Introduction
In the process of antigen recognition, the molecular structure detected by a T cell is never a soluble ligand. Recognition always requires the association between an antigenic peptide and a MHC molecule, present either on the surface of dedicated antigen-presenting cells (APCs, bearing class I and class II MHC) or on the surface of any other type of cell (bearing class I MHC). Thus, antigen recognition starts with a cell–cell interaction. At the interface between a T cell and an APC, concentrations of membrane receptors, intracellular adaptors, cytoskeletal components and enzymes form a structure controlling the communication between the two cells. In particular, this structure controls activation of the T cell. The contact is reminiscent of that observed at synapses of the nervous system, and hence the term ‘immunological synapse’ has been proposed.1,2 The main properties of this structure, which we will call ‘I-synapse’, have already been discussed in excellent reviews.3–5 In the present review, we will focus on how our present knowledge of this synapse is limited by the cells and the methods used to visualize this structure. We will then present a current view of the synapses formed by neurons (N-synapses) and, finally, examine how the more detailed information available for N-synapses can guide our studies of I-synapses.
Characteristics of the I-synapse
Let us first examine the different experimental models which have been used to characterize the I-synapse and the specific information obtained from each of them. We define an I-synapse as a region of membrane apposition between two cells of the immune system. This usage is more restrictive and appropriate than that found in some of the literature, where the term ‘synapse’ is sometimes employed to describe receptor clustering, or capping, induced by cross-linking antibodies.
Immunofluorescent studies of fixed cellular conjugates
More than 15 years ago, Abraham Kupfer and colleagues pioneered the study of T-cell–APC (T–APC) interactions at the single-cell level. Immunofluorescence of fixed cellular conjugates was used to identify the molecules and structures concentrated at the T–APC interface. The first molecular reorganization described was the polarization of the microtubule organizing centre (MTOC) in CD8+ T-cell clones, the MTOC turning towards the target cell.6 Similar observations were then made during antigen-specific interactions between CD4+ T-cell clones and APCs, i.e. B-cell hybridomas or lymphomas7,8 or MHC II-transfected fibroblasts.9 This MTOC polarization was observed only in the T cells but not in the APCs. These initial observations were followed by several reports describing the redistribution of various proteins, such as the coreceptor CD4, the integrin LFA-1 and the cytoskeletal adaptor talin, which all colocalized with the T-cell receptor (TCR) at the T–APC contact zone.10,11 More recently, Kupfer, Monks and collaborators showed that PKC-θ was also recruited at this antigen-specific interface, and that its serine/theonine kinase activity was necessary for TCR-induced NF-κB activation and T-cell proliferation.12,13 The most recent experiments took advantage of an improvement of the imaging system, deblurring after deconvolution of a Z-series of images, giving access to a third dimension of the I-synapse and to further details of its structure. After about 30 min of antigen-specific interaction between a T-cell clone and a B-cell lymphoma, concentrations of several molecules (PKC-θ, CD3, TCR, and two tyrosine kinases, Fyn and Lck) were found at the centre of the I-synapse. This cluster was called the cSMAC (central supra-molecular activation cluster). Talin and LFA-1 surrounded this cSMAC to form the pSMAC ( peripheral supra-molecular activation cluster).14 This molecular organization was antigen-specific, since it was not found when B-cell lymphomas did not present the specific antigen peptides to T-cell clones. However, I-synapse formation did not appear to depend solely upon a TCR-specific signal. Indeed, PKC-θ clustering was observed in T-cell clones but not in T-cell hybridomas.12
