Abstract
Donor T cells are crucial for target organ injury in graft-versus-host disease (GVHD). We examined the effects of donor T cells on the target organs using a parent-into-F1 model of acute and chronic GVHD. Donor T cells showed engraftment in the spleen, small intestine and liver of mice with acute GVHD, causing typical GVHD pathology in these organs. Interferon-γ and Fas ligand expression were up-regulated, and host lymphocytes were depleted in the target organs of these mice. In contrast, donor T cells did not show engraftment in the small intestine of mice with chronic GVHD, and no GVHD pathology was observed in this organ. However, both donor T-cell engraftment and GVHD pathology were observed in the spleen and liver of chronic GVHD mice, along with the up-regulation of interleukin-4 (IL-4) and IL-10 expression plus the expansion of host lymphocytes such as splenic B cells and hepatic natural killer (NK) 1.1+ T cells. Donor anti-host cytotoxic T-lymphocyte activity was observed in spleen cells from mice with acute GVHD, but not in spleen cells from mice with chronic GVHD. Transplantation of Fas ligand-deficient (gld) spleen cells did not induce host lymphocyte depletion in target organs. These results indicate that donor T cells augment type 1 T helper immune responses and deplete the host lymphocytes from target organs mainly by Fas-mediated pathways in acute GVHD, while donor T cells augment type 2 T helper immune responses and expand host splenic B cells and hepatic NK1.1+ T cells in chronic GVHD.
Introduction
Donor T cells are crucial for the development of graft-versus-host disease (GVHD), since depletion of T cells from the donor inoculum effectively prevents GVHD in both clinical and murine studies.1,2 However, the precise role of donor T cells in target organ damage related to GVHD has not been examined. The parent-into-F1 model of GVHD is useful for studying the effect of donor T cells on host tissues and lymphocytes.3 In this model, both acute and chronic GVHD can be created. Acute GVHD is characterized by a severe reduction of host lymphohaematopoietic cells and profound immunodeficiency due to activation of both donor CD4+ and CD8+ T cells in response to host alloantigens.4,5 In contrast, chronic GVHD results from the selective activation of alloreactive donor CD4+ T cells that act as helpers of host B cells, leading to B-cell activation and autoantibody production.6 In both forms of GVHD, alloreactive donor T cells undergo activation and expansion, produce cytokines, and mature into effector cells. However, previous studies have been carried out in the spleen, and not in the other target organs of GVHD, such as the liver or intestine, and a precise analysis of the effect of donor T cells on the hepatic lymphocytes and intestinal intraepithelial lymphocytes (IEL) of the host has not been performed. In view of the similar effects of donor T cells in the target organs of GVHD, we asked whether donor T cells were also responsible for pathological changes and host lymphocyte depletion and activation in the intestine and liver in mice with acute and chronic GVHD. To address this question, we examined the relationship between the composition of donor T cells among host lymphocytes in the target organs such as the spleen, small intestine and liver and the pathological changes of GVHD using the parent-into-F1 model of GVHD. We also examined the effect of donor T cells on host lymphocyte populations as well as on cytokine and Fas ligand (FasL) expression in the target organs. GVHD pathology was observed in organs showing engraftment of donor T cells, such as the spleen, small intestine and liver in mice with acute GVHD. The expression of type 1 T helper (Th1) cytokines in these organs was up-regulated and host lymphocytes were depleted mainly by Fas-mediated pathways. GVHD pathology was also observed in organs showing engraftment by donor T cells, such as the spleen and liver, in mice with chronic GVHD. However, type 2 T helper (Th2) cytokine expression was up-regulated in these organs and there was expansion of host splenic B cells and hepatic natural killer (NK) 1.1+ T cells. These results indicate a crucial role of donor T-cell-mediated immune responses for target organ injuries in both acute and chronic GVHD.
Materials and methods
Mice
C57BL/6 (B6), C57BL/6/gld/gld (B6/gld), DBA/2, and (C57BL/6 × DBA/2) (BDF1) mice were obtained from SLC (Hamamatsu, Japan). Female mice between 6 and 10 weeks old were used in all of the studies. Animal care was in accordance with the guidelines of The Hyogo College of Medicine.
Induction of GVHD
Single-cell suspensions from the spleens of DBA/2 or B6 parental donors were prepared in Hanks' balanced salt solution (HBSS) without phenol. Cell suspensions were filtered through sterile nylon mesh, washed, diluted to a concentration of 108 viable cells/ml, and injected intravenously into non-irradiated BDF1 mice, as described previously.5 Acute GVHD was induced using 50 × 106 spleen cells from either B6 mice or B6/gld mice, and chronic GVHD was induced with 90 × 106 DBA/2 spleen cells.
Preparation of hepatic lymphocytes
Lymphocytes were prepared from the liver as described previously.7 Briefly, livers harvested from mice were minced, pressed through a mesh, and suspended in phosphate-buffered saline (PBS). To remove hepatic parenchymal cells, the cells were resuspended in 33% Percoll containing 100 U/ml heparin and then were centrifuged at 800 g for 10 min at room temperature. The pellet was resuspended in red blood cell (RBC) lysis solution, and then washed three times in PBS.
