Abstract
ZAP-70 deficiency is a rare primary immunodeficiency characterized by the absence of peripheral CD8+ T cells and defects in T-cell receptor (TCR) signalling. T cells in ZAP-70-deficient patients are assumed to have no helper functions for B-cell immunoglobulin synthesis, whereas the patients rarely have antigen-specific antibodies. We experienced a ZAP-70-deficient patient, who had immunoglobulin E (IgE) antibodies specific to food allergens, and we investigated the mechanisms of switching to IgE in the patient. Peripheral blood mononuclear cells from the patient did not proliferate upon stimulation with the antigens but produced distinct levels of interleukin-4 (IL-4). Cell sorting analysis indicated that the cells that produced IL-4 in response to the antigens were enriched in CD4+ T cells. Purified CD4+ T cells from the patient produced IL-4 and expressed CD40L upon stimulation with anti-CD3. Moreover, CD4+ T cells pretreated with anti-CD3 induced mature ε transcript on naive B cells. Since the results indicated that there remained sufficient T-cell receptor (TCR)-signalling in the patient's T cells to exert antigen-specific IgE switching on B cells, we next investigated the expression of the ZAP-70-homologous kinase Syk. Syk was present in high levels in patient's CD4+ T cells and was tyrosine-phosphorylated after TCR stimulation. Inhibition of Syk by piceatannol resulted in decreased production of IL-4 and expression of CD40L on patient's CD4+ T cells. Moreover, Syk was expressed on all human T-cell leukaemia virus (HTLV-1)-transformed T-cell lines derived from peripheral blood of the patient, whereas it was low or undetectable in control lines. It was therefore concluded that specific IgE responses in the patient were most likely to be mediated by Syk-dependent TCR-signalling.
Introduction
ZAP-70 deficiency is a rare type of severe combined immunodeficiency (SCID) characterized by the absence of CD8+ T cells and by the presence of non-functional CD4+ T cells in peripheral blood.1,2 In patients with ZAP-70 deficiency, T cells cannot respond to T-cell receptor (TCR)-mediated stimuli because of mutations in a TCR-associated protein tyrosine kinase, ZAP-70.1–3 Absent proliferation responses in vitro to allogeneic antigens and to mitogenic stimuli mediated through surface TCRs, such as anti-CD3, have been reported in patients with ZAP-70 deficiency. Because T-cell help for immunoglobulin synthesis in B cells is impaired, patients with ZAP-70 deficiency often show hypogammablobulinaemia and usually lack specific antibody production.4,5 However, some patients have normal or increased levels of serum immunoglobulins and rarely have the ability to produce antigen-specific antibodies.6,7
Differentiation of naive B cells into antibody-secreting cells is largely controlled by the direct interaction of B cells with activated T cells expressing the CD40 ligand (CD40L)8–10 and by cytokines secreted from activated T cells. Switching to each isotype of immunoglobulin is strongly influenced by the cytokine milieu where B cells respond with T cells. For example, interleukin-4 (IL-4) is a major cytokine that induces immunoglobulin E (IgE) switching on B cells.11–13
We report here a case of a ZAP-70-deficient patient who had IgE antibodies specific to food allergens. Although peripheral T cells in the patient did not proliferate upon TCR-mediated stimuli in vitro, the cells retained the capacity to produce IL-4 and to express CD40L in response to the stimuli. These two signals were sufficient to induce IgE switching on naive B cells. These findings suggested that there are alternative TCR-signalling pathways independent of ZAP-70. Our speculation is that ZAP-70-homologous kinase Syk is most likely to participate in the alternative TCR-signalling pathway.
Materials and methods
Patient
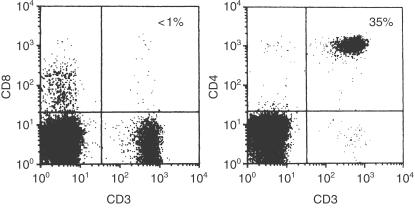
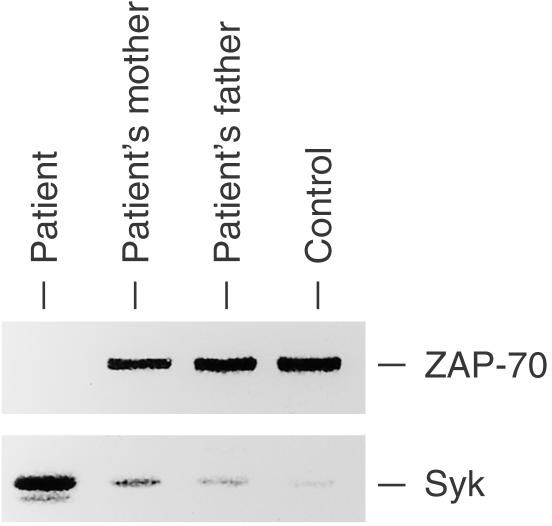
An 8-month-old girl with a history of recurrent respiratory infection was admitted to our hospital. Flow cytometric analysis of her peripheral blood mononuclear cells (PBMNC) revealed a lack of CD8+ T cells (< 1%) but a sufficient number of CD4+ T cells (Fig. 1). The patient's PBMNC did not proliferate upon stimulation with phytohaemagglutinin (PHA), concanavalin A (con A), or anti-CD3 monoclonal antibody (mAb; anti-CD3) but responded to stimulation with phorbol myristate acetate (PMA) plus ionomycin (Table 1). These results suggested that the patient had ZAP-70-deficient SCID, and this was confirmed by the results of Western blots, which showed an absence of ZAP-70 protein in CD4+ T cells (Fig. 2). DNA sequencing revealed that the genomic DNA of the patient contained a G to A homozygous transition at nucleotide position 1603, resulting in an arginine to histidine transition at position 465 of the kinase domain. Serum immunoglobulin levels were normal: 788 mg/dl IgG, 96 mg/dl IgA, 154 mg/dl IgM, and 183 IU/l IgE. At 6 months of age, no IgE antibodies specific to food allergens such as milk, soybean, and egg white were detected (< 0·35 IU/ml); however, IgE responses to egg white (2·46 IU/ml), milk (4·21 IU/ml), soybean (15·0 IU/ml), and wheat (2·43 IU/ml) were detected at 8 months of age. In spite of the presence of IgE antibodies specific to food allergens, she showed no obvious allergic reactions to the foods. Peripheral blood stem cell transplantation from her haploidentical father was performed twice at 9 and 10 months of age but engraftment was not successful.
