Abstract
Both lipopolysaccharide (LPS) and phorbol 12-myristate 13-acetate (PMA) impeded monocyte to macrophage differentiation with respect to typical phenotypic modulation and certain phagocyte-related processes. The down-regulation of the porcine monocyte marker SWC1, and up-regulation of the SWC9 macrophage marker were retarded, but not inhibited, as was the differentiation-associated down-regulation of p53 and myeloperoxidase. Despite this clear impairment of macrophage differentiation, not all cellular functions were equally susceptible. Both agents inhibited phagocytosis, but not low-density lipoprotein receptor-associated endocytosis. Only LPS inhibited tartrate-resistant acid phosphatase up-regulation. In contrast, increase of vacuolar acidification rates was more susceptible to PMA. The activity of certain endosomal/lysosomal enzymes – esterase, nucleotidase, peroxidase and cathepsins – was generally enhanced by both LPS and PMA. This contrasted with autophagosomal activity, detected through the induction of an antiviral state. Disruption of autophagosomes and lysosomes (methionine-O-methyl ester), but not lysosomes alone (glycyl-l-phenylalanine) reversed LPS-induced inhibition of virus replication, without influencing the PMA-induced antiviral effect. Thus, PMA is similar to LPS in inhibiting monocyte to macrophage differentiation, when primary blood monocytes are employed, but not all pathways are equally susceptible. The analyses demonstrate that the pathways modulated during monocyte differentiation function somewhat independently. Moreover, certain functions of monocytic cells are more important with respect to the outcome of virus infection, with autophagosomal activities in particular favouring cell survival.
Introduction
An important step during macrophage (Mφ) development is the transition from blood-borne monocytes into tissue Mφ.1 In vitro, monocyte differentiation into Mφ can be induced by serum factors, being associated with the expression of specific maturation-associated antigens.2–5 These differentiation steps have been reported to simulate in vivo events.3–5 New antigens appearing during differentiation, as well as the loss of those typically expressed by monocytes, have permitted an evaluation of Mφ diversity at the membrane level.2,4–9 Furthermore, cell activity is modulated upon differentiation, with a number of processes apparently evolving in unison.10
A number of substances have been reported to influence the differentiation, maturation and activation of monocytic cells.11 Lipopolysaccharide (LPS), a glycolipid component of Gram-negative bacterial cell walls, binds to its main receptor CD14 and stimulates monocytic cells through action on associated cell surface bystander molecule(s). Intracellular events in response to this stimulation result in the phosphorylation and activation of certain protein kinases, mediating amongst other events cytokine production.12 The protein kinase C activator, phorbol 12-myristate 13-acetate (PMA), will also stimulate Mφ functions including phagocytosis and the secretion of immunomodulatory cytokines.13
Monocytic cell differentiation has been related to the permissiveness of the cells to infection by herpes simplex virus,14 cytomegalovirus15 and African swine fever virus (ASFV).4,10 The effects of LPS and PMA on monocytic cell development have also been utilized to study the relationship between cell activity and virus replication. For instance, the replication of human immunodeficiency virus (HIV) was found to be reduced in LPS-treated monocyte-derived Mφ.16,17 PMA-dependent differentiation of promonocytic cells has also been employed to analyse macrophagotropic HIV-1 entry.18
Accordingly, the present work set out to determine how LPS and PMA influence the functional modulation of monocytic cell differentiation. Considering that these agents signal the cell in different manners, and LPS has been reported to impair whereas PMA promotes differentiation, an elaboration of the relationship between the intracellular processes and differentiation was sought. Such modulation of monocytic cell activity was also related to the susceptibility of these cells to infection by a macrophagotropic virus – ASFV. This virus [reviewed in ref. 19] cannot infect monocytes, and its replication efficiency varies depending on the stage of monocytic cell differentiation.4,10
Materials and Methods
Reagents and cells
Peripheral blood mononuclear cells (PBMC) were isolated over Ficoll-Paque (Pharmacia, Piscataway, NJ), using venous blood – collected into Alsever's solution – from Swiss White Landrace pigs, held under specific pathogen-free conditions, as described elsewhere.4 The PBMC were resuspended in Dulbecco's modified Eagle's medium (DMEM)/10% (v/v) fetal calf serum (FCS) at 2 × 106−4× 106 cells/ml, and non-adherent cells were removed following culture on plastic for 2–3 hr at 37°. Adherent cells were then treated with either LPS (1 µg/ml) or PMA (10 ng/ml) (Sigma, Buchs, Switzerland), and further incubated in growth medium; DMEM/30% (v/v) heparinized porcine plasma, 2 mm l-glutamine, 10 mm HEPES (Life Technologies, Grand Island, NY), at 37° for the times shown in the Results section. These doses of LPS and PMA were chosen following dose–response analyses: 1 µg/ml LPS and 10 ng/ml PMA were the lowest concentrations effecting an optimum modulation of monocytic cell phenotype and function without interference from cytotoxicity (data not shown). Following such culture, the adherent cells were recovered with the aid of 4° Ca2+/Mg2+ free phosphate buffered saline (PBSA)−5 mm ethylenediaminetetraacetic acid (EDTA) treatment. Cells cultured for 3 days in the absence of LPS or PMA were referred to as monocyte-derived macrophages (MDMs), due to the characteristics previously elucidated.4,5,10
Determination of differentiation antigen expression (surface and intracellular)
These analyses employed the FACScan® Flow Cytometer (Becton Dickinson, Mountain View, CA) and the following monoclonal antibodies (mAb) for surface antigen expression: anti-SWC1 (76-6-7; American Type Culture Collection, Rockville, MD) found on porcine monocytes and T lymphocytes;20 anti-SWC3 [DH59; Veterinary Medical Research and Development (VMRD), Pullman, WA] porcine pan-myeloid cell marker;21,22 anti-SWC9 (PM18-7; kindly provided by Dr Y. Kim, Finch University of Health Sciences, Chicago, IL) found on porcine Mφ but not monocytes.4,10,22,23 The tumour suppressor gene product p53 and the myeloperoxidase protein were the differentiation-susceptible intracellular antigens studied,10 detected with fluorescein isothiocyanate (FITC)-conjugated anti-human p53 protein mAb DO-7,24 and FITC-conjugated anti-human myeloperoxidase mAb MPO-725 (Dako, Copenhagen, Denmark). For the antibody labelling, the cells (106/100 µl) were incubated with the mAb for 20 min/4°, followed by washing with CellWash (Becton Dickinson), incubated for 15 min/4° with the appropriate isotype-specific conjugates [FITC or phycoerythrin (PE) conjugated anti-mouse immunoglobulins; Southern Biotechnology Associates, Birmingham, AL], and given a final wash before acquisition. In order to detect the intracellular proteins, cells were fixed with a cell permeabilization kit (Harlan Sera-Lab Ltd, Loughborough, UK) and incubated with the mAb (DO-7 or MPO-7) for 20 min at 25°, then washed with Cell Wash as above.
