Abstract
Interleukin-12 (IL-12) is a crucial cytokine for the generation of a protective immune response against Mycobacterium avium infection. In contrast to infected control mice, IL-12-deficient mice were unable to control bacterial proliferation and their spleen T cells were almost unresponsive in vitro to specific antigens of M. avium. Susceptibility of mice deficient in IL-12 was similar to that of interferon-γ (IFN-γ)-deficient mice. These data indicate a crucial role of IL-12 in the development of a T-cell population able to produce IFN-γ and to mediate protection against M. avium infection. Treatment of M. avium-infected mice with IL-12 induced CD4+ T cells with enhanced capacity to produce IFN-γ as well as to confer increased protection against M. avium.
Introduction
Human infection caused by Mycobacterium avium is rare in immunocompetent individuals and when it occurs it is most often manifested as cervical lymphadenitis in children1,2 or pulmonary disease in patients who usually have predisposing conditions such as chronic lung disease.3 Since the 1980s, with the increase in the number of individuals infected with human immunodeficiency virus (HIV), infections caused by M. avium have become one of the most prevalent opportunistic infections in patients with acquired immune deficiency syndrome (AIDS) in the United States,4–7 causing considerable morbidity and mortality.5,6,8,9 HIV-positive individuals become susceptible to M. avium infection when the number of CD4 T cells is below 100 cells per µl4,10 and dissemination of the infection occurs very quickly, involving multiple organs.4 Disseminated mycobacterial infections, namely by M. avium, were also observed in individuals with point mutations in genes encoding the subunits for the receptors of interferon-γ (IFN-γ)11–14 or interleukin-12 (IL-12),15 as well as in the IL-12 genes,16 leading to receptor deficiency or a non-functional cytokine, respectively.
IL-12 is a cytokine composed of two polypeptide chains, p40 and p35, encoded by separate genes, covalently linked to form a biologically active heterodimer, p70.17,18 Some reports have shown that IL-12 induces proliferation of activated, but not resting, CD4 and CD8 T cells.19–21 Moreover, IL-12 increases the production of IFN-γ by activated NK cells, CD4 and CD8 αβT cells, as well as γδT cells in response to various stimuli17,18,22–25 and is an essential factor for the differentiation of murine naïve T helper cells into type 1 (Th1) cells.17,18,25–29 In several murine models of intracellular infection, endogenous IL-12 has been shown to be crucial for the generation of a protective Th1 response during a primary infection.30–38 Furthermore, treatment of mice with IL-12, either singly during infection26,32,37,39,40 or as an adjuvant in a vaccine40–44 has been shown to protect or prevent infection by these infectious agents.
In previous studies we have demonstrated that IFN-γ is one of the molecules involved in the mechanisms of innate and acquired resistance to M. avium infection.45 The administration of recombinant IL-12 to either immunocompetent or immunodeficient severe combined immunodeficient (SCID) or CD4-depleted mice infected with M. avium 2447 accelerated the production of IFN-γ and the protective effect of the administration of recombinant IL-12 was dependent on the production of IFN-γ.40 In untreated mice, CD4+ T cells, and not CD8+ T cells, are the cell type involved in the mechanisms that control proliferation of M. avium.45 Given the variety of cell types responding to IL-12, including other T-cell populations as well as natural killer (NK) cells, it is important to assess specifically the role of IL-12 in the induction of immunity mediated by CD4+ T cells during M. avium infections.
Materials and methods
Reagents, cytokines and antibodies
Mycobacterial growth media were purchased from Difco (Sparks, MD). Dulbecco's modified Eagle medium (DMEM), RPMI-1640 medium, HEPES buffer, fetal calf serum (FCS), sodium pyruvate, penicillin/streptomycin, l-glutamine and protein G columns were purchased from Gibco Life Technologies (Paisley, UK). Tween-80, oleic acid, 2-mercaptoethanol (2-ME), potassium bicarbonate, bovine serum albumin (BSA), concanavalin A (ConA) and incomplete Freund's adjuvant (IFA) were purchased from Sigma (St. Louis, MO). The IFN-γ-specific immunoglobulin G1 (IgG1) monoclonal antibodies (mAb) were obtained from the hybridomas AN18 (DNAX, CA) and R4-6A2 (American Type Culture Collection; ATCC, Manassas, VA). Hybridomas were grown in ascites in HSD nude mice primed with IFA. Antibodies were purified by affinity chromatography using a protein G–agarose column followed by dialysis against phosphate-buffered saline (PBS). Fluorescein isothiocyanate (FITC)-conjugated rat anti-mouse CD4, phycoerythrin (PE)-conjugated rat anti-mouse CD8 and PE-conjugated rat anti-mouse CD3 were purchased from Pharmingen (San Diego, CA). Recombinant mouse IL-12 (rmIL-12) was kindly supplied by Dr S. Wolf from Genetics Institute (Cambridge, MA).
Mice
Specific pathogen-free BALB/c female mice were purchased from the Gulbenkian Institute for Science (Oeiras, Portugal). IL-12 p40 gene-disrupted (IL-12 p40–/–) and IFN-γ gene-disrupted (IFN-γ–/–) mice were purchased from Jackson Laboratories (Bar Harbor, ME) in a C57BL/6 background. As control, heterozygous mice (IL-12 p40+/–) with a C57BL/6 background or C57BL/6J mice were used. Outbred HSD nude mice were purchased from the Gulbenkian Institute for Science and used to raise ascites from hybridomas. These mice were kept in sterile housing conditions in cages provided with high efficiency particulate air (HEPA) filter-bearing caps. All mice were used at 6–8 weeks of age.
