Abstract
Dendritic cells (DC) are recruited to sites of inflammation for the initiation of immune responses. As the anaphylatoxins C5a and C3a are important mediators of inflammation, we investigated the expression of their receptors (C3aR and C5aR) on human DC. DC were isolated from human skin or generated from purified blood monocytes and were identified by their expression of CD1a or CD83. Freshly isolated or cultured dermal CD1a+ and CD83+ DC bound anti-C5aR and anti-C3aR monoclonal antibodies (mAbs), as detected by flow cytometry. C5a induced calcium fluxes in dermal CD1a+ and CD83+ DC, which could be inhibited by C17/5, an anti-C5a mAb. C3a did not induce calcium fluxes in these cells. Anaphylatoxin receptor expression was down-regulated on dermal DC by adding tumour necrosis factor-α (TNF-α) to the culture medium. On CD1a+ CD83− cells generated from isolated blood monocytes by culture with 6·25 ng/ml of granulocyte–macrophage colony-stimulating factor (GM-CSF) and 125 U/ml of interleukin-4 (IL-4), expression of both C5aR and C3aR was observed. In these cells, both C5a and C3a induced calcium fluxes. After addition of TNF-α to the culture medium, the majority of the CD1a+ cells expressed CD83+. These cells – expressing a phenotype of ‘mature DC’ – down-regulated the expression of the anaphylatoxin receptors and lost their reactivity to the respective ligands. Our results demonstrate the expression of the anaphylatoxin receptors C5aR and C3aR on human skin-derived DC and blood-derived cells expressing the DC-associated membrane molecule, CD1a. Furthermore, the expression of anaphylatoxin receptors on CD83+ dermal DC is indicative of an intermediate stage of maturation of these cells, which was not observed on in vitro-differentiated CD83+ cells.
Introduction
Dendritic cells (DC) are specialized leucocytes capable of initiating and controlling immune reactions.1 After capturing and processing antigens in the periphery, DC migrate to lymphoid organs, where their maturation is completed by the up-regulation of critical cell-surface molecules (e.g. CD80, CD83 and CD86) and the secretion of cytokines (e.g. interleukin [IL]-12), which support stimulation of antigen-specific lymphocytes.2,3 DC can emigrate to the periphery to participate in the immune response reactions.4–7 The migration and activation of leucocytes are regulated by small secreted proteins called chemokines.8 Chemokines are secreted following inflammatory stimuli or are constitutive. Immature DC, characterized by CD1a+ expression, also express receptors for inflammatory chemokines, such as regulated upon activation, normal T expressed and presumably secreted (RANTES), macrophage inflammatory protein (MIP)-1α or monocyte chemotactic protein (MCP-1),9,10 which may guide them to inflammatory sites where antigen sampling can take place and maturation may be induced. The maturation process, which is triggered by inflammatory cytokines, or by bacterial or viral products, leads to the up-regulation of receptors for constitutive chemokines.11–13 These receptors may be involved in the migration of DC to lymphoid organs where secondary lymphoid tissue chemokine (SLC) and Ebl1-ligand chemokine (ELC; MIP-3β) are expressed and specific immune responses are initiated.14–16 Terminal maturation of DC is indicated by the expression of CD83.
C5a, a 74-amino acid peptide cleaved from the complement protein C5, might be one of the putative mediators capable of inducing DC migration. C5a induces a complex pathophysiological response, including cellular migration to inflammatory sites, changes in blood flow and impairment of vascular integrity associated with oedema.17–19 In addition, C5a possesses immunoregulatory activities through the induction of cytokines (tumour necrosis factor [TNF], IL-1, IL-6 and IL-8) in human monocytes.20–22 C5a mediates its effects by binding to a specific, high-affinity receptor (C5aR/CD88), which belongs to the family of seven transmembrane domain receptors that transduce signals via guanosine triphosphate (GTP)-binding regulatory proteins.23,24 C3a is another anaphylatoxin of the complement system. The high-affinity receptor for C3a has recently been cloned.25,26 The effects of C3a include the release of histamine from human mast cells and basophils and the mobilization of intracellular calcium ions, as well as the chemotactic attraction of basophils27–29 and cells of the human mast cell line, HMC-1.30–32
In human skin, C5aR and C3aR have been found on mast cells and dermal macrophages.33–35 Morelli et al. detected C5aR on cultured CD1a+ human Langerhans' cells that had migrated from skin explants into the culture medium.36 Immature human monocyte-derived DC have also been described to express the C5aR.9 To date there is no evidence for C3aR expression on skin-derived DC.
The aim of the present study was to evaluate the expression of anaphylatoxin receptors on human DC in relation to their maturation stage. The investigations were performed on DC isolated from human skin or isolated blood monocytes, which had been treated with low doses of granulocyte–macrophage colony-stimulating factor (GM-CSF) and IL-4.
Materials and methods
Preparation of single-cell suspensions from human skin
Clinical normal human skin was obtained from patients who were undergoing plastic surgery on their breasts. Epidermis and dermis were separated by overnight incubation in dispase (2·4 U/ml; Boehringer Mannheim GmbH, Mannheim, Germany) at 4°. The epidermis was detached from the dermis by using fine forceps and then incubated in Hank's solution containing 0·25% trypsin (Sigma Chemical Co., Munich, Germany) for 20 min at 37°. The digestion was stopped by addition of fetal calf serum (FCS) (Gibco-BRL).
The epidermal sheets were vigorously pipetted in and out of a pipette. An enrichment of Langerhans' cells was achieved by density-gradient centrifugation. Briefly, after filtration through sterile gauze, the epidermal cell suspension was layered onto Lymphoprep (density = 1·077; GIBCO, Life Technologies, Karlsruhe, Germany) at a ratio of 2:1 epidermal cell suspension to Lymphoprep, and then centrifuged at 400 g for 30 min. The cells from the interface were washed twice and cultured for 2 days in RPMI +10% FCS.
