Abstract
The role of T cells in the mouse collagen-induced arthritis (CIA) model for rheumatoid arthritis is not clarified, and different results have been reported concerning the role of CD4 and CD8 T cells. To address this issue, we have investigated B10.Q mice deficient for CD4 or CD8. The mice lacking CD4 were found to be less susceptible to disease, but not completely resistant, whereas the CD8 deficiency had no significant impact on the disease. No difference in the development of late occurring relapses was noted. Interestingly, the CD4-deficient mice had a severely reduced response to the glycosylated form of the immunodominant type II collagen (CII) 256–270 peptide whereas the response to the non-glycosylated peptide was not significantly different. Furthermore, CD4-deficient mice had lower antibody responses to CII, explaining the lower disease susceptibility. In comparison with previously reported results, it is apparent that the lack of CD4 molecules has a different impact on CIA if present on different genetic backgrounds, findings that could possibly be related to the occurrence of different disease pathways of CIA in different mouse strains.
Introduction
The main genetic association of rheumatoid arthritis (RA) is to the major histocompatibility complex (MHC) class II, and particularly human leucocyte antigen (HLA)-DR alleles with the shared epitope DRB1*0401.1–4 In the mouse collagen-induced arthritis (CIA), an RA model, the MHC association has been shown to be mediated by the class II molecule H2-Aq, a molecule that binds the same type II collagen (CII) peptide region as the DR4 (DRB1*0401) molecule.5–7 These associations to the MHC class II complex suggest involvement of a T-cell-mediated autoimmune recognition of joint-specific antigens,4,8,9 and that CII, being the major protein component of articular cartilage, is one of the possible candidates.
The finding that susceptibility to CIA is associated with MHC and furthermore in fact linked to a specific class II molecule, strongly suggests a role for class II restricted T cells in the disease. Further evidence is provided by observations that treatment with anti-CD4 antibodies before the disease onset partially prevents onset of arthritis10–12 and that mice lacking αβ T cells are completely protected from the disease.13 It is likely that one important role of the CII-reactive T cells is to activate B cells to produce pathogenic anti-CII antibodies.14,15 The role of T cells in the later phases of the disease is still unclear, as treatments with antibodies to CD4 or T-cell receptor (TCR) often have no or sometimes reversed effects if given after B-cell priming.16,17
On the other hand, therapeutic effects have been shown by blockage of T-cell costimulatory molecules,18 and treatment with antibodies to the TCR on autoreactive T cells.19 T cells may however, play different roles, as CII-reactive CD4+ T cells have been shown to protect against established disease,20 whereas under other circumstances they may induce mild arthritis.21,22
Recent studies have however, questioned whether there is any role of CD4+ T cells in CIA. In one study using DBA/1 mice deleted of CD4, no reduction in CIA incidence, clinical course or severity could be demonstrated.23 In another study, it was observed that recombinase activating gene (RAG)-deleted DBA/1 mice, lacking T and B cells, develop arthritis after immunization with CII.24
The role of CD8+ T cells in inflammatory diseases is also unclear, and in CIA, there are some possibly contrasting observations.25,26 It has been shown that CD8+ T cells play a role in the onset of diabetes in the non-obese diabetic (NOD) mouse,27 experimental myasthenia gravis,28 experimental allergic encephalomyelitis29,30 and autoimmune myocarditis.31 In the CIA model, depletion of CD8+ T cells has been reported not to have any significant effects32 in the rat, but to suppress arthritis in the mouse.33 Moreover, it has been shown that CII could be recognized by class I restricted T cells.25,34 It has been suggested that CD8+ T cells regulate arthritis,21,23 possibly through an effect on tolerance induction.35
To clarify these issues, we have bred CD4- and CD8-targeted deleted genes into a well-defined genetic background, the B10.Q, which is normally susceptible to CIA. We found that deletion of CD4 clearly suppressed disease susceptibility, lowered the anti-CII antibody responses, and dramatically reduced the T-cell response to the immunodominant glycosylated CII256–270 peptide.
Materials and methods
Mice
The CD4- and CD8-deficient mice were originally obtained by inserting a neomycin resistance gene (neoR) into the CD4 or CD8 gene using homologous recombination, thus disrupting the gene,36,37 and were generously provided by Ahnders Öhrn at Karolinska Institutet, Stockholm, Sweden, when they were backcrossed to C57Bl/6. The mice have subsequently been backcrossed up to 10 generations to the B10.Q strain in our animal facility. During backcrossing to the B10.Q background, the mice were screened for presence of the neoR gene using the polymerase chain reaction (PCR) technique. NeoR+/CD4+ and NeoR+/CD8+ mice were intercrossed once or twice to yield homozygous wild type, heterozygous, or homozygous deficient mice. Heterozygous mice were then intercrossed with deficient littermates in order to yield either heterozygous or homozygous deficient mice.
The mice were screened for CD4 and CD8 expression using a flow cytometry (FC) technique. One or two drops of blood were collected from a tail vein in each mouse. The blood was suspended in polystyrene tubes (Becton Dickinson, San Jose, CA) containing 300 µl FC medium (phosphate-buffered saline (PBS) + 0·5% bovine serum albumin (BSA) + 0·02% NaN3) and 25 µl heparin (Lövens, LeoPharma, Malmö, Sweden; 5000 IU/ml). The erythrocytes were lysed in a buffer containing 0·84% NH4Cl at pH = 7·4 for 3–5 min. The remaining cells were then washed in Dulbecco's modified Eagle's medium (DMEM), and then washed in FC medium before staining with antibodies. The monoclonal antibodies used for CD4 staining were phycoerythrin (PE)-conjugated H129 (Pharmingen, La Jolla, CA) and for CD8 staining FITC-conjugated 53-6.7 (Pharmingen). Each sample of cells was incubated for 25 min at 4° in the dark, with a solution consisting of 100 µl FC medium and an appropriate concentration of antibodies. After washing in FC medium, the cells were resuspended and fixed in 400 µl PBS containing 1% formaldehyde. The cells were then analysed on a FACSort using the CellQuest software (Becton-Dickinson).
All mice were kept in polystyrene cages with wooden shavings at the specific pathogen-free animal facility of the Medical Inflammation Research, at Lund University, Sweden. They were fed standard rodent chow and water ad libitum. They were also routinely screened for pathogens, and found negative for Sendai virus, mouse hepatitis virus, pneumonia virus of mice, and mycoplasma. For a detailed report, see http://net.inflam.lu.se/.