Acquisition of the data employed the following steps in order to gate only monocytic cells. First, the negative marker set-up was established from the reaction obtained with the appropriate isotype control antibodies. Second, a myeloid region was created in the forward scatter/side scatter by gating on SWC3+ cells. From these analyses, it was possible to identify the percentage of positive cells in every test to the right of the negative control. Within this positive region, the geometric mean of the relative fluorescence intensity was measured and that of the control peaks was subtracted, to obtain the relative intensity of the fluorescent signal on positive cells.
Detection of extracellular enzyme activity
Tartrate-resistant acid phosphatase (ACP) was measured with the Sigma Diagnostics kit. Monocytes were cultured over 2 days, and the medium was replaced 24 hr before measurement to detect de novo synthesis of the enzyme on the 3rd day. Samples (1 ml) of media from the cultures were tested according to the manufacturer's instructions, and absorbance at 405 nm was recorded with an LKB Biochrom Ultrospec spectrophotometer (LKB, Rockville, MD). The absorbance values were converted into ACP activity (U/l), and those obtained using medium alone were subtracted.
Phagocytosis and endocytosis assays
BODIPY-fluorescein-Zymosan (Molecular Probes, Leiden, the Netherlands), at 50 particles/cell, was incubated with the monocytic cell cultures for 30 min/37°, before washing twice with CellWash and measuring by flow cytometry.10 Before acquisition, trypan blue (0·04% v/v) was added to quench the signal from zymosan adsorbed on the surface of the phagocytic cells, but not yet internalized.
Low-density lipoprotein from human plasma (BODIPY-fluorescence LDL complex; L-3483, Molecular Probes) was employed to investigate the endocytic activity of the cells. Adherent cells were harvested and left for 20 min at 4°. L-3483 (20 µg/ml) was incubated with the cells for 30 min at 37°, and data were acquired with the FACScan after two washing steps.10
Intracellular vacuolar pH
Dextran conjugated with Cl-NERF (D-3324; Molecular Probes) was employed for the analysis of the pH of the endosomal/lysosomal environment. Cells were harvested and kept on ice for 20 min before incubation with the probe (0·25 mg/ml) for 5 min at 37°, followed by washing for 5 min at 4°.10 The data were acquired in two stages: (I) after 5 min, but before washing; and (II) immediately after a 10-min washing period, yielding a total incubation time of 15 min. This Cl-NERF dextran is sensitive to vacuolar acidification. If the test is performed at 0–2°, in the presence of anti-mannose receptor antibodies, or in the presence of unlabelled mannose, there is no internalization of the dextran, as seen by the sensitivity of the fluorescence signal to quenching by trypan blue. The washing step is essential, because the continued presence of the dextran allows a continuous uptake by the cells, masking the measurement of the pH-sensitive signal of the dextran internalized at a particular time-point. A kinetic study of the uptake of dextran showed that the 5-min and 15-min time-points were the most informative concerning the rate of vacuolar acidification.10
Cell-associated intracellular enzyme activities
The following probes (Molecular Probes) were employed to detect intracellular enzyme activity by flow cytometry:10 5-(and-6)-carboxy 2,7-dichlorofluoresceindiacetate (CCl2FDA; catalogue number C-369), fluorescein di-β-galactopyranoside (FDG; catalogue number F-1179), dihydrorhodamine-6G (DHR-6G; catalogue number D-633), dihydrorhodamine 123 (DHR 123; catalogue number D632) and the 5′-nucleotidase kit (BODIPY FL-AMP; E-6643). For the detection of cathepsin (C and D) activity, the TP-cathepsin (catalogue number 7547105) and VK-cathepsin (catalogue number 7547106) probes were obtained from Beckman Coulter (Fullerton, CA). The latter of these cathepsin probes is no longer available. Consequently, a second probe for cathepsin D was employed (Molecular Probes BODIPY FL-pepstatin A; catalogue number P-12271).
CCl2FDA was employed for the measurement of esterase activity.26 It readily enters cells and its hydrolysis by cellular esterase obeys Michaelis–Menten kinetics, although it can be partly hydrolysed by membrane-bound esterases.26 FDG was used for detecting β-galactosidase.27 DHR-6G was employed for the assays on peroxidase activity,28 the results being confirmed using a second peroxidase probe – DHR 123. The oxidation of the latter probe is reported to detect only the presence of H2O2 and intracellular peroxidases, but not the generation of , with only the peroxidase-containing cells fluorescing.29 BODIPY FL-AMP was used to detect 5′-nucleotidase, but according to the manufacturers (Molecular Probes) this will also measure phosphatase activity. Measurement of cathepsin C employed TP-cathepsin, which is the substrate for cathepsins C and G, threonine-proline-Rho 110. Cathepsin C is a lysosomal cysteine peptidase found in many cell types, whereas cathepsin G is a serine endopeptidase found primarily in polymorphonuclear leucocytes and promonocytes, but reduced in mature monocytes.30 VK-cathepsin was similar to TP-cathepsin, except that it was a substrate for cathepsins D and B. However, it is now an obsolete probe, for which reason detection of cathepsin D activity also employed BODIPY FL pepstatin A, based on the ability of pepstatin to target cathepsin D within the lysosomes of living cells.31
Probes were added to cells immediately before acquisition with the flow cytometer, to serve as negative controls of incorporation. In order to detect esterase activity, the substrate CCl2FDA (3 µm) was added to 106 cells for 1 min at 37°. The reaction was stopped with 1 ml of cold CellWash, followed by two washing cycles and centrifugation for 5 min/4°/350 g. Galactosidase activity was detected with FDG (1 mm), incubated with the cells for 1 min at 37°. The reaction mixture was then diluted 10 times with phosphate-buffered saline (PBS), kept dark at 4° for 45 min, and finally stopped as for the esterase test. For peroxidase activity, DHR-6G or DHR 123 (25 µm) was incubated with the cells for 20 min at 37°. BODIPY FL-AMP (5 µm) was incubated for 20 min at 37° with the cells to detect 5′-nucleotidase activity. The cathepsin probes were added following the manufacturer's instructions for 10 min at 37°.
Virus infection
The KWH isolate of ASFV (kindly provided by Dr P. Wilkinson, BBSRC Institute for Animal Health, Pirbright, UK) – moderately virulent in pigs – was used, after passage once in pigs and twice in cultured swine Mφ. Mock stocks were prepared from uninfected swine Mφ cultures. Virus titres were expressed in immunofluorescence focus-forming units (IFFU) per ml.4 Porcine Mφ cultures (MDMs) were infected at a multiplicity of infection (MOI) of 10 IFFU/cell. After an adsorption period of 2 hr at 37°, the cultures were washed five times with prewarmed PBS/1%(v/v) FCS, to remove non-adsorbed virus, and fed with growth medium. The infected cultures were incubated at 37° for the times shown in the Results section. Virus antigen in infected cells was detected with mAb against virus VP73 (kindly provided by Dr Fernando Alonso, CISA-INIA, Valdeolmos, Spain) by immunofluorescence assay or flow cytometry after cellular permeabilization (see above). Conjugates were FITC-isotype-specific conjugates (Southern Biotechnology), at 10 µg/ml. All labellings employed 30-min incubations at 25°. Immunofluorescent analysis of cells infected with this MOI permitted the detection of de novo virus antigen, but not that initially associated with the cells. Progeny virus titres were calculated from titration of culture supernatants at 2 hr (residual virus), and culture lysates at 24, and 48 hr post-infection (p.i.), on fresh MDMs.
