Abstract
We investigated the role of interleukin-6 (IL-6) in the development of the immune response to a subunit vaccine against tuberculosis consisting of the culture filtrate proteins of Mycobacterium tuberculosis emulsified in the adjuvant dimethyldioctadecylammonium bromide (DDA). C57Bl/6 mice immunized with this vaccine developed a strong T helper 1 (Th1) response characterized by an increased production of interferon-γ (IFN-γ) secreted by CD4+ T cells. Neutralization of IL-6 during in vivo priming resulted in marked reduction in the ability of T cells to secrete IFN-γ and IL-2 and to proliferate. IL-6 gene-disrupted mice primed with the vaccine showed a decrease in the number of IFN-γ-producing cells and an increase in IL-4-secreting cells as compared to control mice. In contrast, neutralization of IL-6 during a boost of the vaccine in previously primed mice did not affect the development of IFN-γ-producing cells but still increased the number of IL-4-producing cells. Our work shows that IL-6 plays a major role in the priming but not in the later expression of a Th1 response to a tuberculosis vaccine.
Introduction
Tuberculosis is expected to kill more than 70 million people over the next three decades.1 Nevertheless, the only method used in humans to prevent this disease is a vaccine known as bacille Calmette–Guérin (BCG) whose protection varies from 0 to 80%.2,3 In order to develop a more effective vaccine against tuberculosis it is important to understand how the protective immune response develops against its causative agent – Mycobacterium tuberculosis – and then define the vaccine components that will elicit it. T cells bearing the CD4 coreceptor molecule (T helper (Th) cells) can be classified as type 1 cells (Th1), if they secrete interferon-γ (IFN-γ) and interleukin (IL)-2 or type 2 cells (Th2), if they secrete IL-4 and IL-5.4 In M. tuberculosis infections Th1 cells have been described as the main protective phenotype (for a review see 4 and 5).
A subunit vaccine based on the culture filtrate proteins of M. tuberculosis (ST-CF) and the adjuvant dimethyldioctadecylammonium bromide (DDA) induced protective immunity at levels similar to BCG.6 We have recently examined the cytokines involved in the immune response to it, showing that both IL-6 and IL-12 are important in the generation of T cells able to secrete IFN-γ and to confer protective immunity.7 Furthermore, we have demonstrated that IL-12 was able to work as a coadjuvant in the vaccine increasing the protection against a M. tuberculosis challenge, in agreement with the key role of this cytokine in the development of Th1 cells.8 Yet, the role of IL-6 in the generation of a Th1 response to this vaccine remained unclear. It has been shown that IL-6 is required for the induction of a protective Th1 response during mycobacterial9–11 as well as listerial infections.12–14 Others have shown that IL-6 is involved in the generation of Th2 cells.15,16 To further understand the immune response against this vaccine it is important to know how IL-6 is involved in the promotion of Th1 cells and how important it is in its establishment.
In previous work7 we showed that IL-6 was required for the in vivo priming of IFN-γ production in mice immunized with the ST-CF vaccine. These data contrasted with the in vitro effects of IL-6 reported earlier17 and confirmed by us.7 We then suggested that IL-6 may play distinct roles according to the state of activation of T cells, with an effect on naive T cells and a distinct and possible opposite effect upon effector T cells. Here, we analysed the role of IL-6 in the priming of an immune response to the ST-CF/DDA vaccine as well as during an effector phase of the immune response.
Materials and methods
Animals
C57Bl/6 female mice, aged 8–12 weeks were purchased from Harlan Interfauna Iberica S.A. (Barcelona, Spain). IL-6 gene-knockout (IL-6-KO) male mice with a C57Bl/6 background (aged 8 weeks) were obtained in our laboratory by backcrossing the original strain (a kind gift from Manfred Kopf18), into a C57Bl/6 background for six generations and then screening the genomic DNA as described.18 C57Bl/6 male mice aged 8 weeks, purchased from Gulbenkian Institute (Oeiras, Portugal), were used as controls.
Reagents
A monoclonal antibody specific for IL-6 was purified from ascitic fluid of nude mice injected intraperitoneally (i.p.) with the hybridoma MP5-20F3 secreting rat immunoglobulin G1 (IgG1) specific for mouse IL-6 (DNAX, Palo Alto, CA). Rat immunoglobulin was obtained from sera of Lewis rats. Both ascites and serum were delipidated with an organic solvent (1 : 4 mixture of 1-butanol and ethyl ether), precipitated with 50% ammonium sulphate and dialysed against phosphate-buffered saline (PBS).