Dermal tissue was incubated in Hank's solution (4 hr at 37°) containing 0·1% collagenase, 20 µg/ml of DNAse, 1·2 U/ml of dispase, 0·1% hyaluronidase and 10% FCS. The cell suspension was filtered through nylon gauze. The cells were washed three times and cultured for 2 days in RPMI +10% FCS.
Preparation of monocyte-derived DC
Peripheral blood mononuclear cells (PBMC) were isolated by Lymphoprep density-gradient centrifugation of heparinized leucocyte-enriched buffy coats. Highly purified monocytes were obtained by elutriation, using a Beckmann E-6B centrifuge equipped with an elutriation rotor. The flow rate of the elution medium was adjusted to 18 ml/min. The centrifugation speed was reduced step-by-step from 975 g to 344 g and the cells were collected in 200-ml fractions. Cells collected as such were analysed by flow cytometry (Becton-Dickinson, Heidelberg, Germany). Most of the monocytes were enriched in the fraction obtained at 381 g Platelets were obtained at 694 g or slower, small lymphocytes at 548 g and a monocyte/lymphocyte mixture at 440 g. The elution medium was Ca2+- and Mg2+-free phosphate-buffered saline (PBS) containing 2% FCS. The purity of CD14+ monocytes obtained by this procedure was greater than 80%; granulocytes could not be detected (< 0·1%). Cells were cultured in six-well plates containing 5 ml of RPMI medium supplemented with 10% FCS, 125 U/ml of IL-4 (R & D Systems, Wiesbaden, Germany) and 6·25 ng/ml of GM-CSF (R & D Systems). Fresh medium and cytokines were added to the cultures every 2–3 days, and non-adherent cells (thereafter termed monocyte-derived DC) were harvested on day 6. In some experiments, cells were cultured for additional days with GM-CSF, IL-4 and 200 U of TNF-α/ml (R & D Systems).
Flow cytometric analyses
Cells were analysed by double-colour immunofluorescence staining using a FACScan flow cytometer. For indirect labelling, cells (2 × 105) were washed and resuspended in PBS containing 0·2% gelatine, 20 mm sodium azide and 10 µg/ml of heat-aggregated human immunoglobulin G (IgG) (Sigma-Aldrich, Munich, Germany). Subsequently, cells were incubated for 1 hr on ice with the anti-C3aR monoclonal antibody (mAb), HC3aRZ1-5, or with the anti-C5aR mAb, P12. In a second step, cells were incubated for a further 1 hr on ice with a fluorescein isothiocyanate (FITC)-conjugated goat anti-mouse IgG antibody (Dianova, Hamburg, Germany). Cells were further treated with 1 mg/ml of mouse IgG (30 min, 4°) (Sigma-Aldrich, Deisenhofen, Germany) to completely saturate all binding sites for the secondary antibody. To identify the anaphylatoxin receptors, the dendritic skin-derived and the blood-derived cells were incubated with phycoerythrin-labelled anti-CD1a mAb (Immunotech, Hamburg, Germany) or anti-CD83 mAb (45 min, 4°) (Immunotech). Stained cells were washed three times and fixed in PBS containing 1% paraformaldehyde. To analyse distinct cell populations, gates were set on CD1a+ and CD83+ cells. The expression of receptors for C3a and C5a was analysed after 3, 4 and 6 days of cell culture.
Measurement of [Ca2+]i by flow cytometry
For the analysis of distinct cell populations, the skin-derived DC, stained with phycoerythrin-labelled anti-CD1a or CD83 mAbs, were incubated at a density of 1 × 107 cells/ml for 25 min at 37° in PBS supplemented with 1 mm calcium, 1 mm magnesium (PBS++), 0·1% bovine serum albumin and 10 µm Fluo-3 AM (Molecular Probes, Eugene, OR). Fluo-3 AM was prediluted in 1% dimethylsulphoxide (DMSO) (v/v) containing 37·5 g/l of Pluronic F-127 (Sigma-Aldrich, Deisenhofen, Germany). Cells were washed twice to remove extracellular Fluo-3 AM. Finally, the cells were adjusted to a density of 2·5 × 106/ml in PBS++ and kept in the dark until used.
Assessment of [Ca2+]i was performed at room temperature using a FACScan flow cytometer (Becton-Dickinson), as described previously.33 The argon laser was set to 488 nm (excitation), and emission was measured at 530 nm using the logarithmic mode. After analysis of the basal fluorescence of the sample, the stimulus was added to the test tube through a 24-gauge needle during the aspiration of the cells into the flow cytometer. The increase in fluorescence, reflecting an increase in single-cell [Ca2+]I, was monitored continuously using the ‘chronys’ software (Becton-Dickinson).
