Introduction
Intrathymic deletion of thymocytes with high affinity for self antigen cells plays a crucial role in contracting the autoreactive T-cell repertoire. However, this is manifestly an incomplete process. Not all self proteins are effectively presented in the thymus, including those that are expressed well after the bulk of the T-cell repertoire has been formed, and it is relatively easy to detect autoreactive T cells following immunization with self antigens. For this reason mechanisms of regulating peripheral T cells with unwanted specificity are crucial to survival. There are several mechanisms of peripheral T-cell unresponsiveness including ignorance, deletion by apoptosis, and cytokine-mediated regulation. The topic of this review is a further mechanism, T-cell anergy. Data will be highlighted, which suggests that the induction of T-cell anergy is an important contributor to peripheral T-cell tolerance, and that anergic T-cells are not passive, but may play an important role as regulatory cells.
Definitions
The term anergy was coined by Nossal and Pick in 19801 to describe the unresponsive state that was induced in B cells following the injection of soluble proteins in vivo. They observed that the antigen-specific B cells were present, but were refractory to subsequent activation by antigen or mitogen. The first observations of antigen-induced T-cell proliferative unresponsiveness were made with human T cell clones following culture with antigen in the absence of added antigen-presenting cells (APC).2 Since that time the term anergy has been used to describe a variety of forms of unresponsiveness, induced in a variety of ways, and mediated by diverse mechanisms. This has led to some confusion, and scepticism as to whether anergy was a useful or informative term.
The definition of anergy that we favour is the following: a state of long-lasting, partial or total unresponsiveness induced by partial activation. This excludes unresponsiveness due to ‘blindness’ or ‘partial sightedness’ resulting from temporary T-cell receptor (TCR) modulation, and the refractory state that can be seen when T cells are prematurely exposed to activating stimuli too soon after their last antigen encounter. The large majority of reports of anergy have described the inhibition of interleukin-2 (IL-2) secretion by T cells that are normally capable of secreting IL-2 upon activation. However, anergy has also been described in T helper 2 (Th2) cells, in which the secretion of the key signature cytokine IL-4 is unaffected, but the ability of the T cells to proliferate in response to IL-4 is inhibited.3 The common theme in these examples is that anergy refers to the inability of T cells to produce or respond to proliferative signals. It is important to note that the unresponsiveness is often partial. This is well illustrated by the observations that Th0 cells continue to secrete IL-4 after the induction of anergy4 and T cells with the capability of secreting IL-2 and mediating cytotoxicity retain their cytotoxic potential after being rendered anergic even though IL-2 secretion is shut down.5
Having attempted to provide a universal definition of anergy, it may well be that different ‘levels’ of anergy exist.6 This is illustrated by the finding that anergy can sometimes be reversed by culture in exogenous IL-2, leading to several rounds of cell division,7 while in other systems this has been found not to be the case.8 These differences are likely to be explained by differences in the state of activation of the starting T-cell populations, and by differences in the partial activation signals that were used to induce anergy. As the intracellular signalling events that accompany anergy become better defined, as discussed below, it may be possible to explain these differences.
Mechanisms of anergy induction in vitro
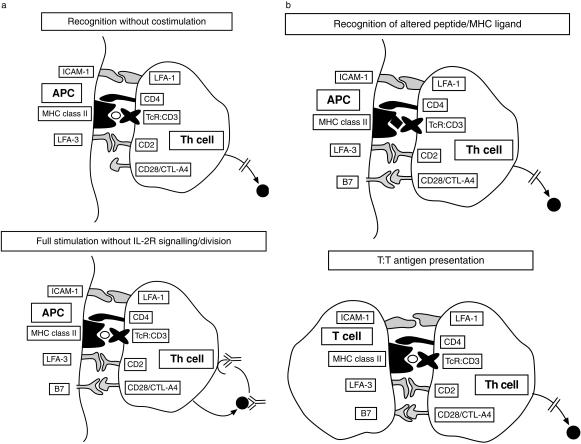
There are at least four distinct sets of circumstances that have been observed to induce T-cell anergy in vitro; these are represented schematically in Fig. 1, and will be discussed in turn.
Figure 1.
The different circumstances that have been observed to induce T-cell anergy in vitro are illustrated.
TCR ligation in the absence of full costimulation
This is the most extensively studied set of conditions that lead to T cell unresponsiveness. The first observations of this kind were made in the artificial, but very precisely defined, system of Quill and Schwartz9 using artificial lipid bilayers, coated onto glass coverslips, and impregnated with H2-Ek molecules together with the appropriate peptide of pigeon cytochrome c. Exposure of T-cell clones specific for this peptide–major histocompatibility complex (MHC) to the impregnated planar membranes not only failed to induce proliferation, but rendered the T cells refractory to further stimulation in response to fully competent APC. In other words they had been rendered ‘anergic’. At the time, the nature of the missing ‘costimulatory’ molecules was completely unknown, however, the identity of one of the key molecules was established four years later when B7 (CD80) was cloned10 and found to ligate the T-cell surface molecule CD28.11 Definition of this major costimulatory pathway spawned an era of experimentation in vitro and in vivo during which the consequences and exploitability of costimulation-deficient antigen presentation were explored. This era has extended up to the present day, in that some of the first clinical trials of costimulatory blockade in the context of autoimmune disease and transplantation are currently taking place.12 The generation of a fusion protein, CTLA4-immunoglobulin, composed of the second receptor for B7 molecules on T cells, namely cytotoxic T-lymphocyte antigen 4 (CTLA-4 function is discussed later), fused to the Fc portion of immunoglobulin, facilitated many of these experiments.
Most of the observations of anergy in response to TCR ligation in the absence of full costimulation have involved established mouse or human T-cell clones.13 In contrast, naïve T cells appear not to be susceptible to the induction of anergy under most of these conditions in vitro.14–16 However, several of the descriptions of anergy in vivo have involved the injection of bacterial superantigens, as discussed in detail below, implying that naïve T cells can be rendered unresponsive in this way.
Exposure to peptide partial agonists
In the process of screening peptides for those with improved binding characteristics to MHC molecules, Sette and colleagues discovered that some analogues, usually differing by a single amino acid from the parent peptide, acted as antagonists.17 These antagonists induced no detectable signalling to the T cell, and had no effect on subsequent T cell reactivity. Soon after this, Sloan-Lancaster and colleagues described amino acid-substituted peptide variants that not only failed to induce T-cell proliferation, but that induced T-cell anergy.18 In contrast with the first conditions that were discovered to induce anergy, the effect of these peptide partial agonists was observed even when the APC provided full costimulation. Similar observations were made by Germain and colleagues using transfectants expressing amino acid-substituted MHC class II molecules as APC.19 As the nature of TCR–CD3-transduced signals was elucidated it became clear that these altered peptide ligands (APL), or altered MHC molecules, induced a characteristic pattern of early signalling events, suggestive of partial activation.20,21 This will be discussed further, below.
