Abstract
Macrophages can process and present exogenous antigens on major histocompatibility complex (MHC) class I molecules through an alternative mechanism involving the internalization of antigens and the secretion of peptides loading MHC class I molecules at the cell surface. In this paper, we found that interferon-γ (IFN-γ) -activated macrophages infected with Salmonella typhimurum secreted peptides able to load empty MHC Kb molecules on co-cultured TAP-2-deficient RMA-S cells, added as targets for peptide loading. The increase in class I Kb on the RMA-S cells, resulting from the macrophage-derived peptides, exhibited a comparable stability as the direct addition of an exogenous Kb-binding peptide (OVA257–264) to the RMA-S cells. In both cases, the Kb complexes were stable for at least 3 hr after separating the RMA-S cells from the macrophages. The endosomal inhibitors, leupeptin and ammonium chloride, did not inhibit the release of peptides and the increase in Kb staining on the RMA-S cells in the co-culture systems. Brefeldin A also had no effect. P815 cells previously co-cultured with Salmonella-infected macrophages became targets for cytotoxic T lymphocytes isolated from Salmonella-infected BALB/c mice. Taken together, our data suggest that IFN-γ-activated macrophages process exogenous antigens in an intracellular compartment where serine proteases generate peptides released to the external environment for loading empty MHC class I molecules at the cell surface. This TAP-independent mechanism for the MHC class I presentation may be involved in priming cytotoxic T lymphocytes against intracellular pathogens in vivo.
Introduction
Macrophages are the host cell of Salmonella and the main site for bacterial proliferation and survival during an infection.1 The ability of the macrophages to kill Salmonella during early infection determines the outcome of the disease.2 Interferon-γ (IFN-γ) potently stimulates a number of macrophage effector activities, including receptor expression, phagocytosis, antigen presentation and oxidative and nitric oxide (NO) burst.3 During Salmonella infection, IFN-γ greatly strengthens the capacity of macrophages to clear circulating bacteria and present bacterial antigens to T cells.4 Another quality of macrophages is their ability to present exogenous antigens on major histocompatibility complex (MHC) class I molecules in order to activate CD8+ T cells.5 Macrophages can use the classical TAP (transporter associated with antigen processing) -dependent endogenous pathway, or a pathway involving the release of antigenic peptides into the external media.6 In the classical pathway, soluble exogenous antigens entering the cytoplasm are processed by the proteasome complex into peptides like other cytosolic proteins.7 Here, the dimer of TAP transports the peptides generated in the cytosol into the endoplasmic reticulum lumen, where loading of the peptide on class I MHC occurs via bridging with tapasin.8,9 After the peptide–heavy chain–β2 microglobulin (β2m) complex is assembled, it is transported to the cell surface. This mechanism is greatly reduced in macrophages from TAP-deficient mice and is inhibited in normal bone marrow macrophages by brefeldin A (BFA) and proteasome inhibitors.7 Nevertheless, TAP-1-deficient macrophages can process Escherichia coli, Salmonella typhimurium, or polystyrene beads containing the OVA(257–264) epitope for presentation on Kb.10 TAP-1-deficient macrophages can also present peptides from the recombinant glycoprotein and nucleoprotein of lymphocytic choriomeningitis virus and the nucleoprotein of vesicular stomatitis virus on their MHC class I, suggesting that alternative TAP-independent presentation pathways exist.11
The pathway leading to secretion of antigenic peptides by macrophages involves sequestration of the internalized antigen in the phagosome; here peptides from the internalized material can be generated and then released to the surface to load empty MHC class I molecules. This pathway has been found to be resistant to BFA and to proteasome inhibitors.5,12,13 Empty class I molecules can be loaded externally with natural or synthetic peptides. They can also be stabilized with β2m found in the serum.14–16 In this secretory pathway it is unclear exactly where antigen degradation takes place, and whether the released peptides are the same as those generated by proteasomes. Another uncertainty is whether the released peptides can be loaded into empty class I MHC on neighbouring cells or in the same cell inside endosomes.
In the study presented here, we provide evidence for the participation of a release pathway in the generation of peptides from internalized Salmonella typhimurium. Our results suggest that when macrophages are activated by IFN-γ and infected with Salmonella typhimurium they are able to secrete peptides from phagocytosed material that load empty class I molecules on the surface of the macrophage or on neighbouring cells. The previously empty molecules loaded with the secreted peptides were stable on the surface of the cells for several hours and were recognized by antigen-specific cytotoxic T cells previously primed in vivo.
Materials and methods
Mice
Female BALB/c mice (8–12 weeks old) were obtained from the Mexican Children's Hospital (Mexico City, Mexico).
Bacteria
Salmonella typhimurium strain LT2 was donated by Dr C. Alpuche (Experimental Medicine, UNAM, Mexico City, Mexico). The bacteria were grown in brain–heart infusion (Difco, Detroit, MI) for 3 hr at 37° until they reached logarithmic phase and were washed twice with phosphate-buffered saline (PBS) before the infection.
Cells
Dr G. J. Hammerling (German Cancer Research Centre, Heidelberg, Germany) donated RMA-S and RMA cells.17 IC21 macrophages (H-2b),18 J774 macrophages (H-2d),19 P815 mastocytoma (H-2d)20 and L929 fibroblast (ATCCCL-1) were all obtained from the American Type Culture Collection (ATCC, Rockville, MD). The L-1210 cells were donated by Dr Cesar Gonzalez (Hospital La Raza, Mexico City). All cells were grown in RPMI-1640 supplemented with 10% fetal calf serum (FCS), 50 µm 2-mercaptoethanol, 2 mm l-glutamine and 1 mm sodium pyruvate (Gibco-BRL, Long Island, NY) without antibiotic. The designation, RP-10, refers to the media mentioned above. Gentamycin (at 20 µg/ml; Boehringer Mannheim, Mexico City) was added to the infected macrophages to control the growth of extracellular bacteria.