Modulation of lysosomal activities
Adherent monocytes were either treated for 2 hr at 37° with LPS (1 µg/ml) or left untreated (control). The medium was replaced before treating with l-methionine-O-methyl ester (MetOMe) or glycyl-l-phenylalanine (GPN)32 at final concentrations of 1 mm and 0·2 mm, respectively, 48 and 72 hr post-plating. The cultures were then infected with ASFV at a MOI of 1 IFFU/cell without further washing. Newly synthesized virus antigen in infected cells was detected with mAb against virus VP73 as described above. In parallel experiments, monocytes were allowed to differentiate for 24 hr (day 1 MDMs) before treatment with the above substrates and infection with ASFV.
Detection of monocyte cytokines
The production of certain cytokines was measured in the medium taken from blood monocytes or MDMs, either without or after treatment with 1 µg/ml LPS. Interleukin-1 (IL-1), IL-6 and tumour necrosis factor (TNF) were measured by bioassay33 or enzyme-linked immunosorbent assay (ELISA) using antibodies specific for the porcine cytokine in question (Endogen, Buhlmann Labs, AG, Switzerland; Serotec, Inotech AG, Switzerland). Prostaglandin E2 (PGE2) detection used an ELISA for the general measurement of PGE2 (Cayman Chemical Company, Ann Arbor, MI33). Type I interferon (IFN) was detected using the VSV plaque-reduction assay.
Results
Cell surface and intracellular antigen expression profiles
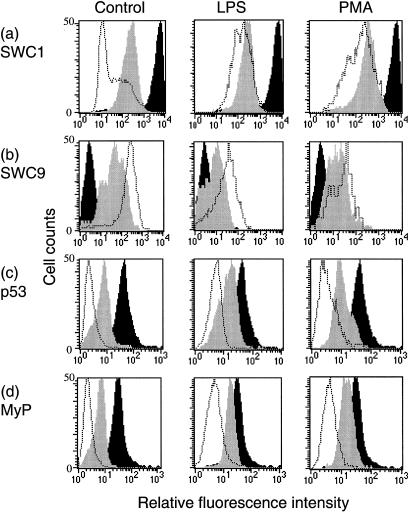
Freshly isolated monocytes were typically SWC1high, but SWC9– (Fig. 1a,b; control, black histograms) – values between 1 and 10 on the y1-axis corresponded to the negative antibody control reaction. With time in culture of the untreated control cells, SWC1 gradually down-regulated (Fig. 1a; control, grey and open histograms), concomitant with the SWC9 Mφ marker becoming up-regulated (Fig. 1b; control, grey and open histograms). Expression of the intracellular proteins p53 and myeloperoxidase (Fig. 1c,d; control) also down-regulated with time in culture. This demonstrated that the monocytes were differentiating towards Mφ.4,5,10
Figure 1.
Flow cytometric analysis of cell surface and intracellular markers on differentiating monocytes. Cells (control and LPS- or PMA-treated) were harvested after 0 (black histograms), 2 (grey histograms), and 4 days (dotted histograms) of culture, and labelled with mAb against SWC1 (a), SWC9 (b), p53 (c) and myeloperoxidase (MyP) (d). In the case of the intracellular proteins (c and d), the cells were fixed and made permeable as described in the Materials and methods. Values for a relative fluorescence intensity between 1 and 10 on the x-axis corresponded to the negative control antibody reaction. The results are representative from a single experiment of three replicates.
The up-regulation of SWC9, as well as the down-regulation of SWC1 and the intracellular proteins were noticeably hindered when monocytes were treated with the predetermined optimum modulatory doses of LPS and PMA (Fig. 1). Comparing the geometric means of marker expression, LPS treatment may seem to be slightly more effective than PMA at retarding phenotypic modulation, but the differences between LPS and PMA were always minimal. LPS was apparently more efficient at inhibiting the morphological characteristics of monocyte to Mφ differentiation, observable under light microscopy (data not shown). Nevertheless, the results would indicate that both the LPS and PMA treatments would have impaired but not abrogated monocytic cell differentiation. There was no indication that either agent promoted differentiation.
Functional characteristics associated with monocytic cell differentiation
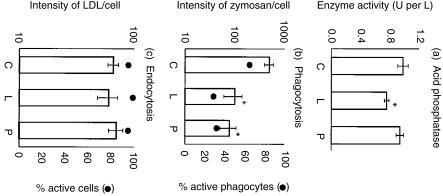
A steady increase in the activity of extracellular tartrate-resistant ACP is associated with monocyte differentiation into Mφ.10 Following LPS/PMA treatment of monocytes, the LPS-treated cells showed a slightly lower (P < 0·05) ACP activity compared with control cells, whereas PMA had no effect (Fig. 2a). Nevertheless, the ACP activity in untreated, LPS-treated and PMA-treated cultures was in all cases notably higher (P < 0·01) than that obtained with the freshly isolated monocytes (data not shown).
Figure 2.
Functional differentiation of LPS- and PMA-treated monocytes. (a) The activity of tartrate-resistant acid phosphatase in differentiating monocytes: untreated control (C), and LPS-treated (L) or PMA-treated (P). Samples (1 ml) from adherent cell cultures were assayed for the secreted enzyme after 3 days of culturing, in which the medium was replaced 24 hr before testing. The data shown are minus the values for culture medium alone, and are expressed as means ± SEM from triplicate experiments. (b) The capacity of differentiating monocytes to phagocytose BODIPY fluorescein-Zymosan (50 particles/cell, 30 min/37°) following LPS or PMA treatment of the monocytes for 72 hr. The values for fluorescence intensity of the phagocytosed zymosan are expressed as means ± SEM from triplicate experiments (y1-axis; histograms); the values for the actual percentage of cells phagocytosing shows means only, the SEM being < 5% (y2-axis; •). (c) Endocytic activity of differentiating monocytes treated with either LPS or PMA for 72 hr was measured by the uptake of 20 µg/ml BODIPY-fluorescein LDL complex for 30 min/37°. The data and values (y1-axis; histograms) for fluorescence intensity of the LDL complex are expressed as in (b) above, and the percentage of cells that incorporated the LDL shows means only, the SEM being < 5% (y2-axis; •). Statistical analyses were performed using the Student's t-test; * indicates P < 0·05 for treatment vs. control.
In addition to ACP, phagocytosis and LDL receptor-mediated endocytosis become elevated during Mφ differentiation.10 The ability of the monocytic cells to phagocytose unopsonized BODIPY-fluorescein-labelled zymosan was employed to measure the phagocytic capacity of differentiating cells (Fig. 2b). Almost 65% of the MDMs which developed in untreated cultures (C) were seen to be phagocytic (Fig. 2b, y2 axis). This capacity was significantly (P < 0·05) and almost equally inhibited in both LPS- and PMA-treated monocytes (Fig. 2b; L and P). Not only was the inhibition noted concerning the number of active phagocytes (Fig. 2b, y2 axis), but also in terms of the quantity of engulfed zymosan per cell (Fig. 2b, y1 axis, histograms).