Preparation of intestinal IEL
Intestinal IEL were prepared as described previously, with some modifications.8 Briefly, the small intestine was surgically removed, flushed with 20 ml of PBS and inverted. Then the intestine was washed again with PBS and cut into four segments, which were stirred in PBS supplemented with 20% fetal calf serum (FCS) for 30 min at room temperature. After debris was removed, the remaining cell suspension was centrifuged. The supernatant was aspirated, the pellet was resuspended in 7 ml of 45% Percoll, and then was layered onto 66·7% Percoll solution, and centrifuged (600 g) at 20° for 20 min. Intestinal IEL were harvested from the interface and were washed twice with PBS.
Flow cytometry
Cell suspensions were prepared in HBSS without phenol red containing 1% FCS and 0·1% sodium azide. The cells were incubated with an anti-Fc receptor monoclonal antibody (mAb) (2.4G2) for 10 min at 4°, and then were incubated with fluorescein isothiocyanate (FITC)-conjugated mAbs and/or phycoerythrin (PE)-conjugated mAbs for 30 min. The stained cells were washed twice, resuspended and analysed using a FACScan (Becton Dickinson, Mountain View, CA). FITC-conjugated anti-mouse H-2Kb mAb (clone AF6-88.5), anti-mouse CD3 mAb (clone 145-2C11), anti-mouse T-cell receptor mAb (TCR), β-chain mAb (clone H57-597) as well as PE-conjugated anti-mouse H-2Kd mAb (clone SF1-1.1), anti-mouse CD3 mAb (clone 145-2C11), anti-mouse NK1.1 mAb (clone PK136), anti-mouse TCR γ-chain mAb (clone GL3), anti-mouse B220 mAb (clone RA3-6B2), were purchased from Pharmingen (San Diego, CA). Multicolour flow cytometry was performed as described previously5 with some modifications. Channel numbers for the analysis were chosen on the basis of the staining pattern of normal spleen cells. Staining of normal F1 spleen cells with anti-major histocompatibility complex (MHC) reagents resulted in a unimodal positive profile when compared with the negative controls. When donor parental cells were detected in the GVHD mice, these cells were seen as subpopulations that were clearly negative (equivalent to non-specific findings) for F1-specific MHC.
Histopathology
The tissues harvested from mice with GVHD were placed in PBS with 10% formalin. The fixed tissues were embedded in paraffin, sectioned, and stained with haematoxylin and eosin.
Ex vivo detection of anti-host cytotoxic T lymphocyte (CTL) activity
Effector CTL activity was tested using freshly harvested spleen cells without in vitro sensitization. CTL activity was tested by the lysis of F1 concanavalin A (ConA) blasts from BDF1 mice in a 4-h 51Cr release assay, as described elsewhere.9 To show anti-host specificity of CTL, ConA blasts from B6 mice were also used as targets. Effector cells were tested in triplicate at four effector : target ratios and the per cent lysis was calculated from counts per minute (c.p.m.) data according to the following formula: [(sample c.p.m. − spontaneous c.p.m.)/(maximum c.p.m. − spontaneous c.p.m.)] × 100%. Results were shown as the mean per cent lysis±SD at a given E : T cell ratio for each treatment group.
Reverse transcription polymerase chain reaction (RT-PCR)
Total RNA was extracted from tissues using a standard guanidine thiocyanate–phenol–chloroform protocol. Following quantification of the isolated RNA by spectrophotometric analysis, 1 µg of total RNA was reverse transcribed in the presence of random hexanucleotide primers (Promega Corp., Madison, WI) and avian myeloblastosis virus reverse transcriptase (Promega). The reaction mixture was incubated at 42° for 1 hr and then at 72° for 5 min to terminate the reaction. From this reaction mixture, one-tenth of the volume was amplified by PCR to generate mouse FasL, interferon-γ (IFN-γ), interleukin-4 (IL-4), IL-10 and β-actin cDNAs. The reaction mixture contained 50 pmol of each primer, 25 mm dATP, dGTP, dCTP and dTTP (Pharmacia, Uppsala, Sweden), 2 ml of 10 × reaction buffer, 1 U of Taq DNA polymerase (Boehringer Mannheim, Indianapolis, IN) and sterile distilled water. PCR amplification was performed at 94° for 1 min, 65° for 1 min and 72° for 1 min in a DNA thermal cycler (Perkin Elmer Cetus Instruments, Norwalk, CT) for 26, 29 and 32 cycles to confirm linearity. A 10-µl aliquot of the reaction mixture was subjected to electrophoresis on 1·5% agarose gel (NuSieve, FMC, Vallensbaek Strand, Denmark) containing ethidium bromide, after which the gel was photographed under ultraviolet light. The 5′ and 3′ PCR primers were obtained from Clontech (Palo Alto, CA) and Bex (Tokyo, Japan), and had the following sequences, respectively: FasL, CGTGAGTTCACCAACCAAAGC and CCCAGTTTCGTTGATCACAAG; IFN-γ, TGGCTGTTTCTGGCTGTTACTG and AATCAGCAGCGACTCCTTTTCC; IL-4, CCAGCTAGTTGTCATCCTGCTCTTCTTTCTCG and CAGTGATGAGGACTTGGACTCATTCATGGTGC; IL-10, ATGCAGGACTTTAAGGGTTACTTGGGTT and ATTTCGGAGAGAGGTACAAACGAGGTTT; β-actin, TGTGATGGTGGGAATGGGTCAG and TTTGATGTCACGCACGATTTCC. Semi-quantitative determination of the PCR products was performed by the procedure described previously.10 A standard curve was obtained from the quantitative relationship between serial 1 : 2 dilutions of the cDNA mixture from 250 ng of total RNA, and the amount of the final PCR product was measured by densitometry. There was a log-linear relation between the amount of cDNA added to the mixture and the PCR products over the range of cDNA dilutions used for the PCR. In addition, the FasL, IFN-γ, IL-4 and IL-10 PCR products were normalized by the β-actin internal control.