Antibodies
Y3 hybridoma (anti-Kb)21 was donated by Dr G. J. Hammerling and M1/70·15.11.5 hybridoma (anti-Mac-1) was obtained from ATCC. Hybridomas were grown in RP-10 with antibiotics (100 µg/ml streptomycin and 100 U/ml penicillin G). The monoclonal antibody (mAb) Y-3 was affinity-purified in a Protein A–Sepharose column (Amersham Pharmacia Biotech, Uppsala, Sweden) and was conjugated to biotin using Sulpho–N–hydroxysuccinimide–Biotin (Pierce, Rockford, IL) in carbonate buffer according to the manufacturer's instructions. Streptavidin–phycoerythrin (PE) conjugate, goat anti-rat antibody-fluorescein isothiocyanate (FITC) conjugate and goat anti-mouse antibody-FITC conjugate were purchased from Gibco-BRL.
Peptides
Salmonella peptides were obtained from an extraction of total membrane and cytosolic proteins22 treated with 1% trypsin as described by Moore et al.23 OVA peptide 257–264 (SIINFEKL) was synthesized by solid-phase Fmoc-peptide synthesis in an ABI 430-A automated synthesizer (Applied Biosystems Inc., Foster City, CA) and purified by reverse-phase high-performance liquid chromatography in a C18 column (Millipore, Bedford, MA).
Macrophage infection and RMA-S co-cultures
In order to activate macrophages, 2 × 105 IC21 or J774 macrophages were cultivated for 48 hr in 12-well Costar plates (Fisher Scientific, Pittsburgh, PA) with 20 U/ml of IFN-γ or 20% of L-1210 supernatant (containing IFN-γ) in antibiotic-free medium. The macrophages were gently washed to remove the IFN-γ from the media and to eliminate any dead cells. The macrophages were then infected by adding a ratio of 100 bacteria (LT2 strain) per macrophage and the plate was centrifuged at 1000 g for 5 min at room temperature.24 The plates were incubated for 30 min at 37°, and the extra cellular bacteria were removed by four washes with warm PBS. RMA-S cells were then added to the macrophage monolayer at a ratio of 1:1. The co-culture was incubated in RP-10 supplemented with 20 µg/ml gentamycin (RP-10–gentamycin) at 37° for 18 hr. Following the incubation period, RMA-S cells were harvested and washed with PBS before immuno-staining.
Co-cultures in the presence of inhibitors
IFN-γ-activated macrophage monolayers were treated with 1 µm of leupeptin (Boehringer Mannheim, Mexico City) or 20 mm of ammonium chloride (Sigma-Aldrich, St. Louis, MI) for 30 min at 37° before infection with Salmonella. All other procedures were performed as described above, except that more leupeptin and ammonium chloride was added with the RMA-S cells to keep the endosome inhibitor concentration constant during 18 hr of incubation time. As control, cells were incubated for 18 hr with the inhibitors at the same concentration. BFA (Boehringer Mannheim) was added to the final co-cultures with RMA-S cells where indicated, at a final concentration of 1 µg/ml. RMA-S control cells were incubated under the same conditions as described above.
Flow cytometric analysis
MHC class I Kb molecules were stained on the surface of RMA-S and IC21 cells using biotin-conjugated Y3 mAb and counter-stained with streptavidin-PE conjugate. Contaminating macrophages in the RMA-S samples were stained with mAb Mac-1 and revealed with a FITC-conjugated goat anti-rat antibody. Cells were analysed using a FACScan™ cytometer and Lysis II™ software (Becton Dickinson, Mountain View, CA). Dead cells were detected using propidium iodide (Sigma-Aldrich) and were gated out during the analysis.
Salmonella labelling and immunoprecipitation of Kb molecules
Salmonella typhimurium LT2 was grown in M9 medium and labelled with 3H-labelled leucine, 3H-labelled alanine, 3H-labelled valine and 3H-labelled phenylalanine (NEN Life Science, Boston, MA) for 1 hr. After three washes with PBS, the labelled bacteria was used to infect 1 × 107 IC21 macrophages followed by co-culture with RMA-S in RP-10–gentamycin as described above. After an 18-hr incubation period, the RMA-S cells and the macrophages were harvested and lysed with 50 mm Tris–HCl pH 8·0, 1% Nonidet P-40, 150 mm NaCl, 1 mm ethylenediamine tetraacetic acid, 1 mm phenylmethylsulphonyl fluoride, leupeptin and aprotonin. Kb class I MHC protein was immunoprecipitated using Y3 mAb (20 µg); W6-32 was used as an irrelevant mAb control. The peptides were eluted from the complexes using 10% acetic acid and boiling for 2 min. The released Kb peptides were separated from the MHC protein by filtration using Centriprep-10 (Millipore), and the amount of radioactivity was determined using a β-scintillation counter (Beckman Instruments, Irvine, CA).
Cytotoxic T lymphocyte assay
BALB/c mice were sublethally infected with Salmonella typhimurium LT2 (10 bacteria/mouse injected intraperitoneally every 10 days for 1 month). Spleens were harvested and isolated splenocytes were stimulated with 100 µg/ml of Salmonella peptides for 5 days in vitro. P815 cells co-cultivated for 18 hr with Salmonella-infected IC21 macrophages were used as cytotoxic T lymphocytes (CTL) targets in a 51Cr-release assay.25 Target cells (1 × 103 cells) were incubated for 4 hr with different numbers of CTL effectors in 96-well round-bottom plates, in a final volume of 200 µl/well of complete medium. Supernatants were harvested mechanically using a supernatant collection system (Skatron Instruments, Sterling VA). Supernatant radioactivity was measured in a gamma-counter (Beckman Instruments). Per cent specific release was calculated from the mean of triplicate cultures according to the following formula: % specific release = 100 × [(experimental release − spontaneous release)/(maximal release − spontaneous release)].
Statistical analysis
The mean fluorescence channel of RMA-S cells from infected and non-infected macrophages was compared by non-parametric Wilcoxon signed-rank test for paired samples using SPPSS 9·0 statistical software.