In contrast to the clear inhibition of phagocytosis, the endocytic activity of the LPS/PMA-treated cells was not affected (Fig. 2c). The expression of LDL receptors on monocytic cells differentiating towards Mφ was employed as a measure of receptor-mediated endocytosis, using the pH-insensitive BODIPY-LDL probes. Figure 2(c) demonstrates that neither LPS nor PMA treatment (L, P) interfered with the development of this activity, with reference to the control cells (C). More than 90% of the cells in all cultures were positive for endocytosis (Fig. 2c, y2 axis), and exhibited a similar capacity for LDL binding/uptake (Fig. 2c, y1 axis, histograms). This LDL uptake was significantly higher in all cultures compared to the original monocytes (data not shown).
The intracellular pH environment of monocytic cells
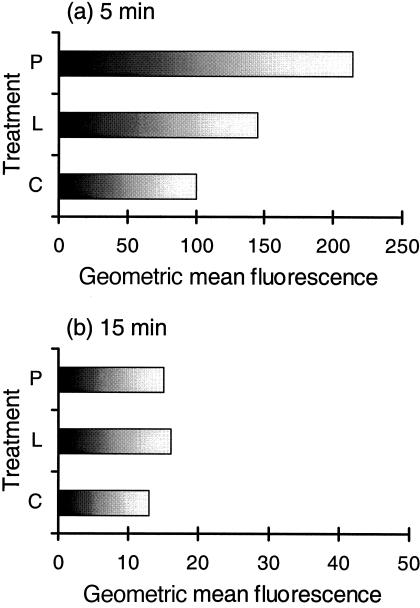
If a virus enters Mφ via phagocytosis or endocytosis, it will encounter the pH modulations associated with the endosomal system. Mφ are known to present a progressively acidifying cytoplasmic vacuolar environment, in order to effect processing of endocytosed or phagocytosed material.10 This was analysed by comparing the loss of fluorescence from pH-sensitive dextran probes internalized by the monocytic cells. It was clear that the probes initially (after 5 min incubation) encountered a lower pH within untreated control cells (Fig. 3a,c) compared with the treated cultures (Fig. 3a, L, P). This is seen as a lower geometric mean obtained with the internalized probe in the untreated controls (Fig. 3a,c). Furthermore, the vacuolar pH within PMA-treated cells was higher than in LPS-treated cells at this 5-min postincubation with the probe (Fig. 3a, P, L). After the second stage, subsequent to the washing step, resulting in the incubation time now being 15 min, it was noted that the environment in which the probe was found continued to acidify (Fig. 3b). The acidification level attained during this time was similar for control, LPS-treated cells and PMA-treated cells (Fig. 3b).
Figure 3.
Flow cytometry analysis of Cl-NERF-dextran (pH indicator) internalization in differentiating monocytes. Monocytic cells were cultured for 3 days, untreated (C), LPS-treated (L), or PMA-treated (P). The cells were left for 40 min on ice before incubation with the probe (0·25 mg/ml) for 5 min at 37°. Data were acquired at two stages: (a) immediately after this 5 min, before washing; and (b) after washing, which increased the incubation period to 15 min (unfilled graphs). The results are representative from a single experiment of three replicates, and the values are shown as geometric means of the fluorescence peak.
Endosomal/lysosomal enzyme activities
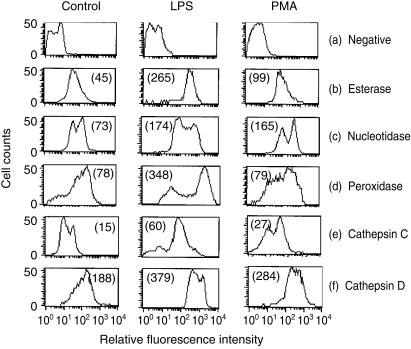
Another important parameter examined in these differentiating monocytes was the cell-associated enzyme activity. MDMs developing in control cultures had a reduced level of cell-associated non-specific esterase activity (Fig. 4b; control) compared with the monocytes from which they were derived (data not shown), as previously reported.10 Both LPS and PMA treatment yielded cells with higher esterase activity than the control cultures (Fig. 4b; LPS, PMA). This higher activity was most obvious with LPS-treated cells, and was almost identical to the monocyte activity.10
Figure 4.
The relative intracellular enzyme activity in monocytic cells after 3 days of culture; untreated control – left column – and LPS or PMA-treated – middle and right columns, respectively. The cells were unlabelled (a) or labelled with probes for esterase (b), 5′-nucleotidase/phosphatase (c), peroxidase (d), cathepsin C (e) and cathepsin D (f). Data acquired are presented as graphs, where the position of the negative control is shown under (a). The results are representative from one experiment out of three replicates, and the numbers noted in each plot are the geometric means of the fluorescent peak values.
A similar observation was noted with respect to the 5′ nucleotidase activity (Fig. 4c), but with the LPS and PMA-treated cultures being more closely related. Again, these levels were similar to that found in the original monocytes (data not shown and ref. 10). It should be noted however, that these results would be influenced by the presence of acid phosphatase, the activity of which would also be detected by the assay kit employed for these analyses.
Cell-associated peroxidase activity (Fig. 4d) was high only in the LPS-treated cultures. In this case, however, there was a double population, whereas the original monocytes were more homogeneous in their peroxidase expression (data not shown and ref. 10). This would suggest that a minor subpopulation of the LPS-treated cells had indeed down-regulated peroxidase activity, akin to the control cells differentiating into Mφ. Alternatively, the LPS may have activated enzyme activity, in this case in the majority of cells in the treated culture. As for the control and PMA-treated cultures, their geometric means for peroxidase activity were practically identical, although there was a notable difference in the shape of the histogram obtained (Fig. 4d; control, PMA). The results shown employed the DHR-6G probe. Similar results were obtained using the DHR 123 probe reported to be particularly specific for H2O2 and peroxidase activity.29
The activities of the lysosomal enzymes cathepsin C and D were also highest in LPS-treated cells and lowest in the untreated control cells (Fig. 4e,f). With both the controls and PMA-treated cells, the down-regulation of cathepsin C gave two main peaks of activity. In LPS-treated cultures, the profile resembled more that of peroxidase – the majority of cells were relatively high in activity, with only a minor population showing the down-regulation. The probe used for the cathepsin D results shown – VK cathepsin – is no longer commercially available. Consequently, a second probe for cathepsin D – BODIPY FL pepstatin A30 – was employed, providing confirmation of the results shown.
The activity of another lysosomal enzyme β-galactosidase activity was almost identical in all cultures (data not shown), but this activity is known not to change significantly as monocytes differentiate into Mφ.10 Glucosidase activity was also unaffected by the LPS or PMA treatment.