In vitro cytokine production by spleen cells, IEL and hepatic lymphocytes
Cells (1 × 105/well/200 µl) were incubated for 72 hr on anti-CD3-coated plates that had been preincubated with 20 µg/ml of 145-2C11 in PBS overnight at 4°. The concentrations of IFN-γ and IL-4 in the culture supernatant were measured by ELISA using anti-mouse IFN-γ and IL-4 mAbs (Genzyme Corp, Cambridge, MA). Each assay was performed according to the manufacturer's protocol.
Statistical analysis
Differences between group mean values were compared using Student's t-test.
Results
GVHD pathology in the target organs
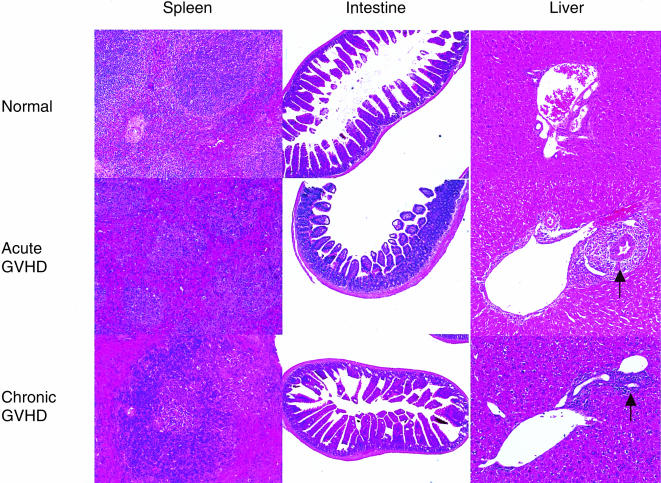
Acute and chronic GVHD were, respectively, induced by injection of 5 × 107 parental B6 spleen cells or 9 × 107 parental DBA/2 spleen cells into non-irradiated BDF1 mice. At 2 weeks after the induction of GVHD, the spleens of mice with acute GVHD showed marked lymphoid atrophy, structural disorganization and focal necrosis. In contrast, the spleens from mice with chronic GVHD showed lymphoid hypertrophy. The small intestines from mice with acute GVHD, but not mice with chronic GVHD, exhibited dilatation, flattening of the villi and elevation of the crypts, which are characteristic features of intestinal GVHD. The livers from mice with both acute and chronic GVHD showed massive periportal infiltration of mononuclear cells (Fig. 1).
Figure 1.
Histopathological features of GVHD. Acute and chronic GVHD were induced as described in the Materials and Methods. Three mice from each group were killed on day 14. Paraffin sections of the spleen, small intestine and liver were stained with hamatoxylin and eosin. Control sections were obtained from age-matched normal BDF1 mice. The livers from mice with acute and chronic GVHD showed cellular infiltration in the periportal area (arrow). Original magnification×40, small intestine; ×100, spleen and liver.
Donor T-cell engraftment in the target organs
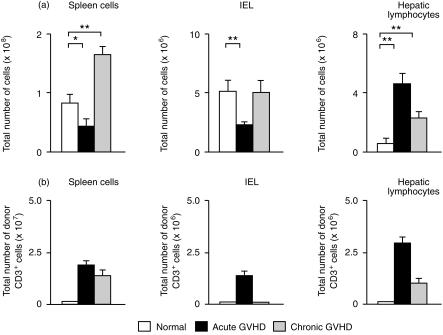
We prepared spleen cells, intestinal IEL and hepatic lymphocytes from mice at 2 weeks after the induction of GVHD. The number of spleen cells and intestinal IEL was decreased, while the number of hepatic lymphocytes was increased in the mice with acute GVHD. In mice with chronic GVHD, both spleen cells and hepatic lymphocytes were increased (Fig. 2a). To investigate donor T-cell engraftment in the target organ of GVHD, we examined the proportion of engrafted donor T cells among spleen cells, intestinal IEL and hepatic lymphocytes by multiplying the total number of mononuclear cells by the fraction of H-2Kd-negative and CD3-positive cells in acute GVHD, or by the fraction of H-2Kb-negative and CD3-positive cells in chronic GVHD. Donor T-cell engraftment was observed among the spleen cells, intestinal IEL and hepatic lymphocytes of mice with acute GVHD. In contrast to acute GVHD, donor T-cell engraftment was observed among the spleen cells and hepatic lymphocytes, but not the intestinal IEL, in mice with chronic GVHD (Fig. 2b). Since neither donor T-cell engraftment nor GVHD pathology were observed in the small intestine of mice with chronic GVHD (Fig. 1), this suggests that donor T-cell engraftment is essential for GVHD to occur in target organs.