Figure 1.
Results of flow cytometry of the patient's PBMNC. PBMNC of the patient were stained with FITC-or PE-conjugated anti-CD3, anti-CD4, and anti-CD8 mAb and then analysed using FACScan.
Table 1.
Proliferation responses of the patient's PBMNC
| Stimulation | Normal control (c.p.m.) | Patient (c.p.m.) |
|---|---|---|
| (–) | 1080 ± 15 | 1250 ± 12 |
| PHA | 39780 ± 785 | 1578 ± 25 |
| PMA + ionomycin | 62570 ± 1428 | 47850 ± 875 |
| Anti-CD3 | 33150 ± 658 | 1625 ± 22 |
| Ovalbumin | NT | 1440 ± 87 |
| Ovomucoid | NT | 1329 ± 54 |
The patient's PBMNC were stimulated with PHA (5 µg/ml), PMA (10 ng/ml) and ionomycin (1 µg/ml), biotinylated anti-CD3 (100 ng/ml) plus streptavidin, ovalbumin (25 µg/ml), or ovomucoid (25 µg/ml) for 3 days. Cell proliferation was assessed by incorporation of 3H-thymidine. Each value represents the mean value for three experiments ±SEM. NT, not tested.
Figure 2.
ZAP-70 and Syk expressions on CD4+ T cells. CD4+ T cells from the patient, her parents, and a normal control were purified from PBMNC using MACS. Cell lysates of the CD4+ T cells (2 × 106 each) were analysed by SDS–PAGE and were subjected to anti-ZAP-70 and anti-Syk blots.
Cell preparation and phenotype analysis
PBMNC were isolated by Ficoll–Hypaque gradient centrifugation. Cells were stained with fluoroscein isothiocyanate (FITC)- or phycoerythrin (PE)-conjugated anti-CD3, anti-CD4, or anti-CD8 (Pharmingen, San Diego, CA) and analysed by two-colour flow cytometry using a FACScan (Becton Dickinson, San Jose, CA). The cells positive for each antibody were separated using a preparative magnetic cell sorter (MACS; Miltenyi Biotec GmbH, Bergisch Gladbach, Germany) according to the manufacturer's instructions. The purity of each cell population was > 95%.
Analysis of ZAP-70 and Syk expression
Cell lysates of purified 1 × 106 CD4+ T cells were analysed by 10% sodium dodecyl sulphate–polyacrylamide gel electrophoresis (SDS–PAGE) and the separated proteins were transferred to polyvinylidene difluoride (PVDF) membranes (Amersham-Pharmacia, Uppsala, Sweden). The blots were incubated with anti-ZAP-70 antibody (Santa-Cruz Biotechnology, Santa-Cruz, CA) and then visualized using ECL (Amersham-Pharmacia) according to the manufacturer's instructions.
Cell proliferation assay
PBMNC (2 × 105/well) were incubated at 37° in 5% CO2 with RPMI-1640 in 96-well round-bottomed tissue culture plates with or without 5 µg/ml PHA, 5 µg/ml con A, 10 ng/ml phorbol 12-myristate 13-acetate (PMA) plus 1 µg/ml ionomycin, 100 ng/ml biotinylated anti-CD3 (Pharmingen) plus streptavidin, 25 µg/ml ovalbumin, or 25 µg/ml ovomucoid. Cell proliferation was assessed by the incorporation of 3H-thymidine after 3 days.
IL-4 production
PBMNC or purified CD4+ T cells (2 × 105/well) were stimulated with various mitogens and antigens as indicated in the cell proliferation assay for 2 days. In some cases, cells were cultured with 10 µm piceatannol14 (Sigma, St. Louis, MO). The levels of IL-4 in culture supernatants were measured by enzyme-linked immunosorbent assay (ELISA; MBL, Nagoya, Japan).
IL-4 mRNA expression
Cells positive for CD3, CD4, CD20, CD56, and FcεRI+ (CRA1; Kyokuto Pharmaceutical, Ibaraki, Japan) were purified from patient's PBMNC using MACS. Cells (106) were cultured with or without 25 µg/ml ovalbumin for 48 hr. After cultivation, total RNA was extracted from sorted cells using ISOGEN (Nippon gene, Tokyo, Japan) and reverse-transcribed with Moloney murine leukaemia virus reverse transcriptase (BRL Live Technologies, Gaithersburg, MD) using oligo (dT) primers (BRL). IL-4 and β-actin cDNA were amplified by polymerase chain reaction (PCR) with specific primers (IL-4 sense primer, 5′-GATCGTTAGCTTCTCCTGATAAACT-3′; IL-4 antisense primer, 5′-AGATTCTATATATACTTTATTTTAT-3′;15 β-actin sense primer, 5′-CGTGACATCAAAGAGAAGCTGTG-3′;16 and β-actin antisense primer, GCTCAGGAGGAGCAATGATCTTGA-3′). Thirty-five cycles of denaturation (94° for 45 s), annealing (60° for 45 s), and elongation (75° for 1·5 min) were performed in a thermocycler (Perkin-Elmer, Norwalk, CT). PCR products were separated by electrophoresis on 2% agarose gels and visualized by ethidium bromide staining.
CD40 ligand expression
Purified CD4+ T cells (2 × 105) were stimulated with biotinylated anti-CD3 plus streptavidin in the presence or absence of 10 µm piceatannol for 3 hr. The cells were then stained with PE-conjugated anti-CD40L mAb (Pharmingen) and analysed using FACScan. The stained cells were sorted into CD40L– and CD40L+ populations using FACS Vantage (Becton Dickinson). Cell lysates of the sorted cells (2 × 106 each) were analysed by SDS–PAGE and were subjected to anti-Syk blots as indicated above.
