Abstract
Murabutide is a safe synthetic immunomodulator derived from muramyl dipeptide, the smallest bioactive unit of bacterial peptidoglycan. Although it is well known that muramyl peptides modulate the functions of monocytes/macrophages, their activity on dendritic cells is poorly documented. We thus investigated the effects of Murabutide on immunophenotype, endocytosis, T-cell stimulatory capacity, and cytokine secretion of human monocyte-derived immature dendritic cells (iDCs). We found that Murabutide triggers immunophenotypic changes as upon treatment, iDCs up-regulate the surface expression of the major histocompatibility complex type II molecule human leucocyte antigen-DR, the co-stimulatory molecules CD80, CD86 and CD40 and the differentiation marker CD83, and down-regulate the expression of the mannose receptor. These phenotypic changes are also mirrored by changes in their biological activity. Subsequent to treatment with the synthetic immunomodulator, DC have a decreased endocytic capacity but exhibit enhanced stimulatory capacity for both allogeneic and autologous T cells. In addition, Murabutide-stimulated iDCs have a greater cytostatic activity toward the tumour cell line THP-1. Furthermore, in the presence of Murabutide, DCs transiently increased the release of macrophage inhibitory protein-1β, tumour necrosis factor-α and interleukin-10, whereas the enhanced production of macrophage-colony stimulating factor was sustained over the 3-day period analysed. In addition, Murabutide triggers the phosphorylation of the three classes of mitogen-activated protein kinases in iDCs. Altogether our results demonstrate that Murabutide triggers the maturation and activation of monocyte-derived iDCs. As this immunomodulator is approved for administration in humans, it could be a useful adjunct to boost the efficacy of DC-based vaccines designed against tumours or virus-infected cells.
Introduction
Dendritic cells (DCs) play a crucial role in the initiation of primary T-cell-mediated immune responses.1 These cells exist in two major stages of maturation associated with different functions. Immature DCs (iDCs) are located in most tissues or in the circulation and are recruited into inflamed sites. They are highly specialized antigen-capturing cells, expressing large amounts of receptors involved in antigen uptake or phagocytosis such as the mannose receptor,2 FcγR,3 and CD36.4 Following antigen capture and processing, iDCs move to local T-cell areas in lymph nodes or spleen.1 During this process, DCs lose their antigen-capturing capacity and the pattern of cell surface molecule expression changes dramatically, turning DCs into immunostimulatory mature DCs (mDCs).3 The mDCs display high levels of major histocompatibility complex (MHC) –peptide complexes at their surface and up-regulate the expression of co-stimulatory and adhesion molecules. Thus these cells are able to activate T lymphocytes potently and so induce an antigen-specific response.1 Maturing DCs also produce a set of chemokines that attract iDCs, monocytes and T cells to the inflamed tissue, thereby amplifying the immune response.5 The importance of DCs in the initiation of the immune response is underscored by the fact that in cancer or chronic viral infections, DC functions are often impaired1,6,7 leading to limited immune responses directed against the tumour or the infected cells. In this context, cell-based immunotherapy aimed at activating DCs ex vivo using tumour-derived products or viral antigens prior to re-injection into patients offers promising avenues.8–11 However, the parallel use of immunostimulatory molecules may be required to obtain DCs that are optimally activated.12,13
Murabutide (MB) is a synthetic immunomodulator derived by chemical modification of muramyl dipeptide (MDP), which is the minimal bioactive structure of bacterial peptidoglycan. Unlike most other members of the muramyl peptide family of molecules, MB is well tolerated by humans14,15 and maintains certain biological effects, acting principally on cells of the reticulo-endothelial system. It possesses strong adjuvant properties in both mice and humans when injected with poorly immunogenic antigens.14,16 In addition, MB favours the release of cytokines in vivo without significant induction of pro-inflammatory mediators.15 Using murine models, it has also been shown that this molecule enhances the host's non-specific resistance to certain bacterial and viral infections.17,18 Moreover, MB enhances the anti-inflammatory and antiviral activities of type I interferons and potentiates the anti-tumour activity of both interleukin-2 (IL-2) and interferon-α.19–21 Recently, we have demonstrated that this synthetic immunomodulator dramatically inhibits HIV-1 replication in acutely infected monocyte-derived macrophages (MDMs) and mDCs.22
In vitro, blood monocytes cultured in granulocyte–macrophage colony-stimulating factor (GM-CSF) and IL-4 give rise to iDCs possessing characteristics of peripheral tissue DCs.3 Further differentiation into mDCs can be triggered by exposure to pro-inflammatory cytokines, such as tumour necrosis factor-α (TNF-α) or IL-1β,2,23 or by bacterial cell wall components, such as lipopolysaccharide (LPS) or mycobacterial cell wall skeleton.2,24 However, the effects on DCs of defined cell wall structures isolated from Gram-positive bacteria have not been reported. Furthermore, although it is well known that muramyl peptides modulate the functions of monocytes/macrophages, their action on DCs is poorly documented. Therefore, we investigated the effects of MB, a synthetic but well-tolerated analogue of MDP, on the phenotype, T-cell stimulatory capacity, cytostatic activity, and cytokine secretion of human monocyte-derived DCs. Furthermore, since signalling via mitogen-activated protein (MAP) kinases plays an important role in cellular responses, such as differentiation and cytokine secretion,25,26 we also examined the involvement of the three classes of MAP kinases in response to MB stimulation.
Materials and Methods
Reagents and antibodies
Recombinant human GM-CSF, IL-4 and TNF-α were from R & D systems, Abingdon, UK. LPS from Escherichia coli strain 026:B6 was purchased from Sigma (Saint Quentin Fallavier, France). MB was prepared as described elsewhere22 and dissolved in phosphate-buffered saline (PBS). The absence of endotoxin contaminant (< 6 × 10−2 EU/ml) was verified by the Limulus amoebocyte lysate assay (BioWhittaker France, Fontenay-sous-bois, France). Fluorescein isothiocyanate (FITC)-dextran (MW 20 000) was purchased from Molecular Probes (Interchim, Montluçon, France) and tetanus toxoid was obtained from the Statens Serum Institute (Copenhagen, Denmark). Fluorochrome-conjugated mouse monoclonal antibodies directed against the following cell surface molecules were used: CD14 (clone RM052), CD40 (clone mAb89), CD25 (clone B1.49.9), CD86 (clone HA5.2B7) and CD83 [clone HB15a, immunoglobulin G2b (IgG2b)] (Immunotech, Marseille, France); mannose receptor (MR) (clone 19), CD80 (clone L307) (Pharmingen, Rungis, France); HLA-DR (clone L243) (Becton-Dickinson, Rungis, France). Antibodies recognizing the phosphorylated form of human p38MAPK, extracellular signal-regulated kinases (ERK), and C-Jun N-terminal kinases (JNK) were from New England Biolabs (Ozyme, Saint-Quentin en Yveline, France), and anti-actin monoclonal antibody was from Sigma.