Results
Macrophages secrete peptides that load empty class I Kb molecules
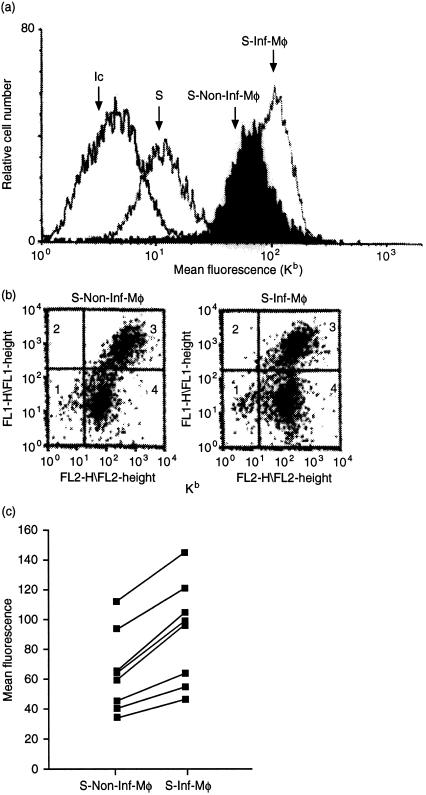
We determined whether IFN-γ-activated macrophages were able to secrete peptides into the external medium. After 48 hr of activation with IFN-γ, the IC21 cells were washed to remove any free IFN-γ and RMA-S cells were added and incubated with the washed macrophages for 18 hr. Class I Kb molecule expression on the surface of the RMA-S cells was then determined by indirect immunofluorescence using the Y3 mAb (recognizing only peptide-loaded Kb molecules). As seen in Fig. 1(a), co-cultures with Salmonella-infected macrophages increased the expression of detectable Kb complexes on the RMA-S cells [mean fluorescence channel (MFC) of 150 compared to a MFC of 17 on RMA-S cells alone]. Any contaminating macrophages (Mac-1 and Kb double-positive stained cells, Fig. 1b, quadrant 3 of each dot plot) within the RMA-S samples were excluded from the analysis. Interestingly, co-culture with non-infected, IFN-γ-activated macrophages also increased RMA-S surface Kb expression but not to the same extent as when the macrophages were previously infected with Salmonella. As seen in Fig. 1(c), this up-regulation of Kb on RMA-S cells showed some variation, probably due to the efficiency of Salmonella infection between experiments. However, according to statistical analysis using the non-parametric Wilcoxon test for paired samples, in fact IFN-γ-activated Salmonella-infected macrophages secreted more peptides than the IFN-γ-activated non-infected macrophages (P = 0·012). No increase in Kb expression was observed when RMA-S cells alone were incubated with bacteria (data not shown). As a control, macrophages were fixed with formaldehyde immediately after the infection with Salmonella; under these conditions no increase in Kb staining in RMA-S cells was found (data not shown). Thus, macrophages co-cultured with RMA-S cells induced an increase in class I MHC Kb molecule expression on the RMA-S cells, especially after bacterial infection, suggesting that peptides secreted from the macrophage load empty class I molecules on neighbouring cells.
Figure 1.
Macrophages secrete peptides that load empty Kb molecules on the surface of RMA-S cells. IC21 macrophages were pretreated with 20 U/ml of IFN-γ for 48 hr and then infected at an ratio of 100 bacteria (Salmonella typhimurium LT2) per macrophage. RMA-S cells were then added to the washed macrophage monolayer at a ratio of 1:1. RMA-S cells were harvested after 18 hr incubation period and immunostained using anti-Kb mAb Y3-biotin and streptoavidin PE. Contaminant macrophages in the RMA-S samples were stained using mAb M1/70.15.11.5 (anti-Mac-1) and anti-rat-FITC (dot plot). (a) The histograms show RMA-S stained with an isotype-control antibody (Ic), Kb expression on RMA-S cultured alone (S) and Kb expression on the gated population of RMA-S cells from co-culture with non-infected macrophages (S-Non-Inf-Mφ) and with infected macrophages (S-Inf-Mφ). (b) The dot blot shows co-culture population: macrophages quadrant 3 and RMA-S gated population on quadrant 4. (c) Graphic lines present the significant increase of mean fluorescence channel of RMA-S from infected macrophages (S-Inf-Mφ) compared to the non-infected macrophage (S-Non-Inf-Mφ) in eight different experiments. (P = 0·012 according to Wilcoxon test for paired samples).
In order to determine whether the increase in Kb staining on RMA-S cells after co-culture with infected macrophages was due to released peptides of bacterial origin, Salmonella were first labelled with 3H-labelled alanine, leucine, valine and phenylalanine (amino acids often used as anchor residues in optimal peptides that bind Kb molecules). The labelled bacteria were washed and used to infect the macrophages. The macrophages infected with radiolabelled bacteria were co-cultured with RMA-S cells as before. Following co-culture, the RMA-S cells and macrophages were isolated separately and class I MHC Kb molecules were immunoprecipitated from lysates of the two cell populations using Y3 mAb; the radioactivity was then determined in the peptides eluted from the immunoprecipitates. The same elution procedure was performed with immunoprecipitates of an isotype-matched non-Kb-specific mAb (W632) as a control and the radioactivity was subtracted from the amount recovered from the peptides eluted from the Y3 immunoprecipitates. In these experiments 300 ± 10 counts per min (c.p.m.) and 1000 ± 40 c.p.m. (average of three experiments) were recovered from RMA-S cells and macrophages, respectively, The background levels of W632 mAb immunoprecipitations were 44 c.p.m. for the RMA-S and 100 c.p.m. for the macrophages (these values were subtracted). Thus, at least some the loaded peptides binding to class I MHC on the TAP-deficient RMA-S cells were of bacterial origin.
IFN-γ activation and Salmonella infection of macrophages promotes the secretion of peptides
We examined whether the activation of macrophages by IFN-γ played a significant role in the observed secretion of class I-loading peptides following Salmonella infection. IC21 cells were treated with or without IFN-γ for 48 hr, washed, and then infected with bacteria before co-culture with RMA-S cells. The activity of the IFN-γ preparation used was confirmed by the increase in class I MHC staining on the macrophages themselves (Table 1). After 18 hr of co-culture, the expression of Kb molecules on the RMA-S cells was evaluated after separation from the macrophages. Non-IFN-γ-activated macrophages, in the absence of Salmonella infection, were unable to induce an increase in Kb expression on the RMA-S cells (Table 1). In contrast, when macrophages were treated with IFN-γ prior to RMA-S co-culture, an increase in Kb molecules was observed, which was significantly enhanced if the macrophages were also infected with Salmonella. However, Salmonella infection of non-IFN-γ-activated macrophages caused only a slight increase in Kb expression on the co-cultured RMA-S cells. Thus, IFN-γ activation was required for macrophages to secrete class I Kb-loading peptides from endocytosed material.