Results
Expression of anaphylatoxin receptors on skin-derived DC
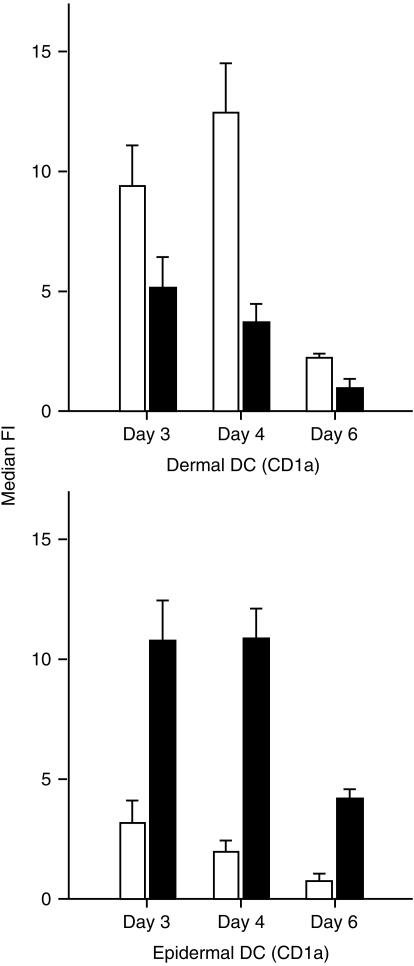
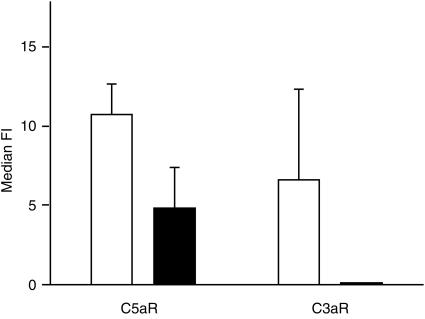
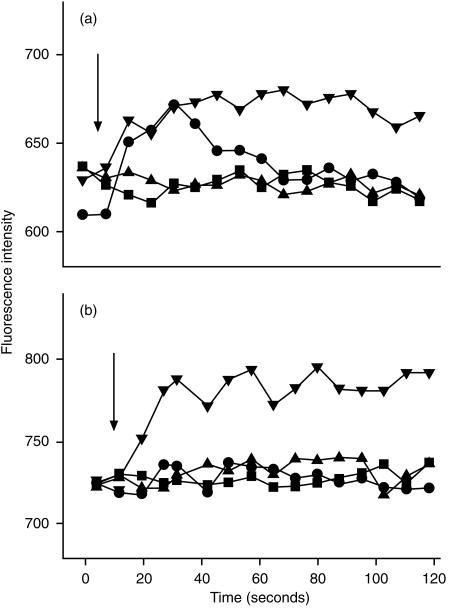
Cultured dermal and epidermal DC showed distinct patterns of receptor expression (Fig. 1). Dermal CD1a+ DC expressed predominately C5aR, whereas epidermal DC expressed predominately C3aR. Freshly isolated CD83+ dermal DC also expressed C5aR and C3aR (results not shown). Expression of the anaphylatoxin receptors on CD83+ skin-derived DC was significantly down-modulated by treatment with 200 U/ml of TNF-α (Fig. 2). C5a led to a transient calcium flux in C5aR-positive dermal CD1a DC, which could be inhibited by preincubation with a 20-fold molar excess of anti-C5a mAb (Fig. 3a). After stimulation with 200 U/ml of TNF-α, most of the CD1a+ cells became CD83+ (data not shown) and no longer showed a calcium flux after stimulation with C5a (Fig. 3b).
Figure 1.
Expression of receptors for C3a (▪) and C5a (□) on skin-derived dermal and epidermal dendritic cells (DC), which were cultured for up to 6 days. Dermal and epidermal cell suspensions were stained for simultaneous detection of anaphylatoxin receptors and CD1a, and analysed by flow cytometry. Results represent the average ±SD of four experiments. Only the median fluorescence intensity (median FI) of the CD1a+ cells is shown. Dermal DC express predominately C5aR, epidermal DC predominately C3aR.
Figure 2.
Expression of receptors for C3a and C5a on skin-derived dendritic cells (DC), with (▪) and without (□) treatment with 200 U/ml of tumour necrosis factor-α (TNF-α). Dermal cell suspensions were cultured for 5 days and then stained for simultaneous detection of anaphylatoxin receptors and CD83 and analysed by flow cytometry. Results represent the average ±SD of four experiments. TNF-α decreases the expression of C5aR as well as of C3aR. Median FI, median fluorescence intensity.
Figure 3.
Increase of cytosolic calcium in skin-derived dendritic cells (DC) upon stimulation with unlabelled C3a and C5a. Cells were cultured for 5 days (a). A proportion was treated with 200 U/ml of tumour necrosis factor-α (TNF-α) (b). After labelling with phycoerythrin-conjugated anti CD1a, DC were loaded with Flou-3 AM, as described in the Materials and methods. C3a (1 µg/ml) (▴) or C5a (1 µg/ml) (•) was then added to suspended cells and [Ca2+] was immediately assessed by flow cytometry. C5a was pretreated with a 20-fold molar excess of C17/5 (an anti-C5a monoclonal antibody [mAb]) in a control experiment (▪). Ca-ionophore was used as positive control (▾). C5a led to a transient calcium influx in C5aR+ dermal CD1a DC, which could be inhibited by preincubation with anti-C5a mAb (a). After stimulation with 200 U/ml of TNF-α, most of the cells became CD83+ and no longer showed calcium influx (b). Arrows refer to the time point when C3a resp. C5a were added to the cell suspension.