There has been considerable interest in the possibility that APL could be used therapeutically.22 However their potential is limited by the fact that a peptide that acts as an APL for one T-cell clone/TCR may well be an agonist or entirely neutral for other clones with the same antigen specificity. Therapeutic use of APL in autoimmune disease or in transplantation would require therefore that the T cell affected by the APL could regulate other T cells with the same autoantigen or alloantigen specificity. The finding that certain viral escape mutants encode APL, in place of a CTL epitope may be taken to indicate the potential of this approach.23,24
Full signalling without IL-2 receptor-driven cell division
The importance of cell division in the maintenance of T-cell reactivity was demonstrated by experiments in which mouse T-cell clones were cultured with costimulation-positive antigen-pulsed APC, but were prevented from dividing by the addition of anti-IL-2 and anti-IL-2 receptor (IL-2R) antibodies.25 This observation could be explained in two ways. First, it could be that cell division is necessary to avoid unresponsiveness. This would be consistent with the suggestion that inhibitors of activation, ‘anergy proteins’, are invariably generated in the context of T-cell activation, but that the T cell escapes from their inhibitory effects by the dilution that results from division. A second possible explanation for the anti-IL-2/R result is that IL-2R signalling is itself important in maintaining T-cell responsiveness. These possibilities have recently been addressed using a drug, rapamycin, that blocks IL-2R signalling and prevents transition from G1 to S phase of the cell cycle. T cells were cultured with immobilized anti-CD3 and anti-CD28 in the presence or absence of rapamycin. Despite TCR/CD3- and CD28-mediated signals the cells cultured in the presence of rapamycin became anergic.26 The additional finding in this study was that cell cycle arrest by addition of hydroxyurea did not have the same effect. This suggests that IL-2R-mediated signals, independent of the induction of G1 to S phase transition prevent the induction of anergy.
TCR ligation in the presence of IL-10
IL-10 is clearly an important regulatory cytokine, and has been implicated in some models of transferrable T-cell tolerance. The first connection between T cell anergy and IL-10 was made by Groux et al.8 who noted that stimulation of human CD4+ T cells in the presence of IL-10 induced a state of profound unresponsiveness, that could not be reversed with IL-2. These findings may be explained by later observation that stimulation of T cells in the presence of IL-10 favoured the emergence of IL-10-secreting T cells. Such cells have regulatory properties, and have been labelled as T regulatory (Tr) cells.27 In retrospect, the description of the IL-10 effect could be re-interpreted as ‘immune deviation’ rather than as the induction of anergy. However, as will be argued below, a spectrum of non-responsive T cells appears to exist, and the distinction between anergy and immune deviation may be semantic.
T–T antigen presentation
The earliest observations of T-cell anergy arose from the culture of human T-cell clones with antigen in the absence of APC, as described above.2,28 Initially this was thought to require high antigen concentrations, however, it was subsequently shown to occur in the presence of the same antigen concentration that induced optimal proliferation when presented by professional APC.29 This is an anomalous finding, in that activated human T cells, as used in these experiments, not only express high levels of MHC class II molecules, but also high levels of B7 family molecules.30–32 The molecular mechanisms responsible for this form of anergy remain to be adequately explained, although some information is available which suggests that TCR–CD3 signalling is altered in some way.33 The possibility that CTLA-4 (CD152) ligation contributes to the effects of T–T interactions has not been fully explored; given that CD152 signalling can alter CD3-transduced signals,34 this is a reasonable candidate explanation for this unusual form of anergy.
Role of CTLA-4 in the induction of anergy
It was discovered in 1991 that the long known T-cell activation antigen, CD152, was a second ligand for the B7 family of molecules.35 It was soon apparent that CD152 had a negative regulatory role, with almost opposite effects to CD28.36,37 The most startling results were from mice in which the CD152-encoding gene was inactivated. These mice died at approximately 3 weeks of age with overwhelming lymphocyte accumulation.38,39
The involvement of CD152 in anergy induction was first suggested by experiments in DO11.10 TCR-transgenic mice.40 It was noted that injection of soluble ovalbumin, the antigen for which the transgenic T cells were specific, induced a cohort of unresponsive cells. If anti-CD152 antibody was coinjected with the ovalbumin, the induction of anergy was prevented. Later, Walunas and Bluestone also showed that CD152 blockade prevents SEB-induced T-cell anergy in vivo.41 CD152–B7 interactions also appear to influence the development of transplantation tolerance. Administration of anti-CD152 monoclonal antibody (mAb) to murine recipients of skin allografts completely reversed the tolerance induced by thymectomy, anti-CD154 (CD40L) antibody and donor-specific blood transfusion (DST).42 Similar findings were reported in a mouse heart graft model in which long-term graft acceptance was achieved by recipient pretreatment with DST in conjunction with CTLA-4-immunoglobulin; administration of anti-CD152 at the time of transplantation significantly reduced graft survival time.43 Whether anti-CD152 mAb in these models is inhibiting the induction of anergy, deletion, or the emergence of regulatory cells has yet to be determined.
Contradictory results have also been reported. In a nasal tolerance model anti-CD152 did not reverse the antigen-induced unresponsiveness; however, it is not clear that the mechanism of unresponsiveness was T-cell anergy.44 In an in vitro system Frauwirth observed that CD8 T cells from CD152-deficient mice were equally susceptible to the induction of anergy by anti-CD3 antibody.45
Relationship between anergy and apoptosis
Until recently the majority view was that anergy was functionally equivalent to a cell's ‘last gasp’ before admitting defeat and dying by apoptosis. Because it was possible, in vitro at least, to maintain cells in this ‘preterminal’ state, the anergic cell could be studied in detail. Although there is clear overlap between anergy and apoptosis it is now clear that they are distinct outcomes of partial activation of a T cell. This was investigated using human T-cell clones rendered unresponsive following T–T peptide presentation, as described above.46 Some clones emerged from these cultures live but unresponsive, i.e. anergic, while other clones underwent apoptosis such that only 10% of T cells survived. The distinction between these two patterns of response appeared to be determined by sensitivity to Fas-mediated death, in that addition of a neutralizing anti-Fas antibody to the apoptosis-prone clone rescued it from death. Interestingly, the rescued clone was then found to be profoundly anergic. The molecular basis for the difference between these sets of clones was not defined.