Induction of arthritis
Arthritis was induced using native rat collagen type II prepared from a rat chondrosarcoma, as previously described.38 It was dissolved in 0·1 m acetic acid, and resuspended 1 : 1 in Freund's complete adjuvant (FCA, Difco, Detroit, MI) to a final concentration of 1 mg/ml. This solution was homogenized, and 100 µl of the homogenate was injected intradermally at the base of the tail of both male and female mice on day 0. The mice were boosted on day 35 with a 50-µl injection intradermally at the base of the tail with a homogenate containing 50 µg collagen type II resuspended in Freund's incomplete adjuvant (FIA, Difco), and prepared as described above. On day 90, four to five mice of each type (female, male, heterozygous and deficient) were selected for a second booster. The selected mice had had severe arthritis, which had ameliorated to a minimum. The booster consisted of a 100-µl injection intradermally at the base of the tail with a homogenate containing 100 µg collagen type II resuspended in FIA. Animal experiments were approved by the local ethical committee.
Anti-CII serum titre measurements
Serum samples were collected from the mice on day 35 after immunization. Enzyme-linked immunosorbent assay (ELISA) plates (Costar Corporation, Cambridge, MA) were coated over night in room temperature with native rat collagen (10 µg/ml) dissolved in 0·1 m acetic acid with 0·02% NaN3. The plates were kept in a moisture chamber at 4° until used for ELISA.
Serum samples were diluted in PBS to an appropriate concentration, and 50 µl was added in duplicates to the plates. 50 µl of anti-collagen type II antiserum of known titre was added to the plates in duplicate, and was used as standard. A 1 : 10 serial dilution of the samples and standard was performed, and the plates were incubated at 4° in a moisture chamber over night.
After washing in a Tris–Tween buffer (0·13 m NaCl, 0·01 m Tris–HCl, 0·1% Tween-20), goat-anti-mouse immunoglobulin G (IgG)(Fc) antibodies (Jackson Immunoresearch Laboratories, Inc., Westgrove, PA), conjugated with alkaline phosphatase, were added. The plates were then incubated at room temperature in a moisture chamber for 2 hr. After washing in Tris–Tween buffer, bound enzyme was detected by adding disodium paranitrophenyl phosphate (Sigma 104 phosphatase substrate tablets, Sigma Aldrich, St Louis, MO) at a concentration of 1 mg/ml in 9·7% diethanolamine containing 0·5 mm MgCl2. The plates were stored in the dark until analysed at 405 nm using a Titretek Multiskan Plus spectrophotometer. Anti-CII titres were calculated as described elsewhere.39
Evaluation of arthritis and statistical analyses
The mice were checked three times per week for macroscopic signs of arthritis, i.e. swelling and erythema, using both a detailed variant and the originally described scoring protocol.40 Briefly, a score of 1 point was given for each swollen toe. Metatarsal swelling yielded an additional 0–5 points depending on severity, and involvement of the heel and ankle/wrist another 0–5 points. This results in a maximum of 15 points per paw. The score for each paw was summed up, to give an arthritic score of maximally 60 points for each mouse. The original protocol gives one point for the first swollen joint, two points for metatarsal involvement and three points for a completely swollen paw, giving a maximum of 12 points per mouse. Any mouse with an arthritic score greater than 0, persisting for more than a week, was considered arthritic.
If inflammation in a joint decreased and after five days or more increased again, it was considered as a relapse. Relapse percentage (relative relapse) in a certain paw was calculated according to the following equation:
rel. relapse = [(max score in a paw during relapse) − (min. score in between relapses)]/[(max score before relapse) − (min score in between relapses)]
P-values for arthritis incidence and relapses were calculated using the χ2 test, whereas both the Mann–Whitney rank sum test and Student's unpaired t-test were used for analysing the anti-CII antibody titres and the day of onset. The severity was evaluated using the maximum score obtained by each arthritic mouse. The severity scores in the groups were evaluated with the Mann–Whitney rank sum test.
T-cell assays and interferon-γ assays
Age matched mice deficient for CD4 and CD8 were immunized in the hind foot pads with 60 µg rat CII mixed 1 : 1 with complete H37 Ra adjuvant (Difco). Popliteal lymph nodes were removed 10 days after immunization, and single-cell suspensions were prepared in DMEM supplemented with 1% fresh mouse serum, HEPES, penicillin, streptomycin and β-mercaptoethanol.
To measure the antigen-specific proliferative response, the lymph node cells were put in cultures in flat-bottomed 96-well plates (Nunc, Roskilde, Denmark), stimulated with antigen for 72 hr before pulsing with 3H-TdR, and harvested 15 hr later in a Filtermate™ cell harvester (Packard Instruments, Meriden, CT). The antigens used were mycobacterial purified protein derivative (PPD), lathyritic CII, the CII peptide 256–270 lacking post-translational modifications and a monoglycosylated peptide (GalCII256–270) (i.e. galactose bound to hydroxylysine). PPD was used at a concentration of 10 µg/ml and all other antigens at 50 µg/ml. Peptides were a kind gift from Prof. J Kihlberg, Umeå University, Umeå, Sweden. The incorporation of 3H-TdR was determined in a matrix 96 Direct Beta Counter (Packard). All experiments were performed with triplicate cultures.
Before harvesting the cell cultures, 75 µl supernatant was removed from each well. Interferon-γ (IFN-γ) concentrations were determined using a sandwich ELISA with the rat-anti-mouse IFN-γ R46-A2 antibody as capturing antibody and the biotin-conjugated rat-anti-mouse AN-18.17.24 antibody as detecting antibody. Both antibodies were purified from culture supernatants and by affinity chromatography on protein G–Sepharose and conjugated. Alkaline phosphatase-conjugated Extravidin (Sigma) was added to the wells at 0·5 µg/ml, and bound enzyme was detected in the same manner as the serum anti-CII antibody concentrations described above.