Bacterial infections
Mycobacterium avium strain 2447 (an AIDS isolate obtained from Dr F. Portaels, Institute of Tropical Medicine, Antwerp, Belgium) was grown in Middlebrook 7H9 medium containing 0·04% Tween-80 at 37° until the mid-log phase of growth. Bacteria were harvested by centrifugation and resuspended in a small volume of saline containing 0·04% Tween-80. The suspension was briefly sonicated with a Branson sonifier (15 seconds at 50 W) to disrupt bacterial clumps, diluted and frozen in aliquots at −70° until use. Mice were infected intravenously (i.v.) with 106 colony-forming units (CFU) of M. avium 2447 through a lateral tail vein. At different time-points, mice were killed by cervical dislocation and the organs were collected, homogenized, serially diluted in a 0·04% Tween-80 solution in distilled water and plated onto Middlebrook 7H10 agar medium. The plates were incubated for 1–2 weeks at 37° and the number of CFU was counted. The data are expressed as the mean of the log10 number of CFU recovered per organ ± standard deviation of the mean (for four or five animals).
Treatment of mice with rmIL-12
Treatment with rmIL-12 was performed by administration of 0·4 µg of rmIL-12 per animal intraperitoneally (i.p.) every other day from the beginning of infection and during the whole period of experimental infection. Control mice were injected i.p. with PBS.
Antigen preparations
Antigen preparations were obtained as described elsewhere.46 Briefly, M. avium 2447 was grown for 2 weeks in Sauton medium supplemented with 0·5% pyruvate and 0·5% glucose, and the culture supernatants were obtained by centrifugation of the bacteria and filtration through 0·45 µm filters. Such culture supernatants were concentrated 100-fold through ultrafiltration using filters with a molecular mass cut-off of 3000. The resulting preparation was then subjected to 80% ammonium sulphate precipitation followed by extensive dialysis against PBS. This reagent was therefore named culture filtrate protein from M. avium (CFP). Mycobacterium avium envelope proteins (MEP) were obtained from bacteria after sonicating the pellet in PBS containing 0·1% Tween-80, 1 mm MgCl2 (Merck, Darmstadt, Germany) and 1 mm benzamidine (Sigma) with pulses of 1 min at maximum power, keeping the sample in ice during the procedure. The sonicate was centrifuged to discard intact mycobacteria (30 min at 2700 g) and the supernatant was dialysed against PBS. The suspension was then ultracentrifuged for 2 hr at 150 000 g and the pellet, containing the envelope proteins, was resuspended in PBS.
Stimulation of spleen cells in vitro
Mice were killed on day 15 or day 60 of infection and spleens were collected. Half of the organ was used to prepare single cell suspensions. Spleen cells were depleted of red blood cells by incubation in haemolytic buffer (155 mm NH4Cl, 10 mm KHCO3, pH 7·2) for 5–10 min at room temperature and thoroughly washed. Spleen cells were resuspended in RPMI-1640 containing 10% heat-inactivated FCS, HEPES (10 mm), penicillin (100 U/ml), streptomycin (100 µg/ml), l-glutamine (1%), and 2-ME (0·05 mm) (complete RPMI) and cultured in triplicate at a density of 2 × 105 cells per well in duplicate 96-well plates either with no further stimulus or in the presence of ConA (5 µg/ml), CFP (4 µg/ml), or MEP (4 µg/ml) at 37° in a 7% CO2 atmosphere. Supernatants were harvested from triplicate cultures after 72 hr and stored at −20° until used for measurement of IFN-γ by enzyme-linked immunosorbent assay (ELISA).
Purification and culture of CD4+ T cells
Pools of nucleated spleen cells (5 × 106 cells/ml) were incubated in tissue culture Petri dishes at 37° in a 7% CO2 atmosphere for 3 hr to remove adherent cells. Non-adherent cells were centrifuged, resuspended in PBS containing 0·1% glucose, 1% BSA and 5 mm ethylenediaminetetraacetic acid (EDTA) pH 7·2 at a concentration of 1 × 108 cells per ml, and incubated with microbeads coated with anti-CD4 mAb (clone L3T4; Miltenyi Biotec, Germany) at 4° for 20 min. Cells were washed, resuspended in PBS/glucose/BSA/EDTA buffer (108 cells/500 µl) and CD4 T spleen cells were positively selected by using MiniMACS separation columns (Miltenyi) as described in the instructions from the manufacturer. CD4+ T cells [85–94% pure on fluorescence activated cell sorter (FACS) analysis] were washed, counted, and resuspended in complete RPMI at 2 × 106/ml. CD4+ T cells were stimulated in 96-well plates with irradiated (3000 rads) nucleated spleen cells as APC (1 × 107 cells/ml) plus ConA (5 µg/ml), CFP (4 µg/ml), or MEP (4 µg/ml) at 37° in 7% CO2 atmosphere. Supernatants were collected from triplicate cultures after 72 hr and frozen at −20° until the measurement of IFN-γ by ELISA.