ST-CF was produced at the Statens Seruminstitut (Copenhagen, Denmark) as described previously.19 Briefly, M. tuberculosis (4 × 106 colony-forming units (CFU)/ml) was grown in modified Sauton medium without Tween-80 on an orbital shaker for 7 days. The culture supernatants were sterile filtered and concentrated on an Amicon YM3 membrane (Amicon, Danvers, MA).
Tissue culture reagents were from Gibco (Paisley, UK) and bacterial culture medium was from Difco (Sparks, MD).
Experimental vaccine
The experimental vaccine consisted of a mixture of ST-CF and DDA (Eastman Kodak Inc., Rochester, NY). DDA was dissolved in double-distilled water, warmed in a water bath at 80° for 10 min, cooled at room temperature, and mixed with an equal volume of ST-CF, so as to inject each animal with 125 µg of DDA and 25 µg of ST-CF in a total volume of 100 µl. A control mixture consisting of PBS and DDA was also prepared admixing a volume of PBS with an equal volume of dissolved DDA, so as to inject each animal with 125 µg of DDA in a total volume of 100 µl.
Immunizations
Mice were injected in each hind footpad with 50 µl of the vaccine (or the control preparation – PBS/DDA) after having been anaesthetized. The monoclonal antibody specific for IL-6 or the control antibody (rat immunoglobulin) were administered i.p. 2–3 hr before the vaccine in a dose of 2 mg per animal.
Lymphocyte cultures
Lymphocytes were obtained by preparing single-cell suspensions from lymph nodes (popliteal and inguinal nodes) by dispersion of the tissue through a sterilized stainless steel mesh. Cells were thoroughly washed and cultured in 96 well microtitre plates containing 2 × 105 cells per well in a volume of 200 µl of RPMI-1640 supplemented with 5 × 10−5 m 2-mercaptoethanol, 100 IU/ml penicillin, 100 µg/ml streptomycin, 2 mm 2-glutamine, and 10% (v/v) of fetal calf serum. ST-CF was used to stimulate cells in a concentration of 4 µg per ml. In some cultures the anti-CD4 (clone GK1.5), anti-CD8 (clone 2.43) or anti-β-galactosidase (clone GL117) monoclonal antibodies were added in a concentration of 10 µg per ml, at the onset of the culture period. Cell proliferation was investigated by pulsing cultures after 48 hr of incubation (0·5 µCi/per well [3H]thymidine). After incubation for 18–20 hr, plates were harvested and processed for liquid scintillation counting. All tests were carried out in triplicate. Supernatants from the cultures were also tested for the determination of cytokines by harvesting parallel cultures after 24 (IL-2) or 48 hr (IFN-γ) of incubation.
For the enzyme-linked immunospot (ELISPOT) assay, cells were cultured in 24-well plates, each well containing 4 × 106 cells in a volume of 1 ml of RPMI-1640 supplemented with 5 × 10−5 m 2-mercaptoethanol, 100 IU/ml penicillin, 100 µg/ml streptomycin, 2 mm 2-glutamine, and 5% (v/v) of fetal calf serum.
Cytokine enzyme-linked immunosorbent assay (ELISA)
The cytokine content in supernatants was determined by ELISA by using the coating/detecting antibody pairs R4-6A2 (American Type Culture Collection, Rockville, MD)/AN18 (DNAX) specific for mouse IFN-γ, and the coating/detecting antibody pairs JES6-1A12 (Pharmingen, San Diego, CA)/JES6-5H4 (Pharmingen) specific for mouse IL-2. The standards were made of recombinant mouse IFN-γ from Genzyme (Cambridge, MA) and recombinant mouse IL-2 from (Pharmingen). The detection limit of the IFN-γ ELISA ranged from 24 to 80 pg per ml and that of the IL-2 ELISA was 20 pg per ml.
ELISPOT technique
The ELISPOT assay was performed as described by Müller et al.20 with minor modifications introduced by Brandt et al.21 Briefly, microtitre plates (Dynatech Immulon, Helsinki, Finland) were coated with 2·5 µg of monoclonal rat anti-mouse IFN-γ (R4-6A2 cell line) per ml and were incubated overnight at 4°. Plates were emptied and blocked for 2 hr, followed by washing with PBS containing 0·05% Tween-20. Analyses were conducted on cells pooled from the lymph nodes (popliteal and inguinal nodes) of four or five mice per group. Cells were stimulated with 4 µg/ml of ST-CF in modified RPMI-1640 for 18–22 hr and subsequently cultured for 6·5 hr directly in the ELISPOT plates. For each group of cultured cells, six serial twofold dilutions were prepared with a starting concentration of 4 × 105 cells (every sample was run in duplicate). Cells were removed by washing the plates, and the site of cytokine secretion was detected by biotin-labelled rat anti-mouse IFN-γ monoclonal antibody (AN-18 cell line) and phosphatase-conjugated streptavidin. The enzyme reaction was developed with 0·9 mg of 5-bromo-4-chloro-3-indolylphosphate (BCIP; Sigma Chemical Co., St Louis, MO) per ml of substrate buffer (0·74 mm MgCl2, 0·1% Triton-X-405, and 9·6% 2-amino-2-methyl-1 propanol, pH 10·25) containing 0·6% agarose. Blue spots were counted microscopically. The relationship between the number of spots developed per well and the number of input cells was determined. The ELISPOT for IL-4 was performed in the same way, using the coating/detecting antibody pairs BVD4-1D11/BVD6-24G2 (DNAX) specific for mouse IL-4 and starting with a concentration of 1 × 106 cells for the serial twofold dilutions. Data are presented as the number of spots per 2 × 105 cells.