Cytokine assays
Lymph node cells from untreated heterozygous and deficient mice and from CII-immunized deficient mice were suspended in DMEM supplemented with HEPES, penicillin, streptomycin, glutamine and 10% fetal calf serum (cell medium). 1·5 × 106 cells/ml were cultured in vitro for 6 days at 37°, 5% CO2 in cell medium containing either the lectin concanavalin A (Con A, 5 µg/ml, Pharmacia, Uppsala, Sweden) or the superantigen Staphylococcus aureus enterotoxin A (SEA, 175 ng/ml, kindly provided by Annette Sundstedt at Pharmacia, Lund, Sweden). The cells were re-stimulated with phorbol 12-myristate 13-acetate (PMA) 50 ng/ml and ionomycin 1 µg/ml for six hours in the presence of 3 µm of the protein transport inhibitor monensin (all from ICN Pharmaceuticals, Costa Mesa, CA) before staining. The cells were washed and resuspended in staining buffer (SB) containing 0·5% BSA (Sigma) and 0·01% NaN3 in PBS. Cells (1–2 × 106) were stained for CD4, CD8 and B220 for 20 min at 4°. The following antibodies were used: anti-CD4–PE (H129·19) and anti-CD8–fluoroscein isothiocyanate (FITC; (53.6.7, Pharmingen); anti-B220 (RA3-6B2)-Tricolor (TC), anti-CD4–TC (CT–CD4), anti-CD8–TC (CT–CD8) (all three from Caltag Laboratories, Burlingame, CA). An anti-FcγRII/IIIε(2.4.G2) purified monoclonal antibody (mAb) was used to inhibit antibody binding to the Fcγ receptors. For the intracellular staining of cytokines, the cells were fixated in 1% formaldehyde/PBS over night at 4°. For permeation, and to inhibit unspecific antibody binding, the cells were incubated with 5% normal rat serum/1% saponin (Sigma) in SB for 30 min at 4°. The cells were washed in intracellular staining buffer (ISB) containing 0·025% digitonin (Sigma), 1% saponin, 2% BSA, 0·01% NaN3, PBS before intracellular staining. Intracellular staining was performed in ISB at 4° for 20 min, using the following antibodies: anti-IFN-γ–FITC (AN-18.17.24 and R46-A2), anti-interleukin (IL)-2–FITC (S4B6) all three purified and conjugated; anti-IL-4–PE (11B11) and anti-IL-10–PE (JES5-16E3), both purchased from Pharmingen. FITC- and PE-conjugated monoclonal antibodies with the same isotype as the anti-cytokine antibodies were used as negative controls. CD3ε (145-2C11) (purified and conjugated) was stained intracellularly after blocking of surface expressed CD3ε as a positive control for the intracellular staining procedure. To further ensure the specificity of the staining procedure CD3ε binding was blocked by molar excess of pure anti-CD3ε antibody before the conjugated antibody was added. The cells were washed twice with ISB and once with SB and resuspended in PBS before analysing them in a FACSort (Becton-Dickinson) using the CellQuest software. The results were analysed statistically by Student's unpaired t-test.
Results
CIA susceptibility in CD4-deficient mice
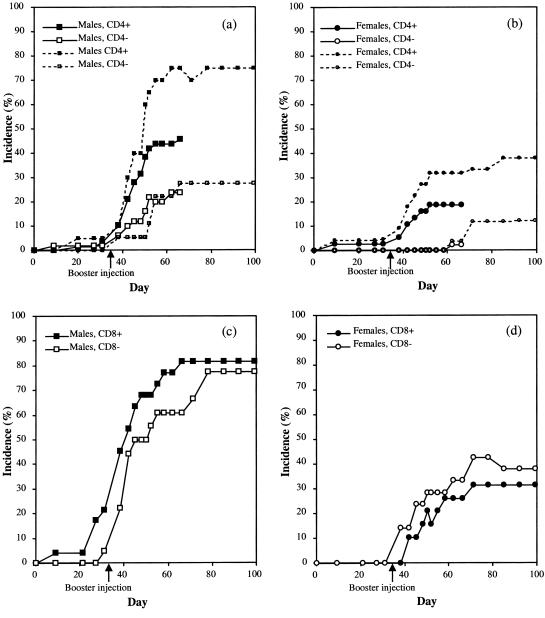
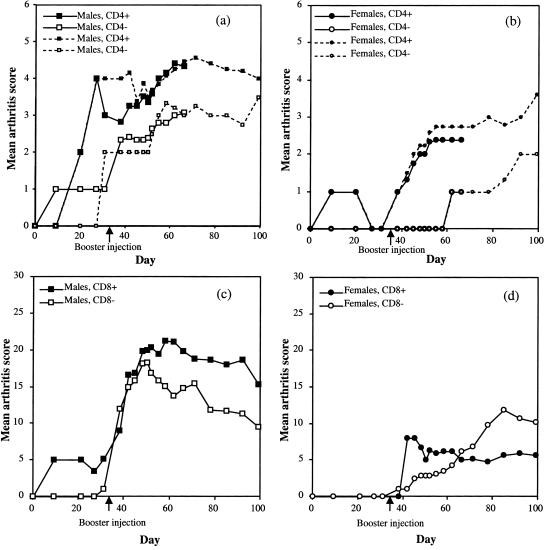
Heterozygous (B10.Q-CD4+/−) mice were mated with homozygous CD4-deficient mice to yield 50% CD4+/− and 50% CD4−/− mice, both of which were immunized with rat CII. Three independent experiments were performed. Arthritis development was followed for 66–99 days and added together (Figs 1 and 2). Mice were bled and boosted on day 35. Males were in general more susceptible than females as has been earlier observed,41 the results are therefore shown separated for the sexes. Both male and female mice deficient for CD4 were less susceptible to arthritis, in that fewer mice developed arthritis as compared with their heterozygous littermates (Table 1, Table 2). Furthermore the severity was lower in CD4 deficient males (Fig. 2a, Table 1). A few arthritic mice with minimal residual inflammation were selected for reimmunization on day 90 and then followed for another 90 days. Arthritis reappearing in previously swollen joints as well as arthritis in previously healthy joints was observed. Relapses occurred in both groups (Table 3). The anti-CII antibody serum titres on day 35 of the experiment differed significantly (P < 0·001) between the CD4-deficient and heterozygous mice (Tables 1 and 2). We also noted that both heterozygous and CD4-deficient male mice, the antibody titres at day 35 were significantly correlated to disease incidence. Arthritic male heterozygous mice (n = 27) had a mean anti-CII titre (± SD) of 246 ± 208 µg/ml serum whereas non-arthritic mice (n = 30), had 109 ± 216 µg/ml serum (P < 0·001). The pattern was similar in CD4-deficient males and in heterozygous females (data not shown). This suggests that an elevated antibody titre may contribute to development of disease at a later stage, and also further supports a role for the CD4 molecule in CIA.
Figure 1.
Incidence of CIA in CD4+/− and CD4−/− male (a) and female (b) mice, and in CD8+/− and CD8−/− male (c) and female (d) mice. The mice were immunized with 100 µg rat collagen type II in Freund's complete adjuvant, and given a booster injection of 50 µg rat collagen type II in Freund's incomplete adjuvant on day 35. (a) A significantly (P < 0·05) lower disease incidence could be seen in the CD4−/− male mice on day 66 (large symbols, solid lines). Results from three independent experiments were pooled. One experiment observed until day 99 presented for reference (small symbols, dashed lines). (b) A decrease in incidence (P < 0·05) could be seen also in the female CD4−/− mice (large symbols, solid lines). Results from two independent experiments were pooled. One experiment observed until day 99 included for reference (small symbols, dashed lines). (c, d) There were no significant differences between the CD8−/− and CD8+/− mice in either day of onset, severity or incidence.
