Abstract
Pregnant animals can generate and maintain immune responses to fetal antigens. This however, does not usually lead to fetal loss. At least two types of immune response are recognized. T helper type 1 (Th1) responses support the generation of cellular cytotoxicity. In contrast, Th2-type responses support the production of non-cytotoxic antibody and suppress the Th1-type. One attempt to explain why the fetus is not generally rejected has been to suggest that during pregnancy Th2-type responses are dominant. These responses rely heavily on interleukin-4 (IL-4) for both functions. This work focuses on maternal immunity to the male antigen H-Y, which is expressed in male fetuses. When injected with male spleen cells, female mice of certain strains mount a cytotoxic immune response to H-Y. However, pregnant females immunized in this way do not deliver litters with fewer males. To help delineate the possible role of IL-4 in such maternal tolerance, female mice genetically deficient in IL-4 were studied. The results show that: (1) deficiency in maternal IL-4 does not affect fertility, (2) deficiency in IL-4 is not associated with selective loss of male offspring in unimmunized mice, (3) pregnancy does not obliterate anti-H-Y reactivity in immunized mice and (4) maternal immunity to H-Y in the absence of IL-4 does not result in loss of male offspring. The results suggest that IL-4-dependent Th2-type responses are not critical to maternal tolerance. Other cytokines must be examined for their role in this phenomenon.
Introduction
The fetal–placental unit is a semi-allogeneic graft that is in direct contact with maternal tissue, yet is normally not rejected by the maternal immune system. Protection of the fetus from immune-mediated destruction is thought to rely on two main mechanisms: the maintenance of a barrier between mother and fetus and down-regulation of the maternal immune system. Data exist from both old1–4 and new5–7 studies which suggest that down-regulation of maternal immunity occurs on a systemic level. However, contradictory older8–11 and newer12,13 evidence also exists. The idea that the most important down-regulation of maternal immunity occurs locally at the maternal–fetal interface14–18 has been used to reconcile these two bodies of evidence.
Studies of the immune response have led to the delineation of at least two classes of T-cell response: T helper type 1 (Th1) and type 2 (Th2).19,20 The classes comprise distinct subsets of CD4 and CD8 T cells, produce a distinct set of cytokines and support different functions. Th1-type immune responses function to generate cells [i.e. natural killer (NK) cells and killer T cells] and factors [i.e. tumour necrosis factor (TNF) and cytotoxic antibodies] whose activity leads to cell death. Th2-type responses function to generate particular antibodies, such as immunoglobulin E (IgE), which are important in the allergic response.
The evidence regarding regulation of class in the immune response gave rise to the hypothesis that pregnant females are not immunosuppressed but are instead restricted to certain class(es) of response. Support for this theory initially came from two groups of investigators21,22 studying cytokines from whole murine placenta placed in culture, or the presence of specific cytokine mRNA in unpurified murine placental cells. Their work suggested that the placenta may be capable of producing Th2-type cytokines and thus direct a pregnant female's immune responses towards this phenotype.23 Further support for this idea came from the study of parasitic diseases in mice24 and humans.25
In contrast, other investigators have found Th1-type cytokines, such as TNF-α and interferon-γ, (supportive of cytotoxic responses) in placenta, decidua and maternal blood in mice22 and in humans.26,27 Moreover, there is evidence in humans that such responses can be recruited to the placenta if needed to combat infections.25,28 Finally, while cytotoxicity to fetal antigens does not necessarily result in fetal loss,10,29 exposure to fetal antigens during or immediately after pregnancy can itself result in detectable, systemic cytotoxic immune responses to human9 and murine12 fetal antigens.
Because of these data, the argument that mothers are restricted to certain classes of response has been refined to suggest that successful pregnancy relies on the local, rather than systemic dominance of Th2-type responses. Two possible mechanisms have been suggested: The specific local down-regulation of Th1-type responses with unmodified local or systemic Th2-type responses30 or, the up-regulation of Th2-type responses, either locally or systemically.31,32
Interleukin-4 (IL-4) is crucial to the development of Th2 cells,33 and participates with other co-factors such as macrophage chemoattractant protein 1 (MCP-1) (reviewed by O'Garra20). This cytokine is produced by a variety of cell types. Mast cells, basophils and eosinophils (reviewed by Brown and Hural34) can release preformed IL-4 on cross-linking of the FcεRI. Specialized NK1.1+ T cells,35 and activated Th2 cells synthesize and release IL-4 after T-cell receptor cross-linking. IL-4 binds to a receptor that has a unique, ligand-specific α-chain and a common γ-chain that is part of the receptor for many other cytokines, including IL-7, IL-236 and IL-13.37 Post-receptor signalling activates the transcription factor STAT6. Activated STAT6, through incompletely understood mechanisms, supports increased IL-4 gene expression and IL-4 production.
Th2 cells, once generated, make cytokines such as IL-4 and IL-5 that are important in immunoglobulin class switching33 and IL-13, thought to be important in the production of IgE.37 IL-4 favours the generation and maintenance of Th2-type (versus Th1-type) responses in many respects. For example, the effects of IL-4 in inducing Th2 development are thought to be dominant over any effects in inducing Th1 development.33 This may be because IL-4 down-regulates IL-12 receptor β2 and renders Th2 cells unresponsive to this Th1-generating cytokine.38 In addition, IL-4 also inhibits Th1 responses by inhibiting production of IL-12 by dendritic cells and macrophages.39,40 Finally, there is evidence41 which suggests that IL-4 can convert cytotoxic T cells to IL-4-producing non-cytolytic cells.