Figure 2.
(a) Effect of GVHD on the number of spleen cells, intestinal IEL and hepatic lymphocytes. On day 14 after induction of GVHD, spleen cells, intestinal IEL and hepatic lymphocytes were prepared as described in the Materials and Methods. The mean±SD for four mice is shown. *P < 0·05, **P < 0·01. (b) Engraftment of donor T cells in the target organs. The number of engrafted donor T cells was determined by multiplying the total number of the cells by the fraction of H-2Kd-negative and CD3-positive cells (acute GVHD) or by the fraction of H-2Kb-negative and CD3-positive cells (chronic GVHD). The mean±SD for four mice is shown.
Effect of donor T cells on host lymphocytes in the target organs
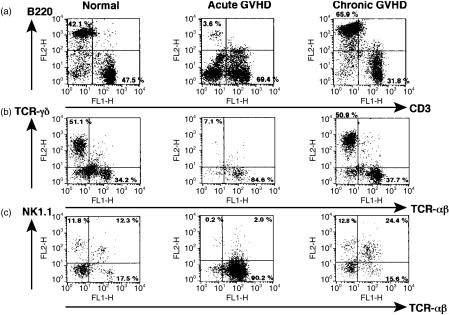
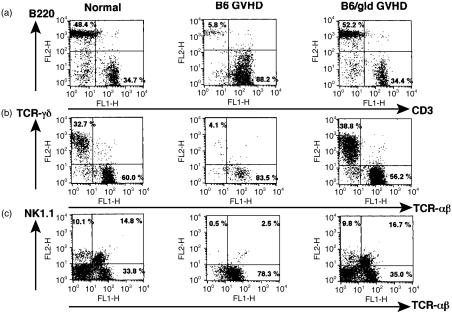
We examined the effect of donor T cells on host lymphocytes in the target organs. γδT cells were depleted from the intestinal IEL in mice with acute GVHD, but not in mice with chronic GVHD (Fig. 3b). Splenic B cells and hepatic NK cells and NK1.1+ T cells were depleted in acute GVHD, whereas splenic B cells and hepatic NK1.1+ T cells were expanded in chronic GVHD (Fig. 3a,c). We next examined whether the expanded splenic B cells and hepatic NK1.1+ T cells in chronic GVHD were derived from host cells or engrafted donor cells. B220-positive spleen cells and NK1.1-positive hepatic lymphocytes were exclusively H-2Kb-positive, indicating that these cells were of host origin (Fig. 4). These results suggest that host lymphocytes in target organs such as splenic B cells, intestinal γδ IEL, hepatic NK cells and hepatic NK1.1+ T cells are depleted in acute GVHD, while host splenic B cells and hepatic NK1.1+ T cells are expanded in chronic GVHD.
Figure 3.
Effect of GVHD on host lymphocytes in target organs. Spleen cells (a), intestinal IEL (b) and hepatic lymphocytes (c) were prepared from normal BDF1 mice, acute GVHD mice and chronic GVHD mice as described in the Materials and Methods, and two-colour staining was done for CD3 and B220, TCR-αβ and TCR-γδ, and TCR-αβ and NK1.1, respectively. The numbers in the figure represent the percentage of fluorescence-positive cells in the corresponding areas.
Figure 4.
Expansion of host splenic B cells and NK1.1+ T cells in mice with chronic GVHD. Spleen cells and hepatic lymphocytes were prepared from normal BDF1 mice and chronic GVHD mice as described in the Materials and Methods, and two-colour staining was performed for B220 and H-2Kb, or NK1.1 and H-2Kb, respectively. The numbers in the figure represent the percentage of fluorescence-positive cells in the corresponding areas.
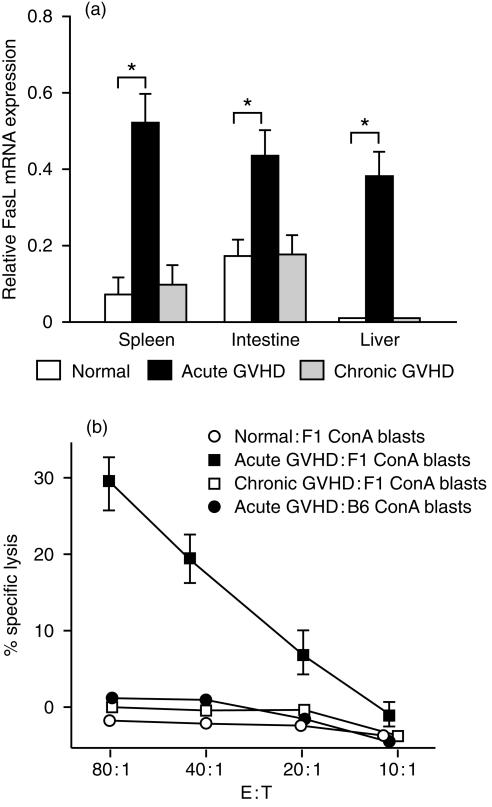
FasL mRNA expression and anti-host CTL activity of lymphocytes in the target organs
To explore the mechanisms underlying host lymphocyte depression in acute GVHD, we examined Fas ligand (FasL) mRNA expression in GVHD target organs. Total RNA was extracted from the spleen, small intestine and liver, and RT-PCR was performed using specific primers for FasL. We found that FasL mRNA expression was increased in the spleen, small intestine and liver in acute GVHD, but not in chronic GVHD (Fig. 5a). Next, we tested the in vitro anti-host CTL activity of spleen cells from mice with acute and chronic GVHD. Spleen cells obtained from mice with acute GVHD demonstrated strong anti-host CTL activity, while cells from mice with chronic GVHD did not show anti-host CTL activity (Fig. 5b). Since FasL contributes to anti-host CTL activity,11 these findings indicate that host lymphocytes were depleted from target organs mainly by Fas-mediated pathways in the mice with acute GVHD, although perforin/granzyme pathways also appear to play a role.