Induction and detection of germline and mature ε transcript on B cells
Surface IgD+ B cells were purified using MACS as described above. One 106/ml sIgD+ naive B cells from the patient were incubated with or without 15 ng/ml IL-4 (Genzyme, Cambridge, MA), 100 ng/ml anti-CD40 antibody (Serotec, Oxford, UK), or 1 × 106/ml CD4+ T cells pretreated with biotinylated anti-CD3 plus streptavidin for 24 hr. After 10 days of cultivation, total RNA was extracted from the cells and subjected to reverse transcription (RT)–PCR analysis using specific primers for germline ε transcripts (sense primer, 5′-AGGCTCCACTGCCCGGCACAGAAAT-3′; antisense primer, 5′-ACGGAGGTGGCATTGGAGGGAATGT-3′), mature ε transcripts (sense primer, 5′-GACACGGCTGTGTATTACTG-3′; antisense primer, 5′-ACGGAGGTGGCATTGGAGGGAATGT-3′)17 and β-actin. PCR products were analysed by electrophoresis on 2% agarose gels followed by ethidium bromide staining.
Expression and tyrosine-phosphorylation of Syk and ZAP-70
Purified CD4+ T cells were stimulated with biotinylated anti-CD3 and streptavidin for 2 min. Cells (106) were lysed in NP-40 lysis buffer (10 mm Tris–HCl, ph 7·4, 5 mm ethylenediamine tetra-acetic acid (EDTA), 150 mm NaCl, 0·1% SDS, 0·1% sodium deoxycholate, 1 mm phenylmethylsulphonyl fluoride (PMSF), 5 µg/ml aprotinin, and 30 mm Na4P2O7). Cell lysates were incubated with anti-ZAP-70, anti-Syk, or anti-TCR ζ antibodies (Santa Cruz Biotechnology Inc., Santa Cruz, CA) and then with protein A-Sepharose (Amersham-Pharmacia). The precipitates were subjected to 10% SDS–PAGE and separated proteins were transferred to PVDF membranes (Amersham-Pharmacia). The blots were incubated with anti-ZAP-70, anti-Syk, anti-TCR ζ, or anti-phosphotyrosine antibodies (Santa-Cruz Biotechnology Inc.) and then visualized using ECL (Amersham-Pharmacia) according to the manufacturer's instructions.
Syk expression on HTLV-1-transformed T-cell lines
HTLV-1 transformation of PBMNC from the patient and normal individuals was performed as described elsewhere.18 Cell lines were maintained in RPMI-1640 medium supplemented with 10% fetal calf serum and IL-2 (20 U/ml). Each HTLV-1 line expressed αβ-TCR and CD4 similarly. Cells (106) were lysed in NP-40 lysis buffer as described above. Cell lysates were analysed by 10% SDS–PAGE and Western blots using anti-Syk antibody.
Results
IL-4 production by PBMNC in response to food allergens
Because IL-4 is one of the main cytokines that induces IgE switching on B cells,11–13 we investigated the levels of IL-4 production by PBMNC taken from the patient. When the PBMNC were stimulated with PMA plus ionomycin over a period of 2 days, high levels of IL-4, comparable to those of controls, were detected in culture supernatants (Table 2). Stimulation of the PBMNC with ovalbumin and ovomucoid, which are major allergens of egg white,19,20 resulted in the production of small but distinct amounts of IL-4, although no proliferation responses of PBMNC were observed by stimulation with these allergens (Table 1).
Table 2.
IL-4 production by PBMNC and CD4+ T cells
| PBMNC (pg/ml) | CD4+ T cells (pg/ml) | |||
|---|---|---|---|---|
| Stimulation | Control | Patient | Control | Patient |
| (–) | < 2·0 | < 2·0 | < 2·0 | < 2·0 |
| PMA + ionomycin | 2542 ± 123 | 2854 ± 151 | 3745 ± 873 | 2538 ± 1025 |
| Anti-CD3 | 1423 ± 151 | 583 ± 54 | 2523 ± 622 | 1785 ± 211 |
| Ovalbumin | NT | 85 ± 12 | NT | NT |
| Ovomucoid | NT | 73 ± 18 | NT | NT |
| Anti-CD3 + | ||||
| Piceatannol | NT | NT | 2055 ± 855 | 173 ± 98 |
The patient's PBMNC and purified CD4+ T cells were stimulated with PMA (10 ng/ml) and ionomycin (1 µg/ml), biotinylated anti-CD3 (100 ng/ml) plus streptavidin, ovalbumin (25 µg/ml), or ovomucoid (25 µg/ml) in the presence or absence of 10 µm piceatannol for 2 days. The amount of IL-4 in culture supernatants was measured by ELISA. Each value represents the mean value for three experiments ±SEM. NT, Not tested.
IL-4 production by CD4+ T cells in response to food allergens
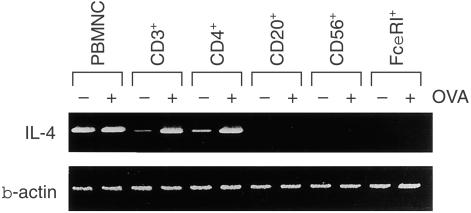
To clarify the lineage of the cells producing IL-4 in response to food allergens in the patient's PBMNC, cell sorting followed by RT–PCR analysis of IL-4 mRNA were performed. When PBMNC were fractionated by their surface marker, transcripts for IL-4 were detected in CD3+ and CD4+ T cells but not in FcεRI+ cells after stimulation with OVA (Fig. 3). Moreover, purified T cells, especially CD4+ T cells, produced IL-4 in response to anti-CD3 (Table 2). These results indicated that CD4+ T cells, but not FcεRI+ cells, produced IL-4 in response to the allergens.