Figure 2.
Mean arthritis score in afflicted CD4+/− and CD4−/− male (a) and female (b) mice, and in CD8+/− and CD8−/− male (c) and female (d) mice. The sharp decrease in the CD4+/− male mice on day 31 was a result of an increase in incidence, but with low scores, thus lowering the average. The CD4−/− female mice did show a tendency to have a lower severity index than the control group, although it could not be shown to be statistically significant, as too few mice had the disease. (c) Although the disease seemed to decline somewhat faster in CD8−/− male mice, this was not a statistically significant difference. (d) Among the female mice, disease in CD8−/− did not decrease over time.
Table 1.
CIA experiment in male CD4–/–, CD8–/– and their heterozygous littermates
| Strain | Incidence (%) | Mean day of onset | Mean maximal severity | Mean anti-CII titre d35 (µg/ml serum) |
|---|---|---|---|---|
| CD4+/– | 46 (n = 57) | 44 | 4·6 | 104 |
| CD4–/– | 24 (n = 50)* | 44 | 3·2* | 32·8*** |
| CD8+/– | 82 (n = 23) | 38 | 22 | 248 |
| CD8–/– | 78 (n = 18) | 50 | 16 | 173 |
The mean maximal severity for the CD4–/– animals are calculated using a 12-graded scoring system, whereas it was calculated using a 60-graded scoring system for the CD8–/– animals. The CD4-deficient mice differ from the control group in incidence and severity as well as anti-CII antibody titres.
(P < 0·05);
(P < 0·001).
Table 2.
CIA experiment in female CD4−/−, CD8−/− and their heterozygous littermates
| Strain | Incidence (%) | Mean day of onset | Mean maximal severity | Mean anti-CII titre d35 (µg/ml serum) |
|---|---|---|---|---|
| CD4+/− | 19 (n = 37) | 39 | 2·3 | 191 |
| CD4−/− | 2·5 (n = 40)* | 62 | 1·0 | 5·08*** |
| CD8+/− | 33 (n = 21) | 53 | 7·7 | 382 |
| CD8−/− | 43 (n = 21) | 51 | 12 | 368 |
The mean maximal severity for the CD4−/− animals are calculated using a 12-graded scoring system, whereas it was calculated using a 60-graded scoring system for the CD8−/− animals. The CD4-deficient mice differ from the control group in incidence as well as anti-CII antibody titres, as compared to the control group.
(P < 0·05);
(P < 0·01);
(P < 0·001).
Table 3.
Difference in relapses between male CD4–/– and CD8–/– mice and their heterozygous littermates
| Mouse type | Mean relapse* start† | Relapse incidence‡ | Mean relative relapse§ | Mice w/new inflammation¶ |
|---|---|---|---|---|
| CD4+ (n = 4) | 35 | 25% | 33% | 50% |
| CD4– (n = 5) | 23 | 20% | 10% | 40% |
| P (no diff.) | 0·10 | |||
| CD8+ (n = 5) | 21 | 80% | 18% | 60% |
| CD8– (n = 4) | 35 | 25% | 6.3% | 25% |
| P (no diff.) | 0·13 | 0·099 | 0·76 |
Increased swelling in a joint at least five days after amelioration was considered a relapse.
Average number of days after second reimmunization before relapses were observed.
The frequency of mice with relapses.
The severity of the new inflammation compared to swelling before amelioration as outlined in Materials and Methods.
The frequency of mice with swelling in previously unaffected joints.
The relapse start, relapse incidence and relative relapse do not differ significantly. The small delay in relapse start and lower relapse incidence in CD8–/– may indicate an activating role for the CD8 molecule in CIA.
CIA susceptibility in CD8-deficient mice
Heterozygous (B10.Q-CD8+/−) mice were mated with homozygous CD8-deficient mice to yield 50% CD8+/− and 50% CD8−/− mice, which were immunized with rat CII. Arthritis development was followed until day 99 (Fig. 1c,d) and severity was assessed using a detailed scale (Fig. 2c,d). Compared to the control group, the CD8-deficient mice did not differ in arthritis susceptibility or severity (Tables 1 and 2). There was no significant difference in disease onset (P = 0·06 as evaluated by the Mann–Whitney rank sum test) between CD8+/− and CD8−/− mice, although a trend towards later disease onset was noted (P = 0·03 as evaluated with Student's unpaired t-test). Mice were selected for reimmunization using the same criteria as for the CD4 mice. No significant difference was found after the boosting of the mice or in the late occurrence of arthritis relapses (Table 3). The anti-CII antibody titres were not different in the CD8-deficient mice as compared to the controls (Tables 1 and 2). As with the CD4-deficient and heterozygous mice, the anti-CII antibody titres on day 35 correlates positively with disease by the end of the experiment for the CD8-heterozygous mice (data not shown).