Adoptive transfer of T cells
Spleens of non-infected mice or mice that had been infected for 3 weeks with M. avium strain 2447 and treated or not with rmIL-12 were aseptically collected and gently teased in RPMI-1640 medium containing 2% FCS (AT medium). Spleen cells were pooled (from five animals), washed and red blood cells were lysed as described above. Purified CD4+ T spleen cells were obtained as described above. Alternatively, T-cell-enriched populations were prepared as described previously.40 Cells were washed twice in AT medium, counted and injected i.v. in 0·5 ml of the same medium into recipient mice (four animals per group) that had been irradiated with a Cs source (500 rads/mouse) 24 hr earlier. After 2 hr, recipient mice were challenged with 106 CFU of the same M. avium strain. Mice were killed 30 days later, and viable counts were performed on the spleens and livers as described above. The protection achieved was calculated by subtracting the geometric mean of CFU in the organs of mice receiving immune CD4+ or enriched T cells from that in mice receiving non-immune CD4+ or enriched T cells. The percentage of CD4+ T spleen cells in the final cell suspension of selected CD4+ cells varied from 80 to 83% and the number of CD4+ T cells infused into recipient animals was approximately 7 × 106 CD4+ T cells per mouse.
Flow cytometry
To analyse the CD4+ T-cell-enriched spleen cells from uninfected and infected mice used in culture experiments and in adoptive transfer, 106 cells from the CD4+ T-cell pools were incubated for 30 min at 4° in a microtitre plate with PE-conjugated rat anti-mouse CD8 or PE-conjugated rat anti-mouse CD3 and FITC-conjugated rat anti-mouse CD4 mAbs. Cells were washed twice with staining medium (PBS containing 0·1% sodium azide and 3% FCS) and resuspended in staining medium containing propidium iodide (PI) to allow exclusion of dead cells. Flow cytometric analysis was performed with a FACScan apparatus (Becton Dickinson, Mountain View, CA) equipped with PCLysisII software. For each sample, 10 000 events were analysed.
Cytokine assays
IFN-γ levels in culture supernatants were quantified by a two-site sandwich ELISA using IFN-γ-specific affinity-purified mAbs (R4-6A2 as capture and biotinylated AN-18 as detecting antibodies) and a standard curve was generated with known amounts of recombinant mouse IFN-γ (Genzyme, CA). The sensitivity of the assay was 80 pg/ml.
Proliferation assay
Cell proliferation was measured by [3H]TdR incorporation. Briefly, proliferative responses were assessed after 48 hr of culture in a humidified atmosphere of 7% CO2 in air. Cultures were pulsed for 18–20 hr before harvesting with 0·5 µCi [3H]TdR (Amersham, UK) per well, and incorporation of [3H]TdR was measured by liquid scintillation. Results are expressed as mean counts per minute (c.p.m.) of triplicate cultures ± 1 standard deviation.
Statistical analysis
Statistical analysis was performed using the Student's t-test.
Results
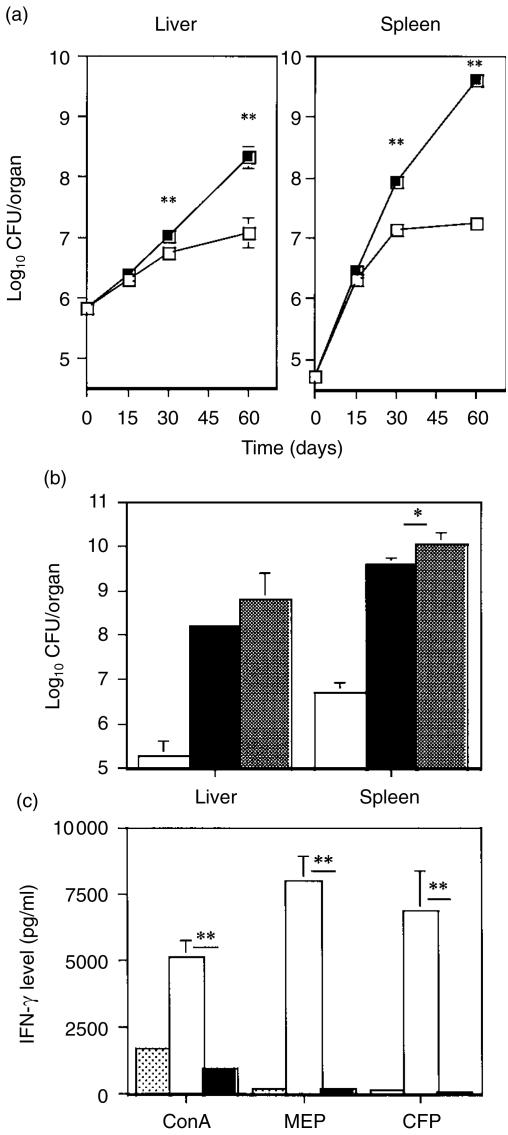
Mice deficient in the p40 subunit of IL-12 (IL-12 p40–/–) and their littermate controls (IL-12 p40+/–) were infected i.v. with 106 CFU of M. avium 2447 and the bacterial load was quantified at different time-points (Fig. 1a). As shown, IL-12 p40–/– mice had no ability to control bacterial proliferation whereas IL-12 p40+/– mice started to restrict mycobacterial growth between 2 and 4 weeks after infection. The susceptibility of the IL-12 p40–/– mice was also compared to that of IFN-γ–/– animals. As shown in Fig. 1(b), mycobacterial loads at 90 days of infection were higher in these two mutant strains than in wild-type C57BL/6 mice (P < 0·01 for both organs and for both strains). There was little difference between bacterial counts in the two mutated strains. At 60 days of infection, spleen cells from IL-12 p40–/– and IL-12 p40+/– mice infected with M. avium as well as non-infected IL-12 p40+/– mice were collected and stimulated in vitro with conA or with specific antigens from M. avium. As shown in Fig. 1(c), ConA-induced secretion of IFN-γ by spleen cells from infected IL-12 p40–/– mice was dramatically reduced when compared to those from heterozygous control mice. Splenocytes from infected IL-12 p40–/– mice were unresponsive in vitro to specific antigens of M. avium (MEP and CFP) whereas cells from infected controls responded with significant production of IFN-γ. The proliferative response to M. avium-specific antigens or ConA was identical for spleen cells from either infected IL-12 p40–/– or IL-12 p40+/– mice (not shown). At 60 days of infection there were no significant differences in spleen cell number between IL-12 p40–/– mice (1·1 × 108 ± 3·8 × 107 cells) and IL-12 p40+/– mice (1·3 × 108 ± 1·9 × 107 cells) or in the levels of IFN-γ found in spleen homogenates of those animals (IL-12 p40–/–, 423·8 ± 84·8 pg/ml; IL-12 p40+/–, 456·2 ± 45·2 pg/ml).