Figure 3.
IL-4 mRNA expressions on various cell populations of the patient's PBMNC. Cells positive for CD3, CD4, CD20, CD56, or FcεRI were purified using MACS. Each cell population was stimulated with (+) or without (–) OVA (25 µg/ml) for 48 hr. Total RNA was extracted from 1 × 106 cells and subjected to RT–PCR analysis for IL-4 and β-actin mRNA expression.
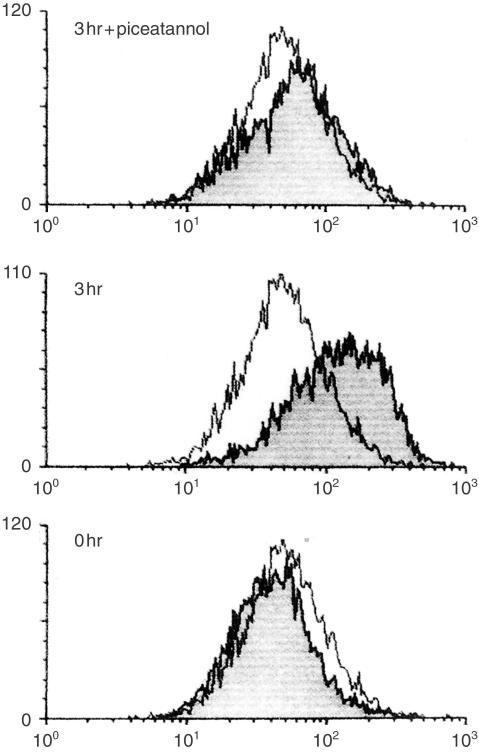
CD40L expression on TCR-stimulated CD4+ T cells
Besides the action of cytokines, switching to IgE requires direct contact between T cells and B cells through interaction with CD40 and CD40L.8–10 We therefore investigated the expression of CD40L on the patient's CD4+ T cells, which were stimulated with anti-CD3 for 3 hr. CD40L expression was recognized after 1 hr of TCR stimulation and continued to increase up to 6 hr. Figure 4 shows the results at 3 hr of cultivation. On the other hand, there were no proliferation responses of T cells as well as CD4+ T cells to TCR stimulation (Table 1). These results indicated that the patient's CD4+ T cells had the capacity to express CD40L and produce IL-4 in response to TCR-mediated stimuli without cell proliferation.
Figure 4.
CD40L expression on TCR-stimulated CD4+ T cells of the patient. Purified CD4+ T cells were stimulated with biotinylated anti-CD3 plus streptavidin for 3 hr with or without 10 µm piceatannol. Cells were stained with PE-conjugated anti-CD40L antibody and were analysed using FACScan. The clear histogram indicated CD40L expression before stimulation, whereas the shaded histogram corresponded to that after stimulation.
Induction of germline and mature ε transcripts on naive B cells by CD4+ T cells
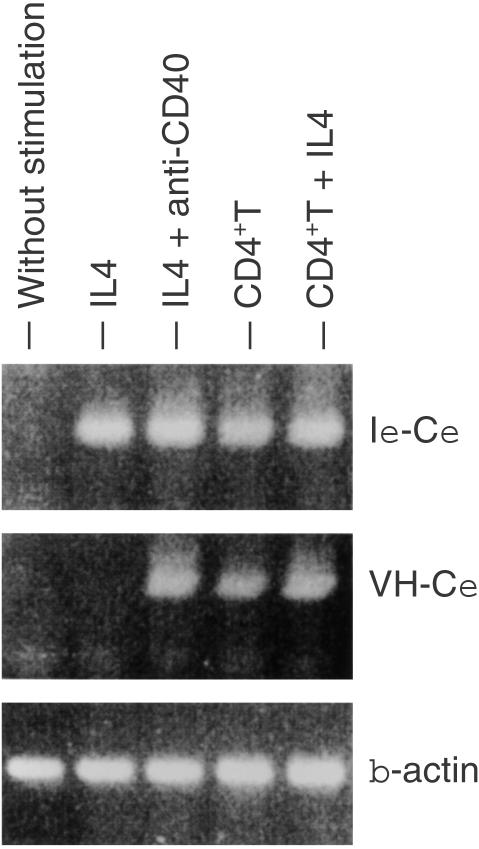
Because these two signals, namely IL-4 production and CD40L expression, are thought to be sufficient to evoke IgE class switching on B cells, we investigated whether TCR-stimulated T cells really induce IgE transcripts in naive B cells. When purified naive sIgD+ B cells were cultured in the presence of IL-4, germline ε transcripts were induced (Fig. 5). A combination of IL-4 and cross-linking of CD40 on B cells by anti-CD40 mAb induced the expression of mature ε transcripts as well as germline ε transcripts. On the other hand, TCR-stimulated CD4+ T cells from the patient could substitute for these two stimuli and could induce germline and mature ε transcripts on naive B cells.
Figure 5.
Induction of germline and mature ε transcripts on naive B cells by the patient's T cells. CD4+ T cells were prestimulated with biotinylated anti-CD3 plus streptavidin for 24 hr. Purified IgD+ naive B cells were cultured with IL-4, anti-CD40, or the preactivated CD4+ T cells for 10 days. Total RNA was extracted from the cells and subjected to RT–PCR for the expression of germline (Iε-Cε) and mature (VH-Cε) ε transcripts.