ASFV infection/replication in MDMs
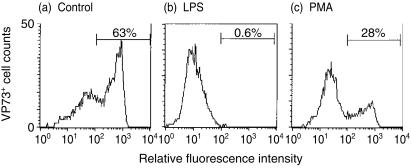
Following the observed characteristic changes in differentiating monocytes after treatment with LPS or PMA, it was interesting to determine the influence of these agents on ASFV infection, which requires monocyte differentiation towards Mφ.4,10 It was also considered that the infection might allow analysis of phagolysosomal activity, which is best studied in porcine Mφ through its inhibition. Monocytes were therefore treated with LPS or PMA, or left untreated to differentiate into MDMs. All cultures were maintained for 3 days at 37° before infection with ASFV. Figure 5 shows the percentage of antigen-positive cells as detected with the anti-virion VP73 mAb at 24 hr p.i. – the method employed detects only de novo virus antigen synthesis at the MOI used. The untreated control culture displayed a high degree of susceptibility to ASFV infection/replication (Fig. 5a). LPS treatment of the monocytes was more effective than the PMA at reducing the susceptibility of the cells to ASFV infection (Fig. 5b,c). This apparent antiviral state was not an irreversible abrogation of virus infection by the LPS or PMA. If analyses were effected at 48 hr p.i., instead of 24 hr p.i., more infected cells were detectable in all cultures (data not shown). Nevertheless, the levels of VP73+ cells were always lower in the LPS-treated cultures than in the controls; the PMA-treated cultures gave results intermediate between these two extremes.
Figure 5.
Infection/replication of ASFV in monocytic cells. Adherent monocytes were untreated (a), LPS-treated (1 µg/ml) (b) or PMA-treated (10 ng/ml) (c) for 3 days before infection with ASFV (10 IFFU/cell), and analysed 24 hr p.i. for the production of de novo ASFV-specific antigen (VP73). The percentage antigen-positive cells detected with the anti-VP73 mAb, and the associated marker shown on each graph, were identified as those cells to the right of the negative control antibody peak. The results are representative from a single experiment of five replicates.
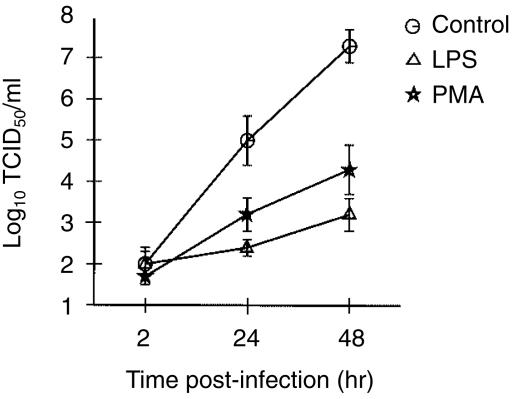
These results correlated with those obtained from analyses on the production of new infectious progeny (Fig. 6). At 2 hr p.i., the residual virus in the supernatant after washing was almost identical for untreated and treated cells. By 24 and 48 hr p.i., the infected untreated (control) cells were clearly the more productive. Although the LPS-treated cells were the least efficient at producing infectious progeny (0·01% of untreated cell levels), it was interesting to note the low productivity of PMA-treated cells (0·1% of untreated cell levels). Infectious centre assay confirmed that the low productivity related to fewer cells capable of supporting a productive virus infection (data not shown).
Figure 6.
Analysis of productive replication by ASFV in monocytic cells which were. untreated (controls), LPS-treated, or PMA-treated, before infection at 3 days post-treatment with an MOI of 10 IFFU/cell. The majority of non-adsorbed virus was removed after 2 hr by serial washing with PBS/1% (v/v) FCS. Progeny virus titres were calculated from titration of culture supernatants at 2 hr (residual virus), and culture lysates at 24 and 48 hr p.i., on fresh MDMs, as described in the Materials and methods. Data are expressed as means ± SEM from triplicate experiments.
Autophagosomal activity in LPS/PMA-treated monocytic cells
Considering the influence of LPS and PMA on the intracellular activities of lysosomal esterases and cathepsins, it was of interest to look at autophagosomal activity, and the role this might be playing in ASFV infection. Furthermore, it is difficult to measure autophagosomal activity in porcine monocytic cells, for which reason it was hoped that the ASFV infection might prove useful as an indicator. Cells were therefore treated with the lysosomal substrates MetOMe and GPN, due to their reported ability to disrupt lysosomal, and in the case of MetOMe autophagosomal functions.32 This interference with the lysosomal function was confirmed by demonstrating that both cathepsin D and esterase enzymes had lower activities in MetOMe- and GPN-treated cells (data not shown).
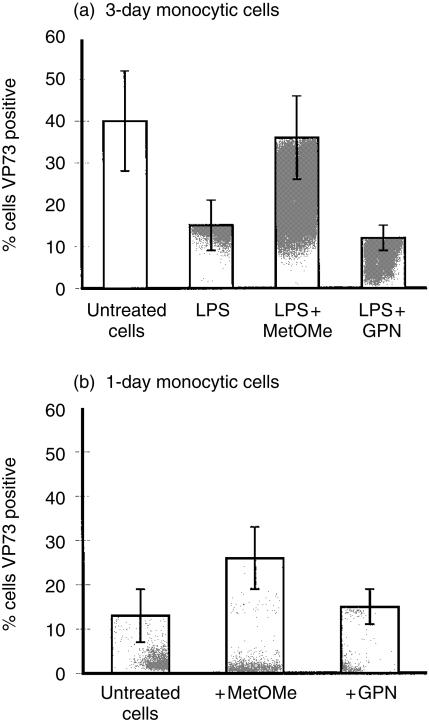
MetOMe was found to reverse the influence of LPS on the infection/replication of ASFV (Fig. 7a). In contrast, the addition of the GPN substrate did not modify the influence of LPS on subsequent ASFV infection. It should be noted that the ‘untreated’ cells in Fig. 7 relate to the ‘control’ cells of Figs 1–6, at 3 days of cultures. With PMA-treated cells, addition of the above substrates did not significantly alter the characteristics of virus infection (data not shown).
Figure 7.
Influence of modulating lysosomal functions on the infection/replication of ASFV in monocytic cells. (a) Adherent monocytic cells were either untreated controls (unfilled histogram), or LPS-treated (filled histogram). In certain LPS-treated cultures, the cells received the lysosomal enzyme substrates MetOMe or GPN, as described in the Materials and methods, followed by infection with ASFV (MOI 1 IFFU/cell). (b) Monocytes were cultured for only 24 hr before treatment with the lysosomal substrates and infection with ASFV, as in (a). Analyses were performed at 24 hr p.i. for the production of the de novo ASFV-specific late structural protein (VP73). Antigen-positive cells, detected with the anti-VP73 mAb, were determined by gating on the cells to the right of the negative antibody control peak, as for Figure 2. The results are expressed as means ± SEM from five replicate experiments.
In order to confirm the influence of MetOMe on ASFV infection, monocytic cells which had been cultured for only 1 day were employed. These cells have a low susceptibility to the ASFV infection compared with the 3 day-old MDMs.4,10 When treated with the above substrates, it was again the MetOMe but not the GPN which permitted an increased de novo synthesis of the virus late structural protein VP73 (Fig. 7b).