Figure 1.
Failure of IL-12 p40–/– mice to control M. avium infection and of their T cells to respond to specific antigens. IL-12 p40–/– mice and their IL-12 p40+/– littermates (a) and (c) or wild-type C57BL/6, IL-12 p40–/– and IFN-γ–/– mice (b) were infected i.v. with 106 CFU of M. avium 2447. (a) Number of viable bacteria present in the organs of IL-12 p40–/– (filled symbols) and IL-12 p40+/– (open symbols) mice. Each time-point represents the geometric mean of the CFU from four or five animals. (b) Number of viable bacteria present in the organs of C57BL/6 (open bars), IL-12 p40–/– (solid bars), and IFN-γ–/– (hatched bars) mice at 90 days of infection. Each value represents the geometric mean of the CFU from four or five animals. (c) In vitro production of IFN-γ by spleen cells from uninfected (stippled bars) or infected mice at 60 days of infection (IL12 p40+/–, open bars; IL12 p40–/–, solid bars) after stimulation with ConA or specific antigens of M. avium (MEP and CFP) for 72 hr. Values from non-stimulated spleen cells were below the detection limit of the assay and were not represented. Data are presented as the means ± SD of triplicates from individual mice for each group (four mice per group). Statistically significant differences between the two groups of infected mice are labelled as **P < 0·01.
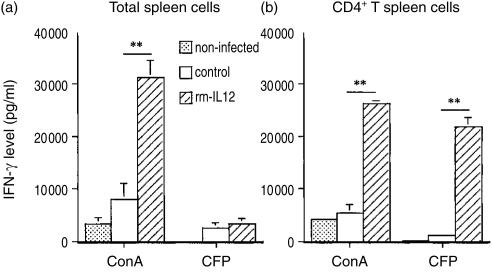
We next assessed the role of IL-12 in the differentiation of CD4+ T cells during M. avium infection. BALB/c mice were inoculated intravenously with 106 CFU of M. avium 2447 and were treated every other day with either rmIL-12 (0·4 µg/animal) or PBS i.p. At 15 days of infection total spleen cells from non-infected mice as well as infected mice, treated or not with rmIL-12, were stimulated in vitro with ConA or specific antigens of M. avium 2447 for 72 hr. Spleen cells from infected mice treated with IL-12 produced 3·9 times more IFN-γ when stimulated in vitro with ConA than those from infected control mice (Fig. 2a). However, the production of IFN-γ in response to specific mycobacterial antigens by spleen cells from the former mice was very low when compared with that in response to ConA. Additionally, the production of IFN-γ induced by CFP was very similar for spleen cells from infected mice regardless of the IL-12 treatment. However, flow cytometric analysis showed that the fraction of CD4+ (13·5%) and CD8+ (6·2%) cells in spleens of infected mice treated with rmIL-12 was lower when compared to those found in spleens of infected control mice (17·8% CD4+ cells and 10·7% CD8+ cells). Thus, the higher percentage of inflammatory cells, namely macrophages, induced by treatment with rmIL-1240 could be interfering negatively with the production of IFN-γ by T cells stimulated with specific mycobacterial antigens. Additionally, the absolute number of responding cells was also lower in the treated group. CD4+ cells were purified from the pools of spleen cells from the previous experiment and cultured with irradiated spleen cells plus ConA or CFP for 72 hr and the levels of IFN-γ in the supernatants were measured. As shown in Fig. 2(b), CD4+ T cells from spleens of IL-12-treated infected mice produced large amounts of IFN-γ in response to both ConA and CFP. In contrast, CD4+ T cells from spleens of infected control mice secreted 4·9 times less IFN-γ in response to ConA and 19 times less in response to CFP. FACS analysis showed that the number of CD4+ T cells in culture was similar for all groups of mice (9·1 × 104 cells for non-infected mice; 8·5 × 104 cells for IL-12-treated infected mice; 9·4 × 104 cells for infected control mice).
Figure 2.