Expression of Syk in CD4+ T cells and its tyrosine-phosphorylation after TCR stimulation
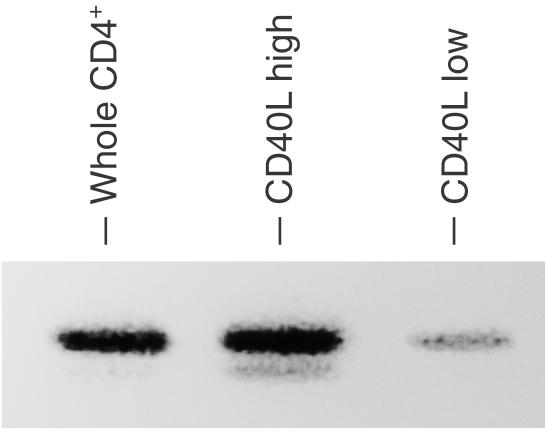
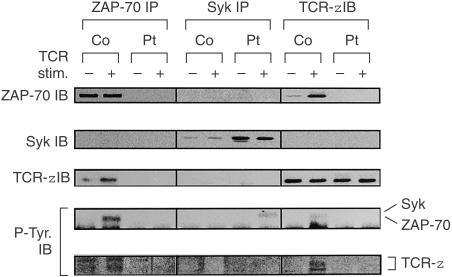
Since it was thought that there remained signalling pathways via TCR in the patient's T cells that bypassed ZAP-70, we investigated the expression of the ZAP-70-homologous kinase Syk. High levels of Syk were detected in the patient's CD4+ T cells by Western blot analysis, whereas faint or undetectable in normal controls (Fig. 2). In CD4+ T cells of the patient, Syk was expressed predominantly in the cells that expressed high levels of CD40L in response to TCR stimulation (Fig. 6). We next investigated the tyrosine-phosphorylation and association with TCR ζ chain of Syk in CD4+ T cells of the patient after TCR stimulation by immunoprecipitation followed by immunoblotting. Syk immunoprecipitates were tyrosine-phosphorylated after TCR stimulation of the patient's CD4+ T cells (Fig. 7). The ζ chain was not tyrosine-phosphorylated after TCR stimulation and there was no association between Syk and the ζ chain.
Figure 6.
Syk expression on patient's CD4+ T cells that express high levels of CD40L. Purified CD4+ T cells of the patient were stimulated with biotinylated anti-CD3 plus streptavidin for 3 hr. The cells were stained with PE-conjugated anti-CD40L antibody and were sorted into CD40L+ (CD40L high) and CD40L– (CD40L low) populations using FACS Vantage. Cell lysates of the sorted cells (2 × 106 each) were analysed by SDS–PAGE and were subjected to anti-Syk blots.
Figure 7.
Expression and tyrosine phosphorylation of Syk, ZAP-70, and TCR ζ chain in the patient's CD4+ T cells. CD4+ T cells obtained from the patient and normal control were cultured with (+) or without (–) biotinylated anti-CD3 plus streptavidin for 2 min. The cells (2 × 106 each) were lysed in 1% NP-40 lysis buffer, and immunoprecipitated with anti-Syk, anti-ZAP-70, and anti-TCR ζ antibodies. Immunopreciptates were resolved on 10% SDS–PAGE and immunoblotted with anti-Syk, anti-ZAP-70, and anti-TCR ζ, and anti-phosphotyrosine (P-Tyr.) antibodies.
Syk expression in T-cell lines derived from the patient
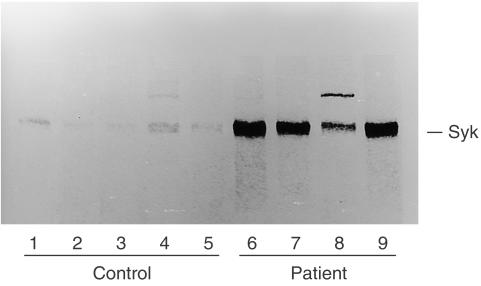
We hypothesized that high levels of Syk expression in the whole lysate of the patient's CD4+ T cells reflect increased numbers of T cells, which expressed high levels of Syk. To test this hypothesis, we established HTLV-1-transformed T-cell lines from the patient and controls, and we studied the Syk expression in each cell line. As shown in Fig. 8, Syk expression was low or undetectable in five control cell lines, whereas every cell line obtained from the patient expressed high levels of Syk. These results suggested that the number of CD4+ T cells that expressed high levels of Syk was increased in the peripheral blood of the patient.
Figure 8.
Syk expression on T-cell lines. HTLV-1-transformed T-cell lines were established from the patient and normal control. Cell lysates of T-cell lines (2 × 106 each) obtained from the patient (lanes 6–9) and control (lanes 1–5) were analysed by SDS–PAGE and were subjected to anti-Syk blot.
Discussion
In the present study, we found that T cells in a ZAP-70-deficient patient have the capacity to induce antigen-specific IgE production from B cells in response to TCR stimulation. CD4+ T cells from our patient produced IL-4 in culture supernatants and expressed CD40L on their surfaces in response to TCR stimulation. These two signals are necessary, and sufficient, for induction of IgE class switching on naive B cells. In fact, co-cultivation of prestimulated CD4+ T cells with naive B cells resulted in the induction of germline and mature ε transcripts. These results suggested that CD4+ T cells in our patient were able to induce switching to IgE on naïve B cells and subsequent IgE production from B cells in vitro. It is unlikely that cells other than CD4+ T cells were engaged in IgE switching, because only CD4+ T cells, among various cells in peripheral blood, produced IL-4 in response to food antigens. As well as T cells, it is known that FcεRI+ cells, namely, basophils, eosinophils and monocytes, have the capacity to induce IgE switching on naïve B cells. These cells can produce IL-421–23 and express CD40L24,25 after cross-linking of FcεRI. Therefore, antigen-specific activation of FcεRI+ cells requires antigen-specific IgE antibodies that are bound to multivalent antigens. In the absence of antigen-specific IgE antibodies, FcεRI+ cells cannot produce IL-4 in response to antigens. The fact that food-specific IgE antibodies in the serum of the patient were not detected at 6 months of age but were detected at 8 months of age suggests that de novo synthesis of antigen-specific IgE occurred after exposure to food allergens. Moreover, IL-4 production by peripheral blood FcεRI+ cells of the patient was not observed by cell sorting followed by RT–PCR. Thus, FcεRI+ cells were not to be candidate helper cells for IgE production in our patient.