Table 1. The macrophages require activation by IFN-γ for secretion of peptides.
| Co-culture conditions | Kb expression on RMA-S | |||||
|---|---|---|---|---|---|---|
| RMA-S | IC-21 | IFN-γ | Salmonella | I | Y3 | Y3–I |
| + | + | − | − | 10·3 | 24·5 | 14·2 |
| + | + | − | + | 10·7 | 48·9 | 38·2 |
| + | + | + | − | 8·8 | 61·2 | 52·4 |
| + | + | + | + | 12·4 | 71·3 | 58·9 |
Results are shown as the Mean Fluorescence Channel (MFC). Co-cultures were carried out as described in Fig. 1. When indicated, 20 U/ml of IFN-γ was used to activated macrophages during 48 hr. I, isotype control antibody; Y3, antibody against Kb molecules. The MFC of RMA-S Kb expression, with and without IFN-γ, was 16·3, that of IC21 Kb expression was 507·30 and after IFN-γ activation it was 602·80.
Endosomal inhibitors do not affect peptide release
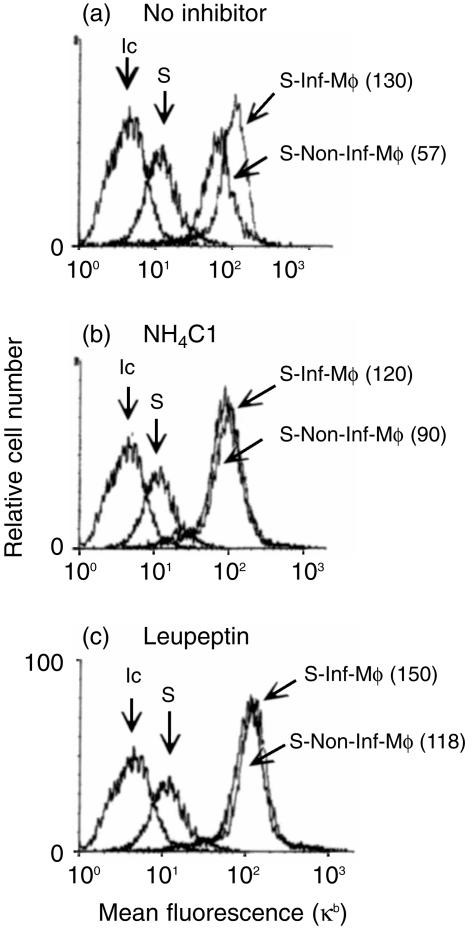
We next determined whether the generation of secreted peptides loading class I Kb molecules following Salmonella infection required proteolytic activity from an endosomal–lysosomal compartment of the macrophages. To test this we treated IC21 macrophages with ammonium chloride (prevents lysosomal functions), or leupeptin (a serine and thiol protease inhibitor) before Salmonella infection and during the co-culture with RMA-S cells. RMA-S culture alone in the presence of these inhibitors did not produce any change in their Kb expression. The increase in RMA-S Kb staining from the co-culture with infected macrophages was not affected by leupeptin or ammonium chloride treatment. Interestingly, the non-infected controls showed increased Kb expression on RMA-S cells (Fig. 2). Thus, leupeptin and ammonium chloride did not affect Salmonella-infected macrophage Kb peptide secretion and seemed to increase the processing of soluble proteins from the culture media.
Figure 2.
Proteolytic endosomal inhibitors partially decrease the secretion of Kb peptides from Salmonella-infected macrophages. IFN-γ-activated macrophage monolayers were treated with: (a) no inhibitor (b) 20 mm ammonium chloride, or (c) 1 mm leupeptin for 30 min at 37° before infection with Salmonella. The co-cultures with RMA-S were performed as described in Fig. 1. Both ammonium chloride and leupeptin were also added at the time of co-culture initiation. As control, RMA-S alone were also treated with the inhibitors for 18 hr. MHC class I Kb expression on RMA-S cells was analysed by FACS staining as described in Fig. 1. The histograms show RMA-S stained with an isotype-control antibody (Ic), Kb expression on RMA-S cultured alone (S) and Kb expression on the gated population of RMA-S cells from co-culture with non-infected macrophages (S-Non-Inf-Mφ) and with infected macrophages (S-Inf-Mφ). RMA-S cells from the co-cultured with macrophages. The results from one of six experiments are shown. Values in parenthesis correspond to mean fluorescence channel.
BFA does not inhibit the generation of Kb-loading peptides following Salmonella infection
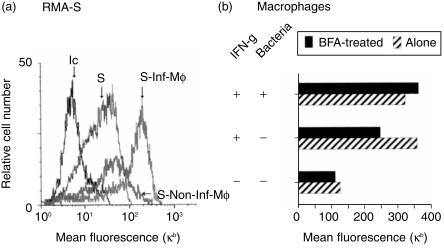
BFA blocks protein transport into the Golgi apparatus. It generates redistribution of the Golgi into the endoplasmic reticulum, inhibiting vesicle traffic to the cell surface. However, it does not inhibit recycling of endosomes at the membrane level (e.g. recycling of transferrin receptor).26 We treated the RMA-S and IFN-γ-activated macrophage co-cultures with BFA for 18 hr before the fluorescence-activated cell sorter (FACS) analysis of Kb molecules. We first asked whether the generation and secretion of peptides by macrophages after Salmonella infection involved the exportation of vesicles coming from the Golgi; and second whether the secreted peptides loaded preformed MHC class I molecules on the surface of the RMA-S. Interestingly, we found that RMA-S cells co-cultured with Salmonella-infected macrophages still showed an increase in Kb molecules in the presence of BFA (Fig. 3a) over that seen with RMA-S control cells treated with BFA or RMA-S cells co-cultured with non-infected macrophages treated with BFA. Thus, the macrophages release Salmonella peptides from the phagosomes and not from material contained in vacuoles coming from the endoplasmic reticulum. In addition, the secreted peptides load preformed class I molecules on the surface of RMA-S.