Overexpression of IL-4 leads to an allergy-like disorder of the eye34 and an early burst of IL-4 in the course of a Leishmania major infection is thought to be responsible for susceptibility to life-threatening disease in BALB/c mice.42
Gene deletion of IL-4 significantly reduces production of Th2 cytokines in stimulated CD4 T cells.43 In addition, IL-4-deficient mice express significantly decreased levels of IgG1 and IgE and a blunted IgE response to Nippostrongylus brasiliensis.44 Disruption of STAT6, important in the IL-4 signalling pathway does not completely abrogate Th2 responses.19
H-Y is an antigen encoded by the Y chromosome.45 Since most litters contain male pups (on average 50% of pups are male), most mothers are potentially exposed to H-Y during pregnancy or birth. This antigen has long been known as the basis for female mouse rejection of same-strain male organ grafts.46 Administration of male spleen cells to female mice of the H-2b major histocompatibility complex (MHC) haplotypes [i.e. C57Bl/6 (B6) or C57Bl/10 (B10)] generates a cytotoxic immune response that can be assayed in vitro by the killing of male targets. Cytotoxic immunity to H-Y in pregnant females does not lead to male pup loss.12
To delineate the role of IL-4 in normal pregnancy, the investigations described below examine the function of IL-4 in fetal protection from cytotoxic maternal immune responses. Accordingly, these experiments focused on male pup survival in IL-4-deficient44 female mice that were primed against H-Y in comparison to a normal, similarly immunized cohort of the same genetic background.
Materials and Methods
Animals
C57Bl/6 (B6) and C57Bl/6-IL4-KO (B6-4KO) mice (now available from Jackson Laboratories, Bar Harbor, ME, with permission from Werner Mueller) were housed under American Association for Accreditation of Laboratory Animal Care (AAALAC)-approved guidelines.
Skin grafting
Tail skin grafts were applied as previously described.47 Briefly, mice were anaesthetized and two small portions of tail skin were removed. In one of the beds created, a male tail skin graft (test) was placed. In the other bed a female graft (negative control) was placed. Grafts were observed regularly and time of rejection (when the graft had completely fallen off) was recorded.
JAM test for cytotoxicity
Effectors
Spleen cells (4 × 106) from responder females were placed in culture for 6 days in 2 ml Iscove's modified Dulbecco's medium (IMDM) with 10% fetal calf serum, l-glutamine, penicillin, streptomycin, gentamycin and β2-mercaptoethanol with 2 × 106 irradiated (1500 Rads) syngeneic male or MHC disparate allogeneic female spleen cells.
Targets
Thirty-six hours before assay, male, allogeneic female and syngeneic female spleen cells were placed in culture with 1·8 µg/ml concanavalin A. The day of the assay these targets were labelled with 5 µCi/ml [3H]thymidine for 4 hr and washed. Responders and targets were incubated together in effector to target (E : T) ratios from 1 : 1 to 270 : 1 in 96-well round-bottom plates. The plates were harvested and specific killing of male or allogeneic targets was calculated as previously described.48 In this study, the reported specific killing is the percentage of male or allogeneic targets killed minus the percentage of female targets killed by responder spleen cells at an E : T ratio where the killing by control mice (B6 primed, not pregnant) spleen cells falls off the plateau.
Statistics
The studies were designed to detect a fall in the proportion of male offspring per litter from the normal average of approximately 0·51–0·33. A power calculation (80% power, level of significance P = 0·05) suggested that approximately eight litters of each group (immunized B6, unimmunized B6, immunized B6-4KO, unimmunized B6-4KO) were needed to detect this level of difference. χ2 analysis was used to compare categorical variables (i.e. tolerant versus not tolerant), while a two tailed t-test was used to compare continuous variables (i.e. fraction of males, specific killing of male targets).
Results
Health and fertility in B6 and B6-4KO mice
Deficiency in certain cytokines, such as transforming growth factor-β (TGF-β), results in a higher incidence of death before reaching the age of reproduction.49 However, B6-4KO mice have a normal life span.44 Ninety-seven per cent of both B6 and B6-4KO survived the course of experiments. Thus the two different strains, under specific pathogen-free conditions appear to be equally healthy.
Several cytokines found at the maternal fetal interface (for example IL-850 or IL-1032) may play a role in either fetal–placental growth and development, or in immune response modulation. Before addressing whether deficiency in IL-4 precipitates or facilitates immune-mediated, antigen-specific fetal loss, the studies examined overall fertility in unimmunized B6 and B6-4KO females mated to B6-4KO males. Table 1 shows the results of this analysis. The fertility in unimmunized B6 mice was 15/36 (0·42 litters/mouse-matings) while that in B6-4KO mice was 17/58 or 0·29 (P > 0·5). Further analysis of the mating data showed that there was no significant difference in litter size (B6 8·2 ± 2·2 and B6-4KO 7·4 ± 2·0) nor in the tendency to eat a litter. Thus the fertility between B6 and B6-4KO females mated to B6-4KO males was not significantly different; suggesting that IL-4 deficiency per se does not significantly impair fetal growth and development.
Table 1.
Fertility of animals used in this study
| B6 females | B6-4KO females | |||
|---|---|---|---|---|
| Unprimed | Primed | Unprimed | Primed | |
| Mice mated | 18 | 26 | 29 | 32 |
| Matings | 36 | 52 | 58 | 64 |
| Pregnant | 11 | 17 | 12 | 17 |
| No. of litters* | 15 | 23 | 17 | 26 |
| Litters included in analysis | 12 | 15 | 16 | 21 |
Primed mice received an intraperitoneal injection of B6-4KO male spleen cells followed by either placement of a B6-4KO male skin graft or a repeat injection of male spleen cells.
There was no significant difference in the fertility in any group of mice as measured by the number of litters/mouse-matings.
Litters not included in the analysis were either eaten by their unprimed (3 B6 and 1 B6-4KO) or primed (6 B6 and 1 B6-4KO) mothers or were born to primed mothers whose anti H-Y CTL activity could not be assessed.