Generation of DCs
Peripheral blood mononuclear cells (PBMCs) were isolated by gradient density centrifugation of buffy coats from healthy donors (Etablissement de Transfusion Sanguine, Lille, France) using Ficoll–Hypaque (Amersham-Pharmacia Biotech, Orsay, France) and were resuspended in RPMI-1640 (Life Technologies, Cergy Pontoise, France) containing 10% heat-inactivated AB serum. Following overnight adherence to tissue culture flasks (Falcon, Le pont de Claix, France) and washes with warm PBS, monocytes were recovered by gentle detachment with a cell scraper. The monocyte-depleted PBMCs were used to purify CD4 T lymphocytes (see below). To generate iDCs, monocytes were cultured in DC medium containing RPMI-1640, 5% AB serum, 800 U/ml GM-CSF, and 500 U/ml IL-4. On days 3 and 5, 90% of the medium was removed and cultures were replenished with an equal volume of fresh medium supplemented with cytokines as described above. After 7 days of differentiation, non-adherent and loosely adherent cells representing the iDCs were harvested, washed and used for subsequent experiments. In some experiments, the differentiation was performed in DC medium supplemented with either 10 µg/ml of MB or 5 ng/ml of TNF-α over a 7-day period.
Stimulation and phenotypic analysis
The iDCs were seeded at 0·5 × 106 cells in 0·5 ml per well of a 48-well plate in DC medium. MB was added at 10 µg/ml, final concentration, and LPS was added at 100 ng/ml. After 24, 48 and 72 hr of stimulation, culture supernatants were collected and stored at −80° for subsequent analysis of secreted cytokines. Cells were then harvested, washed with PBS, and processed for phenotyping. A total of 1 × 105 cells were incubated for 45 min on ice with specific monoclonal antibody or with isotype-matched control diluted 1 : 100 in PBS containing 2% fetal calf serum (Life Technologies). Following one wash in ice-cold PBS, cells were resuspended and fixed in 1% paraformaldehyde in PBS and analysed using a FACSCalibur flow cytometer and the program cellquest (Becton Dickinson). Live cells were gated on their forward and side light scatter characteristics, and the percentage of positive cells and the mean fluorescence intensity (MFI) of the population were recorded.
Endocytosis
MR-mediated endocytosis was measured by the cellular uptake of FITC-dextran and was quantified by flow cytometry. The iDCs, stimulated or not for 24 hr with MB, were incubated in medium containing 1 mg/ml FITC-dextran for 60 min at either 37° or 4° (control). Cells were then washed extensively in ice-cold PBS and the uptake of FITC-dextran was determined by flow cytometry. Results were expressed as percentage inhibition calculated as follows: 100 × [1 − (MFI of MB-treated DCs at 37° − MFI of MB-treated DCs at 4°)/(MFI untreated DCs at 37° − MFI untreated DCs at 4°)].
Mixed lymphocyte reaction and antigen presentation
CD4 T lymphocytes were purified from monocyte-depleted PBMCs by positive selection using magnetic beads coated with a mouse monoclonal anti-CD4 antibody (Dynabeads, Dynal, France) according to the manufacturer's instructions. After selection, cells were cultured overnight in medium containing RPMI-1640 and 10% AB serum to allow bead detachment. The CD4 T cells were either used in mixed lymphocyte reaction (MLR) or stored frozen in liquid nitrogen in RPMI containing 20% AB serum and 10% dimethylsulphoxide until use for antigen presentation. For the MLR, iDCs were treated with 50 µg/ml mitomycin C (Sigma) for 45 min at 37° and washed extensively. Cells were resuspended in DC medium and seeded at 2 × 102−2 × 104 cells per well of a 96-well plate. The allogeneic CD4 T cells were then added at 2 × 105 cells per well in a final volume of 200 µl per well. Where needed, MB or LPS was added to the cultures at 10 µg/ml or 100 ng/ml, respectively. Cells were co-cultured for 6 days and during the last 16 hr of incubation, 0·5 µCi [3H]thymidine (Amersham-Pharmacia Biotech) were added to each well. Incorporation assays were performed in quadruplicate. The following day, cells were harvested onto glass fibre filter plate (Optiplate, Packard, France) and the radioactivity was counted on a liquid scintillation counter (Top count, Packard). Radioactivity incorporated in the presence of unstimulated iDCs at a DC : CD4 T-cell ratio of 1 : 30 was set at 100%. For statistical analysis, the actual count per minute (c.p.m.) values corrected for spontaneous incorporation of [3H]thymidine into CD4 T cells were used. For antigen presentation assay, iDCs were seeded at 1 × 104 cells per well of a 96-well plate in DC medium then pulsed for 20 hr with 10 µg/ml tetanus toxoid (TT). Two hours after the beginning of the pulse, MB, LPS, or TNF-α were added at 10 µg/ml, 100 ng/ml and 5 ng/ml, respectively. After the incubation period, medium was discarded and iDCs were treated with mitomycin C as described above and washed extensively. Autologous CD4 T cells (1 × 105/well) were then added and co-cultured for 6 days. The last 16 hr of incubation, 0·5 µCi [3H]thymidine was added to each well. Incorporation assays were performed in quadruplicate and the amount of radioactivity incorporated into DNA was determined as described above.
Tumoristatic activity
The iDCs were seeded at 1 × 104 or 5 × 104 cells per well of a 96-well plate in DC medium. MB was added at 10 µg/ml final concentration, 150 µl/well final volume, 24 hr before the addition of THP-1 cells. THP-1 cells were added at 1 × 104 cells per well in 200 µl per well final volume to yield ratio of DCs to THP-1 of 1 : 1 or 5 : 1. Cells were co-cultured for 5 days and were pulsed for the last 16 hr of incubation with 0·5 µCi per well [3H]thymidine. Incorporation assays were performed in triplicate and the amount of radioactivity incorporated into DNA was determined as described above. Data were presented as percentage inhibition calculated as follows: % inhibition = (1 − test c.p.m./control c.p.m.) × 100%, where test c.p.m. represents the radioactivity incorporated by tumour cells cultured with DC treated or not with MB, and control c.p.m. is the radioactivity incorporated by THP-1 cells cultured without DCs but in the presence or absence of MB. Incorporation of [3H]thymidine in DCs was negligible compared to the radioactivity incorporated into THP-1 cells (less than 4% compared to THP-1).