Expression of anaphylatoxin receptors on blood-derived DC
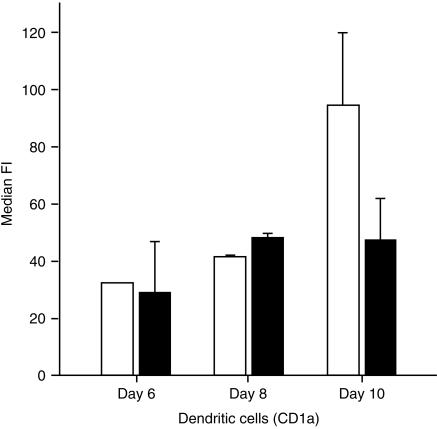
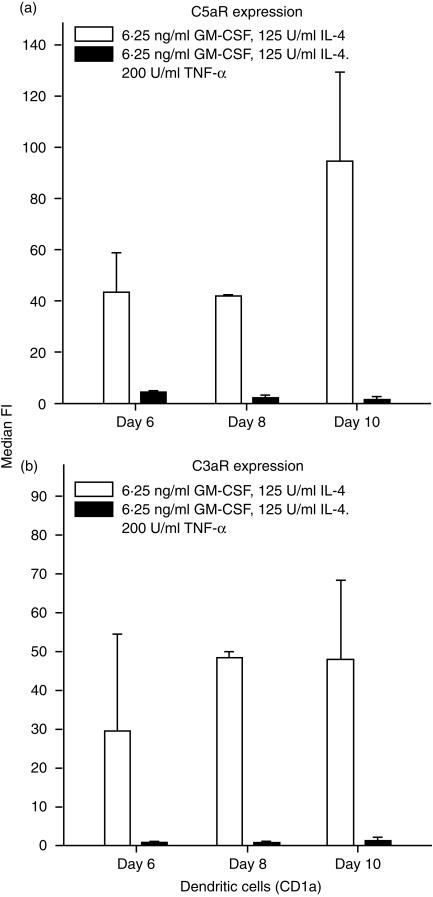
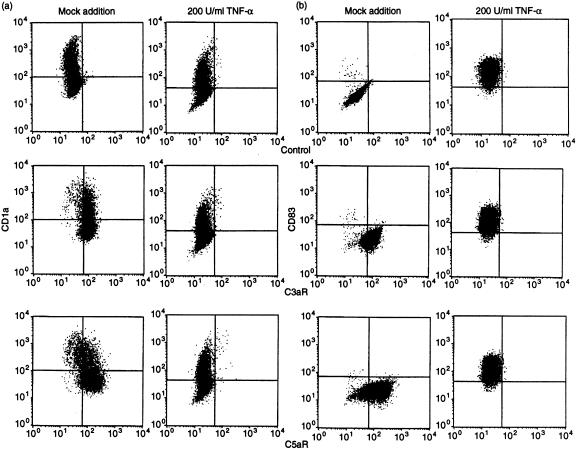
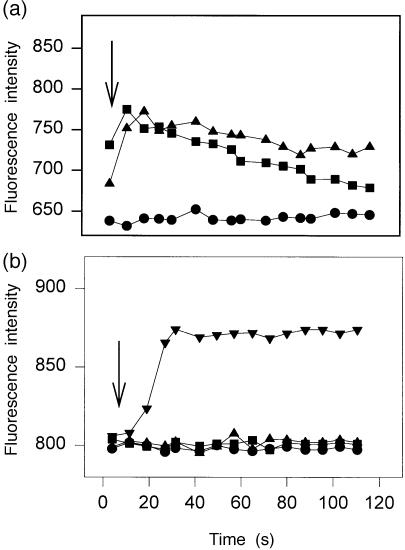
The expression of anaphylatoxin receptors on blood-derived cells was analysed after 6, 8 and 10 days of culture in the presence of IL-4 (125 U/ml) and GM-CSF (6·25 ng/ml). The median fluorescence intensity (median FI), which reflects the level of binding of the specific mAb, was, during days 6–10 of culture, relatively unchanged for C3aR but showed a slight increase for C5aR on day 10 (Fig. 4). Expression of the anaphylatoxin receptors on blood-derived cells was significantly down-modulated by treatment with 200 U/ml of TNF-α (Fig. 5). Figure 6 shows details of the anaphylatoxin receptor expression on CD1a+ cells (Fig. 6a) and CD83+ cells (Fig. 6b), as monitored by flow cytometry. Blood-derived CD1a+ cells, as well as the CD1a– cells, showed expression of both C3aR and C5aR, which was completely down-modulated by treatment with TNF-α (Fig. 6a). These cells, which had been generated in the presence of low levels of GM-CSF (6·25 ng/ml) and IL-4 (125 U/ml), did not express CD83, but did carry C3aR and C5aR (Fig. 6b). The treatment of these cells with TNF-α led to a general expression of CD83 and to the disappearance of the anaphylatoxin receptors. Functional investigations demonstrated an increase of cytosolic calcium in blood-derived cells upon stimulation with unlabelled C3a and C5a (Fig. 7a). After stimulation with 200 U/ml of TNF-α, all cells became CD83+ and a calcium flux could no longer be observed (Fig. 7b).
Figure 4.
Expression of receptors for C3a (▪) and C5a (□) on blood-derived dendritic cells, which were cultured in the presence of interleukin-4 (IL-4) (125 U/ml) and granulocyte–macrophage colony-stimulating factor (GM-CSF) (6·25 ng/ml) for up to 10 days. Cells were stained for simultaneous detection of anaphylatoxin receptors and CD1a, and analysed by flow cytometry. Results represent the average ±SD of three experiments. Only the median fluorescence intensity (median FI) of the CD1a+ cells is shown. Blood-derived dendritic cells express both C5aR and C3aR.
Figure 5.
Expression of receptors for C3a and C5a on blood-derived cells, with (▪) and without (□) treatment with 200 U/ml of tumour necrosis factor-α (TNF-α). Cell suspensions were cultured for 6, 8 or 10 days and then stained for the simultaneous expression of anaphylatoxin receptors and CD1a, and analysed by flow cytometry. Results represent the average ±SD of three experiments. TNF-α inhibits the expression of both C5aR and C3aR. GM-CSF, granulocyte–macrophage colony-stimulating factor.
Figure 6.
Changes in the expression of anaphylatoxin receptors on blood-derived cells following treatment with 200 U/ml of tumour necrosis factor-a (TNF-α). Cells were cultured for 5 days and then processed for double staining using monoclonal antibodies (mAbs) specific for C3aR and C5aR. The presence of C3aR and C5aR was revealed by incubation with fluorescein isothiocyanate (FITC)-conjugated anti-mouse immunoglobulins followed by phycoerythrin-conjugated anti-CD1a (a) or anti-CD83 (b). Both CD1a+ and CD1a– cells showed expression of C3aR and C5aR, which was completely down-modulated by treatment with TNF-α (a). The treatment of these cells with TNF-α led to the expression of CD83 and to the disappearance of the anaphylatoxin receptors (b).
