IFN-γ. However, this possibility remains to be investigated.
The results showing that infected lpr mice also presented significantly decreased apoptosis in comparison to infected WT mice indicate that Fas is involved in apoptosis induction during the acute period of infection. However, the low number of apoptotic leucocytes present in the lpr mice after infection could be related to the absence of elevated NO production after infection in these mice.
In summary, our results underscore the relationships between the induction of apoptosis and the control of cytokine production after infection with T. cruzi, which are crucial for the induction of NO, inhibition of parasite growth and, probably, in disease outcome. In this context, it is tempting to speculate that the Fas–FasL system could also interfere with the pathogeny of T. cruzi infection. If so, impairment of the Fas–FasL interaction would probably result in enhanced myocarditis. Experiments to test this possibility are currently underway. Understanding the involvement of apoptosis in the regulation of cytokine and NO production and its importance in the immune response could be helpful for elucidating the mechanisms underlying the establishment of an efficient response against T. cruzi.
Acknowledgments
This work was supported by Fundação de Amparo à Pesquisa e ao Ensino do Estado de São Paulo (grants 97/11548-2, 96/04304-7 and 96/4118–9), National Institute of Health (grants AI-41752, AI-12770, HL-60665, HL-61550) and Cancer Center Core NIH Grant 5-P30-CA13330. R. N. Kitsis is a Charles and Tamara Krasne Faculty Scholar in Cardiovascular Research at the Albert Einstein College of Medicine. We wish to thank Dr Carl Nathan (Weil Medical College, New York Hospital-Cornell Medical Center) and Dr John Mudgett (Merck Research Laboratories) for the iNOS–/– and 129 svx C57BL/6 mice. We are also grateful to Vicki L. Braunstein and Vitaliy Shtutin for excellent technical assistance and to David Gebhard for his invaluable help with flow cytometry.
References
- 1.World Health Organization. Chagas Disease: 10th Program Report WHO Technical Report Series 1991; No 811. Geneva: WHO; 1991. [Google Scholar]
- 2.DosReis GA. Cell-mediated immunity in experimental Trypanosoma cruzi infection. Parasitol Today. 1997;13:335–42. doi: 10.1016/s0169-4758(97)01073-9. 10.1016/s0169-4758(97)01073-9. [DOI] [PubMed] [Google Scholar]
- 3.Abrahamsohn IA, Coffman RL. Cytokine and nitric oxide regulation of the immunosuppression in Trypanosoma cruzi infection. J Immunol. 1995;155:3955–63. [PubMed] [Google Scholar]
- 4.Martins GA, Cardoso MGA, Aliberti JCS, Silva JS. Nitric oxide-induced apoptotic cell death in the acute phase of Trypanosoma cruzi infection in mice. Immunol Lett. 1998;63:113–20. doi: 10.1016/s0165-2478(98)00066-2. 10.1016/s0165-2478(98)00066-2. [DOI] [PubMed] [Google Scholar]
- 5.Gazzinelli RT, Oswald IP, Hieny S, James SL, Sher A. The microbiocidal activity of interferon-γ treated macrophages against Trypanosoma cruzi involves an l-arginine-dependent, nitrogen oxide-mediated mechanism inhibitable by interleukin-10 and transforming growth factor-β. Eur J Immunol. 1992;22:2501–6. doi: 10.1002/eji.1830221006. [DOI] [PubMed] [Google Scholar]
- 6.Vespa GNR, Cunha FQ, Silva JS. Nitric oxide is involved in control of Trypanosoma cruzi-induced parasitemia and directly kills the parasite in vitro. Infect Immun. 1994;62:5177–82. doi: 10.1128/iai.62.11.5177-5182.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Silva JS, Vespa GNR, Cardoso MAG, Aliberti JCS, Cunha FQ. Tumor necrosis factor alpha mediates resistance to Trypanosoma cruzi infection in mice by inducing nitric oxide production in infected gamma interferon-activated macrophages. Infect Immun. 1995;63:4862–7. doi: 10.1128/iai.63.12.4862-4867.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Holscher C, Kohler G, Muller U, Mossmann H, Schaub GA, Brombacher F. Defective nitric oxide effector functions lead to extreme susceptibility of Trypanosoma cruzi-infected mice deficient in gamma interferon receptor or inducible nitric oxide synthase. Infect Immun. 1998;66:1208–15. doi: 10.1128/iai.66.3.1208-1215.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huang H, Chan J, Wittner M, Jelicks LA, Morris SA, Factor SM, Weiss LM, Braunstein UL et al. Expression of cardiac cytokines and inducible form of nitric oxide synthase (NOS2) in Trypanosoma cruzi-infected mice. J Mol Cell Cardiol. 1999;31:75–88. doi: 10.1006/jmcc.1998.0848. 10.1006/jmcc.1998.0848. [DOI] [PubMed] [Google Scholar]