Figure 3.
(a) Brefeldin A does not inhibit the secretion of Kb-loading peptides. RMA-S and IC21 macrophage co-cultures were treated with 1 µg/ml of BFA for a period of 18 hr. RMA-S controls were treated with BFA as well. Kb expression on harvested RMA-S cells was analysed as described in Fig. 1. The histograms show RMA-S stained with an isotype-control antibody (Ic), Kb expression on RMA-S cultured alone (S) and Kb expression on the gated population of RMA-S cells from co-culture with non-infected macrophages (S-Non-Inf-Mφ) and with infected macrophages (S-Inf-Mφ). (b) Kb expression was also determined on the IC21 macrophages.
We also determined the level of Kb expression on the macrophages used in the experiment shown in Fig. 3(a). In the absence of bacterial infection, IFN-γ induced an increase in Kb expression on the macrophages, as expected. This increase was markedly inhibited with BFA. This confirms that the BFA was active in our culture system inhibiting the endogenous pathway of MHC class I shuttling to the cell surface. Interestingly, infection of macrophages with Salmonella also induced an increase in Kb expression on the macrophages themselves, even in the presence of BFA in the culture. Thus, it seems that the secreted peptides load any possible empty molecules on the surface of the macrophages. Alternatively, the Salmonella inside the phagosomes may prevent the internalization of class I molecules on the surface of the macrophage.
Only phagocytic cells are able to secrete peptides that load empty class I molecules
We determined whether cell types other than macrophages, treated with IFN-γ, were able to induce an increase in Kb expression on RMA-S cells following co-culture. In these experiments (Table 2), only peritoneal macrophages and infected or non-infected macrophage cell lines (J774 and IC21 shown in Fig. 1) were able to induce an increase in Kb expression on co-cultured RMA-S cells. In contrast, L929 fibroblasts and A20 B lymphoma cells did not induce any significant differences in Kb expression. In these experiments we also found that activated peritoneal macrophages in primary culture could also induce an increase in Kb molecules on the surface of co-cultured RMA-S cells after infection with Salmonella (Table 2 and data not shown).
Table 2. Only phagocytic secrete peptides that load MHC class I molecules.
| Kb expression on RMA-S | |||
|---|---|---|---|
| Co-culture | I | Y3 | Y3–I |
| RMA-S | 2·28 | 18·04 | 15·76 |
| RMA-S + l929 | 3·4 | 13·02 | 9·62 |
| RMA-S + infected L929 | 3·05 | 14·14 | 11·09 |
| RMA-S peritoneal macrophages | 4·15 | 33·19 | 29·04 |
| RMA-S + infected peritoneal macrophages | 3·89 | 45·35 | 41·46 |
| RMA-S + J774 | 2·95 | 48·2 | 45·25 |
| RMA-S + infected J774 | 2·67 | 66·31 | 63·64 |
| RMA-S | 7·98 | 30·59 | 22·61 |
| RMA-S + A20 | 9·41 | 29·08 | 19·67 |
| RMA-S + infected A20 | 4·13 | 28·94 | 24·81 |
Results are shown as the mean fluorescence channel. Co-cultures were performed as described in Fig. 1. L929, peritoneal macrophages from BALB/c mice, J774 and A20 were treated with IFN-γ for 48 hr and infected with Salmonella typhimurium at ratio of 100 bacteria per cell. I, isotype control antibody; Y3, antibody against Kb molecules.
Empty class I molecules loaded with peptides secreted by infected macrophages stay on the surface for several hours
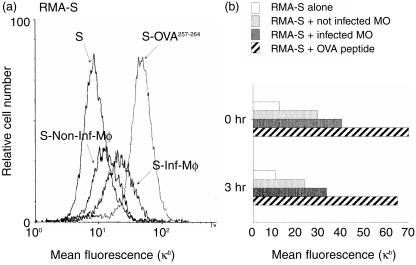
Only added octamer or nonamer peptides which contain anchor-motif amino acids in their sequence are able to stabilize MHC class I molecules on the cell surface.16 Therefore we examined the stability of the increased Kb expression on the surface of RMA-S cells after incubation with infected macrophages. In these experiments the RMA-S cells were isolated after 18 hr co-culture with infected macrophages, washed twice and cultured for an additional 3 hr before determination of Kb expression using flow cytometry. The OVA257–264 peptide was used as a positive control for an octamer Kb-binding peptide. Addition of OVA257–264 led to a large increase in MHC class I Kb expression on the RMA-S cells, which was stable for at least 3 hr after washing the cells to remove unbound peptide (Fig. 4). Similarly, no significant decrease in Kb fluorescence intensity on RMA-S cells was found in co-cultures containing Salmonella-infected macrophages after the additional 3 hr. The overall staining intensity was lower than that obtained with exogenous free OVA peptide (Fig. 4), owing to the high amounts of exogenous OVA peptide added relative to the low amounts of Kb-binding peptides that could be secreted from the bacterial infection. Thus, the class I molecules loaded with secreted peptides stayed on the cell surface and were not internalized or degraded for at least 3 hr.
Figure 4.
(a) The secreted peptides stabilize empty class I molecules. IC21 macrophages were co-cultured with RMA-S cells for 18 hr as described in Fig. 1. Afterward the RMA-S were harvested (0 hr) and washed three times with PBS and cultured alone for three additional hours at 37° (3 hr). MHC class I Kb expression on RMA-S cells was analysed by FACS staining as described in Fig. 1. The histograms show RMA-S stained with an isotype-control antibody (Ic), Kb expression on RMA-S cultured alone (S) and Kb expression on the gated population of RMA-S cells from co-culture with non-infected macrophages (S-Non-Inf-Mφ) and with infected macrophages (S-Inf-Mφ). RMA-S cells cultured with 15 µm/ml of OVA257–264 peptide were used as positive control (S-OVA257–264). (b) Kb expression on RMA-S cell at both experimental times.