Does IL-4 deficiency confer a selective disadvantage to male offspring in the absence of a specific immune response directed against H-Y?
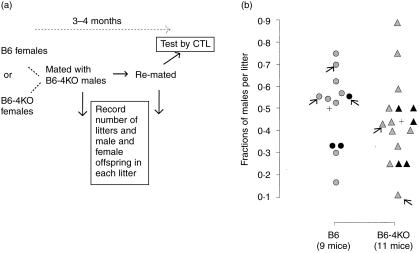
The next experiment was designed to address this possibility. Unimmunized B6 or B6-4KO females were mated with B6-4KO males to create fetal placental units expressing (mothers B6, fathers B6-4KO) or not expressing (mothers B6-4KO, fathers B6-4KO) IL-4. Figure 1(a) shows a schematic of the experiment while Fig. 1(b) shows the data. The proportion of males per litter born under the two conditions was equivalent. Moreover, second litters did not show a significantly decreased proportion of males.
Figure 1.
The absence of IL-4 does not lead to a loss of male offspring in B6 mice. (a) Schema of experiment: 6- to 8-week-old B6 or B6-4KO females were mated twice with B6-4KO males. The numbers of male and female pups born in each litter were recorded. Three months after the first mating, the mice were killed and their spleen cells were assayed in vitro for anti-H-Y and anti-alloantigen reactivity. (b) The experimental data: y-axis, fraction of males born per litter; x-axis, mother's strain. Nine of 11 B6 and 11 of 12 B6-4KO mothers are represented because the litters of the other mice were eaten at birth. Each symbol represents one litter born to a B6 (circle) or B6-4KO (triangle) mother. Grey symbols, first litters; black symbols, second litters. Arrows point to litters born to mothers H-Y-sensitized by pregnancy.
Four of the unimmunized mice developed detectable anti-H-Y cytotoxicity with pregnancy-induced exposure to male fetal tissue. Two B6 mothers had significant anti-H-Y cytotoxicity in their spleen when assayed. One had two litters, each with 56% males. The other had one litter with 70% males. The average percentage of males in these three litters was not different from that found in litters born to B6 control mothers (P > 0·1). Two B6-4KO mothers were also H-Y sensitized by their pregnancy. One bore a litter with 43% males and the other, a litter with 11% males.
Compared to the average of other litters born to B6-4KO mothers these percentages were lower (P < 0·1). These data suggested the possibility that the other mothers with low percentages of male mice in their litters might be H-Y sensitized at a level at, or just below, that detected in our CTL assay. To test the hypothesis that there may be a loss of male pups in the presence of an anti-H-Y immune response and in the absence of IL-4, the next experiment done was to purposefully prime both B6 and B6-4KO females with male spleen cells and then mate them to B6-4KO males.
The immune response to H-Y in B6 and B6-4KO pregnant mice
It was possible that injection of male spleen cells intraperitoneally could create an inflammatory response that would affect overall fertility. Furthermore, it is known that certain cytokines, in presumably high concentrations51 can be directly toxic to placental tissues. Therefore fertility in the two groups of females injected with male spleen cells was examined. Table 1 shows that the fertility of B6 primed females was 23 litters/52 mouse matings (0·44) and for primed B6-4KO females it was 26 litters/64 mouse matings (0·41). Neither of these is significantly different from their unprimed cohort (see above). Thus, merely injecting a potentially immunogenic stimulus does not cause non-specific (i.e. male and female) fetal loss.
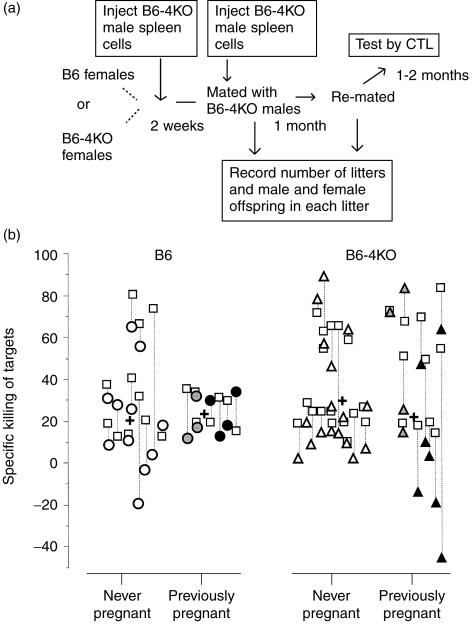
Previous studies in a transgenic model system suggested that pregnancy caused deletion of H-Y-reactive T cells.6 Therefore experiments were performed to determine if mice immunized before pregnancy and boosted at the time of mating retained their ability to mount an anti-H-Y response. Figure 2(a) shows the course of the experiment. B6 or B6-4KO mice were injected with B6-4KO spleen cells and then mated 2 weeks later. At the time of mating they were reinjected with male spleen cells. After two rounds of mating the mice were assayed in vitro for anti-H-Y reactivity. Unmated mice were used as controls. The results from this experiment are depicted in Fig. 2(b). B6 and B6-4KO mice who became pregnant, either once or twice, responded similarly to their never pregnant cohort (B6, 22·6% versus 20·4%; B6-4KO, 21·7 versus 29·6). Thus pregnancy did not appear to impair anti-H-Y cytotoxicity systemically.
Figure 2.