Similar findings have been observed using anti-CD3 mAb stimulation of naïve murine T cells, in the absence of APC.47 Culture of naïve T cells with immobilized anti-CD3 antibody is commonly associated with apoptotic death in approximately 40% of T cells; the surviving T cells emerge with a classically anergic phenotype, being hyper-responsive to exogenous IL-2, but unable to undergo autocrine proliferation in response to full activation stimuli. The difference between the 40% that undergo apoptosis and the remainder that do not is unclear. Similar findings were reported by Frauwirth who noted that T cells from mice transgenic for Bcl-xL under the lck promoter were equally susceptible to the induction of anergy by anti-CD3 antibody.45
Evidence for T-cell anergy in vivo
As mentioned above, for several years after the in vitro phenomenon of T-cell anergy was first described, the majority view was that anergy represented an in vitro artefact, and that anergy was merely a step on the road to death.48 This would appear to be a correct interpretation of anergy in B cells, the cell type in which this phenomenon was first described. Using anti-hen egg lysozyme (HEL) immunoglobulin gene-transgenic mice crossed with HEL-transgenic mice, the life span of anergic B cells has been estimated to be a few hours, justifying the view that B-cell anergy is a preterminal state.49 However, this does not appear to be the case for T cells. Numerous studies have described the persistence of anergic T cells, in vivo, for prolonged periods after the injection of the antigen that was responsible for inducing anergy.
The first description of in vivo T-cell anergy arose from the study of mice transgenic for an H2-E alloantigen under the rat insulin promoter.50 The T cells from these mice were hyporesponsive to the transgene-encoded H2-E when challenged in vitro. Arnold and colleagues have made extensive use of transgenic mice in which the Kb alloantigen is targeted to a variety of peripheral sites using tissue-specific promoters. Reactivity to Kb in these mice was always inhibited; the mechanism of the hyporesponsiveness was determined by the site and level of expression of the Kb molecule. In some cases TCR or CD8 expression was downregulated, in others anergy appeared to be the mechanism.51,52
Many of the in vivo studies have involved the injection of bacterial or Mls (subsequently shown to be murine mammary tumour virus proteins) superantigens (SAg) which enable T cells expressing particular TCR Vβ families to be examined for function without further in vitro manipulation.53–55 In most of these experiments, SAg injection led to a wave of T-cell death, followed by the persistence of T cells expressing the relevant TCR Vβ segment but unable to respond to antigenic challenge in vitro, resembling the results obtained with anti-CD3 antibody treatment in vitro. One feature of the anergy induced in vivo that differs from that induced in vitro was that the anergic T cells were unresponsive to exogenous IL-2, even if they expressed the IL-2 receptor.
The advent of TCR-transgenic mice has enabled similar questions to be addressed using conventional antigen. Two studies have detected anergy in CD8+ TCR-transgenic T cells.56,57 Subsequent experiments were performed with CD4+ TCR transgenic T cells. Lanoue and colleagues studied mice transgenic for a TCR with specificity for a peptide of influenza haemagglutinin.58 It proved to be very difficult to induce unresponsiveness in the transgenic T cells, in situ, in the transgenic mouse. This was only achieved after partial T-cell depletion and repeated injections of antigen. However, in double transgenic mice, generated by crossing the TCR-transgenics with mice transgenic for the influenza haemagglutinin (HA), the T cells that escaped deletion in the thymus were anergic. Similarly, TCR-transgenic T cells that were adoptively transferred into HA-transgenic hosts went through an initial phase of expansion, followed by a wave of deletion, and again the cells that survived were unresponsive. Subsequent studies from this group have observed that the ‘anergic’ T cells from the double transgenic mice secreted large amounts of the regulatory cytokine, IL-10, upon antigen re-challenge, raising questions concerning the mechanisms of unresponsiveness in this system.59
The approach that has been pioneered by Jenkins involves the adoptive transfer of limited numbers of TCR-transgenic CD4+ T cells into normal syngeneic mice. This allows the transgenic T cells to function in a polyclonal environment, and for them to be analysed using clonotypic mAbs, or by labelling the cells before transfer with a fluorescent dye.60 Using this model system it is clear that T-cell anergy is induced following intravenous injection of antigen.61 No evidence for the presence of conventional regulatory cells was obtained in this model.
Several of these in vivo systems have been used to address the lifespan of anergic T cells. In all the cases examined the anergic T cells persisted for a minimum of 1 month.61,62 In the Jenkins adoptive transfer model, recovery of reactivity was observed with time, but the unresponsiveness was maintained if the antigen was injected at weekly intervals. This is reminiscent of observations made in many of the rodent transplantation tolerance models, in which anti-donor reactivity recovered slowly if the transplant was removed.
Does the induction of T-cell anergy have biological significance?