Loading of target cells with secreted peptides from Salmonella-infected macrophages can activate antigen-specific CD8+ T cells
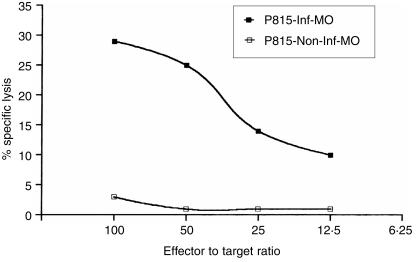
We next determined if the secretion of peptides from activated macrophages and the loading of MHC empty class I molecules on neighbouring cells would create recognition structures for the stimulation of antigen-specific CD8+ T cells. We tested this by employing Salmonella-infected macrophages co-cultured with P815 mastocytoma cells that were later used as target cells for CTL. Spleen cells from BALB/c mice, previously primed with Salmonella, were restimulated in vitro with a mix of peptides from Salmonella proteins for 5 days; this was the source of antigen-specific CTL. As shown in Fig. 5, Salmonella-specific CTL from BALB/c mice lysed P815 cells that were previously cultured with infected macrophages. Thus, the secreted peptides from Salmonella-infected macrophages can generate MHC class I structures that are recognized by specific CD8+ T cells.
Figure 5.
Target cells loaded with secreted peptides from macrophages can activate antigen-specific CD8+ T cells. P815 mastocytoma was co-cultured with non-infected activated IC21 macrophages (P815 + Mφ) or Salmonella-infected IC21 macrophages (P815 + Inf Mφ). After 18 hr, P815 cells were harvested, labelled for 1 hr with 51Cr, and mixed with Salmonella-specific CTL. The source of CTL were spleen cells from Salmonella-infected BALB/c mice. The spleen cells were re-stimulated in vitro for 5 days with 100 µg/ml of Salmonella peptides generated by trypsin digestion before use in the CTL assay.
Discussion
Exogenous antigens can be presented by MHC class I molecules through different antigen-processing pathways.27 Our results agree with previous studies in which release of peptides by macrophages was demonstrated.9,10 However, in these previous studies, it was not clear how the class I molecules were loaded because fixed macrophages were used as recipients of the released peptides. Furthermore, the nature of factors (e.g. IFN-γ) inducing this secretory pathway has not been previously investigated. The size, amount and origin of the antigen have been the principal variables.12,13 In the work presented here, we show that IFN-γ can be a decisive stimulus for the macrophage to induce the secretion of peptides that load empty class I molecules on the surface of neighbouring cells and on the macrophages themselves The dependency of IFN-γ stimulation shown here might explain why a TAP-independent peptide release pathway was detected in some studies but not in others.12,13,28 Peptone-activated macrophages isolated from peritoneal exudates also showed this peptide secretory pathway and IC21 macrophages not treated with IFN-γ were unable to induce the loading and stabilization of RMA-S Kb molecules (Table 1, Table 2 and data not shown). MHC Kb molecules were present on the surface of RMA-S for at least 3 hr in a similar fashion to the octamer OVA257–264 (SIINFEKL). In addition, secreted peptides loaded into class I molecules of P815 target cells can be recognized by CTLs from BALB/c mice primed in vivo with Salmonella. Thus, this secretory antigen presentation pathway can release peptides that can form recognition structures for CTL. Dendritic cells treated with IFN-γ increase their presentation of exogenous antigens by class I molecules using proteasome and TAP endogenous pathways,29 the pathway described here for IFN-γ-activated macrophages may co-operate with dendritic cells during the induction of T-cell responses against foreign antigens. During an inflammatory response tumour necrosis factor-α activates macrophages to produce interleukin-12 and, as a consequence, CD4+ and CD8+ T cells produce IFN-γ.30 These events may induce and prolong the activation of the macrophage in order to recruit specific CD8+ effector T cells in the tissues.
We were able to detect radiolabelled peptides loaded on RMA-S cells after co-culture with activated macrophages infected with radiolabelled Salmonella by Kb immunoprecipitation experiments. Thus, secreted material came from the internalized Salmonella. Interestingly, ammonium chloride and leupeptin did not inhibit the Kb fluorescence increase on RMA-S cells induced by Salmonella infection of IFN-γ-activated macrophages and also induced an increase in the Kb expression induced by the macrophages without bacterial infection. These results suggest that IFN-γ-activated macrophages can cleave exogenous antigens with a set of proteases that can function at neutral pH. Indeed, our results further suggest that the neutralization of the phagosome or the endosomal compartments increases the proteolytic activity of these proteases. The neutralization of the phagosome can be achieved by S. typhimurium, which normally attenuates the acidification of this compartment in order to survive.31 This may be an explanation why there are always more secreted peptides from infected macrophages than from non-infected macrophages.
BFA blocks the transport of proteins from the endoplasmic reticulum to the Golgi apparatus, a step critical for the classical TAP-dependent pathway for the transport of newly synthesized peptide-loaded MHC molecules. Our results suggest that processing and secretion of peptides from exogenous antigens can utilize a BFA-resistant pathway engaging a compartment, which may recycle close to the plasma membrane independent of the Golgi apparatus. Recently, a Fc-γ-receptor-mediated phagocytic pathway mediated by the ARF6 protein was shown to be resistant to BFA in macrophages.32 IFN-γ can induce the expression of two subunits of the proteasome, LMP-2 and LMP-7, as well as the regulator PA28.33–35 It is possible that the IFN-γ activation of the macrophages before the uptake of Salmonella could induce a proteolytic activity inside the phago-lysosome that could generate peptides similar to those generated by the proteasomes.
The peptide release pathway described here needs to be evaluated in vivo. This pathway may be part of the mechanism of ‘cross-priming’ demonstrated by other investigators. In an inflamed tissue, dendritic cells may present peptides released from macrophages and then migrate to the lymph nodes to activate specific T cells, in this way even if the immature dendritic cell is not able to process the antigen, it can be the carrier of peptides for the macrophage.36,37 Alternatively, the release of self-peptides from phagocytosed self-proteins and apoptotic bodies in the tissues could be a mechanism by which activated macrophages could participate in break down of the tolerance and generate autoimmunity.38,39 This mechanism could be dangerous for bystander non-infected cells, however, the activation of CD8+ T cells in the tissues could generate the production of IFN-γ to help macrophage killing of Salmonella. Alternatively, other regulatory mechanisms can control CD8+ T attack in the tissues like CD30–CD30 ligand interactions.40
Acknowledgments
We are grateful for the generous gifts of cell lines from Dr G. J. Hammerling and Dr Kenneth Rock. We thank Dr Lazlo Radvanyi and Dr Celia Alpuche for helpful discussions and careful scrutiny of the manuscript. This work was supported by Research Grant No.3595P-M9608 of Consejo Nacional de Ciencia y Tecnologia (CONACYT), Mexico. N.M.O. is a recipient of a doctoral fellowship from CONACYT.