Pregnancy does not permanently diminish the ability to respond to H-Y in B6 or B6-4KO mice. (a) Schema of experiment: B6 or B6-4KO females were injected with a priming dose of male B6-4KO male spleen cells. Two weeks later, females of both strains were mated with B6-4KO males. Within 3 days of mating, the female mice were given another dose of male spleen cells. They were then mated twice with B6-4KO males. The numbers of male and female pups born in each litter was recorded. Three months after the first mating, the mice were killed and their spleen cells were assayed in vitro for anti-H-Y and anti-alloantigen reactivity. (b) The experimental data: each symbol represents one mouse. Open symbols, mice never pregnant; grey symbols, mice pregnant with one litter; black symbols, mice pregnant with two litters. Y-axis, specific killing of male (circles, killing by B6 females; triangles, killing by B6-4KO females) or allogeneic (squares) targets; x-axis, groups of mice tested. Dotted lines link the data for each mouse. Crosses are the mean values of male-specific killing in each group.
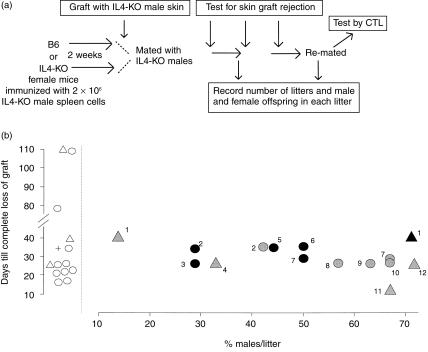
It may still be argued that cytotoxicity against H-Y assayed in the spleen after delivery is not a stringent enough criterion for whether mice can express a significant immune response during pregnancy. Thus, the next experiment was to examine B6 and B6-4KO mice immunized against H-Y for the ability to reject a male skin graft during pregnancy. A diagram for the experiment is presented in Fig. 3(a). B6 or B6-4KO mice were primed with B6-4KO spleen cells and then mated 2 weeks later. At the time of mating, they received a male and a female (as control) skin graft.
Figure 3.
Pregnancy with male pups in B6 or B6-4KO females does not impair the ability to reject a male skin graft, and the ability to reject a male skin graft does not lead to a decrease in normal or IL4-deficient male offspring. (a) Schema of experiment: B6 and B6-4KO females were treated as in Fig. 2, except that instead of being boosted with male spleen cells at the time of mating, they received a male B6-4KO (test) and female (control) tail skin graft. All mice rejected their grafts and within 2 months the mice were re-mated and the resulting litters were examined. Y-axis, days from placement of graft until if completely fell off; the cross refers to the average time for complete rejection in B6 females. X-axis, % males per litter born. Open symbols represent mice which either were never mated or never got pregnant. Solid symbols represent animals which became pregnant. Circles, B6 females (n = 8); triangles, B6-4KO females (n = 4). Grey, first litters; black, second litters. Individual mothers are depicted by number. Thus, immunized mouse no. 2 was mated and grafted, had a litter with 42% males, and completely rejected a male skin graft within 35 days. Her second litter contained approximately 28% males. Mice numbers 3, 5 and 6 ate their first litters at birth.
Figure 3(b) shows that mice that became pregnant were as likely to reject a male skin graft as their cohort which was never mated. Moreover, the data suggest that the proportion of male offspring did not significantly change the time required to reject such grafts.
Does lack of IL-4 render male pups vulnerable to immune attack in female mice primed against H-Y?
From another perspective, the data shown in Fig. 3(b) contains two important pieces of information. First, that ongoing rejection of a male skin graft, either in the presence or in the absence of IL-4, did not translate into increased loss of male fetuses. Second, that second litters born to mice that had rejected a male skin graft were neither more nor less likely to have male pups.
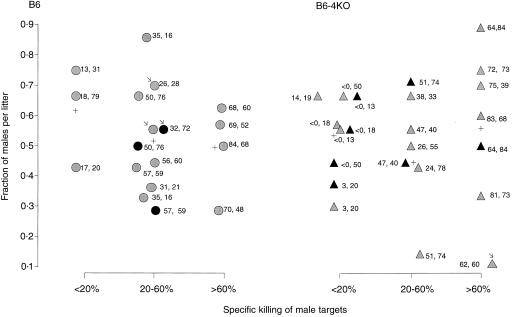
The next step taken was to examine more quantitatively how the level of anti-H-Y cytotoxicity generated in the spleen correlated with the survival of male pups in the presence and absence of IL-4. CTL data from all the primed mice in this study, as well as their pregnancy history (males per litter born), were combined to produce Fig. 4. In this figure, litters from B6 and B6-4KO mice were grouped according to whether their mother's spleen cells contained little (< 20% specific killing of male targets), moderate (20–60% killing), or high (> 60% killing) anti-H-Y cytotoxicity. Moderate or high anti-H-Y cytotoxic activity did not correlate with a decreased proportion of male offspring in IL-4-deficient females (moderate, 0·493; high, 0·555) as compared to that found in normal controls (moderate, 0·517; high, 0·496).
Figure 4.
Survival of male offspring is not altered by sustained systemic anti-H-Y cytotoxicity in B6 or B6-4KO mothers. Test mice in Fig. 3 were examined in vitro for anti-H-Y and anti-alloantigen reactivity. Data from this experiment, the CTL experiments using the mice in Fig. 2 and the mice which were immunized by pregnancy alone were pooled and analysed for comparison of male pup survival to maternal anti-H-Y reactivity in the spleen of B6 and B6-4KO mothers 2 months after the last litter was born. Y-axis, fraction of males in a litter; x-axis, maternal spleen specific killing of male targets, divided into low or no (< 20%), medium (20–60%), or high (> 60%). Each symbol represents a litter. Circles, litters born to B6 mothers; triangles, litters born to B6-4KO females; grey, first litters; black, second litters. Arrows point to litters born to mothers sensitized by pregnancy alone. Numbers beside each litter are the exact values of maternal anti-H-Y and anti-alloantigen reactivity. Crosses represent the average fraction of males per litter in each group.