Figure 5.
(a) FasL mRNA expression in the target organs. Total RNA was isolated from tissue samples and RT-PCR cDNA products were generated and amplified using specific primers for FasL and β-actin. The amount of FasL RT-PCR cDNA product was normalized by the β-actin control. Data represent the mean±SD of the relative expression RT-PCR cDNA products in four mice. *P < 0·05. (b) Anti-host CTL activity of spleen cells in mice with acute and chronic GVHD. Acute and chronic GVHD were induced as described in the Materials and Methods, and anti-host CTL activity was assessed by the lysis of F1 ConA blasts from BDF1 mice on day 10 after parental cell transfer. To show anti-host specificity, ConA blasts from B6 mice were also used for the assessment of CTL activity of spleen cells in mice with acute GVHD. Results are expressed as the mean±SD for three mice per group at four E : T cell ratios.
B6/gld transfer into BDF1 mice
To directly examine whether Fas-mediated pathways have a major role in the depletion of host cells in acute GVHD, BDF1 mice were injected with 5 × 107 spleen cells from either normal B6 mice or FasL-deficient B6/gld mice. At 2 weeks after the induction of GVHD, spleen cells, intestinal IEL and hepatic lymphocytes were prepared. Despite injecting equivalent numbers of B6 or B6/gld donor T cells, host splenic B cells, γδT cells from IEL and hepatic NK cells and NK1.1+T cells were not depleted in BDF1 mice injected with B6/gld mice (Fig. 6). These results indicate that Fas-mediated pathways are important for the depletion of host lymphocytes in acute GVHD.
Figure 6.
B6/gld transfer results in a failure of host lymphocyte depletion. BDF1 mice were injected intravenously with 50 × 106 spleen cells from either B6 mice or B6/gld mice. On day 14 after induction of GVHD, spleen cells (a), intestinal IEL (b) and hepatic lymphocytes (c) were prepared from normal BDF1 mice, B6GVHD mice and B6/gldGVHD mice as described in the Materials and Methods, and two-colour staining was carried out for CD3 and B220, TCR-αβ and TCR-γδ, and TCR-αβ and NK1.1, respectively. The numbers in the figure represent the percentage of fluorescence-positive cells in the corresponding areas.
Th1 and Th2 cytokine expression in the target organs
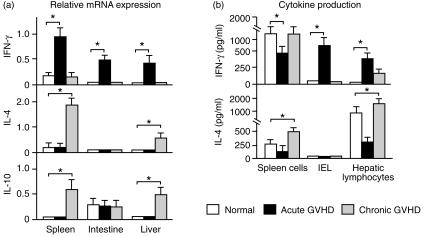
It is generally accepted that Th1 cytokine production is augmented in acute GVHD,5 while chronic GVHD is related to the increased Th2 cytokine production by donor CD4+ T cells,12 resulting in B-cell hyperactivity, formation of autoantibodies and lupus-like disease.6 However, previous studies were carried out in the spleen and not in other target organs of GVHD such as the small intestine and liver. Accordingly, we examined IFN-γ, IL-4 and IL-10 mRNA expression in the spleen, small intestine and liver in the present study. IFN-γ mRNA expression was increased in the spleen, small intestine and liver of mice with acute GVHD, but not in mice with chronic GVHD. In contrast to acute GVHD, IL-4 and IL-10 mRNA expression was increased in the spleen and liver of mice with chronic GVHD (Fig. 7a). Next, we examined the ability of lymphocytes in the target organs to produce Th1 and Th2 cytokines. Spleen cells, intestinal IEL and hepatic lymphocytes were cultured in anti-CD3 mAb-coated plates, and IFN-γ and IL-4 in the culture supernatant were assayed by ELISA. We found that IL-4 production by spleen cells and hepatic lymphocytes was decreased in acute GVHD, but was increased in chronic GVHD. IFN-γ productions by IEL and hepatic lymphocytes was increased in acute GVHD, while spleen cells showed a decrease of IFN-γ production in acute GVHD (Fig. 7b). These results indicated that Th1 cytokine expression was augmented in the target organs of mice with acute GVHD, while Th2 cytokines were increased in chronic GVHD.
Figure 7.