Statistical analysis
Student's t-test was used to compare differences between groups.
Results
In order to follow closely the immune response to the subunit vaccine we chose to immunize C57Bl/6 mice in their footpads and follow the immune response in their popliteal and inguinal lymph nodes. One administration of the vaccine induced an increase in the cellularity of the lymph nodes apparent from day 2, increasing until day 5 or 6 and dropping and stabilizing by day 11 at numbers similar to non-immunized nodes (data not shown).
Early neutralization of IL-6 inhibits priming of CD4+ T cells for IFN-γ secretion
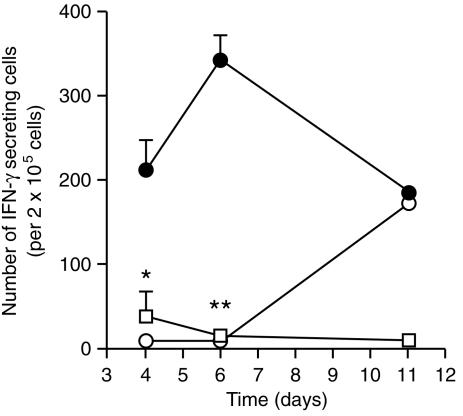
The production of IFN-γ could be detected in the lymph nodes of immunized mice as early as 4 days after immunization (data not shown). The peak for IFN-γ production could be observed between days 5 and 6 but decreased to very low levels after this time. Independently of the day after treatment, or the experiment, the levels of IFN-γ in the group where IL-6 had been neutralized were always near the detection limit (e.g. on day 6 the anti-IL-6 antibody treated group produced approximately 5·6% of the IFN-γ produced by the control group; data not shown). In the immunized animals, the number of lymph node cells secreting IFN-γ increased until day 6 and then disappeared from the lymph nodes (Fig. 1). In contrast, vaccinated animals which received anti-IL-6 antibodies had a delayed appearance of IFN-γ-producing cells that only reached the numbers of the immunized control group by day 11. In order to determine the phenotype of the T-cell populations secreting IFN-γ in response to the vaccine we added a monoclonal antibody against either the CD4 or the CD8 coreceptors to the in vitro stimulated cell cultures from immunized animals. The release of IFN-γ was blocked by the anti-CD4 monoclonal antibody (94% reduction) but not by the anti-CD8 monoclonal antibody (112% of the control response). Flow cytometry of the lymph node cells showed that the relative proportion of CD3+, CD4+, CD8+ and CD19+ cells remained very similar after depleting IL-6 (data not shown).
Figure 1.
IL-6 neutralization blocks IFN-γ priming of T cells in a primary vaccination. C57Bl/6 mice were injected in the hind footpads with PBS in DDA (open squares), ST-CF in DDA plus an irrelevant control antibody (rat globulin) (closed circles) or ST-CF in DDA plus a monoclonal antibody specific for IL-6 (open circles). Popliteal and inguinal lymph nodes were collected on days 4, 6 and 11 and the number of IFN-γ-secreting cells was determined by the ELISPOT technique. Only the results from antigen-stimulated cultures are shown since unstimulated cultures showed numbers of IFN-γ-producing cells below the detection limit (10 in 2 × 105 cells). Statistically significant effects of the antibody treatment are labelled *(for P < 0·05) and **(for P < 0·01), according to Student's t-test.
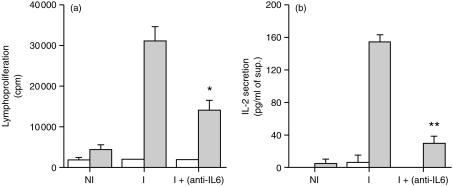
Neutralization of IL-6 hinders cell proliferation and IL-2 production in vaccinated animals
As shown in Fig. 2(a), the early in vivo neutralization of IL-6 reduced the proliferative response of cells from immunized animals by around 50%. Additionally, production of IL-2 was significantly reduced in the immunized animals whose IL-6 had been neutralized (Fig. 2b). Thus, the absence of IL-6 during the immunization with the vaccine led to a reduction in the proliferation of the antigen specific cells probably resulting from the diminished production of IL-2.