Effect of rmIL-12 administration on the ability of spleen cells or purified CD4+ T cells from spleens of M. avium infected mice to produce IFN-γ after stimulation in vitro with M. avium-specific antigens. BALB/c mice were infected i.v. with M. avium and treated i.p. with rmIL-12 (hatched bars) or PBS (open bars) every other day during the 15 days of the experimental infection. At 15 days of infection, total spleen cells (a) or purified CD4+ T spleen cells (b) obtained from uninfected mice or infected mice were cultured in the presence of ConA (5 µg/ml), CFP (4 µg/ml), or medium alone. Supernatants from stimulated and unstimulated cell cultures were harvested at 72 hr and the amount of IFN-γ was measured by ELISA. IFN-γ levels in culture supernatants of unstimulated cells were below the detection limit of the assay and were not represented. Values are reported as means ± SD of triplicates from four individual mice for each group or pool of CD4+ T spleen cells from mice for each group (n = 4). The statistical significance of the differences between rmIL-12 versus PBS treatments is shown as **P < 0·01.
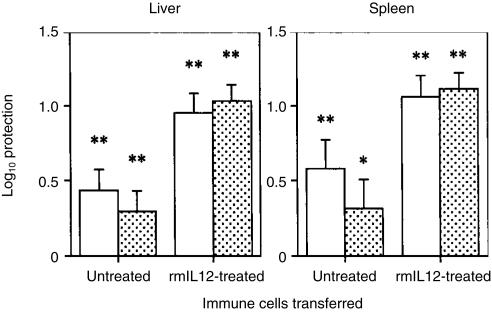
To test the ability of the CD4+ T-cell population developed in spleens of IL-12-treated and infected mice to induce protective immunity, a passive transfer of CD4+ T-cell-enriched spleen cells from infected animals treated or not treated with rmIL-12 was performed and compared to the effects of the transfer of non-selected T-cell-enriched spleen cells. Donor mice were infected i.v. with 106 CFU of M. avium 2447 for 3 weeks. During the period of infection, half of the animals were given rmIL-12 (0·4 µg/animal) and the other half was treated with PBS. At 21 days post-infection, spleen cells from these immune animals as well as spleen cells from non-immune controls were collected, enriched for CD4+ T cells or for whole T cells and infused into irradiated (500 rads) recipient animals. The latter were then challenged with 106 CFU of the same M. avium strain and mycobacterial growth was evaluated 30 days later. As shown in Fig. 3, the CD4+ T-cell population from spleens of immune mice treated with rmIL-12 conferred to recipient animals a significant and substantial protection against M. avium infection, in both liver and in spleen (equal to 1 log) contrasting with the poor protection afforded by the CD4+ T cells from infected mice that were not treated with IL-12. Protection afforded by the former cells was significantly higher than that conferred by the latter cells (P < 0·001 for both organs). The ability of the CD4+ T cells to protect was similar to that of the non-selected whole T-cell populations, suggesting that CD4+ T cells are the most important cell population, if not the only one, to protect against M. avium infection upon adoptive transfer. In independent experiments, depletion of CD8+ T cells did not affect the ability of immune T cells to protect, whereas protection was completely ablated by CD4+ T-cell depletion (data not shown).
Figure 3.
The administration of rmIL-12 enhances the generation of a protective CD4+ T-cell population. The log10 protection afforded by M. avium-immune whole T cells (open bars) or purified CD4+ T cells (stippled bars) from rmIL-12-treated and PBS-treated mice is shown. Five mice were used per group of donors and four were used per group of recipient animals. The total numbers of CD4+ T cells adoptively transferred to the irradiated recipient mice were 6·9 × 106 from uninfected, 6·5 × 106 from PBS-treated infected and 6·9 × 106 from IL-12-treated and infected group. Statistically significant protection (*P < 0·05 and **P < 0·01) was found with all immune cells. No statistically significant differences were found when comparing protection afforded by purified CD4+ T cells versus whole T cells.
Discussion
We used IL-12 gene-disrupted mice to show that IL-12 is a major player in protective immunity to M. avium. Similar data were previously obtained with the use of neutralizing mAbs30,36 and gene-disrupted animals.47 Although the absence of IL-12 and IFN-γ led to similar increases in susceptibility at 90 days of infection, we cannot exclude the possibility that IL-12 may induce additional effector molecules, leading to protective immunity. Indeed, we have already suggested that mechanisms of immunity to M. avium exist that involve signals other than IFN-γ.48 In any case, the protective activity of any additional IL-12-induced molecule or mechanism depends on the concomitant activity of IFN-γ as administration of recombinant IL-12 to IFN-γ–/– mice did not protect or was even slightly deleterious.40 Also, a role for an inducer cytokine in addition to IL-12 may still be envisaged. We believe that such a putative inducer plays a minor role and therefore has no major redundancy with regards to IL-12.
Splenic T cells from mice deficient in IL-12 had an impaired Th1 recall immune response to M. avium-specific antigens in vitro. According to the well known role of IL-12 on T-cell populations,17,19–21,26–29 these results suggest that a lack of endogenous IL-12 in this system leads to an impaired development of M. avium-specific IFN-γ-producing T cells. Given the strict requirement for IFN-γ in the development of protective immunity to M. avium, this defective priming of immune IFN-γ-producing T cells could, on its own, explain the increased susceptibility to infection. However, we cannot exclude that other protective effector functions of the same or distinct T cells may as well be induced by IL-12.