The context in which the induction of anergy might be thought of as having a role in the normal physiology of the immune system is in the induction and maintenance of self tolerance. Although T-cell anergy can be induced in vivo by the injection of SAg, this is unlikely to relate to the induction of self tolerance. The hypothesis that we have promoted is that autoantigen presentation by tissue parenchymal cells, leading to the induction of T-cell anergy, is a key event in the regulation of autoreactive T cells. This hypothesis is predicated on all the observations of anergy induction by costimulation-deficient antigen-presenting cells. Most of these experiments have utilized transfected immortalized cells as APC with or without costimulatory blockade.63 We have reported the ability of primary cultures of interferon-γ-treated, human leucocyte antigen (HLA)-DR+ allogeneic human thyroid and renal tubular epithelial cells to induce allospecific anergy in CD45RO+ (memory) T cells.64,65
Most recently we have exploited clinical transplantation as a model system to explore these events in vivo. We have consistently observed the development of donor-specific hyporesponsiveness in renal and cardiac transplant patients, using limiting dilution analysis (LDA) for cytokine-secreting CD4+ T cell as a read out.66,67 If this was attributable to the in vivo equivalent of the observations that we had made with renal epithelial cells in vitro we predicted that the induction of hyporesponsiveness would be more pronounced in the CD45 RO T-cell subset, the population that can traffic through extralymphoid tissues. In a recent study of renal transplant patients, in whom frequencies were measured on the day, and four months after, transplantation, this prediction was confirmed.68 The final question that we addressed concerning this donor-specific hyporesponsiveness was whether it was due to deletion of anti-donor T cells, or to the induction of anergy. We exploited the fact that T-cell anergy can be reversed in in vitro cultured T cells; after purifying the CD4+ T cells from patients, some were immediately used for an LDA assay, the others were cultured for 3 days in exogenous IL-2 and then rested for 1 day, before being used in LDA. In five of nine hyporesponsive patients a highly significant increase in frequency was seen following culture in IL-2. No change in the anti-third party frequency was seen.69
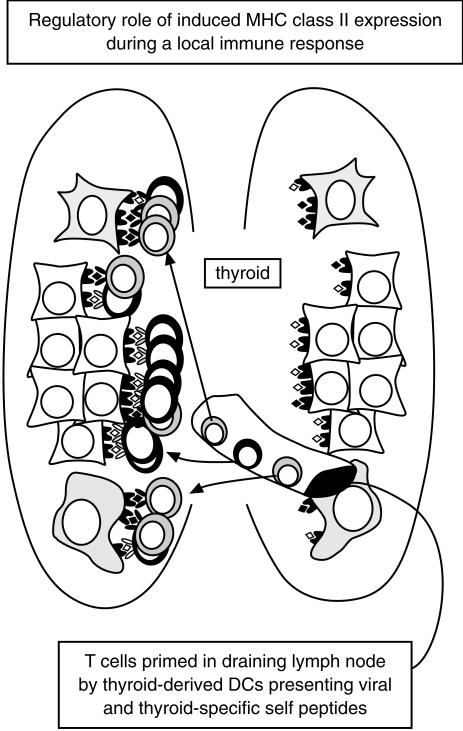
Taking these data together they support the following model concerning the in vivo significance of T-cell anergy induction. The peripheral T-cell repertoire contains autoreactive potential, due to the incomplete nature of thymic deletion and the expression of tissue-sequestered and developmental antigens. When a tissue becomes inflamed, due to a tissue-tropic virus for example, the tissue-resident dendritic cells migrate from the tissue to the draining lymph node, taking with them both the viral antigens and a large number of tissue antigens that were simultaneously internalized. In the lymph node both virus-specific and autoreactive T cells will be primed. The activated T cells will then traffic back to the inflamed tissue due to chemokine release and the inflamed endothelium in the tissue vasculature. Once in the tissue the virus-specific T cells will predominantly meet their peptide antigens on immigrant professional APC that are the most efficient at capturing, processing and presenting exogenous antigen. The autoreactive T cells, in contrast, will predominantly meet their cognate ligands on the mass of parenchymal cells that are by this time expressing MHC class II molecules due to locally produced interferon-γ. The parenchymal cells are the cells making the autoantigen, and are thus ideally placed to process and present it. The parenchymal cell autoantigen presentation will serve to induce anergy in the potentially dangerous T cells, as appeared to be occurring in the T cells with direct anti-donor allospecificity in the transplant patients. This model is illustrated in Fig. 2.
Figure 2.
The possible significance of antigen presentation by tissue parenchymal cells is illustrated. (1) T cells are primed in the lymph node draining an inflamed tissue, by dendritic cells that have migrated from the tissue. (2) Both exogenous antigen-specific, and autoreactive T cells are likely to be primed in response to dendritic cells presentation of both sets of antigen. Upon re-entering the tissue, through the activated endothelium, the T cells will meet their antigen in situ. (3) The exogenous antigen-specific T cells will predominantly be presented by immigrant bone-marrow-derived antigen-presenting cells that are efficient in antigen capture, processing and presentation. This will lead to reactivation of the T cells with exogenous antigen specificity. (4) In contrast, the major cells displaying the autoantigeneic epitopes will be the tissue parenchymal cells, induced to express MHC class II antigens by local interferon-γ, and actively synthesizing the autoantigens.This will lead to the induction of unresponsiveness in these T cells.
Anergic T cells as part of the spectrum of regulatory T cells
The above model may partially address the biological significance of anergic T cells, but it does not provide a reason for keeping the partially activated, potentially dangerous T cell alive. Is there any advantage in maintaining a cohort of unresponsive, apparently inert cells in the system? In the final section of this review we would like to review the evidence that cells with an anergic phenotype have regulatory effects, and that this may justify their existence.
Probably the first illustration of the potential for anergic T cells to act as suppressor cells came from a SAg in vivo model in which Mls-1b mice were injected with Mls-1a cells. The Lyt-1+ cells (probably CD4+) acted as efficient suppressor cells that were antigen-non-specific in their effector function.54,55 More recently we explored the regulatory effects of anergic T cells using clonal populations of human T cells in vitro.70 These cells acted as potent suppressor cells in a dose-dependent and antigen-specific manner. In addition, they were able to effect linked suppression if the APC expressed the antigen for which the anergic and the responsive T cells were specific.71 Most significantly, the suppression could not be observed in a transwell system, and could not be reversed by the addition of antibodies against the regulatory cytokines, IL-4, IL-10, and transforming growth factor-β (TGF-β). These results distinguished this phenomenon from all the other examples of T-cell-mediated regulation which were effected by soluble factors. At the time we interpreted these findings in terms of the passive ‘civil servant’ model of suppression first proposed by Waldmann.72 Subsequently, we reproduced the same results in a murine system, and went on to show that the anergic T cells could prolong skin allograft survival in vivo,73 providing the first evidence that anergic T cells were capable of effecting regulation in vivo.
In our most recent studies74 we have attempted to address the mechanisms whereby anergic T cells mediate their effects. Having observed that they failed to inhibit T-cell responses to combined anti-CD3 and anti-CD28 antibody stimulation, in the absence of APC, we concluded that APC were required for the regulatory effects to be seen. To address directly the effect of anergic T cells on APC, bone-marrow-derived dendritic cells (DCs) were generated in granulocyte–macrophage colony-stimulating factor and incubated with responsive or anergic T cells specific for DC-displayed antigens. After culture with responsive T cells the immunogenicity of the DC was increased. In marked contrast, culture with the anergic T cells rendered the DC almost totally incapable of stimulating T-cell proliferation. This loss of immunogenicity was accompanied by reduced expression of MHC class II and B7 family molecules. These data suggest that inhibition of DC maturation and function may be one of the ways in which anergic T cells exert their regulatory properties. These effects are precisely the opposite of the effects of helper T cells on immature DC, an effect which has been referred to as ‘licensing’. To maintain an alcohol-related terminology, the effect of anergic T cells on DC may be referred to as ‘prohibition’.