LPS induction of cytokine production by porcine monocytes
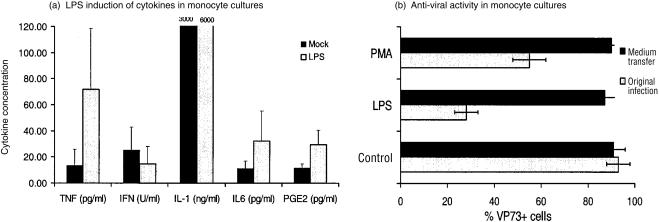
Due to the fact that LPS is known to induce pro-inflammatory cytokines in Mφ, the activity of LPS was therefore confirmed in terms of its capacity to stimulate the production of similar factors by monocytes. As previously reported,10 porcine blood monocytes efficiently produced IL-1, and LPS had only a minimal enhancing effect on this high level productivity (Fig. 8a). In contrast, the levels of spontaneous production for TNF, IL-6 and PGE2 were lower under the culture conditions employed (i.e. conditions that stimulate monocyte differentiation into Mφ4,10). LPS treatment of the monocytes did enhance the production of these cytokines (Fig. 8a). With interferon, on the other hand, LPS had no significant effect on its production by blood monocytes (Fig. 8a). When the monocytes had differentiated into Mφ4 before treatment, the LPS induction of TNF, IL-6 and PGE2 was greater than that obtained with the monocytes (data not shown). This was not the case for IL-1, the production of which by Mφ was poor compared with monocytes, even when treated with LPS.10 Similar results to those obtained with the MDM were forthcoming when lung lavage Mφ were employed (data not shown).
Figure 8.
Cytokine activity associated with porcine blood monocytes after treatment with 1 µg/ml LPS. (a) Detection of cytokines released into the medium by the cells at 24 hr after treatment, detected by specific ELISA for porcine TNF, IL-1 or IL-6, ELISA for PGE2, and the VSV plaque reduction assay for interferon (IFN). (b) Analysis of the capacity of medium taken from LPS-treated monocytes to inhibit ASFV replication. Monocytes were treated for 72 hr with 1 µg/ml LPS. The medium was removed and used to treat other monocytes which had already been cultured for the 3 days to differentiate into Mφ.4 Both the medium-treated Mφ (black bars) and the original LPS-treated cells (grey bars) were infected with ASFV at an MOI of 10 IFFU/cell. The de novo production of virus VP73 was measured at 24 hr p.i. using mAb against the VP73 and flow cytometry.
Considering this LPS-induced cytokine activity by the monocytes, it was interesting to know if this had an influence on the virus infection. Consequently, ASFV infection was attempted in susceptible Mφ, but after addition of supernatants from LPS-treated monocytes, containing the cytokines as shown in Fig. 8(a). The results (Fig. 8b) demonstrated that the relative resistance of the LPS-treated monocytes to ASFV infection could not be transferred by soluble factors secreted by the cells into the medium.
Discussion
Both the phenotype and functional activities of monocytes are modulated as they differentiate into Mφ.10 These results point to a continuous process during differentiation, involving interrelated characteristics linking the more accessory monocyte to the scavenger Mφ. Such modulations were also related to the susceptibility of the cells to monocytotropic virus infection. The porcine pathogen ASFV had a clear preference for differentiating Mφ,4,10 similar to that reported for herpes simplex virus14 and cytomegalovirus.15 Nevertheless, it remained unclear how interrelated were these various differentiation-related processes, and how modulation of the activities of Mφ would influence their interaction with monocytotropic viruses.
LPS has been reported to inhibit human monocyte differentiation to Mφ,11–13,34 whereas PMA has been reported to enhance differentiation, at least of monocytic leukaemia cell lines.13 This was not the case when dealing with primary monocytes. The present work showed that both LPS and PMA retarded the differentiation process. Despite the different signalling processes employed by LPS and PMA,12,13 both agents impeded the concomitant down-regulation of SWC1 and up-regulation of SWC9, as well as the down-regulation of the intracellular antigens p53 and myeloperoxidase. That is, they impeded the development of characteristics typifying porcine Mφ differentiation from monocytes.4,10 There was little difference between LPS and PMA in this effect, but the influence was not absolute: the differentiation process was retarded not abrogated. The treated cells did show some modulation of markers typical of the Mφ differentiation process. The most notable was with SWC9, which is normally only expressed when monocytes begin differentiating into Mφ.4 SWC9 was expressed on LPS- and PMA-treated cells, but to a lower level than on untreated cells.
When the functional activity of the cells was analysed, it became clear that the differentiation process was proceeding, but at a different rate compared to untreated cells. Of particular interest was the observation that the functional processes associated with differentiation were behaving in a manner somewhat independent of one another. LPS and PMA impaired phagocytosis development, whereas LDL receptor-mediated endocytosis remained unaffected. Furthermore, the phagocytosis activity in LPS/PMA-treated cells was higher than that seen with monocytes. Both agents modulated vacuolar enzyme activities important to the outcome of phagocytosis and endocytosis,35–38 the observed up-regulation again relating to impaired differentiation.10 In contrast, only LPS also impaired the development of acid phosphatase activity, demonstrating a disparity in the modulation of the monocytic cells, which may relate to the different signalling pathways induced by LPS and PMA. A disparity was also noted with the influence of the agents on vacuolar acidification. Although both impaired the rate of this acidification, PMA was more effective. Nevertheless, it was only the rate of acidification which was impaired, not the acidification itself – the vacuoles in the treated cells did eventually acidify to a similar degree as those of untreated differentiating monocytic cells. It is possible that the slower vacuolar acidification may have impaired the efficacy of the vacuolar enzymes. Thus, the LPS/PMA modulation of vacuolar enzyme activity may not have been due to impairment of differentiation, but would have indicated an activation of the enzyme activity in compensation for the slower acidification. The latter seems unlikely, due to the fact that PMA was more influential than LPS on vacuolar acidification, but had less effect on enzyme activities. Certainly, although endocytosis was unaffected, LPS and PMA would have impaired both the phagocytic and endocytic processes, due to their effects on vacuolar acidification.
Any difference between PMA and LPS in terms of the phenotype, phagocytosis and enzyme modulations could have been due to the concentrations employed. Although these were considered optimum and of least cytotoxicity, it is certain that lower concentrations of LPS would have brought certain effects induced by the two drugs closer together (data not shown). This cannot be argued for the influence on vacuolar acidification, with which the PMA was the more effective. Differences in modulations here are more probably due to differences in how PMA and LPS signal to the cells. Nevertheless, the PMA effect was still in the direction of impaired differentiation towards Mφ.
LPS and PMA also inhibited infection/replication by the macrophagotropic AFSV, which could be related to the modulation of monocytic cell function, but not to secreted cytokines. This supports the observation that ASFV infection has an obligate requirement for the cytoplasmic vacuolar H+-ATPases39 employed during active phagocytosis and endocytosis.40,41 The reported LPS or PMA inhibition of other monocytotropic virus infections16,17,42–44 may also be due to such functional modulation of monocytic cell activity.
Using virus infection to analyse autophagosome and lysosome activity during Mφ differentiation, it was noted that autophagosomal function was apparently more significant in monocytes than in Mφ, and was up-regulated by LPS. MetOMe, which can rupture lysosomes and autophagosomes,32 reversed LPS impairment of ASFV infection/replication, and increased the infection rate in monocytes, but not Mφ. GPN, which selectively destroys lysosomes without affecting autophagosomes,32 had no effect.