Figure 7.
Increase of cytosolic calcium in blood-derived cells upon stimulation with unlabelled C3a and C5a. Cells were cultured for 5 days (a). A proportion was then stimulated with 200 U/ml of tumour necrosis factor-α (TNF-α) (b). After cell labelling with phycoerythrin-conjugated anti-CD1a, cells were loaded with Flou-3 AM, as described in the Materials and methods. C3a (1 µg/ml) (▴) or C5a (1 µg/ml) (•) was then added to suspended cells and [Ca2+] was immediately assessed by flow cytometry. Ca-ionophore was used as a positive control (▾) and buffer as a negative control (▪). Cytosolic calcium increased in blood-derived cells after stimulation with unlabelled C3a and C5a (a). After stimulation with 200 U/ml of TNF-α, all cells became CD83+ and a calcium influx was no longer observed (b).
Discussion
The aim of the present study was to evaluate the expression of anaphylatoxin receptors on normal human skin cells and blood-derived cells expressing the DC-associated membrane molecules CD1a and CD83, which were induced in the presence of low dose GM-CSF (6·25 ng/ml) and IL-4 (125 U/ml). Using specific mAbs directed against defined complement-binding sites, we were able to show that dermal CD1a+ and CD83+ DC, as well as epidermal CD1a+ DC, which were isolated from normal human skin, expressed anaphylatoxin receptors. Morelli et al.36 found, by flow cytometry, that a small population of epidermal Langerhans' cells expressed C5aR. In accordance with our findings, the majority of the dermal CD1a+ DC expressed C5aR. Morelli et al.36 found an up-regulation of C5aR upon in vitro maturation of epidermal Langerhans' cells after a short-term culture together with keratinocytes in the presence of GM-CSF. The authors, however, did not investigate CD83+ dermal DC, which we found to also express C5aR+. In our experiments, skin-derived DC, which were further treated with TNF-α in vitro, had lost the expression of C5aR or C3aR.
To further investigate the expression of anaphylatoxin receptors at different stages of DC maturation, we generated cells expressing the DC-associated membrane molecules, CD1a and/or CD83, from peripheral blood monocytes. Sallusto & Lanzavecchia6 and Romani et al.37 had demonstrated that, after enrichment of the culture medium with a combination of GM-CSF and IL-4, CD14+ monocytes (≥ 80% pure) turned into DC when cultured for 5–8 days. These cells expressed CD1a, a characteristic of immature DC, and have been used for the identification of the stimuli activity of IL-1, TNF-α, LPS, CD40 ligand (CD40L) and monocyte-conditioned medium.6,38 It has been well established that GM-CSF and TNF-α are involved in DC development and DC trafficking.37,39,40 In our experiments, all CD1a+ cells became CD83+ after culture and treatment with the inflammatory cytokine TNF-α, indicating the terminal maturation of the DC.41 These results support the current concept that immature monocyte-derived DC require a second signal (i.e. TNF-α) for their terminal maturation.42–44 During maturation, in response to TNF-α, the biosynthetic rate of human leucocyte antigen (HLA) class II molecules is up-regulated, resulting in more efficient loading with antigenic peptides. In addition, the half-life of these molecules is markedly increased, resulting in more effective antigen presentation to T cells.45
Interestingly, in the present study, C5aR was expressed by dermal CD83+ DC from skin, but not by monocyte-derived CD83+ cells, in spite of the phenotypically close relationship between dermal DC and monocyte-derived DC described by Grassi et al.46
There is an indication that dermal CD83+ DC in vivo (although they express CD83, the marker for mature DCs) represent an interconverting population in the skin and that the final differentiation depends upon the prevailing cytokine environment.
The reason for the differential expression of C5aR on dermal CD83+ DC and monocyte-derived CD83+ cells is unknown at present. Immature DC, identified by the expression of CD1a and defined by the lack of CD83, have the capacity to produce a large number of cytokines that contribute to T-cell priming (IL-1a, IL-6, IL-15, TNF-α) or to T-cell maturation (IL-12, IL-18, IL-7).47 Lore et al.48 showed that mature DC, as well as Langerhans' cells, have a cytokine production pattern similar to that of immature DC. It has been demonstrated that C5a can stimulate the release of cytokines from inflammatory cells49–51 and it may be postulated that C5a has similar effects on CD83+ dermal DC. Larregina et al. showed that epidermal Langerhans' cells undergo changes in the cytokine receptor repertoire during in vitro maturation.52 Immature DC express a number of receptors for inflammatory cytokines or chemokines, which may attract the cells to sites of inflammation.12 Upon further maturation by stimulation with TNF-α, dermal DC regulate these receptors. This may allow them to emigrate from the inflammatory site. It seems to be that the CD83+ dermal DC are probably immature cells rather than terminal mature cells.
The expression of C5aR and the calcium fluxes observed upon stimulation with C5a indicate a specific response of dermal CD83+ cells to the anaphylatoxin C5a. This is of considerable biological interest because, in experimental models using blood-derived mononuclear cells, C5a has been shown to have immunoregulatory properties. The stimulation of mononuclear cells with lipopolysaccharide (LPS) or IL-1β, together with C5a, leads to an increased synthesis of IL-1, IL-6 and TNF.22,53,54 Furthermore, C5a has been described as a chemoattractant for myeloid cells (such as neutrophils, eosinophils, basophils and monocytes) and for DC generated in vitro by treatment of PBMC with GM-CSF + IL-4.9,19 Another important effect of C5a is the degranulation of monocytes and granulocytes, which liberates many enzymes and rapidly changes the cell phenotype.55 Studies in our laboratory are now in progress to extend these observations to dermal CD83+ cells.