A series of very interesting observations have been made during the last few years by two groups, Sakaguchi's75 and Shevach's,76 regarding a population of pre-existing regulatory cells in the mouse. These cells were first identified in the context of a model of autoimmune disease that arose in mice that were subjected to thymectomy at 3 days. When Sakaguchi compared the 3-day thymectomized with normal mice of the same strain he noted that the manipulated animals lacked a small population of peripheral T cells that were CD4+, CD25+ and CD5hi. Most importantly, when these cells were transferred from a normal to a thymectomized animal they protected the recipient animal from the development of autoimmune disease. Subsequent in vitro studies have revealed some more features of these cells; they do not proliferate in the absence of exogenous IL-2, they do not secrete known cytokines, they require cell–cell contact in order to mediate their suppressive effects, they inhibit IL-2 transcription in the responsive T cells, and they appear to be antigen non-specific in their effector function.77 There are many striking similarities between this cell population and the anergic T cells that are described above. It is tempting to speculate that this CD25+ population arises due to encounter with self antigens, either presented by a non-immunogenic APC, or before the T cell is sufficiently mature to differentiate into a conventional effector cell. Indeed, they may even be generated in the thymus.78,79
At the other end of the spectrum of regulatory cells are those that regulate through the production of inhibitory/anti-inflammatory cytokines such as IL-10.27 One of the best defined in vivo models involving anti-inflammatory cytokine-secreting T cells is that of Powrie,80 in which CD45RBlo T cells from normal animals protect lymphopenic animals from the development of inflammatory bowel disease. It appears that IL-10, and/or TGF-β contribute to the regulation effected by these cells.81
Based on all of the available data, we propose that a spectrum of regulatory cells exists. At one end of the spectrum are cells whose regulatory function is mediated by cytokines that regulate other T cells or APC, at the other end of the spectrum are cells whose regulatory function requires cell-cell contact and is presumably mediated via cell surface molecular contacts involving molecules that have yet to be defined.
Conclusion
In conclusion, anergic T cells are alive and well, albeit in an altered state of health. Not only may the induction of anergy play a role in silencing otherwise pro-inflammatory autoreactive T cells, but their function may extend beyond this to the regulation of other potentially destructive T cells. Once the molecules involved in anergic T-cell mediated regulation are defined, they will be attractive targets for the design of novel and more precisely targeted immunotherapeutic agents.
References
- 1.Nossal GJ, Pick BL. Clonal anergy: persistence in tolerant mice of antigen-binding B lymphocytes incapable of responding to antigen or mitogen. Proc Natl Acad Sci USA. 1980;77:1602–6. doi: 10.1073/pnas.77.3.1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lamb JR, Skidmore BJ, Green N, Chiller JM, Feldman M. Induction of tolerance in influenza virus-immune T lymphocyte clones with synthetic peptides of influenza hemagglutinin. J Exp Med. 1983;157:1434–47. doi: 10.1084/jem.157.5.1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sloan-Lancaster J, Evavold BD, Allen PM. Th2 cell clonal anergy as a consequence of partial activation. J Exp Med. 1994;180:1195–205. doi: 10.1084/jem.180.4.1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mueller DL, Chiodetti L, Bacon PL, Schwartz RN. Clonal anergy blocks the response to IL-4, as well as the production of IL-2 in dual-producing helper T cell clones. J Immunol. 1991;147:4118–25. [PubMed] [Google Scholar]
- 5.Otten GR, Germain RN. Split anergy in a CD8+ T cell receptor-dependent cytolysis in the absence of interleukin-2 production. Science. 1991;251:1228–31. doi: 10.1126/science.1900952. [DOI] [PubMed] [Google Scholar]
- 6.Arnold B, Schonrich G, Hammerling GJ. Multiple levels of peripheral tolerance. Immunol Today. 1993;14:12–4. doi: 10.1016/0167-5699(93)90317-E. [DOI] [PubMed] [Google Scholar]
- 7.Beverly B, Kang SM, Lenardo MJ, Schwartz RH. Reversal of in vitro T cell clonal anergy by IL-2 stimulation. Int Immunol. 1992;4:661–71. doi: 10.1093/intimm/4.6.661. [DOI] [PubMed] [Google Scholar]
- 8.Groux H, Bigler M, de Vries JE, Roncarolo M-G. Interleukin-10 induces a long-term antigen-specific anergic state in human CD4+ T cells. J Exp Med. 1996;184:19–29. doi: 10.1084/jem.184.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Quill H, Schwartz RH. Stimulation of normal inducer T cell clones with antigen presented by purified Ia molecules in planar lipid membranes: specific induction of a long-lived state of proliferative non-responsiveness. J Immunol. 1987;138:3704–9. [PubMed] [Google Scholar]
- 10.Freeman GJ, Freeman AS, Segil JM, Lee G, Whiteman JF, Nadler LM. B7, a new member of the Ig superfamily with unique expression on activated and neoplastic B cells. J Immunol. 1987;143:2714–22. [PubMed] [Google Scholar]
- 11.Linsley PS, Brady W, Urnes M, Grosmarie LS, Damle NK, Ledbetter JA. Binding of the B cell activation antigen B7 to CD28 costimulate T cell proliferation and interleukin-2 mRNA accumulation. J Exp Med. 1991;173:721–30. doi: 10.1084/jem.173.3.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guinan EC, Bussiotis VA, Neuberg D, Brennan L, Hirano N, Nadler LM, Gribben JG. Transplantation of anergic histoincompatible bone marrow allografts. N Engl J Med. 1999;340:1704–14. doi: 10.1056/NEJM199906033402202. [DOI] [PubMed] [Google Scholar]