The T-cell response
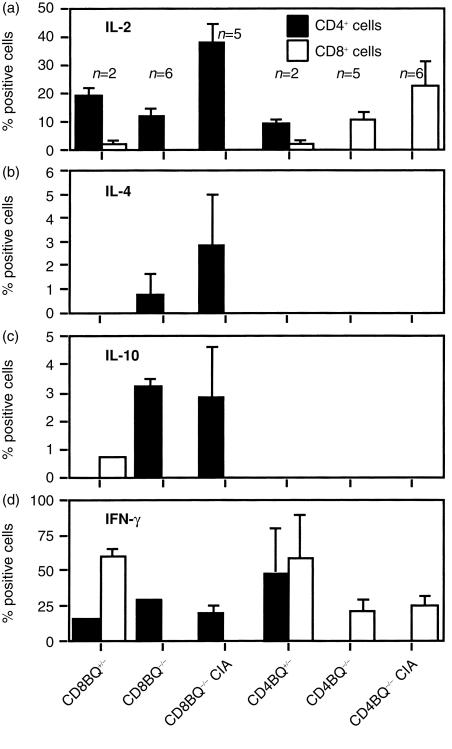
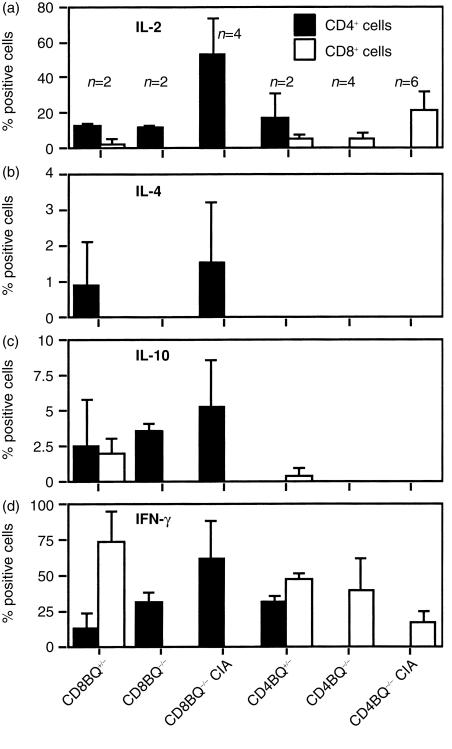
In order to test the T-cell response, we first investigated whether the CD4- and CD8-deficient mice had a skewed T-cell cytokine response. Coreceptor deficient mice were immunized and draining lymph nodes were excised 10 days later. Lymph nodes were also taken from non-immunized mice. Cells were cultured in vitro with Con A or SEA. After culture the cells were stained and the number of cytokine-producing cells were enumerated (Figs 3 and 4). The major difference noted was that the CD8-deficiency led to an increase of CD4+ T cells producing IL-4 and IL-10, which is unusual in the B10.Q background.42 Only a minority of cells produce these cytokines. It is also obvious that CD4+ cells are the major source of IL-2 in our system and that CD8+ cells produce IFN-γ to a large extent. Therefore, in order to compare the T-cell response to CII we selected to measure the IFN-γ secretion and proliferation. Mice were immunized with rat CII in the hind paws and cells from the popliteal lymph nodes were analysed for CII specific immune reactivity by assays of proliferation (Fig. 5) and IFN-γ secretion (Fig. 6) after stimulation with the immunodominant CII peptides in vitro. The immunodominant CII256–270 epitope is variably glycosylated through the hydroxylysine at position 264, which is also the major TCR recognition site. Here, both glycosylated and non-glycosylated forms of the peptide were tested in addition to CII. CD8-deficient lymph node cells and control cells proliferated equally well to the antigens tested. The CD4-deficient lymph node cells had a lower proliferation to the glycosylated peptide but not to the unmodified form (Fig. 5). Furthermore the IFN-γ response was lower to the glycosylated form in CD4-deficient mice. Overall IFN-γ responses appeared weaker both by ELISA and flow cytometry in CD8-deficient mice but effects were not significant either on arthritis or T-cell responses. On the other hand CD4-deficent mice had a lower incidence of arthritis and the disease was milder. These mice had a reduced response to the immunodominant glycopeptide.
Figure 3.
Cytokine production in lymph node cells after ConA stimulation in vitro. Single-cell suspensions were made from lymph nodes from unimmunized CD4+/−, CD4−/−, CD8+/− and CD8−/− mice and from CII-immunized CD4−/− and CD8−/− mice. Cells were cultured for 6 days in Con A-containing medium and were restimulated with PMA and ionomycin before for intracellular staining. Positive cells given in percent ±SD. (a) IL-2-positive cells; (b) IL-4-positive cells; (c) IL-10-positive cells; (d) IFN-γ-positive cells.
Figure 4.
Cytokine production in lymph node cells after SEA stimulation in vitro. Single-cell suspensions were made from lymph nodes from unimmunized CD4+/−, CD4−/−, CD8+/− and CD8−/− mice and from CII-immunized CD4−/− and CD8−/− mice. Cells were cultured for 6 days in SEA-containing medium and were restimulated with PMA and ionomycin before for intracellular staining. Positive cells given in percent ±SD. (a) IL-2-positive cells; (b) IL-4-positive cells; (c) IL-10-positive cells; (d) IFN-γ-positive cells.
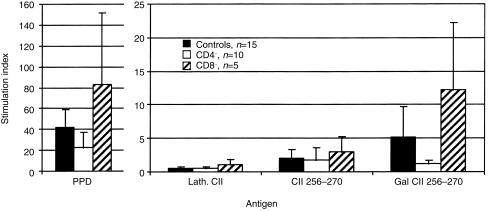
Figure 5.
T-cell stimulation index in male CD4−/−, CD8−/− and wild-type mice. Error bars indicate ± 1 SD. The different deficient mice responded in a similar way to different antigens, with one exception: The CD4-deficient mice responded to a significantly lower extent (P < 0·05) to the GalCII256–270 peptide than the control mice. Single-cell suspensions were made from popliteal lymph nodes of CII-immunized mice. Cells were cultured for 3 days, and T-cell proliferation measured by 3H-TdR incorporation. Background was less than 1000 c.p.m.
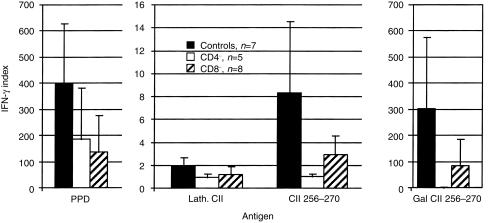
Figure 6.
IFN-γ response in T cell stimulation cultures from male CD4−/−, CD8−/− and wild-type mice. Error bars indicate ± 1 SD. As with the T-cell proliferation response, the CD4-deficient mice secreted significantly less IFN-γ (P < 0·05) in response to the Gal CII256–270 peptide than the control mice. Background was 8 U IFN-γ/ml in the CD4−/− and less than 3 U in the other groups.