Figure 2.
IL-6 neutralization reduces cell proliferation and IL-2 production by T cells during a primary vaccination. C57Bl/6 mice were immunized as indicated in Fig. 1. Popliteal and inguinal lymph nodes were collected on day five and lymph node cells were cultured in vitro with (shaded bars) or without (open bars) the antigen (ST-CF). Cell proliferation was assayed 48 hours after stimulation (a) and IL-2 was measured in the cell culture supernatants 24 hours after stimulation (b). The results are expressed as mean±standard deviation of the results obtained per group. Statistically significant effects of the antibody treatment are labelled *(P < 0·05), **(P < 0·01), according to Student's t-test. NI, non-immunized; I, immunized.
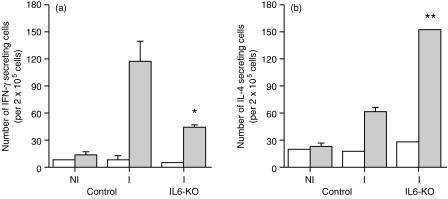
Immunized IL-6-KO mice show increased numbers of IL-4-producing cells
Both IL-6-KO and C57Bl/6 (control) mice were immunized with the vaccine and their lymph nodes collected on day five to detect cytokine-secreting cells using the ELISPOT assay. In agreement with the previous results, immunized IL-6-KO mice had a decreased number of IFN-γ-secreting cells, around one third of the number in control vaccinated mice (Fig. 3a). In contrast, the number of IL-4-secreting cells in IL-6-KO mice was 2·5 times higher than the one observed in the control animals (Fig. 3b).
Figure 3.
IL-6-KO mice show deficient priming for specific IFN-γ production and increased IL-4 secretion during a primary vaccination. C57Bl/6 (control) and IL-6-KO mice (five per group) were injected in the hind footpads with ST-CF in DDA (immune [I]). A group of C57Bl/6 animals was also injected with PBS in DDA (non-immune [NI]). Popliteal and inguinal lymph nodes were collected five days later and pooled lymph node cells were cultured in vitro with (shaded bars) or without (open bars) the antigen (ST-CF). The number of IFN-γ (a) and IL-4 (b) secreting cells was then determined by the ELISPOT. The detection limit for the IFN-γ ELISPOT was 10 and for the IL-4 ELISPOT 12 in 2 × 105 cells. Data were compared using Student's t-test, and significant differences as compared to immune wild-type mice are labelled *(for P < 0·05), **(for P < 0·01).
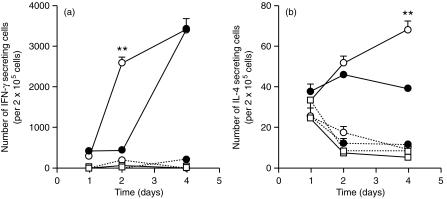
Effects of the neutralization of IL-6 during a secondary immunization
Immunized C57Bl/6 animals were treated with an IL-6-specific monoclonal antibody or a control antibody just before a second immunization with the vaccine. In the days that followed the boost, the number of IFN-γ-producing cells in control mice was up to 10-fold higher than the number found during a primary response (Fig. 4a). In contrast to a primary response, the neutralization of IL-6 accelerated the emergence of IFN-γ-producing cells but still increased the total number of IL-4-secreting cells (Fig. 4).
Figure 4.
Neutralization of IL-6 during a secondary vaccination stimulates both the IFN-γ and the IL-4 responses. C57Bl/6 animals (four per group) were injected in the hind footpads with PBS in DDA (open squares) or were immunized with ST-CF in DDA (circles) twice with a two weeks interval. Half of the immune mice were injected i.p. with a monoclonal antibody specific for IL-6 (open circles) 2 hr before the second immunization, while the other half received an irrelevant control immunoglobulin (closed circles). Popliteal and inguinal lymph nodes were collected on days 1, 2 and 4 and lymph node cells were cultured in vitro with (closed lines) or without (hatched lines) the antigen (ST-CF). The numbers of IFN-γ (a) and IL-4 (b) secreting cells was determined by the ELISPOT technique. Statistically significant effects of the antibody treatment at each time point are labelled **(P < 0·01), according to Student's t-test.