Thus, immunofluorescence experiments show the accumulation of a large number of molecules at the I-synapse. This accumulation is probably facilitated by the formation of a lamellipodium protruding from the T cell towards the APC, most clearly seen in scanning electron microscopy.15 However, one should not conclude that all types of cell surface protein accumulate at this interface. Indeed, the specific exclusion from the I-synapse of CD43 has recently been reported,4,16 while the exclusion of CD45 from the I-synapse is still a matter of controversy.16,17
Imaging the dynamics of I-synapse formation between T and B cells
The monitoring in living cells of fluorescent, GFP-tagged proteins expressed in either APCs or T-cell clones has provided another set of interesting data on the I-synapse. This method was first used to visualize the rearrangement of ICAM-1 during antigen-specific T–APC interaction.18 ICAM-1 accumulation in the presynaptic element, i.e. in a B-cell hybridoma, was detectable a few minutes after the intracellular Ca2+ rise. This study confirmed that polarization of LFA-1 molecules occurring in the postsynaptic (T cell) element was a very early event, probably necessary to sustain Ca2+ signalling.18 The same type of approach allowed Monks and colleagues to show that translocation of T-cell MEK kinase 2 (MEKK2-GFP) occurred within 10 s after exposure of the D10 T-cell line to an antigen-pulsed B-cell line.19 In another study, the movement of CD3ζ-GFP or CD4-GFP molecules was followed during antigen-specific T–APC interactions.20 During the first few minutes, both CD3ζ and CD4 accumulated at the centre of the T-cell–B-cell (T–B) interface, but shortly afterwards, while CD3ζ remained in the central zone, CD4 migrated away from this central spot, suggesting that CD4 was only necessary to trigger initial signalling. Using a slightly different approach, in which beads were attached non-specifically to T cells, Wulfing and Davis provided evidence that the molecular accumulation at the I-synapse was driven by an active cytoskeletal mechanism requiring costimulatory molecules and an intracellular Ca2+ increase.21 However, they did not observe a general movement of proteins on the B-cell surface towards the T–B interface, despite the ICAM-1 accumulation in the B cell described above.
Imaging the dynamics of I-synapse formation between T cells and lipid bilayers
A face-on view of an artificial synapse with an outstanding spatial resolution has been obtained by observing with interference-reflection microscopy (IRM) the contact formed between T cells and a planar bilayer containing glycosylphosphatidylinositol-anchored fluorescent MHC, ICAM-1, CD48 or CD80 molecules.2 In this system, surprisingly, it was LFA-1 which initially accumulated at the centre of the contact and was surrounded by a ring of TCRs. Then, within a few minutes, TCRs and LFA-1 exchanged their respective positions in the centre and periphery, to end up in an arrangement strikingly similar to that of the SMACs described by Kupfer's group. This configuration, which was called a ‘mature immunological synapse’, was stable for at least 1 hr. The addition to the planar bilayer of either CD48 or CD80 molecules did not modify the behaviour of LFA-1. Similar experiments demonstrated that CD2 accumulated at the centre of the interface, inside the LFA-1 ring.
The same approach has been used to analyse the dynamic redistribution of the membrane phosphatase CD45 at the T-cell surface, simultaneously with those of MHC and ICAM-1 in the lipid bilayer.22 Johnson et al. described an initial exclusion of CD45 which subsequently migrated back into the central zone. Their interpretation is that initial CD45 exclusion from the synapse would permit integrin activation, whereas the return of CD45 to the vicinity of the TCR would maintain Src kinase activity for the duration of the TCR engagement.