Previous studies have demonstrated that peripheral blood CD4+ T cells of ZAP-70-deficient patients are not functional cells.2,4 However, we found that the peripheral T cells in our patient were partially functional, although there were no proliferation responses of these cells to TCR-mediated stimuli. The existence of the functional T cells cannot be attributed to engraftment of maternal lymphocytes,26–28 because maternal cells were not detected in the patient's PBMNC by sensitive PCR analysis of polymorphic short tandem repeat markers29 (data not shown). This kind of T-cell activation, namely partial activation without cell proliferation, is observed when T cells respond to ligands as partial agonists.30 Partial agonists have subtle alterations in their peptide sequences and evoke incomplete activation of T cells in the absence of cell proliferation, for example, cytokine production,31,32 expression of surface molecules,33 or induction of anergy.34 Interestingly, tyrosine-phosphorylation of ZAP-70 and the ζ chain are impaired in partial agonism in spite of induction of the mobilization of a small amount of intracellular Ca2+.35 Thus, it is thought that there are other signalling pathways that bypass the ζ chain and ZAP-70 also in normal T cells.
Our results suggest that Syk can compensate for the loss of ZAP-70 not only in thymocytes5 but also in peripheral blood T cells. Syk was present in high levels in peripheral blood T cells as well as T-cell lines that were obtained from the patient. After TCR stimulation, the extent of CD40L induction in patient's T cells was correlated with the levels of Syk expression. TCR stimulation of the patient's T cells resulted in tyrosine phosphorylation of Syk. Moreover, inhibition of Syk by piceatannol14 aborted IL-4 production and CD40L induction on stimulated T cells of the patient. Previous studies have shown that Syk is predominantly expressed on B cells, myeloid cells, and thymocytes but is expressed at only very low levels on peripheral T cells.36 However, the overall low expression of Syk in peripheral blood T cells does not imply the absence of T cells that express high levels of Syk. Actually, it represents the average of the majority of T cells that express low or undetectable levels and a small subpopulation of T cells that express high levels of Syk.37 It is therefore thought that the high levels of Syk expression in the patient's T-cell population reflect an increased number of T cells in the peripheral blood that expressed high levels of Syk. This speculation was supported by the fact that every T-cell line from the patient expressed high levels of Syk, whereas control lines expressed low or undetectable levels of Syk. The roles of Syk in normal T cells are less clear, because the levels of ZAP-70 in the T cells with high Syk expression are comparable to those in T cells with low Syk expression, and therefore the roles of Syk are masked by ZAP-70.37 However, functional similarities between Syk and ZAP-70 are obvious in experiments using mice deficient in Syk and ZAP-70. Syk can restore the development of thymocytes in ZAP-70-deficient mice,38 and ZAP-70 can reconstitute the BCR function in Syk-deficient B cells.39 Accordingly, it was likely that Syk compensated for the loss of ZAP-70 not only in thymocytes but also in peripheral blood T cells in our patient.40
There was no association between Syk and the ζ chain in activated T cells in our patient. Because no association has been found between Syk and the ζ chain in thymocyte lines of ZAP-70-deficient patients,5 this phenomenon does not seem to be unique to peripheral blood T cells in our patient. It is not known how Syk was tyrosine-phosphorylated after TCR stimulation in the absence of phosphorylation of the ζ chain in our patient. One possibility for this phenomenon is that interaction between Syk and the ζ chain is so transient and weak that it cannot be detected by the method we used.5 Another explanation is that there are other TCR-signalling pathways that bypass the ζ chain, such as is observed in partial agonism as described above.40
It is not clear whether the compensation of T-cell functions by Syk is a common phenomenon in ZAP-70-deficient patients. Most ZAP-70-deficient patients lack specific antibody production and some patients show severe hypogammaglobulinaemia, which requires γ-globulin replacement therapy.1,2 Occasionally, ZAP-70-deficient patients show normal or increased levels of serum immunoglobulin and rarely have the ability to produce antigen-specific antibodies.6,7 These studies suggested that the contribution by T cells to antibody production is not a common phenomenon in ZAP-70-deficient patients. On the other hand, Katamura et al. reported a patient with ZAP-70 deficiency who had CD4+ T cells with memory phenotypes in the peripheral blood and in the perivascular area of erythematous skin lesions.41 The T cells in this patient could proliferate in response to a high concentration of PHA and could produce IL-4 and interferon-γ (IFN-γ) in response to TCR stimulation. These data indicate that an alternative signalling pathway, which is independent of ZAP-70, also exists in this patient. Moreover, Noraz et al. recently reported two ZAP-70-deficient patients who had a subset of peripheral T cells that expressed high levels of Syk and could proliferate in vitro.40 The Sykhigh/ZAP-70– T cells had a signalling pathway distinct from conventional T cells. A discrepancy between their data and ours is that Sykhigh/ZAP-70– T cells of our patient could not proliferate upon stimulation of TCR. Although the true reason for this discrepancy is unclear, there is a difference in the characteristics of employed T cells between theirs and ours. We used T cells that were freshly isolated from the patient, whereas they used the cells that were maintained by IL-2 and feeder cells for a long time. Moreover, proliferation responses of Sykhigh/ZAP-70– T cells were markedly decreased compared with control T cells in their report, which was agreed with our observation.
Taken together, it is thought that at least some ZAP-70-deficient patients retain a TCR-signalling pathway via Syk that is insufficient to evoke complete activation of peripheral T cells but is sufficient to induce some T-cell functions such as assistance in specific IgE production.