- 13.Schwartz RH. Models of T cell anergy: is there a common molecular mechanism? J Exp Med. 1996;184:1–8. doi: 10.1084/jem.184.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Davis LS, Lipsky PE. Tolerance induction of human CD4+ T cells: markedly enhanced sensitivity of memory versus naive T cells to peripheral anergy. Cell Immunol. 1993;146:351–61. doi: 10.1006/cimm.1993.1032. 10.1006/cimm.1993.1032. [DOI] [PubMed] [Google Scholar]
- 15.Sagerstorm CG, Kerr EM, Allison JP, Davis MM. Activation and differentiation requirements of primary T cells in vitro. Proc Natl Acad Sci USA. 1993;90:8987–91. doi: 10.1073/pnas.90.19.8987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hayashi R, Loh DY, Kanagawa O, Wang F. Differences between responses of naïve and activated T cells to anergy induction. J Immunol. 1998;60:33–8. [PubMed] [Google Scholar]
- 17.De Magistris MT, Alexander J, Coggeshall M, Altman A, Gaeta FC, Grey HM, Sette A. Antigen analog-major histocompatibility complexes act as antagonists of the T cell receptor. Cell. 1992;68:625–34. doi: 10.1016/0092-8674(92)90139-4. [DOI] [PubMed] [Google Scholar]
- 18.Sloan-Lancaster J, Evavold BD, Allen PM. Induction of T -cell anergy by altered T-cell-receptor ligand on live antigen-presenting cells. Nature. 1993;363:156–9. doi: 10.1038/363156a0. [DOI] [PubMed] [Google Scholar]
- 19.Racioppi L, Ronchese F, Matis LA, Germain RN. Peptide–major histocompatibility complex class II complexes with mixed agonist/antagonist properties provide evidence for ligand-related differences in T cell receptor-dependent intracellular signaling. J Exp Med. 1993;177:1047–60. doi: 10.1084/jem.177.4.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sloan-Lancaster J, Shaw AS, Rothbard JB, Allen PM. Partial T cell signalling: altered phospho-xxx and lack of Zap-70 recruitment in APL-induced T cell anergy. Cell. 1994;9:913–22. doi: 10.1016/0092-8674(94)90080-9. [DOI] [PubMed] [Google Scholar]
- 21.Madrenas J, Wange RL, Wang JL, Isakov N, Samelson LE, Germain RN. Zeta phosphorylation without ZAP-70 activation induced by TCR antagonists or partial agonists. Science. 1995;267:515–8. doi: 10.1126/science.7824949. [DOI] [PubMed] [Google Scholar]
- 22.Frasca L, Tamir A, Jurcevic S, et al. Peptide analogues as a strategy to induce tolerance in T cells with indirect allospecificity. Transplantation. 2000;70:1–10. doi: 10.1097/00007890-200008270-00017. [DOI] [PubMed] [Google Scholar]
- 23.Klenerman P, Rowland-Jone S, McAdam S, et al. Cytotoxic T-cell activity antagonized by naturally occurring HIV-1 Gag variants. Nature. 1994;369:403–7. doi: 10.1038/369403a0. [DOI] [PubMed] [Google Scholar]
- 24.Bertoletti A, Sette A, Chisari FV, Penna A, Levrero M, De Carli M, Fiaccadori F, Ferrari C. Natural variants of cytotoxic epitopes are T-cell receptor antagonists for antiviral cytotoxic T cells. Nature. 1994;369:407–10. doi: 10.1038/369407a0. [DOI] [PubMed] [Google Scholar]
- 25.DeSilva DR, Urdahl KB, Jenkins MK. Clonal anergy is induced by T cell receptor occupancy in the absence of proliferation. J Immunol. 1994;47:3261–7. [PubMed] [Google Scholar]
- 26.Powell JD, Lerner CG, Schwartz RH. Inhibition of cell cycle progression by rapamycin induces T cell clonal anergy even in the presence of costimulation. J Immunol. 1999;162:2775–84. [PubMed] [Google Scholar]
- 27.Groux H, O'Garra A, Bigler M, Rouleau M, Antonenko S, de Vries JE, Roncarolo M-G. A CD4+ T-cell subset inhibits antigen-specific T-cell responses and prevents colitis. Nature. 1997;389:737–42. doi: 10.1038/39614. 10.1038/39614. [DOI] [PubMed] [Google Scholar]
- 28.Lamb JR, Feidmann M. Essential requirement for major histocompatibility complex in recognition in T cell tolerance induction. Nature. 1984;308:72–4. doi: 10.1038/308072a0. [DOI] [PubMed] [Google Scholar]
- 29.Sidhu S, Deacock S, Bal V, Batchelor R, Lombardi G, Lechler R. Human T cells cannot act as autonomous antigen presenting cells but induce tolerance in antigen-specific and alloreactive T cells. J Exp Med. 1992;176:875–80. doi: 10.1084/jem.176.3.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wyss-Coray T, Mauri-Hellweg D, Baumann K, Bettens F, Grunow R, Pichler WJ. The B7 adherent molecule is expressed on activated human T cells: functional involvement in T–T interactions. Eur J Immunol. 1993;23:2175–80. doi: 10.1002/eji.1830230919. [DOI] [PubMed] [Google Scholar]
- 31.Azuma M, Yssl H, Phillips JH, Spits H, Lanier LL. Functional expression of B7/BB1 on activated T lymphocytes. J Exp Med. 1993;177:845–50. doi: 10.1084/jem.177.3.845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sansom DM, Hall ND. B7/BB1, the ligand for CD28, is expressed on repeatedly activated human T cells in vitro. EurJImmunol. 1993:295–8. doi: 10.1002/eji.1830230148. [DOI] [PubMed] [Google Scholar]
- 33.Lombardi G, Hargreaves R, Sidhu S, et al. Antigen presentation by T cells inhibits IL-2 production and induces IL-4 release due to altered cognate signals. J Immunol. 1996;156:2769–75. [PubMed] [Google Scholar]
- 34.Lee KM, Chuang E, Griffin M, et al. Molecular basis of T cell inactivation by CTLA-4. Science. 1998;282:2263–6. doi: 10.1126/science.282.5397.2263. [DOI] [PubMed] [Google Scholar]