The present investigations confirm previous studies describing the expression of C5aR on blood-derived DC and show for the first time that C3aR and C5aR are also expressed on dermal CD83+ DC, with a predominance of C5aR. Further studies should be undertaken to determine the biological function of the anaphylatoxin receptors on mature dermal DC.
Acknowledgments
This work was supported by DFG grants WE 1289/2–1 and Gö 410/7–2.
References
- 1.Bos JD, Kapsenberg ML. The skin immune system: progress in cutaneous biology. Immunol Today. 1993;14:75–8. doi: 10.1016/0167-5699(93)90062-P. [DOI] [PubMed] [Google Scholar]
- 2.Cella M, Scheidegger D, Palmer-Lehmann K, Lane P, Lanzavecchia A, Alber G. Ligation of CD40 on dendritic cells triggers production of high levels of interleukin-12 and enhances T cell stimulatory capacity: T–T help via APC activation. J Exp Med. 1996;184:747–52. doi: 10.1084/jem.184.2.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Koch F, Stanzl U, Jennewein P, Janke K, Heufler C, Kampgen E, Romani N, Schuler G. High level IL-12 production by murine dendritic cells: upregulation via MHC class II and CD40 molecules and downregulation by IL-4 and IL-10. J Exp Med. 1996;184:741–6. doi: 10.1084/jem.184.2.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Steinman RM. The dendritic cell system and its role in immunogenicity. Annu Rev Immunol. 1991;9:271–96. doi: 10.1146/annurev.iy.09.040191.001415. [DOI] [PubMed] [Google Scholar]
- 5.Caux C, Liu YJ, Banchereau J. Recent advances in the study of dendritic cells and follicular dendritic cells. Immunol Today. 1995;16:2–4. doi: 10.1016/0167-5699(95)80061-1. [DOI] [PubMed] [Google Scholar]
- 6.Sallusto F, Lanzavecchia A. Efficient presentation of soluble antigen by cultured human dendritic cells is maintained by granulocyte/macrophage colony-stimulating factor plus interleukin 4 and downregulated by tumor necrosis factor alpha. J Exp Med. 1994;179:1109–18. doi: 10.1084/jem.179.4.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Caux C, Ait-Yahia S, Chemin K, et al. Dendritic cell biology and regulation of dentritic cell trafficking by chemokines. Springer Semin Immunopathol. 2000;22:345–69. doi: 10.1007/s002810000053. [DOI] [PubMed] [Google Scholar]
- 8.Dieu-Nosjean MC, Vicari A, Lebecque S, Caux C. Regulation of dendritic cell trafficking: a process that involves the participation of selective chemokines. J Leukoc Biol. 1999;66:252–62. doi: 10.1002/jlb.66.2.252. [DOI] [PubMed] [Google Scholar]
- 9.Sozzani S, Sallusto F, Luini W, et al. Migration of dendritic cells in response to formyl peptides, C5a, and a distinct set of chemokines. J Immunol. 1995;155:3292–5. [PubMed] [Google Scholar]
- 10.Sozzani S, Luini W, Borsatti A, et al. Receptor expression and responsiveness of human dendritic cells to a defined set of CC and CXC chemokines. J Immunol. 1997;159:1993–2000. [PubMed] [Google Scholar]
- 11.Sozzani S, Allavena P, D'Amico G, et al. Differential regulation of chemokine receptors during dendritic cell maturation: a model for their trafficking properties. J Immunol. 1998;161:1083–6. [PubMed] [Google Scholar]
- 12.Sallusto F, Schaerli P, Loetscher P, et al. Rapid and coordinated switch in chemokine receptor expression during dendritic cell maturation. Eur J Immunol. 1998;28:2760–9. doi: 10.1002/(SICI)1521-4141(199809)28:09<2760::AID-IMMU2760>3.0.CO;2-N. 10.1002/(sici)1521-4141(199809)28:09<2760::aid-immu2760>3.3.co;2-e. [DOI] [PubMed] [Google Scholar]
- 13.Chan VW, Kothakota S, Rohan MC, Panganiban-Lustan L, Gardner JP, Wachowicz MS, Winter JA, Williams LT. Secondary lymphoid-tissue chemokine (SLC) is chemotactic for mature dendritic cells. Arch Dermatol Res. 1999;93:3610–6. [PubMed] [Google Scholar]
- 14.Gunn MD, Tangemann K, Tam C, Cyster JG, Rosen SD, Williams LT. A chemokine expressed in lymphoid high endothelial venules promotes the adhesion and chemotaxis of naive T lymphocytes. Proc Natl Acad Sci USA. 1998;95:258–63. doi: 10.1073/pnas.95.1.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kellermann SA, Hudak S, Oldham ER, Liu YJ, McEvoy LM. The CC chemokine receptor-7 ligands 6Ckine and macrophage inflammatory protein-3 beta are potent chemoattractants for in vitro- and in vivo-derived dendritic cells. J Immunol. 1999;162:3859–64. [PubMed] [Google Scholar]
- 16.Ngo VN, Tang HL, Cyster JG. Epstein–Barr virus-induced molecule 1 ligand chemokine is expressed by dendritic cells in lymphoid tissues and strongly attracts naive T cells and activated B cells. J Exp Med. 1998;188:181–91. doi: 10.1084/jem.188.1.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schulman ES, Post TJ, Henson PM, Giclas PC. Differential effects of the complement peptides, C5a and C5a des Arg on human basophil and lung mast cell histamine release. J Clin Invest. 1988;81:918–23. doi: 10.1172/JCI113403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Werfel T, Oppermann M, Schulze M, Krieger G, Weber M, Götze O. Binding of fluorescein-labeled anaphylatoxin C5a to human peripheral blood, spleen, and bone marrow leukocytes. Blood. 1992;79:152–60. [PubMed] [Google Scholar]