Th1 and Th2 cytokine expression in the target organs. (a) Total RNA was isolated from tissue samples and RT-PCR cDNA products were generated and amplified using specific primers for IFN-γ, IL-4, IL-10 and β-actin. The amounts of the IFN-γ, IL-4 and IL-10 RT-PCR cDNA products were normalized by the β-actin control. Data represent the mean±SD of the relative expression of RT-PCR cDNA products in four mice; *P < 0·05. (b) Spleen cells, intestinal IEL and hepatic lymphocytes were prepared as described in the Materials and Methods. The cells (1 × 105/well/200 µl) were incubated for 72 hr on anti-CD3 mAb-coated plates that had been made by preincubation of each plate with 20 µg/ml of 145-C11 in PBS overnight at 4°. The concentrations of IFN-γ and IL-4 in the culture supernatants were measured by ELISA using anti-mouse-IFN-γ and IL-4 mAbs, respectively. Data represent the mean±SD for four mice. *P < 0·05.
Discussion
In mice with acute GVHD, we found donor T-cell engraftment in the spleen, small intestine and liver (Fig. 2b), and GVHD pathology was also observed in these organs (Fig. 1). In contrast to acute GVHD, the mice with chronic GVHD did not show donor T-cell engraftment (Fig. 2b) or GVHD pathology (Fig. 1) in the small intestine. We also observed donor T-cell engraftment and GVHD pathology in the bone marrow and thymus of mice with acute GVHD but not in mice with chronic GVHD (data not shown), suggesting that GVHD pathology was caused by donor T-cell engraftment in the target organs. The mechanisms that prevent donor T-cell engraftment in organs such as the small intestine, bone marrow and thymus of mice with chronic GVHD are unclear at present. One possible explanation is that the frequency of anti-BDF1 precursor CTL in DBA/2 mice is ninefold lower than in B6 mice.13 Thus, it is more difficult for DBA/2 CD8+ T cells to expand following engraftment in F1 hosts, and these cells may be rejected by host immunocompetent lymphocytes in various organs.
Mice with acute GVHD showed severe lymphocyte hypoplasia in the spleen and small intestine (Fig. 2a), and the number of host splenic B cells and host γδT cells among intestinal IEL was decreased significantly. Although the number of hepatic lymphocytes was increased in acute GVHD, host NK cells and NK1.1+ T cells were depleted from hepatic lymphocytes (Fig. 3). The pathogenesis of acute GVHD involves three elements: a donor Th1-mediated immune response to host alloantigens, activation of pro-inflammatory macrophages and NK cells, and triggering of inflammatory cytokine release by environmental pathogens or their products.14 Host-reactive Th1 cells are crucial for the induction of the anti-host CTL response during acute GVHD, since the Th1-mediated immune response enhances cell-mediated immunity by stimulating CTL differentiation15 and increases the immunogenicity of cells by up-regulation of MHC antigen expression.16 Previous studies have demonstrated that host B cells are eliminated by donor anti-host cytotoxic T lymphocytes (CTL)17 and that Fas–FasL interactions are crucial for the development of donor anti-host CTL11 in the parent-into-F1 model of acute GVHD. Recently, we demonstrated that host haematopoietic progenitor cells in the spleen and bone marrow were eliminated by Fas–FasL interactions in the parent-into-F1 model of acute GVHD.18 The present study demonstrated an anti-host CTL response of spleen cells (Fig. 5b) along with increased IFN-γ and FasL mRNA expression in the small intestine and liver of mice with acute GVHD (Figs 5a and 7a), Furthermore, transplantation of Fas ligand-deficient (gld) spleen cells did not induce host lymphocyte depletion (Fig. 6). These results suggest that Fas–FasL interactions may also be involved in the depletion of host lymphocyte from the small intestine and liver in acute GVHD. We observed an increase of IFN-γ production by intestinal IEL and hepatic lymphocytes from mice with acute GVHD after stimulation of these cells with anti-CD3 mAb, while IFN-γ production by spleen cells from acute GVHD mice was actually decreased (Fig. 7b). The mechanism underlying the discrepancy between the elevated IFN-γ mRNA level and reduced in vitro production of IFN-γ by spleen cells stimulated with anti-CD3 mAb is not completely clear, but it may be related to splenic T-cell dysfunction during acute GVHD.19 An increase of IFN-γ mRNA expression in the spleen may reflect an increase of Th1 cells, the function of which is abolished by acute GVHD.