Discussion
Our study supports a role for CD4-expressing T cells in CIA, as mice deficient for CD4 were less susceptible to arthritis, had a lower anti-CII antibody response, and a lower response to the immunodominant CII glycopeptide than their heterozygous littermates. Possibly because of a lack of statistical power, we were unable to find an influence by CD8 on acute or chronic arthritis, in contrast to earlier studies.23 Based on our results we cannot, however, exclude an influence on CIA exerted by the CD8 molecule, especially as there seemed to be a trend towards later onset in the CD8-deficient mice. Thus, our conclusions concerning the role of CD4 and CD8 in CIA in the B10.Q strain differ from the work by Tada et al.23 who found that CD8-deficient DBA/1 mice had less initial arthritis but pronounced relapses after CII boosting, whereas no effect was found by CD4 deficiency. Our studies were comparable in that CD4-deficient mice were not resistant, and some mice could develop severe arthritis and high antibody responses to CII. A similar situation was found in the experimental allergic encephalomyelitis (EAE) model for multiple sclerosis, which is unquestionable a T-cell dependent disease. These findings show that CD4 is dispensable in the activation of pathogenic T cells. Rahemtulla et al. have shown that CD4–/CD8–/TCRαβ+ cells in CD4 deficient mice are more mature than their counterparts in CD4+ mice, and that these cells have helper T cell activity.43 The CD4 molecule plays a role in signal transduction via the p56Lck protein, and also stabilizes the TCR–MHC-II–peptide complex, thus prolonging the signalling through CD3.44,45 However, these CD4 functions may not always be critical for T-cell activation. In fact, Tada et al.23 elegantly demonstrated that CD4 negative T cells could be CII-specific and MHC class II restricted. There are several important differences between our experiment and previous ones that may have important influence on the results. In most previous studies, the DBA/1 mouse has been used for induction of CIA. Although DBA/1 is highly susceptible for CIA, the reason for its high susceptibility may be that it partly develops a different type of CIA than other mouse strains, like B10.Q. A genetically dependent tendency of the DBA/1 to spontaneously develop enthesopathy in the joint, a pathologic process that is macroscopically similar to the development of autoimmune arthritis, may contribute to this strain difference.46,47 Interestingly, the spontaneous development of enthesopathy is not dependent on T cells and is then most likely unaffected by CD4.47 The same conditions that promote the development of spontaneous enthesopathy also promote arthritis after immunization with CII. The development of CIA in B10.Q, as well as in other strains, is a complex process since it contains different disease pathways involving for example immune complexes, T cells and protease-secreting mesenchymal cells. A similar situation is apparent in the rat, in which the DA rat develops a different type of arthritis after injection of type II collagen emulsified in mineral oil than after injection of mineral oil only.48 If the rat does not respond well to CII, it may get mineral oil-induced arthritis instead of collagen-induced arthritis. In addition to the possibility that enthesopathic responses cause a clinical disease reminiscent of CIA, there are genetic differences between DBA/1 and B10.Q that may show dramatic difference in a situation where CD4 or CD8 are lacking. For example, the B10.Q mice have a lower CD4/CD8 lymph node cell ratio than DBA/1 in particular after activation with T-cell mitogens.42 Clearly, there are several loci outside MHC that affect the disease outcome, as shown by a comparative study on CIA susceptibility in DBA/1 and B10.Q.49 Such a genetically based discrepancy has been demonstrated in studies of arthritis and EAE in mice deficient for several other important genes such as tumour necrosis factor-α (TNF-α), IFN-γ and IL-4.50 In our study T-cell activation was lower in CD4-deficient mice. Both proliferation and IFN-γ secretion were diminished. This lowered T helper activity probably caused the lower antibody response. Both these effects are likely to act in synergy causing the diminished incidence and severity of disease observed. There was a tendency towards a later onset in the CD8-deficient mice. This could be due to lower production of proinflammatory cytokines such as IFN-γ. The antibody response was an important factor because a positive correlation was found between antibody titre and incidence. The effects from antibody response did not override the protective effect of oestrogen seen in female mice. To sort out the precise role of the CD4 and CD8 molecules in a complex process such as arthritis requires more detailed studies both on disease pathways and their specific polygenic control. As a simplified conclusion based on the present and previous findings, it is however, reasonable to postulate that CD4-expressing T cells play an important role in the development of collagen induced arthritis.
Acknowledgments
We wish to express our gratitude towards Prof. Jan Kihlberg and Dr Björn Holm at the department of organic chemistry at Umeå University, Sweden, who synthesized and generously provided the peptides used in the T cell assays. We also wish to thank Lennart Lindström, Carlos Palestro and Marina Persson for taking good care of the animals. This work was supported by a grant from the program ‘Glycoconjugates in Biological Systems’ (GLIBS) sponsored by the Swedish Foundation for Strategic Research and by the Swedish Natural Science research council (NFR).
Abbreviations
- RA
rheumatoid arthritis
- MHC
major histocompatibility complex
- CIA
collagen-induced arthritis
- CII
type II collagen
- TCR
T-cell receptor
- FC
flow cytometry
- DMEM
Dulbecco's modified Eagle's medium
- FCA
Freund's complete adjuvant
- FIA
Freund's incomplete adjuvant
- Con A
concanavalin A
- SEA
Staphylococcus aureus enterotoxin A
- SB
staining buffer
- ISB
intracellular staining buffer
- EAE
experimental allergic encephalomyelitis
- IL
interleukin
- IFN
interferon
- TNF
tumour necrosis factor.
References