Cytokine determination
The amount of macrophage inhibitory protein (MIP) −1β, TNF-α, and interleukin (IL) −10, whereas the enhanced production of macrophage-colony stimulating factor (M-CSF) MIP-1β, TNF-α, M-CSF, IL-10, and IL-12p70 secreted in the culture medium following stimulation of iDCs with MB or LPS was quantified by ELISA as per manufacturer's instructions (Quantikine kits, R and Systems, Abingdon, UK).
Detection of the phosphorylated form of MAP kinases
The iDCs were harvested as described above and incubated in RPMI-1640 without additives for 2 hr at 37°. Cells were then stimulated with 10 µg/ml of Murabutide and, at the indicated time-points, 2 × 106 cells were removed and washed once with ice-cold PBS. The cell pellets were resuspended in boiling 2 × sodium dodecyl sulphate (SDS) sample buffer and incubated for 5 min at 95°. Total proteins were separated by 12% SDS–polyacrylamide gel electrophoresis and blotted onto nitrocellulose membranes (Amersham-Pharmacia Biotech). For the detection of phosphorylated MAP kinases, membranes were processed following the manufacturer's instructions (New England Biolabs) followed by incubation for 1 hr with peroxidase-conjugated secondary antibody. Immunoreactive bands were visualized using enhanced chemiluminescence (ECL, Amersham-Pharmacia Biotech). Membranes were then stripped and reprobed with an anti-actin monoclonal antibody (Sigma) to control for equal loading of proteins.
Statistical analyses
The Student's paired t-test was used to determine the statistical significance of the reported results except for antigen presentation where statistical significance was established using Tukey–Kramer multiple comparisons test and a one-way analysis of variance of all the c.p.m. measurements. P-values < 0·05 were considered statistically significant.
Results
MB-treated DCs up-regulate the expression of co-stimulatory molecules, MHC-II and activation markers
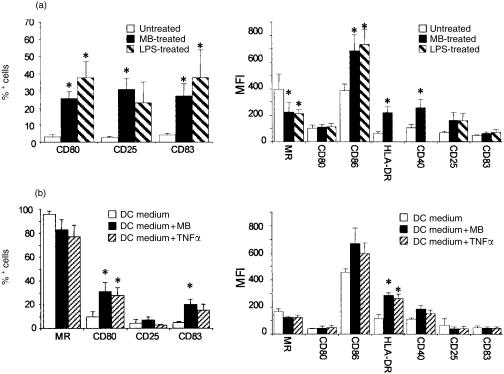
To test the hypothesis that MB activates iDCs, we first investigated by flow cytometry the changes in cell surface expression of several molecules following treatment with MB over 48 hr. iDCs were generated from monocytes following a 7-day differentiation period in medium supplemented with GM-CSF and IL-4 (DC medium). Very few of the resulting iDCs expressed the co-stimulatory molecule CD80, the activation marker CD25 and the differentiation marker CD83 (Fig. 1a), but all stained positively for MR, the co-stimulatory molecule CD86, the major histocompatibility complex class II (MHC-II) molecule human leucocyte antigen (HLA) -DR and CD40. Following a 48-hr stimulation with MB, the number of cells expressing CD80, CD25 and CD83 rose dramatically from 3% to around 30% (Fig. 1a). In addition, following treatment, we observed a steady decline over 48 hr in MR expression as translated by a decrease in MFI (Fig. 1a). Conversely, the expression of CD86, HLA-DR and CD40 significantly increased 48 hr after the onset of stimulation. These changes in the expression of cell surface markers following MB treatment were similar to and not significantly different from those observed following stimulation with LPS (Fig. 1). These results suggest that MB promotes maturation of iDCs. It has been shown that addition of the pro-inflammatory cytokine TNF-α alongside GM-CSF and IL-4 in the DC medium leads to the differentiation of DCs with mature phenotype.27 In order to determine whether MB could substitute for TNF-α, we compared DCs differentiated over 7 days in DC medium supplemented or not with TNF-α or MB. The number of cells expressing the MR tended to be lower in DCs differentiated in the presence of MB or TNF-α (Fig. 1b), suggesting that both molecules induced a maturation process. In addition, differentiation in the presence of either compound led to a significant increase in the number of cells expressing CD80 and CD83 along with an increase in HLA-DR, CD86 and CD40 expression, although only variation in the former marker reached statistical significance. Therefore, MB had the same effect on differentiation as TNF-α, leading to maturation of DCs. Of note, as previously reported for TNF-α,23,27 the presence of either MB or TNF-α in the differentiation medium did not result in an increase in CD25 expression which is in marked contrast with the effect of MB on iDCs. Nevertheless, from these results we concluded that MB activates DCs and promotes the maturation of DCs at least with respect to the expression of cell surface markers. Therefore, we next determined the effects of MB on the biological activities of DCs.
Figure 1.
Murabutide (MB) induces a phenotypic maturation of dendritic cells. (a) Changes in cell surface marker expression following a 48-hr stimulation with MB or LPS. iDCs were positive for MR, CD86, HLA-DR and CD40. Left panel shows the variation in % positive cells, right panel represents the change in MFI. Results are the mean ± SEM of eight independent experiments with MB and of three with LPS. The effect of LPS on the surface expression of HLA-DR and CD40 was not tested. * indicates values significantly different compared with those of unstimulated iDCs (P < 0·05). (b) Differentiation of DCs in the presence of MB or TNF-α. Monocytes were cultured in DC medium, containing MB or TNF-α for 7 days before phenotypic analysis. Left panel shows the variation in % positive cells, right panel represents the change in MFI. Results are the mean ± SEM of four experiments. * indicates significant difference between DCs differentiated in presence of MB or TNF-α and those differentiated in DC medium (P < 0·05).
Effect of MB on endocytosis, MLR and antigen presentation
Antigen capture is a hallmark of iDCs and this ability is down-regulated during the maturation process. Antigen capture can take place via endocytosis mediated by specific receptors such as the MR which is a C-type lectin recognizing glycosylated antigens.2 We thus evaluated the effect of MB treatment on the endocytic capacity of iDCs by measuring the uptake of FITC-dextran. A 24-hr stimulation of iDCs with MB led to a 43% decrease (± 2% SEM, n = 6, P < 0·005) in FITC-dextran uptake as compared to non-stimulated cells, again suggesting that MB promotes the process of maturation of iDCs.