References
- 1.Richter-Dahlfors A, Buchan AMJ, Finlay B. Murine Salmonellosis studied by confocal microscopy: Salmonella typhimurium resides intracellularly inside macrophages and exerts a cytotoxic effect on phagocytes in vivo. J Exp Med. 1997;186:569–80. doi: 10.1084/jem.186.4.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kaufmann SH. Immunity to intracellular bacteria. Annu Rev Immunol. 1993;11:129–63. doi: 10.1146/annurev.iy.11.040193.001021. [DOI] [PubMed] [Google Scholar]
- 3.Boehm U, Klamp T, Groot M, Howard JC. Cellular responses to Interferon-γ. Annu Rev Immunol. 1997;15:749–95. doi: 10.1146/annurev.immunol.15.1.749. [DOI] [PubMed] [Google Scholar]
- 4.Hess J, Ladel C, Miko D, Kaufmann SH. Salmonella typhimurium aroA- infection in gene-targeted immunodeficient mice: major role of CD4+ TCR-αβ cells and IFN-γ in bacterial clearance independent of intracellular location. J Immunol. 1996;156:3321–26. [PubMed] [Google Scholar]
- 5.Pfeifer JD, Wick MJ, Roberts RL, Findlay K, Normark SJ, Harding CV. Phagocytic processing of bacterial antigens for class I MHC presentation to T cells. Nature. 1993;361:359–62. doi: 10.1038/361359a0. [DOI] [PubMed] [Google Scholar]
- 6.Rock KL. A new foreign policy: MHC class I molecules monitor the outside world. Immunol Today. 1996;17:131–7. doi: 10.1016/0167-5699(96)80605-0. 10.1016/0167-5699(96)80605-0. [DOI] [PubMed] [Google Scholar]
- 7.Kovacsovics-Bankowski M, Rock KL. A phagosome-to-cytosol pathway for exogenous antigens presented on MHC class I molecules. Science. 1995;264:243–53. doi: 10.1126/science.7809629. [DOI] [PubMed] [Google Scholar]
- 8.Lehner PJ, Cresswell P. Processing and delivery of peptides presented by MHC class I molecules. Curr Opin Immunol. 1996;8:59–67. doi: 10.1016/s0952-7915(96)80106-3. [DOI] [PubMed] [Google Scholar]
- 9.Sadasivan B, Lehner PJ, Ortmann B, Spies T, Cresswell P. Roles for calreticulin and a novel glycoprotein, tapasin, in the interaction of MHC class I molecules with TAP. Immunity. 1996;5:103–14. doi: 10.1016/s1074-7613(00)80487-2. [DOI] [PubMed] [Google Scholar]
- 10.Wick MJ, Pfeifer JD. Major histocompatibility complex class I presentation of ovalbumin peptide 257-264 from exogenous sources: protein context influences the degree of TAP-independent presentation. Eur J Immunol. 1996;26:2790–99. doi: 10.1002/eji.1830261135. [DOI] [PubMed] [Google Scholar]
- 11.Bachman MF, Oxenius A, Pircher H, Hengartner H, Ashton-Richardt PA, Tonegawa S, Zinkernagel RM. TAP1-independent loading of class I molecules by exogenous viral proteins. Eur J Immunol. 1995;25:1739–43. doi: 10.1002/eji.1830250637. [DOI] [PubMed] [Google Scholar]
- 12.Harding CV, Song R. Phagocytic processing of exogenous particulate antigens by macrophages for presentation by class I MHC molecules. J Immunol. 1994;153:4925–33. [PubMed] [Google Scholar]
- 13.Song R, Harding CV. Roles of proteasomes, transporter for antigen presentation (TAP) and Beta 2-Microglobulin in the processing of exogenous or particulate antigens via an alternate class I MHC processing pathway. J Immunol. 1996;156:4182–90. [PubMed] [Google Scholar]
- 14.Ljunggren HG, Stam NJ, Ohlen C, et al. Empty MHC class I molecules come out in the cold. Nature. 1990;346:476–80. doi: 10.1038/346476a0. [DOI] [PubMed] [Google Scholar]
- 15.Rock KL, Gamble S, Rothstein L, Gramm C, Benacerraf B. Dissociation of β2-Microgobulin leads to the accumulation of a substantial pool of inactive class I MHC heavy chains on the cell surface. Cell. 1991;65:511–20. doi: 10.1016/0092-8674(91)90093-e. [DOI] [PubMed] [Google Scholar]
- 16.Ortiz-Navarrete V, Hammerling GJ. Surface appearance and instability of empty H-2 class I molecules under physiological conditions. Proc Natl Acad Sci USA. 1991;88:3594–7. doi: 10.1073/pnas.88.9.3594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kärre K, Ljunggren HG, Piontek G, Kiessling R. Selective rejection of H-2 deficient lymphoma variants suggests alternative immune defense strategy. Nature. 1986;319:675–9. doi: 10.1038/319675a0. [DOI] [PubMed] [Google Scholar]
- 18.Walker W, Demus A. Antibody-dependent cytolysis of chicken erythrocytes by an in vitro established line of mouse peritoneal macrophages. J Immunol. 1975;114:765–9. [PubMed] [Google Scholar]
- 19.Ralf P, Prichard J, Cohn M. Reticulum cell sarcoma: an effector cell in antibody-dependent cell mediated immunity. J Immunol. 1975;114:895–905. [PubMed] [Google Scholar]
- 20.Plaut M, Lichtenstein LM, Gillespie E, Henney C. Studies on the mechanism of lymphocyte-mediated cytolysis. J Immunol. 1973;111:389–94. [PubMed] [Google Scholar]
- 21.Ozato K, Sachs DH. Monoclonal antibodies to mouse MHC antigens. III. Hybridoma antibodies reacting to antigens of the H-2b haplotype reveal genetic control of isotype expression. J Immunol. 1981;126:317–21. [PubMed] [Google Scholar]