- 1.Wordsworth BP, Lanchbury JS, Sakkas LI, Welsh KI, Panayi GS, Bell JI. HLA-DR4 subtype frequencies in rheumatoid arthritis indicate that DRB1 is the major susceptibility locus within the HLA class II region. Proc Natl Acad Sci USA. 1989;86:10049–53. doi: 10.1073/pnas.86.24.10049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stastny P, Ball EJ, Dry PJ, Nunez G. The human immune response region (HLA-D) and disease susceptibility. Immunol Rev. 1983;70:112–53. doi: 10.1111/j.1600-065x.1983.tb00712.x. [DOI] [PubMed] [Google Scholar]
- 3.Campbell RD, Milner CM. MHC genes in autoimmunity. Curr Opin Immunol. 1993;5:887–93. doi: 10.1016/0952-7915(93)90101-w. [DOI] [PubMed] [Google Scholar]
- 4.Devereux D, O'Hehir RE, McGuire J, van Schooten WC, Lamb JR. HLA-DR4Dw4-restricted T cell recognition of self antigen (s) in the rheumatoid synovial compartment. Int Immunol. 1991;3:635–40. doi: 10.1093/intimm/3.7.635. [DOI] [PubMed] [Google Scholar]
- 5.Holmdahl R, Jansson L, Andersson M, Larsson E. Immunogenetics of type II collagen autoimmunity and susceptibility to collagen arthritis. Immunology. 1988;65:305–10. [PMC free article] [PubMed] [Google Scholar]
- 6.Holmdahl R, Andersson ME, Goldschmidt TJ, Jansson L, Karlsson M, Malmstrom V, Mo J. Collagen induced arthritis as an experimental model for rheumatoid arthritis. Immunogenetics, pathogenesis and autoimmunity. Apmis. 1989;97:575–84. doi: 10.1111/j.1699-0463.1989.tb00446.x. [DOI] [PubMed] [Google Scholar]
- 7.Brunsberg U, Gustafsson K, Jansson L, Michaëlsson E, Ährlund-Richter L, Pettersson S, Mattsson R, Holmdahl R. Expression of a transgenic class II Ab gene confers susceptibility to collagen-induced arthritis. Eur J Immunol. 1994;24:1698–702. doi: 10.1002/eji.1830240736. [DOI] [PubMed] [Google Scholar]
- 8.Albani S, Roudier J. Molecular basis for the association between HLA DR4 and rheumatoid arthritis. From the shared epitope hypothesis to a peptidic model of rheumatoid arthritis. Clin Biochem. 1992;25:209–12. doi: 10.1016/0009-9120(92)90328-p. [DOI] [PubMed] [Google Scholar]
- 9.Wordsworth P, Pile KD, Buckely JD, Lanchbury JS, Ollier B, Lathrop M, Bell JI. HLA heterozygosity contributes to susceptibility to rheumatoid arthritis. Am J Hum Genet. 1992;51:585–91. [PMC free article] [PubMed] [Google Scholar]
- 10.Ranges GE, Sriram S, Cooper SM. Prevention of type II collagen-induced arthritis by in vivo treatment with anti-L3T4. J Exp Med. 1985;162:1105–10. doi: 10.1084/jem.162.3.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ranges GE, Cooper SM, Sriram S. In vivo modulation by monoclonal anti-L3T4. I. Effects on humoral and cell-mediated immune response. Cell Immunol. 1987;106:163. doi: 10.1016/0008-8749(87)90159-6. [DOI] [PubMed] [Google Scholar]
- 12.Pelegri C, Morante MP, Castellote C, Franch A, Castell M. Treatment with an anti-CD4 monoclonal antibody strongly ameliorates established rat adjuvant arthritis. Clin Exp Immunol. 1996;103:273–8. doi: 10.1046/j.1365-2249.1996.d01-624.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Corthay A, Johansson A, Vestberg M, Holmdahl R. Collagen-induced arthritis development requires alpha beta T cells but not gamma delta T cells: studies with T cell-deficient (TCR mutant) mice. Int Immunol. 1999;11:1065–73. doi: 10.1093/intimm/11.7.1065. 10.1093/intimm/11.7.1065. [DOI] [PubMed] [Google Scholar]
- 14.Stuart JM, Dixon FJ. Serum transfer of collagen induced arthritis in mice. J Exp Med. 1983;158:378–92. doi: 10.1084/jem.158.2.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Svensson L, Jirholt J, Holmdahl R, Jansson L. B cell-deficient mice do not develop type II collagen-induced arthritis (CIA) Clin Exp Immunol. 1998;111:521–6. doi: 10.1046/j.1365-2249.1998.00529.x. 10.1046/j.1365-2249.1998.00529.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maeda T, Saikawa I, Hotokebuchi T, Sugioka Y, Eto M, Murakami Y, Nomoto K. Exacerbation of established collagen-induced arthritis in mice treated with an anti-T cell receptor antibody. Arthritis Rheum. 1994;37:406–13. doi: 10.1002/art.1780370315. [DOI] [PubMed] [Google Scholar]
- 17.Goldschmidt TJ, Holmdahl R. Therapeutic effects of monoclonal antibodies to alpha beta TCR but not to CD4 on collagen-induced arthritis in the rat. Cell Immunol. 1994;154:240–8. doi: 10.1006/cimm.1994.1072. 10.1006/cimm.1994.1072. [DOI] [PubMed] [Google Scholar]
- 18.Webb LM, Walmsley MJ, Feldmann M. Prevention and amelioration of collagen-induced arthritis by blockade of the CD28 co-stimulatory pathway: requirement for both B7-1 and B7-2. Eur J Immunol. 1996;26:2320–8. doi: 10.1002/eji.1830261008. [DOI] [PubMed] [Google Scholar]
- 19.Goldschmidt TJ, Holmdahl R. Anti-T cell receptor antibody treatment of rats with established autologous collagen-induced arthritis: suppression of arthritis without reduction of anti-type II collagen autoantibody levels. Eur J Immunol. 1991;21:1327–30. doi: 10.1002/eji.1830210536. [DOI] [PubMed] [Google Scholar]
- 20.Kakimoto K, Katsuki M, Hirofuji T, Iwata H, Koga T. Isolation of T cell line capable of protecting mice against collagen-induced arthritis. J Immunol. 1988;140:78–83. [PubMed] [Google Scholar]
- 21.Kadowaki KM, Matsuno H, Tsuji H, Tunru I. CD4+ T cells from collagen-induced arthritic mice are essential to transfer arthritis into severe combined immunodeficient mice. Clin Exp Immunol. 1994;97:212–8. doi: 10.1111/j.1365-2249.1994.tb06070.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Holmdahl R, Klareskog L, Rubin K, Larsson E, Wigzell H. T lymphocytes in collagen II-induced arthritis in mice. Characterization of arthritogenic collagen II-specific T-cell lines and clones. Scand J Immunol. 1985;22:295–306. doi: 10.1111/j.1365-3083.1985.tb01884.x. [DOI] [PubMed] [Google Scholar]
- 23.Tada Y, Ho A, Koh DR, Mak TW. Collagen-induced arthritis in CD4- or CD8-deficient mice: CD8+ T cells play a role in initiation and regulate recovery phase of collagen- induced arthritis. J Immunol. 1996;156:4520–6. [PubMed] [Google Scholar]
- 24.Plows D, Kontogeorgos G, Kollias G. Mice lacking mature T and B lymphocytes develop arthritic lesions after immunization with type II collagen. J Immunol. 1999;162:1018–23. [PubMed] [Google Scholar]
- 25.Gao X-M, McMichael AJ. Cytotoxic T lymphocytes specific for murine type II collagen do not trigger arthritis in B10 mice. Clin Exp Immunol. 1996;103:89–93. doi: 10.1046/j.1365-2249.1996.00921.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chiocchia G, Boissier MC, Manoury B, Fournier C. T cell regulation of collagen-induced arthritis in mice. II. Immunomodulation of arthritis by cytotoxic T cell hybridomas specific for type II collagen. Eur J Immunol. 1993;23:327–32. doi: 10.1002/eji.1830230204. [DOI] [PubMed] [Google Scholar]