- 19.Frank MM, Fries LF. The role of complement in inflammation and phagocytosis. Immunol Today. 1991;12:322–6. doi: 10.1016/0167-5699(91)90009-I. [DOI] [PubMed] [Google Scholar]
- 20.Cavaillon JM, Fitting C, Haeffner-Cavaillon N. Recombinant C5a enhances interleukin 1 and tumor necrosis factor release by lipopolysaccharide-stimulated monocytes and macrophages. Eur J Immunol. 1990;20:253–7. doi: 10.1002/eji.1830200204. [DOI] [PubMed] [Google Scholar]
- 21.Schindler R, Gelfand JA, Dinarello CA. Recombinant C5a stimulates transcription rather than translation of interleukin-1 (IL-1) and tumor necrosis factor: translational signal provided by lipopolysaccharide or IL-1 itself. Arch Dermatol Res. 1990;76:1631–8. [PubMed] [Google Scholar]
- 22.Montz H, Koch KC, Zierz R, Götze O. The role of C5a in interleukin-6 production induced by lipopolysaccharide or interleukin-1. Immunology. 1991;74:373–9. [PMC free article] [PubMed] [Google Scholar]
- 23.Gerard NP, Gerard C. The chemotactic receptor for human C5a anaphylatoxin. Nature. 1991;349:614–7. doi: 10.1038/349614a0. [DOI] [PubMed] [Google Scholar]
- 24.Boulay F, Mery L, Tardif M, Brouchon L, Vignais P. Expression cloning of a receptor for C5a anaphylatoxin on differentiated HL-60 cells. Biochemistry. 1991;30:2993–9. doi: 10.1021/bi00226a002. [DOI] [PubMed] [Google Scholar]
- 25.Ames RS, Li Y, Sarau HM, Nuthulaganti P, et al. Molecular cloning and characterization of the human anaphylatoxin C3a receptor. J Biol Chem. 1996;271:20231–4. doi: 10.1074/jbc.271.34.20231. [DOI] [PubMed] [Google Scholar]
- 26.Crass T, Raffetseder U, Martin U, Grove M, Klos A, Kohl J, Bautsch W. Expression cloning of the human C3a anaphylatoxin receptor (C3aR) from differentiated U-937 cells. Eur J Immunol. 1996;26:1944–50. doi: 10.1002/eji.1830260840. [DOI] [PubMed] [Google Scholar]
- 27.Bischoff FR, Maier G, Tilz G, Ponstingl H. A 47-kDa human nuclear protein recognized by antikinetochore autoimmune sera is homologous with the protein encoded by RCC1, a gene implicated in onset of chromosome condensation. Proc Natl Acad Sci USA. 1990;87:8617–21. doi: 10.1073/pnas.87.21.8617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.el Lati SG, Dahinden CA, Church MK. Complement peptides C3a- and C5a-induced mediator release from dissociated human skin mast cells. J Invest Dermatol. 1994;102:803–6. doi: 10.1111/1523-1747.ep12378589. [DOI] [PubMed] [Google Scholar]
- 29.Kretzschmar T, Jeromin A, Gietz C, Bautsch W, Klos A, Kohl J, Rechkemmer G, Bitter-Suermann D. Chronic myelogenous leukemia-derived basophilic granulocytes express a functional active receptor for the anaphylatoxin C3a. Eur J Immunol. 1993;23:558–61. doi: 10.1002/eji.1830230239. [DOI] [PubMed] [Google Scholar]
- 30.Nilsson G, Johnell M, Hammer CH, Tiffany HL, Nilsson K, Metcalfe DD, Siegbahn A, Murphy PM. C3a and C5a are chemotaxins for human mast cells and act through distinct receptors via a pertussis toxin-sensitive signal transduction pathway. J Immunol. 1996;157:1693–8. [PubMed] [Google Scholar]
- 31.Hartmann K, Henz BM, Kruger-Krasagakes S, et al. C3a and C5a stimulate chemotaxis of human mast cells. Arch Dermatol Res. 1997;89:2863–70. [PubMed] [Google Scholar]
- 32.Zwirner J, Götze O, Sieber A, Kapp A, Begemann G, Zuberbier T, Werfel T. The human mast cell line HMC-1 binds and responds to C3a but not C3a (desArg) Scand J Immunol. 1998;47:19–24. doi: 10.1046/j.1365-3083.1998.00250.x. [DOI] [PubMed] [Google Scholar]
- 33.Werfel T, Zwirner J, Oppermann M, Sieber A, Begemann G, Drommer W, Kapp A, Götze O. CD88 antibodies specifically bind to C5aR on dermal CD117+ and CD14+ cells and react with a desmosomal antigen in human skin. J Immunol. 1996;157:1729–35. [PubMed] [Google Scholar]
- 34.Werfel T, Oppermann M, Begemann G, Götze O, Zwirner J. C5a receptors are detectable on mast cells in normal human skin and in psoriatic plaques but not in weal and flare reactions or in uticaria pigmentosa by immunohistochemistry. Arch Dermatol Res. 1997;289:83–6. doi: 10.1007/s004030050159. 10.1007/s004030050159. [DOI] [PubMed] [Google Scholar]
- 35.Zwirner J, Götze O, Moser A, Sieber A, Begemann G, Kapp A, Elsner J, Werfel T. Blood- and skin-derived monocytes/macrophages respond to C3a but not to C3a (desArg) with a transient release of calcium via a pertussis toxin-sensitive signal transduction pathway. Eur J Immunol. 1997;27:2317–22. doi: 10.1002/eji.1830270928. [DOI] [PubMed] [Google Scholar]