References
- 1.Elder ME, Lin D, Clever J, Chan AC, Hope TJ, Weiss A, Parslow TG. Human severe combined immunodeficiency due to a defect in ZAP-70, a T cell tyrosine kinase. Science. 1994;264:1596–9. doi: 10.1126/science.8202712. [DOI] [PubMed] [Google Scholar]
- 2.Arpaia E, Shahar M, Dadi H, Cohen A, Roifman CM. Defective T cell receptor signaling and CD8+ thymic selection in humans lacking zap-70 kinase. Cell. 1994;76:947–58. doi: 10.1016/0092-8674(94)90368-9. [DOI] [PubMed] [Google Scholar]
- 3.Chan AC, Kadlecek TA, Elder ME, Filipovich AH, Kuo WL, Iwashima M, Parslow TG, Weiss A. ZAP-70 deficiency in an autosomal recessive form of severe combined immunodeficiency. Science. 1994;264:1599–601. doi: 10.1126/science.8202713. [DOI] [PubMed] [Google Scholar]
- 4.Elder ME, Hope TJ, Parslow TG, Umetsu DT, Wara DW, Cowan MJ. Severe combined immunodeficiency with absence of peripheral blood CD8+ T cells due to ZAP-70 deficiency. Cell Immunol. 1995;165:110–7. doi: 10.1006/cimm.1995.1193. 10.1006/cimm.1995.1193. [DOI] [PubMed] [Google Scholar]
- 5.Gelfand EW, Weinberg K, Mazer BD, Kadlecek TA, Weiss A. Absence of ZAP-70 prevents signaling through the antigen receptor on peripheral blood T cells but not on thymocytes. J Exp Med. 1995;182:1057–65. doi: 10.1084/jem.182.4.1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Monafo WJ, Polmar SH, Neudorf S, Mather A, Filipovich AH. A hereditary immunodeficiency characterized by CD8+ T lymphocyte deficiency and impaired lymphocyte activation. Clin Exp Immunol. 1992;90:390–3. doi: 10.1111/j.1365-2249.1992.tb05856.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Roifman CM, Hummel D, Martinez-Valdez H, Thorner P, Doherty PJ, Pan S, Cohen F, Cohen A. Depletion of CD8+ cells in human thymic medulla results in selective immune deficiency. J Exp Med. 1989;170:2177–82. doi: 10.1084/jem.170.6.2177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jabara HH, Fu SM, Geha RS, Vercelli D. CD40 and IgE: synergism between anti-CD40 monoclonal antibody and interleukin 4 in the induction of IgE synthesis by highly purified human B cells. J Exp Med. 1990;172:1861–4. doi: 10.1084/jem.172.6.1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gascan H, Gauchat JF, Aversa G, Van Vlasselaer P, de Vries JE. Anti-CD40 monoclonal antibodies or CD4+ T cell clones and IL-4 induce IgG4 and IgE switching in purified human B cells via different signaling pathways. J Immunol. 1991;147:8–13. [PubMed] [Google Scholar]
- 10.Spriggs MK, Armitage RJ, Strockbine L, Clifford KN, Macduff BM, Sato TA, Maliszewski CR, Fanslow WC. Recombinant human CD40 ligand stimulates B cell proliferation and immunoglobulin E secretion. J Exp Med. 1992;176:1543–50. doi: 10.1084/jem.176.6.1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lebman DA, Coffman RL. Interleukin 4 causes isotype switching to IgE in T cell-stimulated clonal B cell cultures. J Exp Med. 1988;168:853–62. doi: 10.1084/jem.168.3.853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gauchat JF, Lebman DA, Coffman RL, Gascan H, de Vries JE. Structure and expression of germline epsilon transcripts in human B cells induced by interleukin 4 to switch to IgE production. J Exp Med. 1990;172:463–73. doi: 10.1084/jem.172.2.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Punnonen J, Aversa G, Cocks BG, de Vries JE. Role of interleukin-4 and interleukin-13 in synthesis of IgE and expression of CD23 by human B cells. Allergy. 1994;49:576–86. doi: 10.1111/j.1398-9995.1994.tb00122.x. [DOI] [PubMed] [Google Scholar]
- 14.Robert L, Geahle L, Jerry L, et al. Piceatannol (3,4,3′,5′-tetrahydroxy-trans-stilbene) is a naturally occurring protein–tyrosine kinase inhibitor. Biochem Biophys Res Commun. 1989;165::241–245. doi: 10.1016/0006-291x(89)91060-7. [DOI] [PubMed] [Google Scholar]
- 15.Yokota T, Otsuka T, Mosmann T, et al. Isolation and characterization of a human interleukin cDNA clone, homologous to mouse B-cell stimulatory factor 1, that expresses B-cell- and T-cell-stimulating activities. Proc Natl Acad Sci USA. 1986;83:5894–8. doi: 10.1073/pnas.83.16.5894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ponte P, Ng SY, Engel J, Gunning P, Kedes L. Evolutionary conservation in the untranslated regions of actin mRNAs: DNA sequence of a human beta-actin cDNA. Nucl Acids Res. 1984;12:1687–96. doi: 10.1093/nar/12.3.1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Durandy A, Hivroz C, Mazerolles F, et al. Abnormal CD40-mediated activation pathway in B lymphocytes from patients with hyper-IgM syndrome and normal CD40 ligand expression. J Immunol. 1997;158:2576–84. [PubMed] [Google Scholar]
- 18.Sugamura K, Sakitani M, Hinuma Y. Microplate method for retrovirus-induced transformation of normal human T cells. J Immunol Methods. 1984;73:379–85. doi: 10.1016/0022-1759(84)90413-7. [DOI] [PubMed] [Google Scholar]
- 19.Cooke SK, Sampson HA. Allergenic properties of ovomucoid in man. J Immunol. 1997;159:2026–32. [PubMed] [Google Scholar]