- 10.Brockhaus F, Brüne B. U937 apoptotic cell death by nitric oxide: Bcl-2 downregulation and caspase activation. Exp Cell Res. 1998;238:33–41. doi: 10.1006/excr.1997.3778. 10.1006/excr.1997.3778. [DOI] [PubMed] [Google Scholar]
- 11.Brune B, Von Knethen A, Sandau KB. Nitric oxide and its role in apoptosis. Eur J Pharmacol. 1998;351:261–72. doi: 10.1016/s0014-2999(98)00274-x. 10.1016/s0014-2999(98)00274-x. [DOI] [PubMed] [Google Scholar]
- 12.Fukuo K, Hata S, Suhara T, Nakohashi T, Shinto Y, Tsujimoto Y, Morinoto S, Ogigara T. Nitric oxide induces upregulation of Fas and apoptosis in vascular smooth muscle. Hypertension. 1996;27:823–6. doi: 10.1161/01.hyp.27.3.823. [DOI] [PubMed] [Google Scholar]
- 13.Kim JM, Eckman L, Savidge TC, Lowe DC, Witthoft T, Kagnoff MF. Apoptosis of human intestinal epithelial cells after bacterial invasion. J Clin Invest. 1998;102:1815–23. doi: 10.1172/JCI2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Richardson JO, Forsyth LM, Brown CG, Preston PM. Nitric oxide causes the macroschizonts of Theileria annulata to disappear and host cells to become apoptotic. Vet Res Commun. 1998;22:31–45. doi: 10.1023/a:1005983111138. [DOI] [PubMed] [Google Scholar]
- 15.Rojas M, Oliver M, Gros P, Barrera LF, Garcia LF. TNF-α and IL-10 modulate the induction of apoptosis by virulent Mycobacterium tuberculosis in murine macrophages. J Immunol. 1999;162:6122–31. [PubMed] [Google Scholar]
- 16.Penninger JM, Kroemer G. Molecular and cellular mechanisms of T lymphocyte apoptosis. Adv Immunol. 1998;68:51–144. doi: 10.1016/s0065-2776(08)60558-1. [DOI] [PubMed] [Google Scholar]
- 17.Steller H. Mechanisms and genes of cellular suicide. Science. 1995;267:1445–9. doi: 10.1126/science.7878463. [DOI] [PubMed] [Google Scholar]
- 18.Ashkenzi A, Dixit VM. Apoptosis control by death and decoy receptors. Curr Opin Cell Biol. 1999;11:255–60. doi: 10.1016/s0955-0674(99)80034-9. 10.1016/s0955-0674(99)80034-9. [DOI] [PubMed] [Google Scholar]
- 19.Martins GA, Vieira LQ, Cunha FQ, Silva JS. Interferon-gamma modulates CD95 (Fas) and CD95 ligand (Fas-L) expression and nitric oxide-induced apoptosis during the acute phase of Trypanosoma cruzi infection: a possible role in immune response control. Infect Immun. 1999;67:3864–71. doi: 10.1128/iai.67.8.3864-3871.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nunes MP, Andrade RM, Lopes MF, DosReis GA. Activation-induced T cell death exacerbates Trypanosoma cruzi replication in macrophages co-cultured with CD4+ T lymphocytes from infected hosts. J Immunol. 1998;160:1313–9. [PubMed] [Google Scholar]
- 21.Boyer MH, Hoff R, Kipnis TL, Murphy ED, Roths JB. Trypanosoma cruzi: susceptibility in mice carrying mutant gene lpr (lymphoproliferation) Parasite Immunol. 1983;5:135–42. doi: 10.1111/j.1365-3024.1983.tb00731.x. [DOI] [PubMed] [Google Scholar]
- 22.Lopes MF, Nunes MP, Henriques-Pons A, Giese N, Morse HC, Davidson WF, Araújo-Jorge TC, DosReis GA. Increased susceptibility of Fas ligand-deficient gld mice to Trypanosoma cruzi infection due to a Th2-biased host immune response. Eur J Immunol. 1999;29:81–9. doi: 10.1002/(SICI)1521-4141(199901)29:01<81::AID-IMMU81>3.0.CO;2-Y. 10.1002/(sici)1521-4141(199901)29:01<81::aid-immu81>3.3.co;2-p. [DOI] [PubMed] [Google Scholar]