Thus, the genetic absence of IL-4, either locally at the maternal fetal interface or systemically did not result in male fetal loss although systemic, functional anti-male cytotoxicity existed.
Discussion
Maternal tolerance of the fetus remains an issue of fundamental biological importance. Successful pregnancy has two requirements: protection of the fetus against infection, and protection of the fetus against a possible harmful immunological attack from the mother.
The experiments described here focus on maternal immunity to the male antigen H-Y, and on maternal tolerance of male fetuses. Previously, it has been found that increasing numbers of pregnancies render females increasingly tolerant of male skin grafts, with less than three pregnancies providing no tolerance52 and tolerance in 100% of mice tested requiring on the order of 8–11 pregnancies.53 It was also found that some, but not all, multiparous females show decreased ability to mount an anti-H-Y response when given a priming dose of male spleen cells.54 Moreover, anti-H-Y T-cell receptor transgenic mice lose T cells from the spleen and lymph nodes during pregnancy and those which remain in the spleen are functionally non-responsive.6 However, it is also possible to generate anti-H-Y reactivity during pregnancy.12
A potential way to bring together these findings is to start with the idea that activation or tolerance for an individual T cell depends on the cell presenting antigen to it.55 Interaction with an inappropriate antigen-presenting cell (for example, one not possessing the right co-stimulatory signals56) will cause inactivation, while recognition of antigen on a professional antigen-presenting cell will result in activation. Thus, the overall outcome for a population of T cells is the result of a competition between appropriate, productive antigen interactions and inappropriate, potentially detrimental ones.57
For maternal H-Y-specific T cells, this view predicts that while multiple pregnancies may increase the frequency of interactions between T cells and cells presenting H-Y inappropriately, tolerance to H-Y in any given pregnancy may not be predestined. It also predicts that exposure to a priming dose of male cells before pregnancy may protect the pool of H-Y-specific T cells during pregnancy.
Data from the experiments described in this study, where mice were primed against H-Y both before and at the beginning of pregnancy, refute the idea that fetal antigen-specific T cells are lost (deleted) or irreversibly suppressed during pregnancy. These data also confirm prior reports (for example ref. 12) that pregnancy itself can prime for an anti-fetal cytotoxic immune response.
In this context, this study begins to address three questions. First, given any antigen exposure in the ‘right’ context during pregnancy, will a Th2-type response predominate? Second, if the maternal immune system is primed for a cytotoxic response against a fetal antigen prior to pregnancy, will the presence of Th2-mediated cytokines modify or ameliorate this response? Third, are such mechanisms paramount in the survival of the fetus?
The current study examines the critical nature of IL-4 in supporting successful pregnancy, not only because of its ability to support antibody responses but because of its ability to suppress cytotoxic ones. The data suggest that the state of pregnancy either does not critically rely on IL-4, or that other cytokines and or mechanisms can be used to adapt to its absence.
If the data suggesting that IL-4 is indeed critical20,58 to Th2-type responses are accepted, then the data present in this paper cast serious doubt on the assertion that such responses are required for maternal tolerance. Because Th2-type responses can be detected in mice deficient in IL-4,44 it has been proposed that another cytokine, IL-13 is also important, particularly because it supports IgE production.37 However, it is thought that IL-13 is not crucial to the generation of Th2-type responses and does not participate in the dominance of such responses through suppression of the Th1-type.19
Another caveat to the hypothesis that Th2-type responses are crucial is the evidence that down-regulation of Th1-type responses can be achieved through the generation of regulatory cells, which appear to be dependent on IL-1059,60 and TGF-β.61 Thus, in the context of maternal tolerance, such cytokines must be considered.32,62–64 A defined role for these substances in fetal protection from anti-fetal maternal immune responses has yet to be elucidated. For example, it is not clear if such substances are responsible for particular manifestations, such as down-regulation of T-cell receptor expression5 or loss from the spleen5,6 of fetal antigen-specific T cells during pregnancy.
An evolutionarily important process such as pregnancy must rely on several mechanisms acting in concert. It is thus useful to study substances that may be important to various proposed mechanisms of functional maternal tolerance. The challenge however, will not only be to rule in or out one factor or the other, but to understand how important factors themselves interact to support successful pregnancy. What we stand to gain through careful study of such molecules is a better general understanding of tissue-specific tolerance and immunity.
Acknowledgments
I would like to thank Polly Matzinger for teaching, encouragement and help with the skin grafting experiment, Werner Mueller for permission to use the B6-4KO mice, Juanita Onyekwuluje for technical assistance with some of the cytotoxic T lymphocyte assays, and Denise Raynor, Hal King and Melissa Brown for critical review of the manuscript. This work was supported by the National Institutes of Health (E.A.B. Medical Staff fellowship), the Emory Medical Care Foundation and the Emory University Research Committee.