In conclusion, both LPS and PMA retard primary monocyte differentiation to Mφ. Of particular interest was the observation that vacuolar acidification was more influenced by the PMA-induced signalling, whereas the other differentiation-related processes were more related in their responses to LPS and PMA. However, neither LPS nor PMA could impair all aspects of Mφ differentiation, demonstrating that the processes modulated during Mφ differentiation10 are not always interdependent on one another. These characteristics also gave further insight into the capacity of a macrophagotropic virus to infect. High phagocytosis and rapid vacuolar acidification were seen to be important for the infection, whereas proteolytic enzyme and autophagosome activities would favour the cell in its struggle with the virus.
Acknowledgments
This work was supported by the Swiss National Science Foundation (grant no.31–47239.96; to SB), and the Swiss federal veterinary Office (to SK). The authors are grateful to H. Gerber for preparing the monoclonal antibodies and D. Brechbühl for animal bleedings.
References
- 1.van Furth R, Cohn ZA. The origin and kinetics of mononuclear phagocytes. J Exp Med. 1968;128:415. doi: 10.1084/jem.128.3.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andreesen R, Brugger W, Scheibenbogen C, Kreutz M, Leser HG, Rehm A, Lohr GW. Surface phenotype analysis of human monocyte to macrophage maturation. J Leukoc Biol. 1990;47:490–7. doi: 10.1002/jlb.47.6.490. [DOI] [PubMed] [Google Scholar]
- 3.Kreutz M, Krause SW, Hennemann B, Rehm A, Andreesen R. Macrophage heterogeneity and differentiation: defined serum-free culture conditions induce different types of macrophages in vitro. Res Immunol. 1992;143:107–15. doi: 10.1016/0923-2494(92)80087-2. [DOI] [PubMed] [Google Scholar]
- 4.McCullough KC, Basta S, Knötig S, Gerber H, Schaffner R, Kim YB, Saalmüller A, Summerfield A. Intermediate stages in monocyte-macrophage differentiation modulate phenotype and susceptibility to virus infection. Immunology. 1999;98:203–12. doi: 10.1046/j.1365-2567.1999.00867.x. 10.1046/j.1365-2567.1999.00867.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McCullough KC, Schaffner R, Kim Y, Natale VAI, Summerfield A. The phenotype of porcine monocytic cells: modulation of surface molecule expression upon monocyte differentiation into macrophages. Vet Immunol Immunopathol. 1997;58:265–75. doi: 10.1016/s0165-2427(97)00045-7. 10.1016/s0165-2427(97)00045-7. [DOI] [PubMed] [Google Scholar]
- 6.Biondi A, Rossing TH, Bennett J, Todd RF. Surface membrane heterogeneity among human mononuclear phagocytes. J Immunol. 1984;132:1237–43. [PubMed] [Google Scholar]
- 7.Waldrep JC, Kaplan AM, Mohanakumar T. Human mononuclear phagocyte-associated antigens. III. Relationship of cell surface antigen phenotype between cultured monocytes and tissue macrophages. J Reticuloendothel Soc. 1983;34:323–30. [PubMed] [Google Scholar]
- 8.Anegon I, Blottiere H, Cuturi MC, Lenne Y, Trinchieri G, Faust J, Perussia B. Characterization of a human monocyte antigen, B148.4, regulated during cell differentiation and activation. J Leukoc Biol. 1993;53:390–8. doi: 10.1002/jlb.53.4.390. [DOI] [PubMed] [Google Scholar]
- 9.Prieto J, Eklund A, Patarroyo M. Regulated expression of integrins and other adhesion molecules during differentiation of monocytes into macrophages. Cell Immunol. 1994;156:191–211. doi: 10.1006/cimm.1994.1164. 10.1006/cimm.1994.1164. [DOI] [PubMed] [Google Scholar]
- 10.Basta S, Knoetig SM, Spagnuolo-Weaver M, Allan G, McCullough KC. Modulation of monocytic cell activity and virus susceptibility during differentiation into macrophages. J Immunol. 1999;162:3961–9. [PubMed] [Google Scholar]
- 11.Rutherford MS, Witsell A, Schook LB. Mechanisms generating functionally heterogeneous macrophages: chaos revisited. J Leukoc Biol. 1993;53:602–18. doi: 10.1002/jlb.53.5.602. [DOI] [PubMed] [Google Scholar]
- 12.Chow CW, Grinstein S, Rotstein OD. Signaling events in monocytes and macrophages. New Horiz. 1995;3:342–51. [PubMed] [Google Scholar]
- 13.Asseffa A, Dickson LA, Mohla S, Bremner TA. Phorbol myristate acetate-differentiated THP-1 cells display increased levels of MHC class I and class II mRNA and interferon-gamma-inducible tumoricidal activity. Oncol Res. 1993;5:11–18. [PubMed] [Google Scholar]
- 14.Bruun T, Kristoffersen AK, Rollag H, Degré M. Interaction of herpes simplex virus with mononuclear phagocytes is dependent on the differentiation stage of the cells. APMIS. 1998;106:305–14. doi: 10.1111/j.1699-0463.1998.tb01351.x. [DOI] [PubMed] [Google Scholar]
- 15.Ibanez CE, Schrier R, Ghazal P, Wiley C, Nelson JA. Human cytomegalovirus productively infects primary differentiated macrophages. J Virol. 1991;65:6581–8. doi: 10.1128/jvi.65.12.6581-6588.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bernstein MS, Tong-Starksen SE, Locksley RM. Activation of human monocyte-derived macrophages with lipopolysaccharide decreases human immunodeficiency virus replication in vitro at the level of gene expression. J Clin Invest. 1991;88:540–5. doi: 10.1172/JCI115337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kornbluth RS, Oh PS, Munis JR, Cleveland PH, Richman DD. Interferons and bacterial lipopolysaccharide protect macrophages from productive infection by human immunodeficiency virus in vitro. J Exp Med. 1989;169:1137–51. doi: 10.1084/jem.169.3.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moriuchi H, Moriuchi M, Fauci AS. Differentiation of promonocytic U937 subclones into macrophage-like phenotypes regulates a cellular factor (s) which modulates fusion/entry of macrophagetropic human immunodeficiency virus type 1. J Virol. 1998;72:3394–400. doi: 10.1128/jvi.72.4.3394-3400.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vinuela E. African swine fever virus. Curr Top Microbiol Immunol. 1985;116:151–70. doi: 10.1007/978-3-642-70280-8_8. [DOI] [PubMed] [Google Scholar]
- 20.Saalmüller A, Aasted B, Canals A, et al. Analysis of monoclonal antibodies reactive with the porcine SWC1. Vet Immunol Immunopathol. 1994;43:255–8. doi: 10.1016/0165-2427(94)90145-7. [DOI] [PubMed] [Google Scholar]