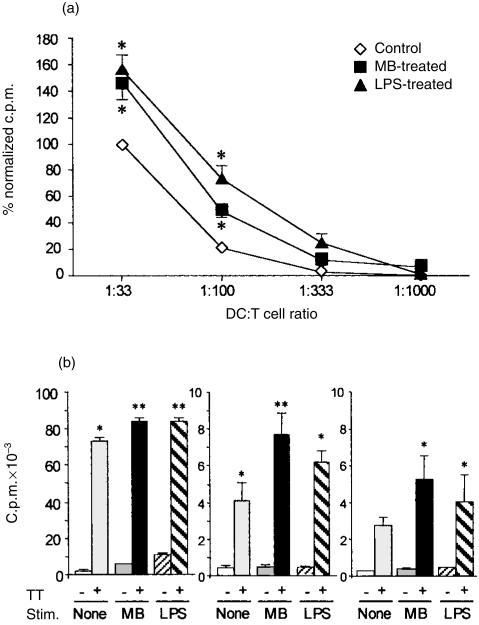
DCs are potent stimulators of either allogeneic or autologous T lymphocytes in a MLR or antigen presentation assay, respectively. To ascertain the effect of MB on the MLR, allogeneic CD4 T lymphocytes were added to graded concentrations of mitomycin C-treated iDCs in the presence or absence of MB or LPS. As previously reported, LPS had a marked stimulatory effect on the MLR (Fig. 2a). Compared to untreated iDCs, the addition of MB resulted in a substantial increase in the proliferation of allogeneic CD4 T cells that was statistically significant at DC to T-cell ratios between 1 : 30 and 1 : 100. There was no significant difference between the effect of MB and of LPS although, the latter tended to be more pronounced. Of note, MB on its own had no effect on T-cell proliferation (not shown).
Figure 2.
Murabutide (MB)-activated DCs show enhanced T-cell stimulatory capacity. (a) MB stimulates allogeneic T-cell proliferation in a MLR. Mitomycin C-treated iDCs were seeded at graded doses then allogeneic CD4 T cells were added in the absence or presence of MB or LPS. The mean c.p.m. given by CD4 T cells incubated in presence of untreated iDCs at a ratio of DCs to T cells of 1 : 30 was arbitrarily set to 100%. Results are the mean of four experiments, when bars are not visible, SEM is smaller than the symbol. * indicates significant difference between MB- or LPS-activated DCs compared to unstimulated DCs (P < 0·05). (b) MB stimulates antigen presentation. iDCs from three different donors were pulsed or not with TT. Two hours after the beginning of the pulse, MB or LPS was added. Twenty-four hours later, iDCs were washed and autologous CD4 T cells were added. Cells were co-cultured for 6 days and were pulsed with [3H]thymidine for the last 16 hr. Results are presented as mean c.p.m. ± SEM of quadruplicate wells. Statistical significance was established using Tukey–Kramer multiple comparisons test at the level of P < 0·05 and a one-way analysis of variance of all the c.p.m. measurements. * indicates significant difference in CD4 T-cell proliferation triggered by pulsed DCs compared to unpulsed DCs whereas ** indicates significant difference in proliferation triggered by MB- or LPS-activated and pulsed DCs compared to unstimulated but pulsed DCs.
We next evaluated in three independent experiments the effect of MB on soluble antigen presentation. To this aim, we compared the proliferative response of autologous CD4 T cells in the presence of iDCs pulsed with TT and treated or not with MB or LPS. As shown in Fig. 2(b), untreated iDCs pulsed with TT increased the proliferative response of CD4 T cells compared to unpulsed and untreated iDCs, thus demonstrating antigen presentation by iDCs. Addition of MB 2 hr after the beginning of the pulse of iDCs with TT led to a significant increase in the proliferation of autologous T cells compared to unpulsed but treated iDCs. Moreover, in two out of three experiments, the T-cell response was significantly enhanced by TT-pulsed, MB-stimulated iDCs compared to pulsed and untreated iDCs. LPS had a similar effect to MB although the difference in T-cell proliferation triggered by pulsed and LPS-treated iDCs compared to that triggered by pulsed but untreated iDCs reached significance in only one experiment.
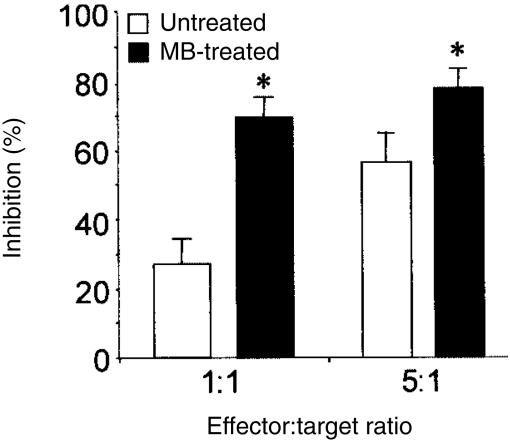
Besides, it has recently been shown that monocyte-derived DCs possess the ability to inhibit the growth of several tumour cell lines in vitro.28 Therefore, we evaluated the effect of MB on the cytostatic activity of DCs. With this aim, iDCs at two different densities were pretreated with MB or not before the addition of the human tumour cell line THP-1. As shown in Fig. 3, addition of MB significantly potentiated the tumoristatic activity of DCs at an effector-to-target ratio as low as 1 : 1. From all these results, we concluded that MB stimulates the biological functions of iDCs in vitro.
Figure 3.
Murabutide (MB) enhances the cytostatic activity of iDCs. iDCs at either 1 × 104 or 5 × 104 cells per well were treated with MB or left unstimulated. After 24 hr, THP-1 cells were added at 1 × 104/well to yield effector to target ratios of 1 : 1 and 5 : 1. Five days later, co-cultures were pulsed with [3H]thymidine for the last 16 hr. The results are presented as the mean % inhibition of triplicate wells obtained with DCs from five separate donors. * indicates significant difference between MB-activated DCs compared to unstimulated DCs (P < 0·05).