In contrast to acute GVHD, mice with chronic GVHD did not develop lymphocyte hypoplasia in their target organs (Fig. 2a), but the number of host splenic B cells and hepatic NK1.1+ T cells was increased (Figs 3 and 4). In chronic GVHD, there is selective activation of CD4+ T cells in the spleen as well as augmentation of Th2 immune responses and humoral immune responses.5,12 We observed an increase of Th2 cytokine mRNA expression as well as increased production of IL-4 and IL-10 in the spleen (Fig. 7), suggesting that Th2 cytokines may stimulate B cells to expand in the spleen, resulting in lupus-like disease in mice with chronic GVHD. We also observed an increase of Th2 cytokine mRNA expression as well as increased Th2 cytokine production in the liver, suggesting the possible involvement of Th2 cytokines in hepatic injury related to chronic GVHD. NK1.1+ T cells are a specialized lymphoid population that co-express a restricted repertoire of TCRαβ and NK lineage receptors.20 It has been reported that NK1.1+ T cells specifically recognize α-galactosylceramide or parasite glycosylphosphatidylinositols, both of which are presented by a monomorphic class Ib molecule, CD1d.21 This population of cells has been shown to mediate the rejection of allografted bone marrow,22 to control the development of autoimmune diseases,23 and to initiate anti-tumour responses.24 However, if these cells are incorrectly activated, self-reactive clones develop that mediate cytotoxicity, resulting in tissue injury and autoimmune disease.25 ConA-induced hepatitis is considered to be an experimental murine model of human autoimmune hepatitis.26 Recently, it was reported that NK1.1+ T cells are involved in ConA-induced hepatitis,27 and that NK1.1+ T cells showed significant expression of IL-4 receptors as well as augmented cytotoxicity in the presence of IL-4.28 Thus, the finding of an increased number of NK1.1+ T cells and increased Th2 cytokine expression in the livers of mice with chronic GVHD suggests that IL-4-stimulated NK1.1+ T cells undergo expansion in the liver and then play a role in immune-mediated hepatic damage.
In conclusion, we demonstrated that donor T cells augmented the Th1-mediated immune response and depleted host lymphocytes from target organs mainly by Fas–FasL interactions in mice with acute GVHD, while donor T cells augmented the Th2-mediated immune response as well as expanding host splenic B cells and hepatic NK1.1+ T cells in mice with chronic GVHD. Our results suggest that donor T-cell-mediated immune responses in the target organs are important for both acute and chronic GVHD.
Abbreviations
- ConA
concanavalin A
- CTL
cytotoxic T lymphocyte
- GVHD
graft-versus-host disease
- IEL
intraepithelial lymphocytes
- Th
T helper.
References
- 1.Prentice HG, Blacklock HA, Janossy G, Bradstock KF, Skeggs D, Goldstein G, Hoffbrand AV. Use of anti-T cell monoclonal antibody OKT3 to prevent acute graft-versus-host disease in allogeneic bone marrow transplantation for acute leukemia. Lancet. 1982;1:310–318. doi: 10.1016/s0140-6736(82)92619-8. [DOI] [PubMed] [Google Scholar]
- 2.Blazer BR, Taylor PA, Snover DC, Bluestone JA, Vallera DA. Nonmitogenic anti-CD3F (ab′) 2 fragments inhibit lethal murine graft-versus-host disease induced across the major histocompatibility barrier. J Immunol. 1993;150:265–77. [PubMed] [Google Scholar]
- 3.Hakim FT, Mackall CL. The immune system: Effector and target of graft-versus-host disease. In: Ferrara JLM, Deeg HJ, Burakoff SJ, editors. Graft-Vs-Host Disease. 2. New York: Marcel Dekker, Inc.; 1997. pp. 257–89. [Google Scholar]
- 4.Rozendaal L, Pals ST, Schilham M, Melief CJ, Gleichmann E. Allosuppression of B cells in vitro by graft-versus-host reaction-derived T cells is caused by cytotoxic T lymphocytes. Eur J Immunol. 1989;19:1669–75. doi: 10.1002/eji.1830190922. [DOI] [PubMed] [Google Scholar]
- 5.Rus V, Svetic A, Nguyen PH, Gause WC, Via CS. Kinetics of Th1 and Th2 cytokine production during the early course of acute and chronic murine graft-versus-host disease: Regulatory role of donor CD8+ T cells. J Immunol. 1995;155:2396–406. [PubMed] [Google Scholar]
- 6.Gleichmann E, Pals ST, Rolink AG, Radaszkiewicz T, Gleichmann H. Graft-versus-host reactions: clues to the etiopathology of spectrum of immunological disease. Immunol Today. 1984;5:324–9. doi: 10.1016/0167-5699(84)90126-9. [DOI] [PubMed] [Google Scholar]
- 7.Hashimoto W, Takeda K, Anzai R, Ogasawara K, Sakihara H, Sugiura K, Seki S, Kumagai K. Cytotoxic NK1.1 Ag+ αβ T cells with intermediate TCR induced in the liver of mice by IL-12. J Immunol. 1995;154:4333–40. [PubMed] [Google Scholar]
- 8.Murosaki S, Yoshikai Y, Ishida A, Nakamura T, Matsuzaki G, Takimoto H, Yuuki H, Nomoto K. Failure of T cell receptor V beta negative selection in murine intestinal intra-epithelial lymphocytes. Int Immunol. 1991;3:1005–13. doi: 10.1093/intimm/3.10.1005. [DOI] [PubMed] [Google Scholar]