Discussion
It has been previously shown that IL-6 is needed for the development of protective T cells against intracellular parasite infections such as M. avium,9 L. monocytogenes13 and M. tuberculosis.10 In addition, it has been shown that despite being necessary for the development of such cells, once the cells are differentiated the cytokine is no longer needed.9,11,14 In some of these works, IL-6 was shown to be necessary for the development of a Th1 response as assessed by the ability of the antigen-specific T cells to secrete IFN-γ.11,14 We have previously demonstrated that the presence of IL-6 was necessary for the development of a T-cell response to a subunit vaccine able to protect against tuberculosis.7 Here we show that the emergence of IFN-γ-secreting CD4+ T cells during the first days that followed a primary immunization was severely hampered in the absence of IL-6. This was paralleled by decreased proliferative responses and a diminished ability to secrete IL-2. It is thus likely that IL-6 may promote the proliferation of CD4+ T cells through IL-2 secretion as previously shown.22 Reduced T-cell proliferation in the absence of IL-6 could be the reason for the reduced number of IFN-γ-secreting cells and consequently for the lower levels of this cytokine produced by lymph node cells. However, the role of IL-6 in clonal expansion of CD4+ T cells is controversial23 and the decreased IFN-γ production in the absence of IL-6 could instead be caused by a failure of these cells to differentiate.
The exposure of naive CD4+ T cells to IL-4 at the initiation of an immune response leads to their differentiation into Th2 cells.24–28 This cytokine is also able to inhibit Th1 development by decreasing the expression of the β 2 chain of the IL-12 receptor, thereby preventing the action of IL-12 on the naive CD4+ T cells and consequently their differentiation into the Th1 phenotype.8,29,30 In our model, immunized IL-6-KO mice had an increased number of IL-4-secreting cells. It is possible that the development of IL-4-producing cells in the absence of IL-6 may have impaired or delayed the development of the Th1 population.
We confirmed here that immunization with a vaccine consisting of ST-CF in DDA induces naive T cells to predominantly differentiate into a Th1 phenotype.31 In the absence of IL-6, however, there was an increase in the ability of immune cells to secrete IL-4. We did not assess whether IL-4 and IFN-γ were produced by the same or distinct types of cells. However, an increase in the IL-4-producing cells was also observed when IL-6 was neutralized at a second immunization. It is known that upon a second antigenic challenge, cytokine coexpression patterns become more Th1- or Th2-like rather than one that produces IL-4 and IFN-γ simultaneously.32 Whenever T-cell differentiation leads to the development of cells producing both IL-4 and IFN-γ, these represent a very small percentage of the total cell population.32 Furthermore, when T cells differentiate into the Th1 phenotype, they are not able to revert to the Th2 phenotype.33–35 Thus, we favour that the majority of IFN-γ- and IL-4-producing cells represent distinct cell types.
Neutralization of IL-6 had different consequences when it was done during the primary as compared to the secondary immunizations. The early neutralization decreased IFN-γ responses whereas the neutralization immediately before the boost did not. In contrast, neutralization of IL-6 always increased the IL-4 responses. Given the anamnestic increase in number of IFN-γ-secreting cells during the secondary response, we suggest that the majority of these latter IFN-γ-producing cells are probably generated from the same population that results from the first immunization and that expand upon restimulation. It is known that the cytokine environment determines the phenotype of the Th cell that will develop in response to an antigen.36,37 In this milieu, the Th2-inducing effect of IL-4 dominates over other cytokines, so that if IL-4 levels reach a certain threshold at the beginning of the immune response, Th2 differentiation is initiated and IL-4 production increases progressively preventing Th1 development.26,37,38 We have seen here that the presence of IL-6, in the beginning of the immune response to the ST-CF/DDA vaccine, can avoid the development of a Th2 phenotype. Thus, the effect of IL-6 neutralization on the lack of expansion of IFN-γ-secreting cells may be explained through the presence of IL-4. Whereas naive cells are susceptible to inhibition by IL-4 during a primary response, they become resistant to IL-4 after differentiation thence the differential effects of IL-6 neutralization at the two stages of the vaccination.
Our data, pointing to a role of IL-6 in T-cell differentiation namely inducing Th1 cytokines and decreasing Th2 cytokines, contrast with observations made by others which suggested that IL-6 is an inducer of Th2 responses.15,39,40 The possibility that our antibodies might be enhancing the activity of IL-6 rather than inhibiting it41 and thus explain these contradictory results was excluded given the similar data obtained with our antibodies and IL-6-KO mice. Furthermore, other in vivo models, namely studies on inflammatory autoimmune diseases, have shown that IL-6 upregulated the Th1 response and downregulated the production of the Th2 cytokine, IL-4.42–44 The reasons for the discrepancy in the data generated by all these groups are not clear. Some possible explanations may relate to the use of in vitro15,17 versus in vivo models, the use of different microbes such as the agents of schistosomiasis,16 borreliosis,39 or leishmaniasis40 in contrast to mycobacteria (7, 9–11, and our present results) or listeria,12–14 or the study of early differentiation steps as compared to already differentiated cells. Clearly, this is still open to much research before a clear view of the effects of IL-6 on T-cell differentiation can be obtained.