Cytokine production
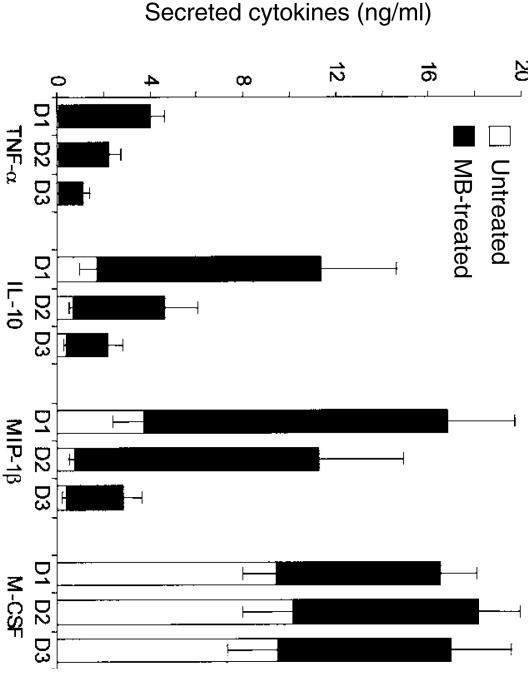
In response to maturation signals, DCs secrete several cytokines that serve to attract other effector cells and to organize the immune response.1,5 Therefore, we measured the effect of MB stimulation on the profile of cytokines secreted by iDCs over a 3-day period. Resting iDCs secreted detectable amounts of MIP-1β, and IL-10 but minute amounts of TNF-α (Fig. 4). On the other hand, iDCs secreted large amounts of M-CSF over the 3-day culture (ranging from 8·6 ng/ml to 10 ng/ml). Upon treatment with MB, there was a sharp increase in the release of MIP-1β, IL-10 and TNF-α (Fig. 4). Secretion of M-CSF only increased less than twofold due to the high constitutive secretion of this cytokine by iDCs. The level of secreted cytokines dropped by day 2 of MB stimulation except for M-CSF which remained at roughly the same level over the 3-day period. The differences in cytokine secretion between MB-activated DCs and untreated DCs remained statistically significant over the 3-day period analysed. When we compared the levels of cytokine released by DCs following stimulation with LPS or MB, we did not find any statistically significant differences between the two immunomodulators (n = 4, not shown). Of note, we also failed to detect any IL-12 p70 in culture supernatants from either unstimulated or MB- or LPS-treated iDCs.
Figure 4.
Profile of cytokines released over a period of 3 days in culture supernatants of iDCs stimulated or not with MB for 3 days. Results are presented as mean ± SEM of 10 experiments. Differences in cytokine release between untreated and MB-treated iDCs were statistically significant at the three time-points tested (P < 0·05).
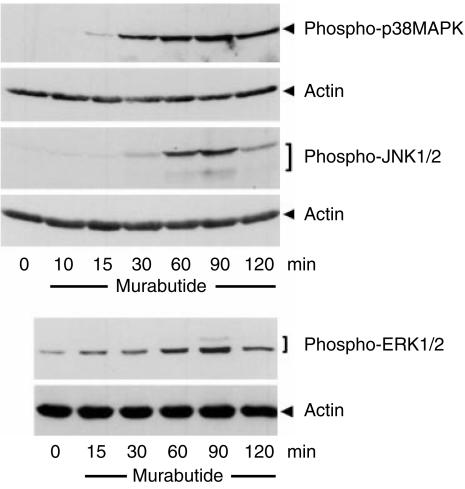
Murabutide induces the phosphorylation of MAP kinases
It has been recently shown that multiple signalling pathways, including p38MAPK and ERK, were activated during the LPS-induced maturation of iDCs.29 Thus, to understand better the maturation process triggered by MB, we investigated the activation of MAP kinases in whole DC lysates by immunoblotting with antibodies specifically recognizing the phosphorylated, and hence the activated, form of these kinases. As shown in Fig. 5, within 15–30 min of treatment with MB, there was a marked increase in p38MAPK phosphorylation, levelling off 60–90 min after the onset of stimulation and declining thereafter. On the other hand, we detected at later time-points (30–60 min) an increase in the phosphorylation of both ERK1/2 and JNK1/2 isoforms following stimulation with MB. Besides, the phosphorylation of these two families of MAP kinase was more transient than p38MAPK phosphorylation.
Figure 5.
Murabutide (MB) induces the phosphorylation of p38MAPK, JNK and ERK. Whole cell lysates from iDCs exposed to MB for various lengths of time were probed using phosphorylation specific antibodies by Western blotting. Blots were first probed with anti-phosphoMAP kinase antibodies then stripped and reprobed with anti-actin to control for equal loading of samples. Results are representative of three identical experiments.
Discussion
Muramyl peptides, derived by chemical modification of MDP, possess many biological activities modulating the functions of monocytes/macrophages. Although they have been studied for several years, little is known about the interaction of muramyl peptides with DCs, cells that play a key role in the initiation of T-cell mediated immunity. In this report, we demonstrate that MB, a synthetic muramyl peptide approved for use in humans, triggers the maturation of DCs, enhances the T-cell stimulatory activities of DCs, induces a distinct pattern of cytokine release, and induces the phosphorylation of the major classes of MAP kinases.
We first addressed the question whether MB had any effect on the spectrum of molecules expressed at the surface of iDCs. Our results clearly show that this muramyl peptide increases the expression of CD83 and CD25 which are considered as markers of DC maturation and activation, respectively.27,30,31 However, it is unlikely that MB induces full maturation of DCs as after 48 hr of treatment only 30% of DCs expressed CD83. On the other hand, there was a marked increase in the cell surface expression of HLA-DR and co-stimulatory molecules, turning MB-treated DCs into DCs with high T-cell stimulatory capacity. This is illustrated by the increased proliferation of allogeneic T cells in the presence of MB-treated DCs. These phenotypic changes and enhancement of MLR have been observed following treatment of iDCs with pro-inflammatory cytokines, ATP, LPS, or bacterial cell wall skeleton.2,23,24,32 However this is the first report demonstrating that a muramyl peptide can trigger the maturation of DCs.
In keeping with a maturation process, iDCs treated for 24 hr with MB have a decreased capacity to take up dextran, a surrogate marker of endocytosis. Again, this decrease has been observed in maturing DCs following stimulation with pro-inflammatory mediators. However, the effect of a maturation-inducing agent on antigen uptake can be a biphasic process as a transient increase in dextran uptake was observed shortly after the addition of ATP followed by a marked inhibition 24 hr later.32
It has been reported that treatment of iDCs with pro-inflammatory cytokines prior to antigen pulsing leads to a decrease in their capacity to stimulate autologous CD4 lymphocyte proliferation, reflecting a decrease in antigen presentation.3 However, another report has shown that CD40 ligand or interferon-γ enhances the DC-induced CD4 T-cell proliferation when added shortly after the beginning of the pulse with recall antigens.33 The enhancement in T-cell stimulation following MB treatment may be explained by the transient increase in antigen uptake immediately following the addition of some maturation promoting agents,32 and also by the increased expression of HLA-DR, CD40 and co-stimulatory molecules at the cell surface of MB-treated iDCs.