- 9.Shustov A, Nguyen P, Finkelman F, Elkon KB, Via CS. Differential expression of Fas and Fas ligand in acute and chronic graft-versus-host disease: Upregulation of Fas and Fas ligand requires CD8+ T cell activation and IFN-γ production. J Immunol. 1998;161:2848–55. [PubMed] [Google Scholar]
- 10.Becker S, Quay J, Soukup J. Cytokine (tumor necrosis factor, IL-6, and IL-8) production by respiratory syncytial virus-infected human alveolar macrophage. J Immunol. 1991;147:4307–12. [PubMed] [Google Scholar]
- 11.Via CS, Nguyen P, Shustov A, Drappa J, Elkon KB. A major role for the Fas pathway in acute Graft-versus-host disease. J Immunol. 1996;157:5387–93. [PubMed] [Google Scholar]
- 12.De Wit D, Van Mechelen M, Zanin C, et al. Preferential activation of Th2 cells in chronic graft-versus-host reaction. J Immunol. 1993;150:361–6. [PubMed] [Google Scholar]
- 13.Via CS, Sharrow SO, Shearer GM. Role of cytotoxic T lymphocytes in the prevention of lupus-like disease occurring in a murine model of graft-versus-host disease. J Immunol. 1987;139:1840–9. [PubMed] [Google Scholar]
- 14.Hill GR, Crawford JM, Cooke KR, Brinson YS, Pan L, Ferrara JLM. Total body irradiation and acute graft-versus-host disease: The role of gastrointestinal damage and inflammatory cytokines. Blood. 1997;90:3204–13. [PubMed] [Google Scholar]
- 15.Via CS, Rus V, Gately MK, Finkelman FD. IL-12 stimulate the development of acute graft-versus-host disease in mice that normally would develop chronic, autoimmune graft-versus-host disease. J Immunol. 1994;153:4040–7. [PubMed] [Google Scholar]
- 16.Kelly VE, Fiers W, Storm TB. Cloned human interferon-γ, but not interferon-β or -α, induces expression of HLA-DR determinants by fetal monocytes and myeloid leukemic cell lines. J Immunol. 1984;132:240–5. [PubMed] [Google Scholar]
- 17.Via CS. Kinetics of T cell activation in acute and chronic forms of murine graft-versus-host disease. J Immunol. 1991;146:2603–9. [PubMed] [Google Scholar]
- 18.Iwasaki T, Hamano T, Saheki K, et al. Graft-versus-host-disease-associated donor cell engraftment in an F1 hybrid model is dependent upon the Fas pathway. Immunology. 2000;99:94–100. doi: 10.1046/j.1365-2567.2000.00919.x. 10.1046/j.1365-2567.2000.00919.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bendelac AM, Rivera MN, Park SH, Roark JH. Mouse CD1-specific NK1T cells: development, specificity, and function. Annu Rev Immunol. 1997;15:535–62. doi: 10.1146/annurev.immunol.15.1.535. [DOI] [PubMed] [Google Scholar]
- 19.Joseph LJ, Iwasaki T, Malek TR, Shearer GM. Interleukin 2 receptor dysfunction in mice undergoing a graft-vs-host reaction. J Immunol. 1985;135:1846–50. [PubMed] [Google Scholar]
- 21.Schofield L, McConville MJ, Hansen D, Campbell AS, Fraser-Reid B, Grusby MJ, Tachado SD. CD1d-restricted immunoglobulin G formation to GPI-anchored antigens mediated by NKT cells. Science. 1999;283:225–9. doi: 10.1126/science.283.5399.225. 10.1126/science.283.5399.225. [DOI] [PubMed] [Google Scholar]
- 22.Takeda K, Dennert G. The development of autoimmunity in C57BL/6 lpr mice correlates with the disappearence of natural killer type-I-positive cells: evidence for their suppressive action on bone marrow stem cell proliferation, B cell immunoglobulin secretion, and autoimmune symptoms. J Exp Med. 1993;177:155–64. doi: 10.1084/jem.177.1.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mieza MA, Ito T, Cui JQ, et al. Selective reduction of Vα14+ NKT cells associated with disease development in autoimmune-prone mice. J Immunol. 1996;156:4035–40. [PubMed] [Google Scholar]
- 24.Cui J, Shin T, Kawano T, et al. Requirement for Vα14 NKT cells in IL-12-mediated rejection of tumors. Science. 1997;278:1623–6. doi: 10.1126/science.278.5343.1623. 10.1126/science.278.5343.1623. [DOI] [PubMed] [Google Scholar]
- 25.Watanabe T, Kawamura T, Kawamura H, Haga M, Shirai K, Watanabe H, Eguchi S, Abo T. Intermediate T-cell receptor cells in mouse lung. Their effector function to induce pneumonitis in mice with autoimmune-like graft-versus-host disease. J Immunol. 1997;158:5805–14. [PubMed] [Google Scholar]
- 26.Tiegs G, Hentschel J, Wendel A. A T cell-dependent experimental liver injury in mice inducible by concanavalin A. J Clin Invest. 1992;90:196–203. doi: 10.1172/JCI115836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Toyabe S, Seki S, Iiai T, et al. Requirement of IL-4 and liver NK1+ T cells for concanavalin A-induced hepatic injury in mice. J Immunol. 1997;159:1537–42. [PubMed] [Google Scholar]
- 28.Kaneko Y, Harada M, Kawano T, Yamashita M, Shibata Y, Gejyo F, Nakayama T, Taniguchi M. Augmentation of Vα14 NKT cell-mediated cytotoxicity by interleukin 4 in an autocrine mechanism resulting in the development of concanavalin A-induced hepatitis. J Exp Med. 2000;191:105–14. doi: 10.1084/jem.191.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]