We specifically assessed that IL-12 acts on the CD4+ T-cell subset. This cell population had a higher capacity to secrete IFN-γ and conferred higher levels of protection upon adoptive transfer when exogenous recombinant IL-12 was given in vivo during its induction by infection. Thus, IL-12 can specifically promote specific immunity dependent on CD4+ T cells in addition to other possible effects on the innate immune system, namely NK cells,49 or on bystander responses.50 These data are particularly pertinent with regards to the possible use of this cytokine in vaccination protocols.40,51
In summary, our results highlight the potential use of IL-12 in modulating protective immunity to mycobacteria by acting on the major cell population responsible for mediating immunity against this opportunistic pathogen, the CD4+ T lymphocyte.
Acknowledgments
The authors are indebted to Dr S. Wolf and the Genetics Institute for the gift of recombinant IL-12 and to Dr T. F. Pais for supplying mycobacterial antigens. This work was supported by contract 13232/1998 from the PRAXIS XXI programme (Lisbon).
References
- 1.Wolinsky E. Mycobacterial diseases other than tuberculosis. Clin Infect Dis. 1992;15:1–10. doi: 10.1093/clinids/15.1.1. [DOI] [PubMed] [Google Scholar]
- 2.Wolinsky E. Mycobacterial lymphadenitis in children: a prospective study of 105 nontuberculous cases with long-term follow-up. Clin Infect Dis. 1995;20:954–63. doi: 10.1093/clinids/20.4.954. [DOI] [PubMed] [Google Scholar]
- 3.Prince DS, Peterson DD, Steiner RM, Gottlieb JE, Scott R, Israel HL, Figueroa WG, Fish JE. Infection with Mycobacterium avium complex in patients without predisposing conditions. N Engl J Med. 1989;321:863–8. doi: 10.1056/NEJM198909283211304. [DOI] [PubMed] [Google Scholar]
- 4.Horsburgh CR. Mycobacterium avium complex infection in the acquired immunodeficiency syndrome. N Engl J Med. 1991;324:1332–8. doi: 10.1056/NEJM199105093241906. [DOI] [PubMed] [Google Scholar]
- 5.Jacobson MA, Hopewell PC, Yajko DM, et al. Natural history of disseminated Mycobacterium avium complex infection in AIDS. J Infect Dis. 1991;164:994–8. doi: 10.1093/infdis/164.5.994. [DOI] [PubMed] [Google Scholar]
- 6.Nightingale SD, Byrd LT, Southern PM, Jockusch JD, Cal SX, Wynne BA. Incidence of Mycobacterium avium-intracellulare complex bacteremia in human immunodeficiency virus-positive patients. J Infect Dis. 1992;165:1082–5. doi: 10.1093/infdis/165.6.1082. [DOI] [PubMed] [Google Scholar]
- 7.Young LS, Inderlied CB, Berlin OG, Gottlieb MS. Mycobacterial infections in AIDS patients, with an emphasis on the Mycobacterium avium complex. Rev Infect Dis. 1986;8:1024–33. doi: 10.1093/clinids/8.6.1024. [DOI] [PubMed] [Google Scholar]
- 8.Benson CA. Disease due to the Mycobacterium avium complex in patients with AIDS. epidemiology and clinical syndrome. Clin Infect Dis. 1994;18:S218–22. doi: 10.1093/clinids/18.supplement_3.s218. [DOI] [PubMed] [Google Scholar]
- 9.Horsburgh CR, Havlik JA, Ellis DA, Kennedy E, Fann SA, Dubois RE, Thompson SE. Survival of patients with acquired immune deficiency syndrome and disseminated Mycobacterium avium complex infection with and without antimycobacterial chemotherapy. Am Rev Respir Dis. 1991;144:557–9. doi: 10.1164/ajrccm/144.3_Pt_1.557. [DOI] [PubMed] [Google Scholar]
- 10.Havlik JA, Horsburgh CR, Metchock B, Williams PP, Fann SA, Thompson SE. Disseminated Mycobacterium avium complex infection: clinical identification and epidemiologic trends. J Infect Dis. 1992;165:577–80. doi: 10.1093/infdis/165.3.577. [DOI] [PubMed] [Google Scholar]
- 11.Dorman SE, Holland SM. Mutation in the signal-transducing chain of the interferon-gamma receptor and susceptibility to mycobacterial infection. J Clin Invest. 1998;101:2364–9. doi: 10.1172/JCI2901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jouanguy E, Altare F, Lamhamedi S, et al. Interferon-gamma-receptor deficiency in an infant with fatal bacille Calmette-Guérin infection. N Engl J Med. 1996;335:1956–61. doi: 10.1056/NEJM199612263352604. [DOI] [PubMed] [Google Scholar]
- 13.Jouanguy E, Lamhamedi-Cherradi S, Lammas D, et al. A human IFNGR1 small deletion hotspot associated with dominant susceptibility to mycobacterial infection. Nature Genet. 1999;21:370–8. doi: 10.1038/7701. [DOI] [PubMed] [Google Scholar]
- 14.Newport MJ, Huxley CM, Huston S, Hawrylowicz CM, Oostra BA, Williamson R, Levin M. A mutation in the interferon-gamma-receptor gene and susceptibility to mycobacterial infection. N Engl J Med. 1996;335:1941–9. doi: 10.1056/NEJM199612263352602. [DOI] [PubMed] [Google Scholar]
- 15.de Jong R, Altare F, Haagen IA, et al. Severe mycobacterial and Salmonella infections in interleukin-12 receptor-deficient patients. Science. 1998;280:1435–8. doi: 10.1126/science.280.5368.1435. 10.1126/science.280.5368.1435. [DOI] [PubMed] [Google Scholar]