Indirect information on the structure of the I-synapse
Although our knowledge of the molecules that accumulate at the I-synapse has improved considerably, simple questions related to the structure of the I-synapse are still unresolved. In particular, what is the distance between the membranes of the T cell and those of the APC at the I-synapse? The direct answer to this question will come from electron microscopy experiments (Fig. 1). Despite this lack of direct information, immunologists tend to favour a scheme in which the synaptic cleft is approximately 15 nm at the centre of the synapse, where the TCR and MHC are assumed to interact with each other, the size of these molecules being around 7 nm. In the immediate vicinity of these molecules, one could easily imagine the presence of molecules of the same size, i.e. CD2, CD28 and their ligands. In contrast, large molecules such as LFA-1 and ICAMs and the even larger CD45 and CD43 could hardly be the immediate neighbours of TCRs at the I-synapse. All these predictions fit neatly with the observed exclusion of CD43 from the synapse, and with the detrimental effects on synapse formation of the expression in a T cell of large chimeric CD2 molecules.23 Mike Dustin and Anton van der Merwe have thoroughly discussed these points and even proposed that formation of the synapse could be driven to a large extent by size-based segregation of receptors and adhesion molecules. They have discussed an additional point, related to the low affinity of CD2 for its ligand when measured in solution. When CD2 (for example) is restricted to a surface, it is automatically concentrated and dissociation reactions are less favoured. Thus, when expressed at the cell membrane, even low-affinity receptors like CD2 might well play an important role in synapse formation/stabilization when their surface expression is large enough to result in a significant 2D affinity.5,24 This is probably the case for CD2, the density of which has been calculated to range between 200 and 300 molecules/µm2 in Jurkat cells.25
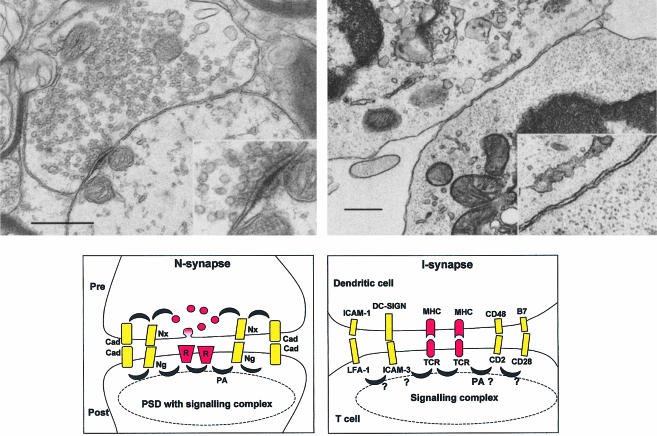
Figure 1.
Comparison between N- and I-synapses. Top: electron micrographs of glycinergic synapse (left, courtesy of Dr A. Triller) and of a T–DC synapse (right, courtesy of Dr G. Raposo). Scale: 0·5 µm (0·25 µm for the insets). The inset of the N-synapse highlights the presence of a PSD. Bottom: schematic summary of molecules involved at the N- and I-synapses (left and right). R, neurotransmitter receptor; Nx, neurexin; Ng, neuroligin; Cad, cadherin; PA, first row adaptors; PSD, postsynaptic density; TCR, T-cell receptor; MHC, major histocompatibility complex.
Thus, strong predictions about the structure of the I-synapse have been derived from theoretical arguments based on the size, ligand binding affinity and abundance of a number of molecules. However, we have seen that only some of them have been tested experimentally, most often indirectly.
Provisional conclusions
Immunofuorescence studies of fixed cell-conjugates have provided important information on the identity of several molecules included within or excluded from the I-synapse. An increased spatial resolution was achieved after image deconvolution. Initial studies provided information on the state of the synapse after 15–30 min of interaction, but more recently briefer interactions have been examined, and, in principle, kinetic information could be obtained from the statistical analysis of sets of data obtained at different times after the initiation of the T–APC contact. In addition, the use of GFP-tagged proteins has given access to direct dynamic information and shown some subtle movements, for instance that of CD4, which is only transiently in a position to interact with TCR–CD3, in a T/B interaction. Finally, by far the best spatio-temporal information is obtained in the IRM–lipid bilayer system, but at the expense of using a surrogate APC quite different from true APCs.
T–DC synapse
The fact that many studies have been performed on model synapses is often overlooked and the results extrapolated to all I-synapses. However, some important I-synapses have not been studied directly. A significant example is the synapse formed between naive T cells and dentritic cells (DCs).
Several factors appear to contribute to the unique ability of DCs to activate naive T cells. In an initial differentiation stage, DCs avidly capture antigen in the periphery of the organism (in the skin or various mucosae). They then leave the antigen capture spots to migrate to the T zone of the secondary lymphoid organs, a meeting point that is visited daily by circulating naive T cells. DCs there have partially lost their ability to capture antigen, and can now present to T cells the antigens previously captured in the periphery.26
DCs at this stage are the only cells able to make conjugates with T cells in an antigen-independent manner,27,28 a property which very probably favours antigen recognition when only scarce specific MHC–peptide complexes are available at the APC surface. We shall use the term ‘presynapse’ for the structure that forms at the T–DC interface either in the absence of antigen or, presumably, just before the antigen is recognized.