We provide preliminary evidence showing that MB-activated DCs have a greater capacity than unstimulated DCs to inhibit the proliferation of the tumour cell line THP-1. A similar effect has been previously reported for LPS.28 These authors have also shown that the continuous presence of LPS in the co-culture medium was dispensable as LPS-pretreated DCs are as effective in blocking the growth of various tumour cells. It remains to be determined whether MB-treated DCs are active toward other tumour cell lines, and whether this tumoristatic activity is cytokine-mediated or requires cell contact, as is the case for LPS.28
Analysis of cytokine secretion by resting and MB-treated iDCs yielded some unexpected results. Firstly, unstimulated iDCs secreted large amounts of M-CSF over the 3-day period studied. It has been previously reported that iDCs constitutively secrete high levels of M-CSF and that this was due to the presence of GM-CSF in the DC medium and to the activation of the phosphatidylinositol 3-kinase pathway.34 Secondly, despite this high basal level, there was a long-lasting induction of M-CSF following stimulation with Murabutide. This is at variance with the other cytokines detected for which we observed a transient induction and, at present, we cannot provide any explanation for this intriguing finding. In addition, MB-treated DCs secreted large amounts of pro-inflammatory mediators. This is in contrast to MB-stimulated MDMs which released over 20-fold less TNF-α and seven-fold less MIP-1β (unpublished results). On the other hand, in MB-treated DCs we detected a high secretion of IL-10, a cytokine with anti-inflammatory properties, which was not detected in MB-stimulated MDMs. It has been proposed that, in the tissue, secretion of MIP-1β, TNF-α and M-CSF by maturing DCs may contribute to the recruitment of other iDCs but also of effector cells such as T lymphocytes, granulocytes and macrophages.5 However, the high IL-10 and M-CSF secretion upon MB stimulation would stop the maturation of incoming iDCs as these two cytokines have been found to exert a profound inhibitory effect on DC maturation in vitro.1,35 Also, the presence of M-CSF after iDCs activation by MB would favour the differentiation of monocytes having crossed the endothelium into macrophages rather than iDCs.35,36 On the other hand, it has been shown in vivo that the co-delivery of DNA vaccine along with a gene coding for M-CSF resulted in increased cytotoxic T-lymphocyte responses and was also associated with increased recruitment of DCs at the site of injection and MIP-1β production.37 Hence, it is possible that high M-CSF and MIP-1β by MB-activated DCs may bring about strong cytotoxic T-lymphocyte responses in vivo. We could not detect any secretion of IL-12 following either MB or LPS stimulation. IL-12 is a notoriously difficult cytokine to detect and indeed, there are conflicting data in the literature regarding its secretion by DCs. While many groups have reported a moderate to high IL-12 secretion by activated DCs,38–40 others have shown that secretion of this cytokine by DCs requires either the presence of antigen-specific T cells41 or a second maturation signal.42 Thus, it would be of interest to determine whether MB synergizes with other immunomodulators to stimulate IL-12 production in vitro or increases the production of this cytokine by DCs in the presence of antigen-specific T cells.
There is scant information about the signalling pathways involved in the maturation of iDCs. To bring further insights into the mechanism of the maturation process triggered by MB, we studied the phosphorylation of the three classes of MAP kinases and we have shown that they were phosphorylated and hence, activated in MB-stimulated iDCs. This is in stark contrast to the situation in MDMs where only ERKs phosphorylation could be detected in response to MB treatment (Vidal et al., submitted). Interestingly, although MDMs and iDCs are derived from the same precursor cells, they react differently to muramyl peptides. Hence, the differentiation events leading to iDCs have brought about changes in the way cells respond to this signal. Also, the strong MAP kinase phosphorylation may partly explain the differences in cytokine release observed between MB-treated iDCs and MDMs. Moreover, it has recently been demonstrated that stimulants leading to iDC maturation such as LPS or TNF strongly activate MAP kinases.29,43 In comparison to MB, LPS activates p38MAPK and ERKs more rapidly. This difference may be due to a difference in the early steps of recognition taking place at the cell surface as we have shown that MB does not signal through the LPS receptor Toll-like receptor 4 (Vidal et al., submitted). Nevertheless, the identification of the muramyl peptide receptor will be instrumental in better understanding the molecular events leading to maturation in MB-stimulated iDCs.
Recently, DCs have attracted a lot of attention as potential immunotherapeutic tools especially as tumour vaccines (see refs 9 and 11 for reviews) and it has been proposed that the use of mDCs may lead to more effective immunization against tumour antigens.12 In this context, MB, which is already approved for human use, could be valuable to boost the efficacy of DC-based vaccine as MB-treated DCs have a higher antigen presentation capacity in vitro. Also, the profile of cytokines secreted by DCs stimulated by this immunomodulator may prove useful in mounting a strong immune response against tumours or virus-infected cells.
Acknowledgments
We are grateful to E. Darcissac for expert assistance with flow cytometry.