- 16.Altare F, Lammas D, Revy P, et al. Inherited interleukin 12 deficiency in a child with bacille Calmette-Guérin and Salmonella enteritidis disseminated infection. J Clin Invest. 1998;102:2035–40. doi: 10.1172/JCI4950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Trinchieri G. Interleukin-12: a cytokine at the interface of inflammation and immunity. Adv Immunol. 1998;70:83–243. doi: 10.1016/s0065-2776(08)60387-9. [DOI] [PubMed] [Google Scholar]
- 18.Trinchieri G. Interleukin-12: a proinflammatory cytokine with immunoregulatory functions that bridge innate resistance and antigen-specific adaptive immunity. Annu Rev Immunol. 1995;13:251–76. doi: 10.1146/annurev.iy.13.040195.001343. [DOI] [PubMed] [Google Scholar]
- 19.Bertagnolli MM, Lin BY, Young D, Herrmann SH. IL-12 augments antigen-dependent proliferation of activated T lymphocytes. J Immunol. 1992;149:3778–83. [PubMed] [Google Scholar]
- 20.Gately MK, Desai BB, Wolitzky AG, et al. Regulation of human lymphocyte proliferation by a heterodimeric cytokine, IL-12 (cytotoxic lymphocyte maturation factor) J Immunol. 1991;147:874–82. [PubMed] [Google Scholar]
- 21.Perussia B, Chan SH, D'Andrea A, et al. Natural killer (NK) cell stimulatory factor or IL-12 has differential effects on the proliferation of TCR-alpha beta+, TCR-gamma delta+ T lymphocytes, and NK cells. J Immunol. 1992;149:3495–502. [PubMed] [Google Scholar]
- 22.Chan SH, Perussia B, Gupta JW, et al. Induction of interferon gamma production by natural killer cell stimulatory factor: characterization of the responder cells and synergy with other inducers. J Exp Med. 1991;173:869–79. doi: 10.1084/jem.173.4.869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Croft M, Carter L, Swain SL, Dutton RW. Generation of polarized antigen-specific CD8 effector populations: reciprocal action of interleukin (IL) -4 and IL-12 in promoting type 2 versus type 1 cytokine profiles. J Exp Med. 1994;180:1715–28. doi: 10.1084/jem.180.5.1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gazzinelli RT, Hieny S, Wynn TA, Wolf S, Sher A. Interleukin 12 is required for the T-lymphocyte-independent induction of interferon gamma by an intracellular parasite and induces resistance in T-cell-deficient hosts. Proc Natl Acad Sci USA. 1993;90:6115–19. doi: 10.1073/pnas.90.13.6115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sad S, Marcotte R, Mosmann TR. Cytokine-induced differentiation of precursor mouse CD8+ T cells into cytotoxic CD8+ T cells secreting Th1 or Th2 cytokines. Immunity. 1995;2:271–9. doi: 10.1016/1074-7613(95)90051-9. [DOI] [PubMed] [Google Scholar]
- 26.Heinzel FP, Schoenhaut DS, Rerko RM, Rosser LE, Gately MK. Recombinant interleukin 12 cures mice infected with Leishmania major. J Exp Med. 1993;177:1505–9. doi: 10.1084/jem.177.5.1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hsieh CS, Macatonia SE, Tripp CS, Wolf SF, O'Garra A, Murphy KM. Development of TH1, CD4+ T cells through IL-12 produced by Listeria–induced macrophages. Science. 1993;260:547–9. doi: 10.1126/science.8097338. [DOI] [PubMed] [Google Scholar]
- 28.Macatonia SE, Hsieh CS, Murphy KM, O'Garra A. Dendritic cells and macrophages are required for Th1 development of CD4+ T cells from alpha beta TCR transgenic mice: IL-12 substitution for macrophages to stimulate IFN-gamma production is IFN-gamma-dependent. Int Immunol. 1993;5:1119–28. doi: 10.1093/intimm/5.9.1119. [DOI] [PubMed] [Google Scholar]
- 29.Manetti R, Parronchi P, Giudizi MG, Piccinni MP, Maggi E, Trinchieri G, Romagnani S. Natural killer cell stimulatory factor (interleukin 12 [IL-12]) induces T helper type 1 (Th1) -specific immune responses and inhibits the development of IL-4-producing Th cells. J Exp Med. 1993;177:1199–204. doi: 10.1084/jem.177.4.1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Castro AG, Silva RA, Appelberg R. Endogenously produced IL-12 is required for the induction of protective T cells during Mycobacterium avium infections in mice. J Immunol. 1995;155:2013–19. [PubMed] [Google Scholar]
- 31.Cooper AM, Magram J, Ferrante J, Orme IM. Interleukin 12 (IL-12) is crucial to the development of protective immunity in mice intravenously infected with Mycobacterium tuberculosis. J Exp Med. 1997;186:39–45. doi: 10.1084/jem.186.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cooper AM, Roberts AD, Rhoades ER, Callahan JE, Getzy DM, Orme IM. The role of interleukin-12 in acquired immunity to Mycobacterium tuberculosis infection. Immunology. 1995;84:423–32. [PMC free article] [PubMed] [Google Scholar]