In summary, we show that IL-6 needs to be present in the early phases of immunization with a tuberculosis subunit vaccine to allow the differentiation of Th1 cells, presumably by preventing the emergence of a population of IL-4-producing cells. At later stages of vaccination, IL-6 has no effect on the clonal expansion of an already differentiated Th1 population.
Acknowledgments
This work was supported by grants from the Commission of the European Communities STD3 programme (contract TS3*-CT/94–0313) and INCO/DC programme (contract ERBIC18CT970254). I.S.L. received a fellowship from the PRAXIS XXI programme (Lisbon). The authors are grateful to Dr M. Kopf for supplying breeders of IL-6-KO mice and to Ana Isabel Correia for helpful assistance.
References
- 1.Murray CJL, Salomon JA. Modeling the impact of global tuberculosis control strategies. Proc Natl Acad Sci USA. 1998;95:13881–6. doi: 10.1073/pnas.95.23.13881. 10.1073/pnas.95.23.13881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fine PEM. The BCG story: lessons from the past and implications for the future. Rev Infect Dis. 1989;11:S353–S359. doi: 10.1093/clinids/11.supplement_2.s353. [DOI] [PubMed] [Google Scholar]
- 3.Verhoef J. The BCG contoversy. Int J Antimicrob Agents. 1994;4:291–5. doi: 10.1016/0924-8579(94)90028-0. [DOI] [PubMed] [Google Scholar]
- 4.Mosmann TR, Coffman RL. Th1 and Th2 cells: Different patterns of lymphokine secretion lead to different functional properties. Annu Rev Immunol. 1989;7:145–73. doi: 10.1146/annurev.iy.07.040189.001045. [DOI] [PubMed] [Google Scholar]
- 5.Kaufmann SHE, Andersen P. Immunity to mycobacteria with emphasis on tuberculosis: implications for rational design of an effective tuberculosis vaccine. Chem Immunol. 1998;70:21–59. doi: 10.1159/000058699. [DOI] [PubMed] [Google Scholar]
- 6.Andersen P. Effective vaccination of mice against Mycobacterium tuberculosis infection with a soluble mixture of secreted mycobacterial proteins. Infect Immun. 1994;62:2536–44. doi: 10.1128/iai.62.6.2536-2544.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Leal IS, Smedegård B, Andersen P, Appelberg R. Interleukin-6 and interleukin-12 participate in induction of a type 1 protective T-cell response during vaccination with a tuberculosis subunit vaccine. Infect Immun. 1999;67:5747–54. doi: 10.1128/iai.67.11.5747-5754.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gately MK, Renzetti LM, Magram J, Stern AS, Adorini L, Gubler U, Presky DH. The interleukin-12/interleukin-12-receptor system: role in normal and pathologic immune responses. Annu Rev Immunol. 1998;16:495–521. doi: 10.1146/annurev.immunol.16.1.495. [DOI] [PubMed] [Google Scholar]
- 9.Appelberg R, Castro AG, Pedrosa J, Minóprio P. Role of interleukin-6 in the induction of protective T cells during mycobacterial infections in mice. Immunology. 1994;82:361–4. [PMC free article] [PubMed] [Google Scholar]
- 10.Ladel CH, Blum C, Dreher A, Reifenberg K, Kopf M, Kaufmann SHE. Lethal tuberculosis in interleukin-6-deficient mutant mice. Infect Immun. 1997;65:4843–9. doi: 10.1128/iai.65.11.4843-4849.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Saunders BM, Frank AA, Orme IM, Cooper AM. Interleukin-6 induces early gamma interferon production in the infected lung but is not required for generation of specific immunity to Mycobacterium tuberculosis infection. Infect Immun. 2000;68:3322–6. doi: 10.1128/iai.68.6.3322-3326.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu Z, Simpson RJ, Cheers C. Recombinant interleukin-6 protects mice against experimental bacterial infection. Infect Immun. 1992;60:4402–6. doi: 10.1128/iai.60.10.4402-4406.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu Z, Simpson RJ, Cheers C. Role of IL-6 in activation of T cells for acquired cellular resistance to Listeria monocytogenes. J Immunol. 1994;152:5375–80. [PubMed] [Google Scholar]