Abbreviations
- DCs
dendritic cells
- ERK
extracellular-signal regulated kinase
- IL
interleukin
- JNK
C-Jun N-terminal kinase
- MAPK
mitogen-activated protein kinase
- MB
Murabutide
- M-CSF
macrophage-colony stimulating factor
- MDP
muramyl dipeptide
- MIP-1β
macrophage inhibitory protein-1β
- TNF-α
tumour necrosis factor-α
References
- 1.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–52. doi: 10.1038/32588. 10.1038/33116. [DOI] [PubMed] [Google Scholar]
- 2.Sallusto F, Cella M, Danieli C, Lanzavecchia A. Dendritic cells use macropinocytosis and the mannose receptor to concentrate macromolecules in the major histocompatibility complex class II compartment: downregulation by cytokines and bacterial products. J Exp Med. 1995;182:389–400. doi: 10.1084/jem.182.2.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sallusto F, Lanzavecchia A. Efficient presentation of soluble antigen by cultured human dendritic cells is maintained by granulocyte/macrophage colony-stimulating factor plus interleukin 4 and downregulated by tumor necrosis factor alpha. J Exp Med. 1994;179:1109–18. doi: 10.1084/jem.179.4.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Albert ML, Pearce SF, Francisco LM, Sauter B, Roy P, Silverstein RL, Bhardwaj N. Immature dendritic cells phagocytose apoptotic cells via alphavbeta5 and CD36, and cross-present antigens to cytotoxic T lymphocytes. J Exp Med. 1998;188:1359–68. doi: 10.1084/jem.188.7.1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sallusto F, Palermo B, Lenig D, et al. Distinct patterns and kinetics of chemokine production regulate dendritic cell function. Eur J Immunol. 1999;29:1617–25. doi: 10.1002/(SICI)1521-4141(199905)29:05<1617::AID-IMMU1617>3.0.CO;2-3. 10.1002/(sici)1521-4141(199905)29:05<1617::aid-immu1617>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 6.Kanto T, Hayashi N, Takehara T, et al. Impaired allostimulatory capacity of peripheral blood dendritic cells recovered from hepatitis C virus-infected individuals. J Immunol. 1999;162:5584–91. [PubMed] [Google Scholar]
- 7.Macatonia SE, Lau R, Patterson S, Pinching AJ, Knight SC. Dendritic cell infection, depletion and dysfunction in HIV-infected individuals. Immunology. 1990;71:38–45. [PMC free article] [PubMed] [Google Scholar]
- 8.Kundu SK, Engleman E, Benike C, Shapero MH, Dupuis M, van Schooten WC, Eibl M, Merigan TC. A pilot clinical trial of HIV antigen-pulsed allogeneic and autologous dendritic cell therapy in HIV-infected patients. AIDS Res Hum Retroviruses. 1998;14:551–60. doi: 10.1089/aid.1998.14.551. [DOI] [PubMed] [Google Scholar]
- 9.Nouri-Shirazi M, Banchereau J, Fay J, Palucka K. Dendritic cell based tumor vaccines. Immunol Lett. 2000;74:5–10. doi: 10.1016/s0165-2478(00)00243-1. 10.1016/s0165-2478(00)00243-1. [DOI] [PubMed] [Google Scholar]
- 10.Weissman D, Ni H, Scales D, et al. HIV gag mRNA transfection of dendritic cells (DC) delivers encoded antigen to MHC class I and II molecules, causes DC maturation, and induces a potent human In vitro primary immune response. J Immunol. 2000;165:4710–17. doi: 10.4049/jimmunol.165.8.4710. [DOI] [PubMed] [Google Scholar]
- 11.Timmerman JM, Levy R. Dendritic cell vaccines for cancer immunotherapy. Annu Rev Med. 1999;50:507–29. doi: 10.1146/annurev.med.50.1.507. [DOI] [PubMed] [Google Scholar]
- 12.Schuler-Thurner B, Dieckmann D, Keikavoussi P, et al. Mage-3 and influenza-matrix peptide-specific cytotoxic T cells are inducible in terminal stage HLA-A2.1+ melanoma patients by mature monocyte-derived dendritic cells. J Immunol. 2000;165:3492–6. doi: 10.4049/jimmunol.165.6.3492. [DOI] [PubMed] [Google Scholar]
- 13.Shimizu K, Fields RC, Giedlin M, Mule JJ. Systemic administration of interleukin 2 enhances the therapeutic efficacy of dendritic cell-based tumor vaccines. Proc Natl Acad Sci USA. 1999;96:2268–73. doi: 10.1073/pnas.96.5.2268. 10.1073/pnas.96.5.2268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Telzak E, Wolff SM, Dinarello CA, et al. Clinical evaluation of the immunoadjuvant murabutide, a derivative of MDP, administered with a tetanus toxoid vaccine. J Infect Dis. 1986;153:628–33. doi: 10.1093/infdis/153.3.628. [DOI] [PubMed] [Google Scholar]
- 15.Bahr GM, Darcissac E, Bevec D, Dukor P, Chedid L. Immunopharmacological activities and clinical development of muramyl peptides with particular emphasis on murabutide. Int J Immunopharmacol. 1995;17:117–31. doi: 10.1016/0192-0561(94)00094-5. 10.1016/0192-0561(94)00094-5. [DOI] [PubMed] [Google Scholar]
- 16.Przewlocki G, Audibert F, Jolivet M, Chedid L, Kent SB, Neurath AR. Production of antibodies recognizing a hepatitis B virus (HBV) surface antigen by administration of murabutide associated to a synthetic pre-S HBV peptide conjugated to a toxoid carrier. Biochem Biophys Res Commun. 1986;140:557–64. doi: 10.1016/0006-291x(86)90768-0. [DOI] [PubMed] [Google Scholar]
- 17.Chedid LA, Parant MA, Audibert FM, Riveau GJ, Parant FJ, Lederer E, Choay JP, Lefrancier PL. Biological activity of a new synthetic muramyl peptide adjuvant devoid of pyrogenicity. Infect Immun. 1982;35:417–24. doi: 10.1128/iai.35.2.417-424.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chomel JJ, Simon-Lavoine N, Thouvenot D, Valette M, Choay J, Chedid L, Aymard M. Prophylactic and therapeutic effects of murabutide in OF1 mice infected with influenza A/H3N2 (A/Texas/1/77) virus. J Biol Response Mod. 1988;7:581–6. [PubMed] [Google Scholar]
- 19.Bahr GM, Darcissac E, Pouillart PR, Chedid LA. Synergistic effects between recombinant interleukin-2 and the synthetic immunomodulator murabutide: selective enhancement of cytokine release and potentiation of antitumor activity. J Interferon Cytokine Res. 1996;16:169–78. doi: 10.1089/jir.1996.16.169. [DOI] [PubMed] [Google Scholar]
- 20.Bahr GM, Pouillart PR, Chedid LA. Enhancement in vivo of the antiinflammatory and antitumor activities of type I interferon by association with the synthetic immunomodulator murabutide. J Interferon Cytokine Res. 1996;16:297–306. doi: 10.1089/jir.1996.16.297. [DOI] [PubMed] [Google Scholar]
- 21.Pouillart PR, Audibert FM, Chedid LA, Lefrancier PL, Bahr GM. Enhancement by muramyl peptides of the protective response of interferon-alpha/beta against encephalomyocarditis virus infection. Int J Immunopharmacol. 1996;18:183–92. doi: 10.1016/0192-0561(96)00005-7. 10.1016/0192-0561(96)00005-7. [DOI] [PubMed] [Google Scholar]