- 36.Morelli A, Larregina A, Chuluyan I, Kolkowski E, Fainboim L. Expression and modulation of C5a receptor (CD88) on skin dendritic cells. Chemotactic effect of C5a on skin migratory dendritic cells. Immunology. 1996;89:126–34. doi: 10.1046/j.1365-2567.1996.d01-701.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Romani N, Gruner S, Brang D, et al. Proliferating dendritic cell progenitors in human blood. J Exp Med. 1994;180:83–93. doi: 10.1084/jem.180.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bender A, Sapp M, Schuler G, Steinman RM, Bhardwaj N. Improved methods for the generation of dendritic cells from nonproliferating progenitors in human blood. J Immunol Methods. 1996;196:121–35. doi: 10.1016/0022-1759(96)00079-8. 10.1016/0022-1759(96)00079-8. [DOI] [PubMed] [Google Scholar]
- 39.Witmer-Pack MD, Olivier W, Valinsky J, Schuler G, Steinman RM. Granulocyte/macrophage colony-stimulating factor is essential for the viability and function of cultured murine epidermal Langerhans' cells. J Exp Med. 1987;166:1484–98. doi: 10.1084/jem.166.5.1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Caux C, Dezutter-Dambuyant C, Schmitt D, Banchereau J. GM-CSF and TNF-alpha cooperate in the generation of dendritic Langerhans' cells. Nature. 1992;360:258–61. doi: 10.1038/360258a0. [DOI] [PubMed] [Google Scholar]
- 41.Zhou LJ, Tedder TF. CD14+ blood monocytes can differentiate into functionally mature CD83+ dendritic cells. Proc Natl Acad Sci USA. 1996;93:2588–92. doi: 10.1073/pnas.93.6.2588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Girolomoni G, Ricciardi-Castagnoli P. Dendritic cells hold promise for immunotherapy. Immunol Today. 1997;18:102–4. doi: 10.1016/s0167-5699(97)01030-x. 10.1016/s0167-5699(97)01030-x. [DOI] [PubMed] [Google Scholar]
- 43.Caux C, Vanbervliet B, Massacrier C, et al. CD34+ hematopoietic progenitors from human cord blood differentiate along two independent dendritic cell pathways in response to GM-CSF+TNF alpha. J Exp Med. 1996;184:695–706. doi: 10.1084/jem.184.2.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Flores-Romo L, Bjorck P, Duvert V, van Kooten C, Saeland S, Banchereau J. CD40 ligation on human cord blood CD34+ hematopoietic progenitors induces their proliferation and differentiation into functional dendritic cells. J Exp Med. 1997;185:341–9. doi: 10.1084/jem.185.2.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cella M, Engering A, Pinet V, Pieters J, Lanzavecchia A. Inflammatory stimuli induce accumulation of MHC class II complexes on dendritic cells [see comments] Nature. 1997;388:782–7. doi: 10.1038/42030. 10.1038/42030. [DOI] [PubMed] [Google Scholar]
- 46.Grassi F, Dezutter-Dambuyant C, McIlroy D, et al. Monocyte-derived dendritic cells have a phenotype comparable to that of dermal dendritic cells and display ultrastructural granules distinct from Birbeck granules. J Leukoc Biol. 1998;64:484–93. doi: 10.1002/jlb.64.4.484. [DOI] [PubMed] [Google Scholar]
- 47.Saint-Vis B, Fugier-Vivier I, Massacrier C, et al. The cytokine profile expressed by human dendritic cells is dependent on cell subtype and mode of activation. J Immunol. 1998;160:1666–76. [PubMed] [Google Scholar]
- 48.Lore K, Sonnerborg A, Spetz AL, Andersson U, Andersson J. Immunocytochemical detection of cytokines and chemokines in Langerhans' cells and in vitro-derived dendritic cells. J Immunol Methods. 1998;218:173–87. doi: 10.1016/s0022-1759(98)00171-9. 10.1016/s0022-1759(98)00171-9. [DOI] [PubMed] [Google Scholar]
- 49.Okusawa S, Yancey KB, van der Meer JW, et al. C5a stimulates secretion of tumor necrosis factor from human mononuclear cells in vitro. Comparison with secretion of interleukin 1 beta and interleukin 1 alpha. J Exp Med. 1988;168:443–8. doi: 10.1084/jem.168.1.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Goodman MG, Chenoweth DE, Weigle WO. Induction of interleukin 1 secretion and enhancement of humoral immunity by binding of human C5a to macrophage surface C5a receptors. J Exp Med. 1982;156:912–7. doi: 10.1084/jem.156.3.912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Larregina AT, Morelli AE, Kolkowski E, Sanjuan N, Barboza ME, Fainboim L. Pattern of cytokine receptors expressed by human dendritic cells migrated from dermal explants. Immunology. 1997;91:303–13. doi: 10.1046/j.1365-2567.1997.00250.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Larregina A, Morelli A, Kolkowski E, Fainboim L. Flow cytometric analysis of cytokine receptors on human Langerhans' cells. Changes observed after short-term culture. Immunology. 1996;87:317–25. doi: 10.1046/j.1365-2567.1996.451513.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schindler R, Gelfand JA, Dinarello CA. Recombinant C5a stimulates transcription rather than translation of interleukin-1 (IL-1) and tumour necrosis factor: translational signal provided by lipopolysaccharide or IL-1 itself. Blood. 1990;76:1631–8. [PubMed] [Google Scholar]
- 54.Morgan EL, Sanderson S, Scholz W, Noonan DJ, Weigle WO, Hugli TE. Identification and characterization of the effector region within human C5a responsible for stimulation of IL-6 synthesis. J Immunol. 1992;148:3937–42. [PubMed] [Google Scholar]
- 55.Goldstein IM. Inflammation. Basic Principles and Clinical Correlates. New York: Raven Press; 1992. [Google Scholar]