- 21.Lunney JK. Characterization of swine leukocyte differentiation antigens. Immunol Today. 1993;14:147–8. doi: 10.1016/0167-5699(93)90227-C. [DOI] [PubMed] [Google Scholar]
- 22.Saalmüller A. Characterization of swine leukocyte differentiation antigens. Immunol Today. 1996;17:352–4. doi: 10.1016/S0167-5699(96)90273-X. 10.1016/0167-5699(96)30019-4. [DOI] [PubMed] [Google Scholar]
- 23.Dominguez J, Ezquerra A, Alonso F, et al. Workshop studies with monoclonal antibodies identifying a novel porcine differentiation antigen, SWC9. Vet Immunol Immunopathol. 1998;60:343–9. doi: 10.1016/s0165-2427(97)00110-4. 10.1016/s0165-2427(97)00110-4. [DOI] [PubMed] [Google Scholar]
- 24.Vojtesek B, Bartek J, Midgley CA, Lane DP. An immunochemical analysis of the human nuclear phosphoprotein p53. New monoclonal antibodies and epitope mapping using recombinant p53. J Immunol Methods. 1992;151:237–44. doi: 10.1016/0022-1759(92)90122-a. [DOI] [PubMed] [Google Scholar]
- 25.Weil SC, Rosner GL, Reid MS, et al. Translocation and rearrangement of myeloperoxidase gene in acute promyelocytic leukemia. Science. 1988;240:790–2. doi: 10.1126/science.2896388. [DOI] [PubMed] [Google Scholar]
- 26.Dive C, Cox H, Watson JV, Workman P. Polar fluorescein derivatives as improved substrate probes for flow cytoenzymological assay of cellular esterases. Mol Cell Probes. 1988;2:131–45. doi: 10.1016/0890-8508(88)90035-7. [DOI] [PubMed] [Google Scholar]
- 27.Berger CN, Tan SS, Sturm KS. Simultaneous detection of beta-galactosidase activity and surface antigen expression in viable haematopoietic cells. Cytometry. 1994;17:216–23. doi: 10.1002/cyto.990170305. [DOI] [PubMed] [Google Scholar]
- 28.Wersto RP, Rosenthal ER, Crystal RG, Spring KR. Uptake of fluorescent dyes associated with the functional expression of the cystic fibrosis transmembrane conductance regulator in epithelial cells. Proc Natl Acad Sci USA. 1996;93:1167–72. doi: 10.1073/pnas.93.3.1167. 10.1073/pnas.93.3.1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Henderson LM, Chappell JB. Dihydrorhodamine 123: a fluorescent probe for superoxide generation? Eur J Biochem. 1993;217:973–80. doi: 10.1111/j.1432-1033.1993.tb18328.x. [DOI] [PubMed] [Google Scholar]
- 30.Thiele DL, Lipsky PE. Mechanism of l-leucyl-l-leucine methyl ester-mediated killing of cytotoxic lymphocytes: dependence on a lysosomal thiol protease, dipeptidyl peptidase I, that is enriched in these cells. Proc Natl Acad Sci USA. 1990;87:83–7. doi: 10.1073/pnas.87.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen CS, Chen WN, Zhou M, Arttamangkul S, Haugland RP. Probing the cathepsin D using a BODIPY FL-pepstatin A. applications in fluorescence polarization and microscopy. J Biochem Biophys Meth. 2000;42:137–51. doi: 10.1016/s0165-022x(00)00048-8. [DOI] [PubMed] [Google Scholar]
- 32.Berg TO, Stromhaug PE, Berg T, Seglen PO. Separation of lysosomes and autophagosomes by means of glycyl-phenylalanine-naphthylamide, a lysosome- disrupting cathepsin-C substrate. Eur J Biochem. 1994;221:595–602. doi: 10.1111/j.1432-1033.1994.tb18771.x. [DOI] [PubMed] [Google Scholar]
- 33.Knoetig SM, Summerfield A, Spagnuolo-Weaver M, McCullough KC. Immunopathogenesis of Classical Swine Fever. Role of monocytic cells. Immunology. 1999;97:359–66. doi: 10.1046/j.1365-2567.1999.00775.x. 10.1046/j.1365-2567.1999.00775.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brugger W, Reinhardt D, Galanos C, Andreesen R. Inhibition of in vitro differentiation of human monocytes to macrophages by lipopolysaccharides (LPS): phenotypic and functional analysis. Int Immunol. 1991;3:221–7. doi: 10.1093/intimm/3.3.221. [DOI] [PubMed] [Google Scholar]
- 35.Guagliardi LE, Koppelman B, Blum JS, Marks MS, Cresswell P, Brodsky FM. Co-localization of molecules involved in antigen processing and presentation in an early endocytic compartment. Nature. 1990;343:133–9. doi: 10.1038/343133a0. [DOI] [PubMed] [Google Scholar]
- 36.Peters PJ, Neefjes JJ, Oorschot V, Ploegh HL, Geuze HJ. Segregation of MHC class II molecules from MHC class I molecules in the Golgi complex for transport to lysosomal compartments. Nature. 1991;349:669–76. doi: 10.1038/349669a0. [DOI] [PubMed] [Google Scholar]
- 37.Lemansky P, Hasilik A, von Figura K, Helmy S, Fishman J, Fine RE, Kedersha NL, Rome LH. Lysosomal enzyme precursors in coated vesicles derived from the exocytic and endocytic pathways. J Cell Biol. 1987;104:1743–8. doi: 10.1083/jcb.104.6.1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Czajkowska B, Ptak M, Bobek M, Bryniarski K, Szczepanik M. Different isoenzyme patterns of nonspecific esterases and the level of IL6 production as markers of macrophage functions. Folia Histochem Cytobiol. 1995;33:111–5. [PubMed] [Google Scholar]
- 39.Natale VAI, McCullough KC. Macrophage lysosomal pH gradients and vacular H+-ATPase activities relative to virus infection. J Leukoc Biol. 1998;6:302–10. doi: 10.1002/jlb.64.3.302. [DOI] [PubMed] [Google Scholar]
- 40.Yamashiro DJ, Fluss V, Maxfield FR. Acidification of endocytic vesicles by an ATP-dependent proton pump. J Cell Biol. 1983;9:929–34. doi: 10.1083/jcb.97.3.929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lukacs GL, Rotstein OD, Grinstein S. Phagosomal acidification is mediated by a vacuolar-type H(+)-ATPase in murine macrophages. J Biol Chem. 1990;26:21 099–107. [PubMed] [Google Scholar]
- 42.Duan X, Nauwynck HJ, Pensaert MB. Effects of origin and state of differentiation and activation of monocytes/macrophages on their susceptibility to porcine reproductive and respiratory syndrome virus (PRRSV) Arch Virol. 1997;14:2483–97. doi: 10.1007/s007050050256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mufson RA, Myers C, Turpin JA, Meltzer M. Phorbol ester reduces constitutive nuclear NF kappa B and inhibits HIV-1 production in mature human monocytic cells. J Leukoc Biol. 1992;52:637–44. doi: 10.1002/jlb.52.6.637. [DOI] [PubMed] [Google Scholar]
- 44.Chen YC, Wang SY, King CC. Bacterial lipopolysaccharide inhibits dengue virus infection of primary human Monocytes/Macrophages by blockade of virus entry via a CD14-dependent mechanism. J Virol. 1999;73:2650–7. doi: 10.1128/jvi.73.4.2650-2657.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]