References
- 1.Clark DA, McDermott MR, Szewczuk MR. Impairment of host-versus-graft reaction in pregnant mice II. Selective suppression of cytotoxic T-Cell generation correlates with soluble suppressor activity and with successful allogeneic pregnancy. Cell Immunol. 1980;52:106–18. [PubMed] [Google Scholar]
- 2.Billingham RES. Studies on the tolerance of the Y chromosome antigen in mice. J Immunol. 1960;85:14–26. [PubMed] [Google Scholar]
- 3.Kaliss N, Dagg MK. Immune response engendered in mice by mulitiparity. Transplantation. 1964;2:416–25. doi: 10.1097/00007890-196405000-00011. [DOI] [PubMed] [Google Scholar]
- 4.Breyere EJ, Barrett MK. Tolerance induced by parity in mice incompatible at the H-2 locus. J Natl Can Inst. 1961;27:409–12. [Google Scholar]
- 5.Tafuri A, Alferink J, Moller P, Hammerling GJ, Arnold B. T Cell awareness of paternal alloantigens during pregnancy. Science. 1996;270:630–3. doi: 10.1126/science.270.5236.630. [DOI] [PubMed] [Google Scholar]
- 6.Jiang SP, Vacchio MS. Multiple mechanisms of peripheral T cell tolerance to the fetal ‘allograft’. J Immunol. 1998;160:3086–90. [PubMed] [Google Scholar]
- 7.Smith RN, Powell AE. The adoptive transfer of pregnancy-induced unresponsiveness to male skin grafts with thymus dependent cells. J Exp Med. 1994;146:899–904. doi: 10.1084/jem.146.3.899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van Rood JJ, Eernisse JG, van Leeuwen A. Leucocyte antibodies in the sera from pregnant women. Nature. 1958;181:1735–6. doi: 10.1038/1811735a0. [DOI] [PubMed] [Google Scholar]
- 9.Overweg J, Engelfriet CP. Cytotoxic leucocyte iso-antibodies formed during the first pregnancy. Vox Sang. 1969;16:97–104. doi: 10.1111/j.1423-0410.1969.tb04722.x. [DOI] [PubMed] [Google Scholar]
- 10.Smith JA, Burton RC, Barg M, Mitchell GF. Maternal alloimunisation in pregnancy. Transplantation. 1978;25:216–20. [PubMed] [Google Scholar]
- 11.Wegmann TG, Waters CA, Drell DW, Carlson GA. Pregnant mice are not primed but can be primed to fetal alloantigens. Proc Natl Acad Sci USA. 1979;76:2410–14. doi: 10.1073/pnas.76.5.2410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bonney EA, Matzinger P. The maternal immune system's interaction with circulating fetal cells. J Immunol. 1997;158:40–7. [PubMed] [Google Scholar]
- 13.Faas MM, Schuiling GA, Linton EA, Sargent IL, Redman CWG. Activation of peripheral leukocytes in rat pregnancy and experimental pre-eclampsia. Am J Obstet Gynecol. 2000;182:351–7. doi: 10.1016/s0002-9378(00)70223-7. [DOI] [PubMed] [Google Scholar]
- 14.Hunziker RD, Wegmann TG. Placental immunoregulation. Crit Rev Immunol. 1986;6:245–85. [PubMed] [Google Scholar]
- 15.Bulmer JN, Johnson PM. The T lymphocyte population in first trimester human decidua does not express the IL-2 receptor. Immunology. 1986;58:685–7. [PMC free article] [PubMed] [Google Scholar]
- 16.Ober C, Hyslop T, Elias S, Weitkamp LR, Hauck WW. Human leukocyte antigen matching and fetal loss: results of a 10 year prospective study. Hum Reprod. 1998;13:33–8. doi: 10.1093/humrep/13.1.33. 10.1093/humrep/13.1.33. [DOI] [PubMed] [Google Scholar]
- 17.Hunt JS, Vassmer D, Ferguson TA, Miller L. Fas ligand is positioned in mouse uterus and placenta to prevent trafficking of activated leukocytes between the mother and conceptus. J Immunol. 1997;158:4122–8. [PubMed] [Google Scholar]
- 18.Kruse A, Merchant MJ, Hallman R, Butcher EC. Evidence of specialized leukocyte–vascular homing interactions at the maternal/fetal interface. Eur J Immunol. 1999;29:1116–26. doi: 10.1002/(SICI)1521-4141(199904)29:04<1116::AID-IMMU1116>3.0.CO;2-4. 10.1002/(sici)1521-4141(199904)29:04<1116::aid-immu1116>3.3.co;2-w. [DOI] [PubMed] [Google Scholar]
- 19.Murphy KM, Ouyang W, Farrar JD, Yang J, Ranganath S, Asnagli H, Afarian M, Murphy TL. Signaling and transcription in T helper development. Annu Rev Immunol. 2000;18:451–94. doi: 10.1146/annurev.immunol.18.1.451. [DOI] [PubMed] [Google Scholar]
- 20.O'Garra A. Cytokines induce the development of functionally heterogeneous T helper cell subsets. Immunity. 1998;8:275–83. doi: 10.1016/s1074-7613(00)80533-6. [DOI] [PubMed] [Google Scholar]
- 21.Delassus S, Coutinho GC, Saucier C, Darche S, Kourilsky P. Differential cytokine expression in maternal blood and placenta during murine gestation. J Immunol. 1994;152:2411–20. [PubMed] [Google Scholar]
- 22.Lin H, Mosmann TR, Guilbert L, Tuntipopipat S, Wegmann TG. Synthesis of T-Helper 2-Type cytokines at the maternal–fetal interface. J Immunol. 1993;151:4562–73. [PubMed] [Google Scholar]
- 23.Wegmann TG, Lin H, Guilbert L, Mosmann T. Bidirectional cytokine interactions in the maternal-fetal relationship: is successful pregnancy a Th2 phenomenon? Immunol Today. 1993;14:353–6. doi: 10.1016/0167-5699(93)90235-D. [DOI] [PubMed] [Google Scholar]