- 23.Kierzenbaum F. Chagas' disease and the autoimmunity hypothesis. Clin Microbiol Rev. 1999;12:21–3. doi: 10.1128/cmr.12.2.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.MacMicking JD, North RJ, LaCourse R, Mudgett JS, Shah SK, Nathan CF. Identification of nitric oxide synthase as a protective locus against tuberculosis. Proc Natl Acad Sci USA. 1997;94:5243–8. doi: 10.1073/pnas.94.10.5243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Green LC, Luzuriaga KR, Wagner DA, Rand W, Istan N, Young VR, Tannenbaum SR. Nitrate biosynthesis in man. Proc Natl Acad Sci USA. 1981;78:7764–8. doi: 10.1073/pnas.78.12.7764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schmid I, Uittenbogaart CH, Keld B, Giorgi J. A rapid method for measuring apoptosis and dual-color immunofluorescence by single laser flow cytometry. J Immunol Methods. 1994;170:145–57. doi: 10.1016/0022-1759(94)90390-5. [DOI] [PubMed] [Google Scholar]
- 27.Ferreira BR, Silva JS. Successive tick infestations selectively promote a T-helper 2 cytokine profile in mice. Immunology. 1999;96:434–9. doi: 10.1046/j.1365-2567.1999.00683.x. 10.1046/j.1365-2567.1999.00683.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Conceição-Silva F, Hahne M, Schröter M, Louis J, Tschopp J. The resolution of lesions induced by Leishmania major in mice requires a functional Fas (APO-1, CD95) pathway of cytotoxicity. Eur J Immunol. 1998;28:237–45. doi: 10.1002/(SICI)1521-4141(199801)28:01<237::AID-IMMU237>3.0.CO;2-O. 10.1002/(sici)1521-4141(199801)28:01<237::aid-immu237>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 29.Liesenfeld O, Kosek JC, Suzuki Y. Gamma interferon induces Fas-dependent apoptosis of Peyer's patch T cells in mice following peroral infection with Toxoplasma gondii. Infect Immun. 1997;65:4682–9. doi: 10.1128/iai.65.11.4682-4689.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Monack DM, Mecsas J, Bouley D, Falkow S. Yersinia-induced apoptosis in vivo aids in the establishment of a systemic infection of mice. J Exp Med. 1998;188:2127–37. doi: 10.1084/jem.188.11.2127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lopes MF, Veiga VF, Santos AR, Fonseca MEF, DosReis GA. Activation-induced CD4+ T cell death by apoptosis in experimental Chagas' disease. J Immunol. 1995;154:744–52. [PubMed] [Google Scholar]
- 32.Zhang X, Brunner T, Carter L, Duton RW, Rogers P, Bradley L, Sato T, Reed JC et al. Unequal death in T helper (Th) 1 and Th2 effectors: Th1, but not Th2, effectors undergo rapid Fas/FasL-mediated apoptosis. J Exp Med. 1997;185:1837–49. doi: 10.1084/jem.185.10.1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Watanabe N, Arase H, Kurasawa K, Iwamoto I, Kayagaki N, Okumura H, Miyatake S, Saito T. Th1 and Th2 subsets equally undergo Fas-dependent and -independent activation-induced cell death. Eur J Immunol. 1997;27:1858–64. doi: 10.1002/eji.1830270807. [DOI] [PubMed] [Google Scholar]
- 34.Das G, Vohra H, Rao K, Saha B, Mishra GC. Leishmania donovani infection of a susceptible host results in CD4+ T-cell apoptosis and decreased Th1 cytokine production. Scand J Immunol. 1999;49:307–10. doi: 10.1046/j.1365-3083.1999.00486.x. 10.1046/j.1365-3083.1999.00486.x. [DOI] [PubMed] [Google Scholar]
- 35.Das G, Vohra H, Saha B, Agrewala JN, Mishra GC. Apoptosis of Th1-like cells in experimental tuberculosis (TB) Clin Exp Immunol. 1999;115:324–8. doi: 10.1046/j.1365-2249.1999.00755.x. 10.1046/j.1365-2249.1999.00755.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Karupiah G, Chen JH, Mahalingam S, Nathan CF, MacMicking JD. Rapid interferon gamma-dependent clearance of influenza A virus and protection from consolidating pneumonitis in nitric oxide synthase 2-deficient mice. J Exp Med. 1998;188:1541–6. doi: 10.1084/jem.188.8.1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Young-Myeong K, Talanian RV, Li J, Billiar TR. Nitric oxide prevents IL-1β and IFN-γ-inducing factor (IL-18) release from macrophages by inhibiting caspase-1 (IL-1β converting enzyme) J Immunol. 1998;161:4122–8. [PubMed] [Google Scholar]
- 38.Ghayur T, Banerjee S, Hugunin M, Butter D, Herzog L, Carter A, Quintal L, Sekert L. Caspase-1 processes IFN-γ-inducing factor and regulates LPS-induced IFN-γ production. Nature. 1997;386:619–21. doi: 10.1038/386619a0. [DOI] [PubMed] [Google Scholar]