- 27.Wang B, Gonzalez A, Benoist C, Mathis D. The role of CD8+ T cells in the initiation of insulin-dependent diabetes mellitus. Eur J Immunol. 1996;26:1762–9. doi: 10.1002/eji.1830260815. [DOI] [PubMed] [Google Scholar]
- 28.Zhang GX, Xiao BG, Bakhiet M, van der Meide P, Wigzell H, Link H, Olsson T. Both CD4+ and CD8+ T cells are essential to induce experimental autoimmune myasthenia gravis. J Exp Med. 1996;184:349–56. doi: 10.1084/jem.184.2.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Koh DR, Fung-Leung WP, Ho A, Gray D, Acha-Orbea H, Mak TW. Less mortality but more relapses in experimental allergic encephalomyelitis in CD8−/− mice. Science. 1992;256:1210–3. doi: 10.1126/science.256.5060.1210. [DOI] [PubMed] [Google Scholar]
- 30.Jiang H, Zhang SI, Pernis B. Role of CD8+ T cells in murine experimental allergic encephalomyelitis. Science. 1992;256:1213–5. doi: 10.1126/science.256.5060.1213. [DOI] [PubMed] [Google Scholar]
- 31.Penninger JM, Neu N, Timms E, Wallace VA, Koh DR, Kishihara K, Pummerer C, Mak TW. The induction of experimental autoimmune myocarditis in mice lacking CD4 or CD8 molecules [corrected] [published erratum appears in J Exp Med 1994; 179 (1): 317] J Exp Med. 1993;178:1837–1842. doi: 10.1084/jem.178.5.1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Larsson P, Goldschmidt TJ, Klareskog L, Holmdahl R. Oestrogen-mediated suppression of collagen-induced arthritis in rats. Studies on the role of the thymus and of peripheral CD8+ T lymphocytes. Scand J Immunol. 1989;30:741–7. doi: 10.1111/j.1365-3083.1989.tb02484.x. [DOI] [PubMed] [Google Scholar]
- 33.Arai K, Yamamura S, Hanyu T, Takahashi HE, Umezu H, Watanabe H, Abo T. Extrathymic differentiation of resident T cells in the joints of mice with collagen-induced arthritis. J Immunol. 1996;157:5170–7. [PubMed] [Google Scholar]
- 34.Chiocchia G, Boissier MC, Ronziere MC, Herbage D, Fournier C. T cell regulation of collagen-induced arthritis in mice. I. Isolation of Type II collagen-reactive T cell hybridomas with specific cytotoxic function. J Immunol. 1990;145:519–25. [PubMed] [Google Scholar]
- 35.Zhang L, Shannon J, Sheldon J, Teh HS, Mak TW, Miller RG. Role of infused CD8+ cells in the induction of peripheral tolerance. J Immunol. 1994;152:2222–8. [PubMed] [Google Scholar]
- 36.Rahemtulla A, Fung-Leung WP, Schilham MW, et al. Normal development and function of CD8+ cells but markedly decreased helper cell activity in mice lacking CD4. Nature. 1991;353:180–4. doi: 10.1038/353180a0. [DOI] [PubMed] [Google Scholar]
- 37.Fung-Leung WP, Schilham MW, Rahemtulla A, Kundig TM, Vollenweider M, Potter J, van Ewijk W, Mak TW. CD8 is needed for development of cytotoxic T cells but not helper T cells. Cell. 1991;65:443–9. doi: 10.1016/0092-8674(91)90462-8. [DOI] [PubMed] [Google Scholar]
- 38.Michaelsson E, Malmstrom V, Reis S, Engstrom A, Burkhardt H, Holmdahl R. T cell recognition of carbohydrates on type II collagen. J Exp Med. 1994;180:745–9. doi: 10.1084/jem.180.2.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Holmdahl R, Klareskog L, Andersson M, Hansen C. High antibody response to autologous type II collagen is restricted to H-2q. Immunogenetics. 1986;24:84–9. doi: 10.1007/BF00373114. [DOI] [PubMed] [Google Scholar]
- 40.Holmdahl R, Carlsén S, Mikulowska A, et al. Human Genome Methods. New York: CRC Press; 1998. Genetic analysis of murine models for rheumatoid arthritis; pp. 215–38. [Google Scholar]
- 41.Holmdahl R, Jansson L, Andersson M. Female sex hormones suppress development of collagen-induced arthritis in mice. Arthritis Rheum. 1986;29:1501–9. doi: 10.1002/art.1780291212. [DOI] [PubMed] [Google Scholar]
- 42.Johansson ÅCM, Vestberg M, Holmdahl R. Non-MHC dependent variations in lymphocyte activity between inbred mouse strains susceptible to various autoimmune diseases. Scand J Immunol. 2000;52:21–9. doi: 10.1046/j.1365-3083.2000.00738.x. 10.1046/j.1365-3083.2000.00738.x. [DOI] [PubMed] [Google Scholar]
- 43.Rahemtulla A, Kundig TM, Narendran A, et al. Class II major histocompatibility complex-restricted T cell function in CD4-deficient mice. Eur J Immunol. 1994;24:2213–8. doi: 10.1002/eji.1830240942. [DOI] [PubMed] [Google Scholar]
- 44.Cantrell D. T cell antigen receptor signal transduction pathways. Annu Rev Immunol. 1996;14:259–74. doi: 10.1146/annurev.immunol.14.1.259. [DOI] [PubMed] [Google Scholar]
- 45.Granja CB, Gozashti CS, Dasgupta JD. CD4-independent signal transduction through the T-cell receptor (TCR/CD3) Immunology. 1994;83:414–9. [PMC free article] [PubMed] [Google Scholar]
- 46.Holmdahl R, Andersson M, Goldschmidt TJ, Gustafsson K, Jansson L, Mo JA. Type II collagen autoimmunity in animals and provocations leading to arthritis. Immunol Rev. 1990;118:193–232. doi: 10.1111/j.1600-065x.1990.tb00817.x. [DOI] [PubMed] [Google Scholar]
- 47.Corthay A, Hansson AS, Holmdahl R. T lymphocytes are not required for the spontaneous development of entheseal ossification leading to marginal ankylosis in the DBA/1 mouse. Arthritis Rheum. 2000;43:844–51. doi: 10.1002/1529-0131(200004)43:4<844::AID-ANR15>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 48.Holmdahl R, Kvick C. Vaccination and genetic experiments demonstrate that adjuvant oil induced arthritis and homologous type II collagen induced arthritis in the same rat strain are different diseases. Clin Exp Immunol. 1992;88:96–100. doi: 10.1111/j.1365-2249.1992.tb03045.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yang HT, Jirholt J, Svensson L, Sundvall M, Jansson L, Pettersson U, Holmdahl R. Identification of genes controlling collagen-induced arthritis in mice: striking homology with susceptibility loci previously identified in the rat. J Immunol. 1999;163:2916–21. [PubMed] [Google Scholar]
- 50.Hultgren O, Kopf M, Tarkowski A. Outcome of Staphylococcus aureus-triggered sepsis and arthritis in IL-4-deficient mice depends on the genetic background of the host. Eur J Immunol. 1999;29:2400–5. doi: 10.1002/(SICI)1521-4141(199908)29:08<2400::AID-IMMU2400>3.0.CO;2-E. 10.1002/(sici)1521-4141(199908)29:08<2400::aid-immu2400>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]