- 20.Honma K, Kohno Y, Saito K, et al. Allergenic epitopes of ovalbumin (OVA) in patients with hen's egg allergy: inhibition of basophil histamine release by haptenic ovalbumin peptide. Clin Exp Immunol. 1996;103:446–53. doi: 10.1111/j.1365-2249.1996.tb08301.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ben-Sasson SZ, Le Gros G, Conrad DH, Finkelman FD, Paul WE. Cross-linking Fc receptors stimulate splenic non-B, non-T cells to secrete interleukin 4 and other lymphokines. Proc Natl Acad Sci USA. 1990;87:1421–5. doi: 10.1073/pnas.87.4.1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Seder RA, Plaut M, Barbieri S, Urban J, Jr, Finkelman FD, Paul WE. Purified Fc epsilon R+ bone marrow and splenic non-B, non-T cells are highly enriched in the capacity to produce IL-4 in response to immobilized IgE, IgG2a, or ionomycin. J Immunol. 1991;147:903–9. [PubMed] [Google Scholar]
- 23.Paul WE, Seder RA, Plaut M. Lymphokine and cytokine production by Fc epsilon RI+ cells. Adv Immunol. 1993;53:1–29. [PubMed] [Google Scholar]
- 24.Gauchat JF, Henchoz S, Mazzei G, et al. Induction of human IgE synthesis in B cells by mast cells and basophils. Nature. 1993;365:340–3. doi: 10.1038/365340a0. [DOI] [PubMed] [Google Scholar]
- 25.Gauchat JF, Henchoz S, Fattah D, et al. CD40 ligand is functionally expressed on human eosinophils. Eur J Immunol. 1995;25:863–5. doi: 10.1002/eji.1830250335. [DOI] [PubMed] [Google Scholar]
- 26.Thompson LF, O'Connor RD, Bastian JF. Phenotype and function of engrafted maternal T cells in patients with severe combined immunodeficiency. J Immunol. 1984;133:2513–7. [PubMed] [Google Scholar]
- 27.Conley ME, Nowell PC, Henle G, Douglas SD. XX T cells and XY B cells in two patients with severe combined immune deficiency. Clin Immunol Immunopathol. 1984;31:87–95. doi: 10.1016/0090-1229(84)90192-2. [DOI] [PubMed] [Google Scholar]
- 28.Pollack MS, Kirkpatrick D, Kapoor N, Dupont B, O'Reilly RJ. Identification by HLA typing of intrauterine-derived maternal T cells in four patients with severe combined immunodeficiency. New Engl J Med. 1982;307:662–6. doi: 10.1056/NEJM198209093071106. [DOI] [PubMed] [Google Scholar]
- 29.Hammond HA, Jin L, Zhong Y, Caskey CT, Chakraborty R. Evaluation of 13 short tandem repeat loci for use in personal identification applications. Am J Hum Genet. 1994;55:175–89. [PMC free article] [PubMed] [Google Scholar]
- 30.Sloan-Lancaster J, Allen PM. Altered peptide ligand-induced partial T cell activation: molecular mechanisms and role in T cell biology. Annu Rev Immunol. 1996;14:1–27. doi: 10.1146/annurev.immunol.14.1.1. [DOI] [PubMed] [Google Scholar]
- 31.Evavold BD, Allen PM. Separation of IL-4 production from Th cell proliferation by an altered T cell receptor ligand. Science. 1991;252:1308–10. doi: 10.1126/science.1833816. [DOI] [PubMed] [Google Scholar]
- 32.Racioppi L, Ronchese F, Matis LA, Germain RN. Peptide-major histocompatibility complex class II complexes with mixed agonist/antagonist properties provide evidence for ligand-related differences in T cell receptor-dependent intracellular signaling. J Exp Med. 1992;177:1047–60. doi: 10.1084/jem.177.4.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen YZ, Matsushita S, Nishimura Y. Response of a human T cell clone to a large panel of altered peptide ligands carrying single residue substitutions in an antigenic peptide: characterization and frequencies of TCR agonism and TCR antagonism with or without partial activation. J Immunol. 1996;157:3783–90. [PubMed] [Google Scholar]
- 34.Sloan-Lancaster J, Evavold BD, Allen PM. Induction of T-cell anergy by altered T-cell-receptor ligand on live antigen-presenting cells. Nature. 1993;363:156–9. doi: 10.1038/363156a0. [DOI] [PubMed] [Google Scholar]
- 35.Kersh EN, Shaw AS, Allen PM. Fidelity of T cell activation through multistep T cell receptor zeta phosphorylation. Science. 1998;281:572–5. doi: 10.1126/science.281.5376.572. [DOI] [PubMed] [Google Scholar]
- 36.Chan AC, van Oers NS, Tran A, Turka L, Law CL, Ryan JC, Clark EA, Weiss A. Differential expression of ZAP-70 and Syk protein tyrosine kinases, and the role of this family of protein tyrosine kinases in TCR signaling. J Immunol. 1994;152:4758–66. [PubMed] [Google Scholar]
- 37.Chu DH, Morita CT, Weiss A. The Syk family of protein tyrosine kinases in T-cell activation and development. Immunol Rev. 1998;165:167–80. doi: 10.1111/j.1600-065x.1998.tb01238.x. [DOI] [PubMed] [Google Scholar]
- 38.Gong Q, White L, Johnson R, White M, Negishi I, Thomas M, Chan AC. Restoration of thymocyte development and function in zap-70–/– mice by the Syk protein tyrosine kinase. Immunity. 1997;7:369–77. doi: 10.1016/s1074-7613(00)80358-1. [DOI] [PubMed] [Google Scholar]
- 39.Kong G, Dalton M, Wardenburg JB, Straus D, Kurosaki T, Chan AC. Distinct tyrosine phosphorylation sites in ZAP-70 mediate activation and negative regulation of antigen receptor function. Mol Cell Biol. 1996;16:5026–35. doi: 10.1128/mcb.16.9.5026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Noraz N, Schwarz K, Steinberg M, et al. Alternative TCR signaling in T cells derived from ZAP-70-deficient patients expressing high levels of Syk. J Biol Chem. 2000;275:15832–8. doi: 10.1074/jbc.M908568199. [DOI] [PubMed] [Google Scholar]
- 41.Katamura K, Tai G, Tachibana T, et al. Existence of activated and memory CD4+ T cells in peripheral blood and their skin infiltration in CD8 deficiency. Clin Exp Immunol. 1999;115:124–30. doi: 10.1046/j.1365-2249.1999.00759.x. 10.1046/j.1365-2249.1999.00759.x. [DOI] [PMC free article] [PubMed] [Google Scholar]