- 33.Gazzinelli RT, Wysocka M, Hayashi S, Denkers EY, Hieny S, Caspar P, Trinchieri G, Sher A. Parasite-induced IL-12 stimulates early IFN-gamma synthesis and resistance during acute infection with Toxoplasma gondii. J Immunol. 1994;153:2533–43. [PubMed] [Google Scholar]
- 34.Heinzel FP, Rerko RM, Ahmed F, Pearlman E. Endogenous IL-12 is required for control of Th2 cytokine responses capable of exacerbating leishmaniasis in normally resistant mice. J Immunol. 1995;155:730–9. [PubMed] [Google Scholar]
- 35.Romani L, Mencacci A, Tonnetti L, Spaccapelo R, Cenci E, Puccetti P, Wolf SF, Bistoni F. IL-12 is both required and prognostic in vivo for T helper type 1 differentiation in murine candidiasis. J Immunol. 1994;153:5167–75. [PubMed] [Google Scholar]
- 36.Saunders BM, Zhan Y, Cheers C. Endogenous interleukin-12 is involved in resistance of mice to Mycobacterium avium complex infection. Infect Immun. 1995;63:4011–15. doi: 10.1128/iai.63.10.4011-4015.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sypek JP, Chung CL, Mayor SE, Subramanyam JM, Goldman SJ, Sieburth DS, Wolf SF, Schaub RG. Resolution of cutaneous leishmaniasis: interleukin 12 initiates a protective T helper type 1 immune response. J Exp Med. 1993;177:1797–802. doi: 10.1084/jem.177.6.1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tripp CS, Gately MK, Hakimi J, Ling P, Unanue ER. Neutralization of IL-12 decreases resistance to Listeria in SCID and C.B-17 mice. Reversal by IFN-gamma. J Immunol. 1994;152:1883–7. [PubMed] [Google Scholar]
- 39.Kobayashi K, Yamazaki J, Kasama T, Katsura T, Kasahara K, Wolf SF, Shimamura T. Interleukin (IL) -12 deficiency in susceptible mice infected with Mycobacterium avium and amelioration of established infection by IL-12 replacement therapy. J Infect Dis. 1996;174:564–73. doi: 10.1093/infdis/174.3.564. [DOI] [PubMed] [Google Scholar]
- 40.Silva RA, Pais TF, Appelberg R. Evaluation of IL-12 in immunotherapy and vaccine design in experimental Mycobacterium avium infections. J Immunol. 1998;161:5578–85. [PubMed] [Google Scholar]
- 41.Afonso LC, Scharton TM, Vieira LQ, Wysocka M, Trinchieri G, Scott P. The adjuvant effect of interleukin-12 in a vaccine against Leishmania major. Science. 1994;263:235–7. doi: 10.1126/science.7904381. [DOI] [PubMed] [Google Scholar]
- 42.Miller MA, Skeen MJ, Ziegler HK. Nonviable bacterial antigens administered with IL-12 generate antigen-specific T cell responses and protective immunity against Listeria monocytogenes. J Immunol. 1995;155:4817–28. [PubMed] [Google Scholar]
- 43.Mougneau E, Altare F, Wakil AE, et al. Expression cloning of a protective Leishmania antigen. Science. 1995;268:563–6. doi: 10.1126/science.7725103. [DOI] [PubMed] [Google Scholar]
- 44.Mountford AP, Anderson S, Wilson RA. Induction of Th1 cell-mediated protective immunity to Schistosoma mansoni by co-administration of larval antigens and IL-12 as an adjuvant. J Immunol. 1996;156:4739–45. [PubMed] [Google Scholar]
- 45.Appelberg R, Castro AG, Pedrosa J, Silva RA, Orme IM, Minoprio P. Role of gamma interferon and tumor necrosis factor alpha during T-cell-independent and -dependent phases of Mycobacterium avium infection. Infect Immun. 1994;62:3962–71. doi: 10.1128/iai.62.9.3962-3971.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pais TF, Cunha JF, Appelberg R. Antigen specificity of T-cell response to Mycobacterium avium infection in mice. Infect Immun. 2000;68:4805–10. doi: 10.1128/iai.68.8.4805-4810.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Doherty TM, Sher A. IL-12 promotes drug-induced clearance of Mycobacterium avium infection in mice. J Immunol. 1998;160:5428–35. [PubMed] [Google Scholar]
- 48.Flórido M, Gonçalves AS, Silva RA, Ehlers S, Cooper AM, Appelberg R. Resistance of virulent Mycobacterium avium to interferon-γ-mediated antimicrobial activity suggests additional signals for induction of mycobacteriostasis. Infect Immun. 1999;67:3610–18. doi: 10.1128/iai.67.7.3610-3618.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bermudez LE, Wu M, Young LS. Interleukin-12-stimulated natural killer cells can activate human macrophages to inhibit growth of Mycobacterium avium. Infect Immun. 1995;63:4099–104. doi: 10.1128/iai.63.10.4099-4104.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Letmemongkolchai G, Cai G, Hunter CA, Bancroft GJ. Bystander activation of CD8+ T, cells contributes to the rapid production of IFNγ in response to bacterial pathogens. J Immunol. 2001;166:1097–105. doi: 10.4049/jimmunol.166.2.1097. [DOI] [PubMed] [Google Scholar]
- 51.Silva RA, Pais TF, Appelberg R. Effects of interleukin-12 in the long-term protection conferred by a Mycobacterium avium sub-unit vaccine. Scand J Immunol. 2000;52:531–3. doi: 10.1046/j.1365-3083.2000.00816.x. 10.1046/j.1365-3083.2000.00816.x. [DOI] [PubMed] [Google Scholar]