- 22.Isibasi A, Ortiz-Navarrete V, Vargas M, Paniagua J, Gonzalez C, Moreno J, Kumte J. Protection against Salmonella typhi infection in mice after immunization with outer membrane proteins isolated from Salmonella typhi 9,12, d, Vi. Infect Immun. 1988;56:2953–9. doi: 10.1128/iai.56.11.2953-2959.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moore MW, Carbone FR, Bevan MJ. Introduction of soluble protein into the class I pathway of antigen processing and presentation. Cell. 1988;54:777–85. doi: 10.1016/s0092-8674(88)91043-4. [DOI] [PubMed] [Google Scholar]
- 24.Buchemeier NA, Heffron F. Intracellular survival of wild type Salmonella typhimurium and macrophage-sensitive mutants in diverse populations of macrophages. Infect Immun. 1989;57:1–7. doi: 10.1128/iai.57.1.1-7.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wunderlich J, Shearer G. Induction and measurements of cytotoxic T lymphocyte activity. In: Coligan JE, Kruisbeek AM, Margulies D, Shevach EM, Strober W, editors. Current Protocols in Immunology. 2. Vol. 1. New York, NY: Greene Publishing Associates, Inc and John Wiley & Sons, Inc; 1992. 3.11.1. [Google Scholar]
- 26.Lippincott-Schwartz J, Yuan L, Tipper C, Amherdt M, Orci L, Klausner RD. Brefeldin A's effects on endosomes, lysosomes, and the TGN suggests a general mechanism for regulating organelle structure and membrane traffic. Cell. 1991;67:601–16. doi: 10.1016/0092-8674(91)90534-6. [DOI] [PubMed] [Google Scholar]
- 27.Jondal M, Schirmbeck R, Reimann J. MHC class I-restricted CTL responses to exogenous antigens. Immunity. 1996;5:259–302. doi: 10.1016/s1074-7613(00)80255-1. [DOI] [PubMed] [Google Scholar]
- 28.Reis e Sousa C, Germain RN. Major histocompatibility complex class I presentation of peptides derived from soluble exogenous antigen by a subset of cells engaged in phagocytosis. J Exp Med. 1995;182:841–51. doi: 10.1084/jem.182.3.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brossart P, Bevan MJ. Presentation of exogenous protein antigens on major histocompatibility complex class I molecules by dendritic cells: pathway of presentation and regulation by cytokines. Blood. 1997;90:1594–99. [PMC free article] [PubMed] [Google Scholar]
- 30.Trinchieri G. Cytokines acting on or secreted by macrophages during intracellular infection (IL-10, IL-12, IFN-γ) Curr Opin Immunol. 1997;9:17–23. doi: 10.1016/s0952-7915(97)80154-9. [DOI] [PubMed] [Google Scholar]
- 31.Alpuche ACM, Swanson JA, Loomis WP, Miller SI. Salmonella typhimurium activates virulence gene transcription within acidified macrophages phagosomes. Proc Natl Acad Sci USA. 1992;89:10079–83. doi: 10.1073/pnas.89.21.10079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang Q, Cox D, Tseng CC, Donaldson JG, Greenberg S. A requirement for ARF6 in Fcγ receptor-mediated phagocytosis in macrophages. J Biol Chem. 1998;273:19977–81. doi: 10.1074/jbc.273.32.19977. [DOI] [PubMed] [Google Scholar]
- 33.Fruh K, Yang Y. Antigen presentation by MHC class I and its regulation by interferon γ. Curr Opin Immunol. 1999;11:76–81. doi: 10.1016/s0952-7915(99)80014-4. 10.1016/s0952-7915(99)80014-4. [DOI] [PubMed] [Google Scholar]
- 34.Ortiz-Navarrete V, Seeling A, Gernold M, Frentzel S, Kloetzel PM, Hammerling GJ. Subunit of “20S” proteasome (multicatalytic proteinase) encoded by the major histocompatibility complex. Nature. 1991;353:662–4. doi: 10.1038/353662a0. [DOI] [PubMed] [Google Scholar]
- 35.Gaczynska M, Rock KL, Goldberg AL. Gamma-interferon and expression of MHC genes regulate peptide hydrolysis by proteasomes. Nature. 1993;365:264–7. doi: 10.1038/365264a0. [DOI] [PubMed] [Google Scholar]
- 36.Kurts C, Heath WR, Carbone FR, Allison J, Miller JFAP, Kosaka H. Constitutive Class I-restricted exogenous presentation of self antigens in vivo. J Exp Med. 1996;184:923–30. doi: 10.1084/jem.184.3.923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Carbone FR, Kurts C, Bennet SRM, Miller JFAP, Heath WR. Cross-presentation: a general mechanism for CTL immunity and tolerance. Immunol Today. 1998;19:368–75. doi: 10.1016/s0167-5699(98)01301-2. 10.1016/s0167-5699(98)01301-2. [DOI] [PubMed] [Google Scholar]
- 38.Platt N, Da Silva RP, Gordon S. Recognizing death: the phagocytosis of apoptotic cells. Trends Cell Biol. 1998;8:365–16. doi: 10.1016/s0962-8924(98)01329-4. 10.1016/s0962-8924(98)01329-4. [DOI] [PubMed] [Google Scholar]
- 39.Steinman RM, Turley S, Mellman I, Inaba K. The induction of tolerance by dendritic cells that have captured apoptotic bodies. J Exp Med. 2000;191:411–16. doi: 10.1084/jem.191.3.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Heath WR, Kurts C, Caminschi I, Carbone FR, Miller JF. CD30 prevents T-cell responses to non lymphoid tissues. Immunol Rev. 1999;169:23–9. doi: 10.1111/j.1600-065x.1999.tb01303.x. [DOI] [PubMed] [Google Scholar]