- 24.Krishnan L, Guilbert LJ, Russell AS, Wegmann TG, Mosmann TR, Belosevic M. Pregnancy impairs resistance of C57Bl.6 mice to Leishmania major infection and causes decreases antigen-specific IFN-γ responses and increased production of T helper cytokines. J Immunol. 1996;156:644–52. [PubMed] [Google Scholar]
- 25.Fried M, Muga RO, Misore AO, Duffy PE. Malaria elicits type 1 cytokines in the human placenta: IFN-gamma and TNF-alpha associated with pregnancy outcomes. J Immunol. 1998;160:2523–30. [PubMed] [Google Scholar]
- 26.Boehm KD, Kelley MF, Ilan J. The interleukin 2 gene is expressed in the syncytiotrophoblast of the human placenta. Proc Natl Acad Sci USA. 1989;86:656–60. doi: 10.1073/pnas.86.2.656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen H-L, Yang Y, Hu X-L, Yelavarthi KK, Fishback JL, Hunt JS. Tumor necrosis factor alpha mRNA and protein are present in human placental and uterine cells at early and late stages of gestation. Am J Pathol. 1991;139:327–0. [PMC free article] [PubMed] [Google Scholar]
- 28.Moore JM, Nahlen BL, Misore A, Lal AA, Udhayakumar V. Immunity to placental malaria I. Elevated production of interferon-gamma by placental blood mononuclear cells is associated with protection in an area with high transmission of malaria. J Inf Dis. 1999;179:1218–25. doi: 10.1086/314737. [DOI] [PubMed] [Google Scholar]
- 29.Lanman JT, Dinerstein J, Fikrig S. Homograft immunity in pregnancy: lack of harm to the fetus from sensitization of the mother. Ann N Y Acad Sci. 1962;99:706–16. doi: 10.1111/j.1749-6632.1962.tb45355.x. [DOI] [PubMed] [Google Scholar]
- 30.Nahmias AJ, Abramosky C, Dobronyi I, Ibegbu C, Henderson S. Infection and immunity at the maternal–placental–fetal interface: Focus on HIV-1. Troph Res. 1998;12:103–24. [Google Scholar]
- 31.Hansen G, Berry G, DeKruyff RH, Umetsu DT. Allergen specific Th1 cells fail to counterbalance Th2 cell induced airway hyperactivity but cause severe airway inflammation. J Clin Invest. 1999;103:175–83. doi: 10.1172/JCI5155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chaouat G, Meliani AA, Martal J, Raghupathy R, Eliot J, Mosmann T, Wegmann TG. IL-10 prevents naturally occurring fetal loss in the CBAxDBA/2 mating combination, and local defect in IL-10 production in the abortion-prone combination is corrected by in vivo injection of IFN-τ. J Immunol. 1995;154:4261–8. [PubMed] [Google Scholar]
- 33.Seder RA, Paul WE. Acquisition of lymphokine-producing phenotype by CD4+ T cells. Annu Rev Immunol. 1994;12:635–73. doi: 10.1146/annurev.iy.12.040194.003223. [DOI] [PubMed] [Google Scholar]
- 34.Brown MA, Hural J. Functions of IL-4 and control of its expression. Crit Rev Immunol. 1997;17:1–32. doi: 10.1615/critrevimmunol.v17.i1.10. [DOI] [PubMed] [Google Scholar]
- 35.Yoshimoto T, Bendelac A, Watson C, Hu-Li J, Paul WE. Role of NK1.1+ T cells in a Th2 response and in immunoglobulin E production. Science. 1995;270:1845–7. doi: 10.1126/science.270.5243.1845. [DOI] [PubMed] [Google Scholar]
- 36.Noguchi M, Nakamura Y, Russell S, Ziegler SF, Tsang M, Cao X, Leonard WJ. Interleukin-2 receptor ‘gamma’ chain: a functional component of the interleukin-7 receptor. Science. 1993;262:1877–80. doi: 10.1126/science.8266077. [DOI] [PubMed] [Google Scholar]
- 37.Shirakawa I, Deichmann KA, Izuhara I, Mao I, Adra CN, Hopkin JM. Atopy and asthma: genetic variants of IL-4 and IL-13 signaling. Immunol Today. 2000;21:60–4. doi: 10.1016/s0167-5699(99)01492-9. 10.1016/s0167-5699(99)01492-9. [DOI] [PubMed] [Google Scholar]
- 38.Szabo SJ, Dighe AS, Gubler U, Murphy KM. Regulation of the interleukin (IL)-12R beta subunit expression in developing T helper 1 (Th1) and Th2 cells. J Exp Med. 1997;185:817–24. doi: 10.1084/jem.185.5.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Koch F, Stanzl U, Jennewein P, Janke K, Heufler C, Kampgen E, Romani N, Schuler G. High level IL-12 production by murine dendritic cells: Upregulation via MHC Class II and CD40 molecules and downregulation by IL-4 and IL-10. J Exp Med. 1996;184:741–6. doi: 10.1084/jem.184.2.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hsieh CS, Heimberger AB, Gold JS, O'Garra A, Murphy KM. Differential regulation of T helper phenotype development by interleukins 4 and 10 in an alpha beta T-cell-receptor T cell transgenic system. Proc Natl Acad Sci, USA. 1992;89:6065. doi: 10.1073/pnas.89.13.6065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Erard F, Wild M-T, Garcia-Sanz JA, Le Gros G. Switch of CD8 T cells to noncytolytic CD8–CD4– cells that make Th2 cytokines and help B cells. Science. 1993;260:1802–5. doi: 10.1126/science.8511588. [DOI] [PubMed] [Google Scholar]
- 42.Sadick MD, Heinzel FP, Holaday BJ, Pu RT, Dawkins RS, Locksley RM. Cure of murine leishmaniasis with anti-interleukin 4 monoclonal antibody. Evidence for a T cell-dependent, interferon gamma-independent mechanism. J Exp Med. 1990;171:115–27. doi: 10.1084/jem.171.1.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kopf M, Le Gros G, Bachmann M, Lamers MC, Bluethmann H, Kohler G. Disruption of the murine IL-4 gene blocks Th2 cytokine responses. Nature. 1993;362:245–8. doi: 10.1038/362245a0. [DOI] [PubMed] [Google Scholar]