- 35.Linsley PS, Brady W, Urnes M, Grosmaire LS, Damle NK, Ledbetter JA. CTLA-4 is a second receptor for the B-cell activation antigen B7. J Exp Med. 1991;174:561–9. doi: 10.1084/jem.174.3.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Walunas TL, Lenschow DL, Bakker CY, Linsley PS, Freeman GJ, Green JM, Thompson CB, Bluestone JA. CTLA-4 can function as a negative regulator of T cell activation. Immunity. 1994;1:405–13. doi: 10.1016/1074-7613(94)90071-x. [DOI] [PubMed] [Google Scholar]
- 37.Krummel MF, Allison JP. CD28 and CTLA-4 have opposing effects on the response of T cells to stimulation. J Exp Med. 1995;182:459–65. doi: 10.1084/jem.182.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Waterhouse P, Penninger JM, Timms E, et al. Lymphoproliferative disorders with early lethality in mice deficient in CTLA-4. Science. 1995;270:985–8. doi: 10.1126/science.270.5238.985. [DOI] [PubMed] [Google Scholar]
- 39.Tivol EA, Borriello F, Schweitzer AN, Lynch WP, Bluestone JA, Sharpe AH. Loss of CTLA-4 leads to massive lymphoproliferative and fatal multiorgan tissue destruction, revealing a critical negetive regulatory role of CTLA-4. Immunity. 1995;3:541–7. doi: 10.1016/1074-7613(95)90125-6. [DOI] [PubMed] [Google Scholar]
- 40.Perez VL, Parijs LV, Biuckians A, Zhang XX, Strom TB, Abbas AK. Induction of peripheral T cell tolerance in vivo requires CTLA-4 engagement. Immunity. 1997;6:411–7. doi: 10.1016/s1074-7613(00)80284-8. [DOI] [PubMed] [Google Scholar]
- 41.Walunas TL, Bluestone JA. CTLA-4 regulates tolerance induction and T cell differentiation in vivo. J Immunol. 1998;160:3855–60. [PubMed] [Google Scholar]
- 42.Zheng XX, Markees TG, Hancock WW, et al. CTLA-4 siganls are required to optimally induce allograft tolerance with combined donor-specific transfusion and anti-CD154 monoclonal antibody treatment. J Immunol. 1999;162:4983–90. [PubMed] [Google Scholar]
- 43.Judge TA, Wu Z, Zhang XG, Sharpe AH, Sayegh MH, Turka LA. The role of CD80, CD86, and CTLA-4 in alloimmune response and the induction of long-term allograft survival. J Immunol. 1999;162:1947–51. [PubMed] [Google Scholar]
- 44.Tsitoura DC, DeKruyff RH, Lamb JR, Umetsu DT. Intransal exposure to protein antigen induces immunological tolerance mediated by functionally disabled CD4+ Tcells. J Immunol. 1999;163:2592–600. [PubMed] [Google Scholar]
- 45.Frauwirth KA, Alegre ML, Thompson CB. Induction of T cell anergy in the absence of CTLA-4/B7 interaction. J Immunol. 2000;164:2987–93. doi: 10.4049/jimmunol.164.6.2987. [DOI] [PubMed] [Google Scholar]
- 46.Hargreaves RG, Borthwick NJ, Montani MSG, Piccolella E, Carmichael P, Lechler RI, Akbar AN, Lombardi G. Dissociation of T cell anergy from apoptosis by blockade of Fas/Apo-1 (CD95) signaling. J Immunol. 1997;158:3099–107. [PubMed] [Google Scholar]
- 47.Chai JG, Lechler RI. Immobilized anti-CD3 mAb induces anergy in murine naive and memory CD4+ T cells. Int Immunol. 1997;9:935–44. doi: 10.1093/intimm/9.7.935. 10.1093/intimm/9.7.935. [DOI] [PubMed] [Google Scholar]
- 48.Matzinger P. Tolerance, danger, and the extended family. Annu Rev Immunol. 1994;12:991–1045. doi: 10.1146/annurev.iy.12.040194.005015. [DOI] [PubMed] [Google Scholar]
- 49.Cyster JG, Hartley SB, Goodnow CC. Competition for follicular niches excludes self-reactive B cells from the recirculating B-cell repertoire. Nature. 1994;371:389–95. doi: 10.1038/371389a0. [DOI] [PubMed] [Google Scholar]
- 50.Burkly LC, Lo D, Kanagawa O, Brinster RL, Flavell RA. T cell tolerance by clonal anergy in transgenic mice with nonlymphoid expression of MHC class II I-E. Nature. 1989;342:564–6. doi: 10.1038/342564a0. [DOI] [PubMed] [Google Scholar]
- 51.Schonrich G, Kalinke U, Momburg F, Malissen M, Schmitt-Verhulst AM, Malissen B, Hammerling GJ, Arnold B. Down-regulation of T cell receptors on self-reactive T cells as a novel mechanism for extrathymic tolerance induction. Cell. 1991;65:293–304. doi: 10.1016/0092-8674(91)90163-s. [DOI] [PubMed] [Google Scholar]
- 52.Schonrich G, Momburg F, Malissen M, Schmitt-Verhulst AM, Malissen B, Hammerling GJ, Arnold B. Distinct mechanisms of extrathymic T cell tolerance due to differential expression of self-antigen. Int Immunol. 1992;4:581–90. doi: 10.1093/intimm/4.5.581. [DOI] [PubMed] [Google Scholar]
- 53.Rammensee HG, Kroschewski R, Frangoulis B. Clonal anerg induced in mature V beta 6+ T lymphocytes on immunizing Mls-1b mice with Mls-1a expressing cells. Nature. 1989;339:541–4. doi: 10.1038/339541a0. [DOI] [PubMed] [Google Scholar]
- 54.Kawabe Y, Ochi A. Selective anergy of vb8+ CD4+ T cells in staphylococcus enterotoxin B-primed mice. J Exp Med. 1990;172:1065–70. doi: 10.1084/jem.172.4.1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rellahan BL, Jones LA, Kruisbeek AM, Fry AM, Matis LA. In vivo induction of anergy in peripheral Vb8+ T cells by staphylococcal enterotoxin B. J Exp Med. 1990;172:1091–100. doi: 10.1084/jem.172.4.1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kyburz D, Aichele P, Speiser DE, Hengartner H, Zinkernagel RM, Pircher H. T cell immunity after a viral infection verus T cell tolerance induced by soluble viral peptides. Eur J Immunol. 1993;23:1956–62. doi: 10.1002/eji.1830230834. [DOI] [PubMed] [Google Scholar]
- 57.Rocha B, Grandien A, Freitas AA. Anergy and exhaustion are independent mechanisms of peripheral T cell tolerance. J Exp Med. 1995;181:993–84. doi: 10.1084/jem.181.3.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lanoue A, Bona C, von Boehmer H, Sarukhan A. Conditions that induce tolerance in mature CD4+ T cells. J Exp Med. 1997;185:405–14. doi: 10.1084/jem.185.3.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Buer J, Lanoue A, Franzke A, Garcia C, von Boehmer H, Sarukhan A. Intereleukin-10 secretion and impaired effector function of major histocompatibility complex class II-restricted T cells anergized in vivo. J Exp Med. 1998;187:177–83. doi: 10.1084/jem.187.2.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kearney ER, Pape KA, Loh DY, Jenkins MK. Visualization of peptide-specific T cell immunity and peripheral tolerance induction in vivo. Immunity. 1994;1:27–339. doi: 10.1016/1074-7613(94)90084-1. [DOI] [PubMed] [Google Scholar]