- 14.Liu Z, Simpson RJ, Cheers C. Role of interleukin-6 in T-cell activation during primary and secondary infection with Listeria monocytogenes. Infect Immun. 1995;63:2790–2. doi: 10.1128/iai.63.7.2790-2792.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rincón M, Anguita J, Nakamura T, Fikrig E, Flavell RA. Interleukin (IL)-6 directs the differentiation of IL-4-producing CD4+ T cells. J Exp Med. 1997;3:461–9. doi: 10.1084/jem.185.3.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Flamme ACL, MacDonald AS, Pearce EJ. Role of IL-6 in directing the initial immune response to schistosome eggs. J Immunol. 2000;164:2419–26. doi: 10.4049/jimmunol.164.5.2419. [DOI] [PubMed] [Google Scholar]
- 17.VanHeyningen TK, Collins HL, Russell DG. IL-6 produced by macrophages infected with Mycobacterium species suppresses T cell responses. J Immunol. 1997;158:330–7. [PubMed] [Google Scholar]
- 18.Kopf M, Baumann H, Freer G, et al. Impaired immune and acute-phase responses in interleukin-6-deficient mice. Nature. 1994;368:339–42. doi: 10.1038/368339a0. [DOI] [PubMed] [Google Scholar]
- 19.Andersen P, Askgaard D, Ljungqvist L, Bentzon MW, Heron I. T-cell proliferative response to antigens secreted from Mycobacterium tuberculosis. Infect Immun. 1991;59:1558–63. doi: 10.1128/iai.59.4.1558-1563.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Müller I, Kropf P, Louis JA, Milon G. Expansion of gamma interferon-producing CD8+ T cells following secondary infection of mice immune to Leishmania major. Infect Immun. 1994;62:2575–81. doi: 10.1128/iai.62.6.2575-2581.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brandt L, Oettinger T, Holm A, Andersen ÅB, Andersen P. Key epitopes on the ESAT-6 antigen recognized in mice during the recall of protective immunity to Mycobacterium tuberculosis. J Immunol. 1996;157:3527–33. [PubMed] [Google Scholar]
- 22.Joseph SB, Miner KT, Croft M. Augmentation of naive, Th1 and Th2 effector CD4 responses by IL-6, IL-1 and TNF. Eur J Immunol. 1998;28:277–89. doi: 10.1002/(SICI)1521-4141(199801)28:01<277::AID-IMMU277>3.0.CO;2-8. 10.1002/(sici)1521-4141(199801)28:01<277::aid-immu277>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 23.Pape KA, Khoruts A, Mondino A, Jenkins MK. Inflammatory cytokines enhance the in vivo clonal expansion and differentiation of antigen-activated CD4+ T cells. J Immunol. 1997;159:591–8. [PubMed] [Google Scholar]
- 24.Gros GL, Ben-Sasson SZ, Seder R, Finkelman FD, Paul WE. Generation of interleukin 4 (IL-4)-producing cells in vivo and in vitro: IL-2 and IL-4 are required for in vitro generation of IL-4-producing cells. J Exp Med. 1990;172:921–9. doi: 10.1084/jem.172.3.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hsieh C-S, Heimberger AB, Gold JS, O'Garra A, Murphy KM. Differential regulation of T helper phenotype development by interleukins 4 and 10 in an αβ T-cell-receptor transgenic system. Proc Natl Acad Sci USA. 1992;89:6065–9. doi: 10.1073/pnas.89.13.6065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.O'Garra A. Cytokines induce the development of functionally heterogeneous T helper cell subsets. Immunity. 1998;8:275–83. doi: 10.1016/s1074-7613(00)80533-6. [DOI] [PubMed] [Google Scholar]
- 27.Swain SL, Weinberg AD, English M, Huston G. IL-4 directs the development of Th2-like helper effectors. J Immunol. 1990;145:3796–806. [PubMed] [Google Scholar]