- 44.Kuhn R, Rajewsky K, Muller W. Generation and analysis of interleukin-4 deficient mice. Science. 1991;254:707–10. doi: 10.1126/science.1948049. [DOI] [PubMed] [Google Scholar]
- 45.Greenfield A, Scott D, Pennisi D, Ehrmann I, Ellis P, Cooper L, Simpson E, Koopman P. An H-Y Db epitope is encoded by a novel mouse Y chromosome gene. Nature Genet. 1996;14:474–8. doi: 10.1038/ng1296-474. [DOI] [PubMed] [Google Scholar]
- 46.Simpson E, Scott D, Chandler P. The male-specific histocompatibility antigen, H-Y: a history of transplantation, immune response genes, sex determination, and expression cloning. Annu Rev Immunol. 1997;15:39–61. doi: 10.1146/annurev.immunol.15.1.39. [DOI] [PubMed] [Google Scholar]
- 47.Bailey DW. An assay for histocompatibility gene mutations in mice. Genetics. 1979;92(Suppl.):s59–s62. [PubMed] [Google Scholar]
- 48.Matzinger P. The JAM test. A simple assay for DNA fragmentation and cell death. J Immunol Meth. 1991;145:185–92. doi: 10.1016/0022-1759(91)90325-a. [DOI] [PubMed] [Google Scholar]
- 49.Letterio JJ, Geiser AG, Kulkarni AB, Roche NS, Sporn MB, Roberts AB. Maternal rescue of transforming growth factor-β1 null mice. Science. 1994;264:1936–8. doi: 10.1126/science.8009224. [DOI] [PubMed] [Google Scholar]
- 50.Elliot CL, Kelly RW, Critchley H, Riley SC, Calder AA. Regulation of interleukin 8 production in the term human placenta during labor and by antigestagens. Am J Obstet Gynecol. 1998;179:215–20. doi: 10.1016/s0002-9378(98)70275-3. [DOI] [PubMed] [Google Scholar]
- 51.Hill JA, Polgar K, Harlow BL, Anderson DJ. Evidence of embryo and trophoblast toxic cellular immune response(s) in women with recurrent spontaneous abortion. Am J Obstet Gynecol. 1992;166:1044–52. doi: 10.1016/s0002-9378(11)90589-4. [DOI] [PubMed] [Google Scholar]
- 52.Lengerova A, Vojtiskova M. Postpartum reactivity of female mice to male specific antigens. Fol Biol. 1962;8:21–6. [Google Scholar]
- 53.Billingham RE, Silvers WK, Wilson DB. A second study on the H-Y transplantation antigen in mice. Proc R Soc Lond Series B. 1965;163:61–89. doi: 10.1098/rspb.1965.0060. [DOI] [PubMed] [Google Scholar]
- 54.Simpson E, Benjamin D, Chandler P. Nonresponsiveness to H-Y: tolerance in H-2b mice. Trans Proc. 1981;13:1880–3. [PubMed] [Google Scholar]
- 55.Jenkins MK, Schwartz RH. Antigen presentation by chemically modified splenocytes induces antigen-specific T cell unresponsiveness in vitro and in vivo. J Exp Med. 1987;165:302–19. doi: 10.1084/jem.165.2.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fuchs EJ, Matzinger P. B cells turn off virgin but not memory T cells. Science. 1992;258:1156–9. doi: 10.1126/science.1439825. [DOI] [PubMed] [Google Scholar]
- 57.Ridge JP, Fuchs EJ, Matzinger P. Neonatal tolerance revisited: turning on newborn T cells with dendritic cells. Science. 1997;271:1726–8. doi: 10.1126/science.271.5256.1723. [DOI] [PubMed] [Google Scholar]
- 58.Reiner SL, Seder RA. T helper cell differentiation in immune responses. Cur Opin Immunol. 1995;7:360–6. doi: 10.1016/0952-7915(95)80111-1. [DOI] [PubMed] [Google Scholar]
- 59.Powrie F, Leach MW, Mauze S, Caddle LB, Coffman RL. Phenotypically distinct subset of CD4+ T cells induce or protect from chronic intestinal inflammation in C.B-17 scid mice. Int Immunol. 1993;5:1461–71. doi: 10.1093/intimm/5.11.1461. [DOI] [PubMed] [Google Scholar]
- 60.Powrie F, Correa-Oliveira R, Mauze S, Coffman RL. Regulatory interactions between CD45RBhigh and CD45RBlow CD4+T cells are important for the balance between protective and pathogenic cell-mediated immunity. J Exp Med. 1994;179:589–600. doi: 10.1084/jem.179.2.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chen Y, Kuchroo VK, Inobe J, Hafler DA, Weiner HL. Regulatory T Cell clones induced by oral tolerance: suppression of autoimmune encephalomyelitis. Science. 1994;265:1237–40. doi: 10.1126/science.7520605. [DOI] [PubMed] [Google Scholar]
- 62.Lea RG, Flanders KC, Harley CB, Manuel J, Banwatt D, Clark DA. Release of a transforming growth factor (TGF)-beta 2-related suppressor factor from postimplantation murine decidual tissue can be correlated with the detection of a subpopulation of cells containing RNA for TGF-beta 2. J Immunol. 1995;148:778–87. [PubMed] [Google Scholar]
- 63.Daya S, Clark DA, Devlin CC, Jarrell J, Chaput A. Suppressor cells in human decidua. Am J Obstet Gynecol. 1985;151:267–70. doi: 10.1016/0002-9378(85)90024-9. [DOI] [PubMed] [Google Scholar]
- 64.Daya S, Rosenthal KL, Clark DA. Immunosuppressive factor (s) produced by decidua-associated suppressor cells: a proposed mechanism for fetal allograft survival. Am J Obstet Gynecol. 1987;156:344–50. doi: 10.1016/0002-9378(87)90281-x. [DOI] [PubMed] [Google Scholar]