- 22.Darcissac EC, Truong MJ, Dewulf J, Mouton Y, Capron A, Bahr GM. The synthetic immunomodulator murabutide controls human immunodeficiency virus type 1 replication at multiple levels in macrophages and dendritic cells. J Virol. 2000;74:7794–802. doi: 10.1128/jvi.74.17.7794-7802.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Reddy A, Sapp M, Feldman M, Subklewe M, Bhardwaj N. A monocyte conditioned medium is more effective than defined cytokines in mediating the terminal maturation of human dendritic cells. Blood. 1997;90:3640–6. [PubMed] [Google Scholar]
- 24.Tsuji S, Matsumoto M, Takeuchi O, Akira S, Azuma I, Hayashi A, Toyoshima K, Seya T. Maturation of human dendritic cells by cell wall skeleton of mycobacterium bovis bacillus calmette-Guerin: involvement of toll-like receptors. Infect Immun. 2000;68:6883–90. doi: 10.1128/iai.68.12.6883-6890.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Martin-Blanco E. p38 MAPK signalling cascades: ancient roles and new functions. Bioessays. 2000;22:637–45. doi: 10.1002/1521-1878(200007)22:7<637::AID-BIES6>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 26.Tibbles LA, Woodgett JR. The stress-activated protein kinase pathways. Cell Mol Life Sci. 1999;55:1230–54. doi: 10.1007/s000180050369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhou LJ, Tedder TF. CD14+ blood monocytes can differentiate into functionally mature CD83+ dendritic cells. Proc Natl Acad Sci USA. 1996;93:2588–92. doi: 10.1073/pnas.93.6.2588. 10.1073/pnas.93.6.2588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chapoval AI, Tamada K, Chen L. In vitro growth inhibition of a broad spectrum of tumor cell lines by activated human dendritic cells. Blood. 2000;95:2346–51. [PubMed] [Google Scholar]
- 29.Ardeshna KM, Pizzey AR, Devereux S, Khwaja A. The PI3 kinase, p38 SAP kinase, and NF-kappaB signal transduction pathways are involved in the survival and maturation of lipopolysaccharide-stimulated human monocyte-derived dendritic cells. Blood. 2000;96:1039–46. [PubMed] [Google Scholar]
- 30.Romani N, Reider D, Heuer M, Ebner S, Kampgen E, Eibl B, Niederwieser D, Schuler G. Generation of mature dendritic cells from human blood. An improved method with special regard to clinical applicability. J Immunol Methods. 1996;196:137–51. doi: 10.1016/0022-1759(96)00078-6. 10.1016/0022-1759(96)00078-6. [DOI] [PubMed] [Google Scholar]
- 31.Bender A, Sapp M, Schuler G, Steinman RM, Bhardwaj N. Improved methods for the generation of dendritic cells from nonproliferating progenitors in human blood. J Immunol Methods. 1996;196:121–35. doi: 10.1016/0022-1759(96)00079-8. 10.1016/0022-1759(96)00079-8. [DOI] [PubMed] [Google Scholar]
- 32.Schnurr M, Then F, Galambos P, Scholz C, Siegmund B, Endres S, Eigler A. Extracellular ATP and TNF-alpha synergize in the activation and maturation of human dendritic cells. J Immunol. 2000;165:4704–9. doi: 10.4049/jimmunol.165.8.4704. [DOI] [PubMed] [Google Scholar]
- 33.Kuniyoshi JS, Kuniyoshi CJ, Lim AM, Wang FY, Bade ER, Lau R, Thomas EK, Weber JS. Dendritic cell secretion of IL-15 is induced by recombinant huCD40LT and augments the stimulation of antigen-specific cytolytic T cells. Cell Immunol. 1999;193:48–58. doi: 10.1006/cimm.1999.1469. 10.1006/cimm.1999.1469. [DOI] [PubMed] [Google Scholar]
- 34.RieSeries C, Ramoner R, Bock G, Deo YM, Holtl L, Bartsch G, Thurnher M. Human monocyte-derived dendritic cells produce macrophage colony-stimulating factor: enhancement of c-fms expression by interleukin-10. Eur J Immunol. 1998;28:2283–8. doi: 10.1002/(SICI)1521-4141(199808)28:08<2283::AID-IMMU2283>3.0.CO;2-X. 10.1002/(sici)1521-4141(199808)28:08<2283::aid-immu2283>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 35.Becker S, Warren MK, Haskill S. Colony-stimulating factor-induced monocyte survival and differentiation into macrophages in serum-free cultures. J Immunol. 1987;139:3703–9. [PubMed] [Google Scholar]
- 36.Randolph GJ, Beaulieu S, Lebecque S, Steinman RM, Muller WA. Differentiation of monocytes into dendritic cells in a model of transendothelial trafficking. Science. 1998;282:480–3. doi: 10.1126/science.282.5388.480. [DOI] [PubMed] [Google Scholar]
- 37.Kim JJ, Yang JS, Lee DJ, et al. Macrophage colony-stimulating factor can modulate immune responses and attract dendritic cells in vivo. Hum Gene Ther. 2000;11:305–21. doi: 10.1089/10430340050016049. 10.1089/10430340050016049. [DOI] [PubMed] [Google Scholar]
- 38.Langenkamp A, Messi M, Lanzavecchia A, Sallusto F. Kinetics of dendritic cell activation: impact on priming of TH1, TH2 and nonpolarized T cells. Nat Immunol. 2000;1:311–16. doi: 10.1038/79758. 10.1038/79758. [DOI] [PubMed] [Google Scholar]
- 39.Demeure CE, Tanaka H, Mateo V, Rubio M, Delespesse G, Sarfati M. CD47 engagement inhibits cytokine production and maturation of human dendritic cells. J Immunol. 2000;164:2193–9. doi: 10.4049/jimmunol.164.4.2193. [DOI] [PubMed] [Google Scholar]
- 40.Thoma-Uszynski S, Kiertscher SM, Ochoa MT, et al. Activation of toll-like receptor 2 on human dendritic cells triggers induction of IL-12, but not IL-10. J Immunol. 2000;165:3804–10. doi: 10.4049/jimmunol.165.7.3804. [DOI] [PubMed] [Google Scholar]
- 41.Schlienger K, Craighead N, Lee KP, Levine BL, June CH. Efficient priming of protein antigen-specific human CD4 (+) T cells by monocyte-derived dendritic cells. Blood. 2000;96:3490–8. [PubMed] [Google Scholar]
- 42.Mosca PJ, Hobeika AC, Clay TM, Nair SK, Thomas EK, Morse MA, Lyerly HK. A subset of human monocyte-derived dendritic cells expresses high levels of interleukin-12 in response to combined CD40 ligand and interferon-gamma treatment. Blood. 2000;96:3499–504. [PubMed] [Google Scholar]
- 43.Sato K, Nagayama H, Tadokoro K, Juji T, Takahashi TA. Extracellular signal-regulated kinase, stress-activated protein kinase/c-Jun N-terminal kinase, and p38mapk are involved in IL-10-mediated selective repression of TNF-alpha-induced activation and maturation of human peripheral blood monocyte-derived dendritic cells. J Immunol. 1999;162:3865–72. [PubMed] [Google Scholar]