- 61.Pape KA, Merica R, Mondino A, Khoruts A, Jenkins MK. Direct evidence that functionally impaired CD4+ T cells persist in vivo following induction of peripheral tolerance. J Immunol. 1998;160:719–4729. [PubMed] [Google Scholar]
- 62.Alferink J, Schittek B, Schonrich G, Hammerling GJ, Arnold B. Long life span of tolerant T cells and the role of antigen in maintenance of peripheral tolerance. Int Immunol. 1995;7:331–6. doi: 10.1093/intimm/7.2.331. [DOI] [PubMed] [Google Scholar]
- 63.Boussiotis VA, Freeman GJ, Gray G, Gribben J, Nadler LM. B7 but not intercellular adhesion molecule-1 prevents the induction of human alloantigen-specific tolerance. J Exp Med. 1993;178:1753–63. doi: 10.1084/jem.178.5.1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Marelli-Berg F, Weetman A, Frasca F, Deacock S, Imami N, Lombardi G, Lechler RI. Antigen presentation by epithelial cells induces anergic immunoregulatory CD45RO+ T cells and deletion of CD45RA cells. J Immunol. 1997;159:5853–61. [PubMed] [Google Scholar]
- 65.Frasca L, Marelli-Berg F, Imami N, Potolicchio I, Carmicheal P, Lombardi G, Lechler RI. Gamma-interferon treated renal tubular epithelial cells induce allospecific tolerance. Kidney Int. 1998;53:679–89. doi: 10.1046/j.1523-1755.1998.00800.x. 10.1046/j.1523-1755.1998.00800.x. [DOI] [PubMed] [Google Scholar]
- 66.Mason PD, Robinson CM, Lechler RI. Detection of donor-specific hyporesponsiveness following late failure of human renal allografts. Kidney Int. 1996;50:1019–25. doi: 10.1038/ki.1996.404. [DOI] [PubMed] [Google Scholar]
- 67.Hornick PI, Mason PD, Yacoub MH, Rose ML, Batchelor R, Lechler RI. Assessment of the contribution that direct allorecognition makes to the progression of chronic cardiac transplant rejection in HmanCirculation. 1998. pp. 1257–63. [DOI] [PubMed]
- 68.Baker R, Hernandez-Fuentes M, Brookes P, Chaudhury A, Lechler R. The role of the allograft in the induction of donor-specific T cell hyporesponsiveness. Transplantation. 2001. in press. [DOI] [PubMed]
- 69.Ng WF, Baker RJ, Hernandez-Fuentes M, Chaudhury A, Lechler R. The role of T cell anergy in the maintenance of donor-specific hyporesponsiveness in renal transplant recipients. Transplant Proc. 2001;33:154–5. doi: 10.1016/s0041-1345(00)01951-5. [DOI] [PubMed] [Google Scholar]
- 70.Lombardi G, Sidhu S, Batchelor R, Lechler RI. Anergic T cells act as suppressor cells in vitro. Science. 1994;264:1587–9. doi: 10.1126/science.8202711. [DOI] [PubMed] [Google Scholar]
- 71.Frasca L, Carmichael P, Lechler R, Lombardi G. Anergic T cells effect linked suppression. Eur J Immunol. 1997;27:3191–7. doi: 10.1002/eji.1830271216. [DOI] [PubMed] [Google Scholar]
- 72.Waldamnn H, Qin S, Cobbold S. Monoclonal antibodies as agents to reinduce tolerance in autoimmunity. J Autoimmun. 1992;5:93–102. doi: 10.1016/0896-8411(92)90024-k. [DOI] [PubMed] [Google Scholar]
- 73.Chai JG, Bartok I, Chandler P, Vendetti S, Antoniou A, Dyson J, Lechler R. Anergic T cells act as suppressor cells in vitro and in vivo. Eur J Immunol. 1999;29:686–92. doi: 10.1002/(SICI)1521-4141(199902)29:02<686::AID-IMMU686>3.0.CO;2-N. 10.1002/(sici)1521-4141(199902)29:02<686::aid-immu686>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 74.Vendetti S, Chai JG, Dyson J, Simpson E, Lombardi G, Lechler R. Anergic T cells inhibit the antigen-presenting function of dendritic cells. J Immunol. 2000;165:1175–81. doi: 10.4049/jimmunol.165.3.1175. [DOI] [PubMed] [Google Scholar]
- 75.Sakaguchi S, Toda M, Asano M, Itoh M, Morse SS, Sakaguchi N. T cell-mediated maintenance of natural self-tolerance: its breakdown as possible cause of various autoimmune diseases. J Autoimmun. 1996;9:211–20. doi: 10.1006/jaut.1996.0026. 10.1006/jaut.1996.0026. [DOI] [PubMed] [Google Scholar]
- 76.Suri-Payer E, Amar AZ, Thornton AM, Shevach EM. CD4+ CD25+ T cells inhibit both the induction and effector function of autoreactive T cells and represent a unique lineage of immunoregulatory cells. J Immunol. 1998;160:1212–8. [PubMed] [Google Scholar]
- 77.Thornton AM, Shevach EM. Suppressor effector function of CD4+ CD25+ immunoregulatory T cells is antigen nonspecific. J Immunol. 2000;164:183–90. doi: 10.4049/jimmunol.164.1.183. [DOI] [PubMed] [Google Scholar]
- 78.Sakaguchi S. Regulatory T cells: key controllers of immunologic self-tolerance. Cell. 2000;101:455–8. doi: 10.1016/s0092-8674(00)80856-9. [DOI] [PubMed] [Google Scholar]
- 79.Seddon B, Mason D. The third function of the thymus. Immunol Today. 2000;21:95–9. doi: 10.1016/s0167-5699(99)01559-5. 10.1016/s0167-5699(99)01559-5. [DOI] [PubMed] [Google Scholar]
- 80.Asseman C, Mauze S, Leach MW, Coffman RL, Powrie F. An essential role for interleukin 10 in the function of regulatory T cells that inhibit intestinal inflammation. J Exp Med. 1999;190:995–1004. doi: 10.1084/jem.190.7.995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Read S, Malmstrom V, Powrie F. Cytotoxic T lymphocyte-associated antigen 4 plays an essential role in the function of CD25 (+) CD4 (+) regulatory cells that control intestinal inflammation. J Exp Med. 2000;192:295–302. doi: 10.1084/jem.192.2.295. [DOI] [PMC free article] [PubMed] [Google Scholar]