- 28.Seder RA, Paul WE, Davis MM, Groth BFDS. The presence of interleukin 4 during in vitro priming determines the lymphokine-producing potential of CD4+ T cells from T cell receptor transgenic mice. J Exp Med. 1992;176:1091–8. doi: 10.1084/jem.176.4.1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Szabo SJ, Dighe AS, Gubler U, Murphy KM. Regulation of the interleukin (IL)-12Rβ2 subunit expression in developing T helper 1 (Th1) and Th2 cells. J Exp Med. 1997;185:817–24. doi: 10.1084/jem.185.5.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rogge L, Barberis-Maino L, Biffi M, Passini N, Presky DH, Gubler U, Sinigaglia F. Selective expression of an interleukin-12 receptor component by human T helper 1 cells. J Exp Med. 1997;185:825–31. doi: 10.1084/jem.185.5.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lindblad EB, Elhay MJ, Silva R, Appelberg R, Andersen P. Adjuvant modulation of immune responses to tuberculosis subunit vaccines. Infect Immun. 1997;65:623–9. doi: 10.1128/iai.65.2.623-629.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Openshaw P, Murphy EE, Hosken NA, Maino V, Davis K, Murphy K, O'Garra S. Heterogeneity of intracellular cytokine synthesis at the single-cell level in polarized T helper 1 and T helper 2 populations. J Exp Med. 1995;182:1357–67. doi: 10.1084/jem.182.5.1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Coffman RL, Mocci S, O'Garra A. The stability and reversibility of Th1 and Th2 populations. Curr Top Microbiol Immunol. 1999;238:1–12. doi: 10.1007/978-3-662-09709-0_1. [DOI] [PubMed] [Google Scholar]
- 34.Mocci S, Coffman RL. The mechanism of in vitro T helper cell type 1 to T helper cell type 2 switching in highly polarized Leishmania major-specific T cell populations. J Immunol. 1997;158:1559–64. [PubMed] [Google Scholar]
- 35.Perez VL, Lederer JA, Lichtman AH, Abbas AK. Stability of Th1 and Th2 populations. Int Immunol. 1995;7:869–75. doi: 10.1093/intimm/7.5.869. [DOI] [PubMed] [Google Scholar]
- 36.Paul WE, Seder RA. Lymphocyte responses and cytokines. Cell. 1994;76:241–51. doi: 10.1016/0092-8674(94)90332-8. [DOI] [PubMed] [Google Scholar]
- 37.Abbas AK, Murphy KM, Sher A. Functional diversity of helper T lymphocytes. Nature. 1996;383:787–93. doi: 10.1038/383787a0. [DOI] [PubMed] [Google Scholar]
- 38.Hsieh C-S, Macatonia SE, Tripp CS, Wolf SF, O'Garra A, Murphy KM. Development of Th1, CD4+ T cells through IL-12 produced by Listeria induced macrophages. Science. 1993;260:547–549. doi: 10.1126/science.8097338. [DOI] [PubMed] [Google Scholar]
- 39.Anguita J, Rincón M, Samanta S, Barthold SW, Flavell RA, Fikrig E. Borrelia burgdorferi-infected, interleukin-6-deficient mice have decreased Th2 responses and increased lyme arthritis. J Infect Dis. 1998;178:1512–5. doi: 10.1086/314448. 10.1086/314448. [DOI] [PubMed] [Google Scholar]
- 40.Saha B, Saini A, Germond R, Perrin PJ, Harlan DM, Davis TA. Susceptibility or resistance to Leishmania infection is dictated by the macrophages evolved under the influence of IL-3 or GM-CSF. Eur J Immunol. 1999;29:2319–29. doi: 10.1002/(SICI)1521-4141(199907)29:07<2319::AID-IMMU2319>3.0.CO;2-3. 10.1002/(sici)1521-4141(199907)29:07<2319::aid-immu2319>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 41.May LT, Neta R, Moldawer LL, Kenney JS, Patel K, Sehgal PB. Antibodies chaperone circulating IL-6. Paradoxical effects of anti-IL-6 ‘neutralizing’ antibodies in vivo. J Immunol. 1993;151:3225–36. [PubMed] [Google Scholar]
- 42.Ohshima S, Saeki Y, Mima T, et al. Interleukin 6 plays a key role in the development of antigen-induced arthritis. Proc Natl Acad Sci USA. 1998;95:8222–6. doi: 10.1073/pnas.95.14.8222. 10.1073/pnas.95.14.8222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Okuda Y, Sakoda S, Fujimura H, Saeki Y, Kishimoto T, Yanagihara T. IL-6 plays a crucial role in the induction phase of myelin oligodendrocyte glycoprotein 35–55 induced experimental autoimmune encephalomyelitis. J Neuroimmunol. 1999;101:188–96. doi: 10.1016/s0165-5728(99)00139-3. 10.1016/s0165-5728(99)00139-3. [DOI] [PubMed] [Google Scholar]
- 44.Okuda Y, Sakoda S, Fujimura H, Saeki Y, Kishimoto T, Yanagihara T. Enhancement of Th2 response in IL-6-deficient mice immunized with myelin oligodendrocyte glycoprotein. J Neuroimmunol. 2000;105:120–3. doi: 10.1016/s0165-5728(00)00192-2. 10.1016/s0165-5728(00)00192-2. [DOI] [PubMed] [Google Scholar]