Putative adhesion molecules involved in presynapse formation
Despite the large number of adhesion and coactivation molecules expressed on DCs and T cells, the identities of the surface molecules that initiate the contact between T and DCs remain elusive. Candidate molecules must fulfil two conditions. First, the molecules initiating this interaction must have a sufficient avidity, or 2D affinity, for their ligand. Secondly, they must have the appropriate subcellular distribution. Antigen-independent adhesion visualized by transmission electron microscopy reveals that the initial contact between T cells and DCs occurs through villus–villus interactions.27 This situation is reminiscent of the initial interaction between leukocytes and endothelial cells during the homing process, an interaction mediated by selectins and α4 integrins, preferentially expressed at the tips of microvilli.29 Such a distribution is expected to facilitate contact between the two cells.30,31
As already mentioned, the TCR is unlikely to promote the initial T–DC adhesion. Experimental evidence for this is that the initial T–DC adhesion is antigen-independent. Also, from a theoretical viewpoint, the expected avidity of trace levels of specific MHC–peptide complexes (∼10–100 complexes/APC, corresponding to an average surface density of 0·1 molecules/µm2)27,32,33 does not fulfil the conditions described above.
The integrin LFA-1 is likely to participate in the formation of stable T–DC conjugates. Thus, T cells from LFA-1 deficient mice show reduced cluster formation with antigen-pulsed APCs.34 However, LFA-1 is not a good candidate for initiating T–DC interaction. In resting T cells, LFA-1 is inactive and unable to bind its ligand35 (otherwise, T cells expressing LFA-1 would bind to any ICAM-expressing cell, including T cells). This is due to cytoskeletal restraints that reduce the lateral diffusion of LFA-1.36–38 However, certain external stimuli free LFA-1 from its cytoskeletal anchorage, allowing it to diffuse freely and to form high-avidity clusters. The cross-linking of a number of surface molecules (including CD3, CD2 and CD28) can lead to LFA-1 activation.39–41 Thus, the binding of LFA-1 to ICAM-1 can strengthen cell–cell adhesion, but is not expected to initiate it.
The recent description of a novel C-type lectin, DC-SIGN, exclusively expressed on DCs has attracted much attention.42 Human DC-SIGN binds well to ICAM-3, a large adhesion molecule which extends above the glycocalix and is expressed by several cell types including naive T cells. ICAM-3 ligation with antibodies triggers an increase in LFA-1 avidity for ICAM-1.43 Thus, DC-SIGN is an interesting candidate for the initiation of T–DC interaction, though it probably does not act alone.
We prefer the idea that initiation of interaction relies on a redundant system involving a number of different molecules. This would be consistent with experiments in which blocking antibodies were used to analyse the contribution of adhesion molecules to antigen-independent T–DC interaction.44 At least four adhesion receptors (including CD2, CD28, ICAM-3 and LFA-1) seem to contribute to this adhesion, although none of the blocking antibodies presented strong inhibitory effects. Preliminary experiments performed with T cells purified from CD2- or CD28-deficient mice led to the same conclusions, since the absence of one of these two molecules did not affect the ability of DCs to form stable conjugates with T cells (Patrick Revy, unpublished results).
From the presynapse to the T–DC synapse
In the absence of antigen, several T-cell responses elicited by DCs have been detected.27,45,46 Antigen-independent contact triggers marked morphological alterations of T cells and small Ca2+ responses. In addition, recruitment of several surface and signalling molecules to the T–DC interface has been observed in T cells contacting unpulsed DCs (Revy et al., submitted). These findings show that, in the absence of antigen-specific interactions, DCs have the ability to form a presynapse. This could allow the T cell to scan the surface of the DC for cognate MHC–peptide complexes. On a longer time-scale, these experiments also revealed that a weak but reproducible T-cell proliferation is induced by DCs27 (Revy et al., submitted). Intracellular signalling pathways controlling these responses are not yet known.
It was recently reported that antigen-specific T–DC interactions taking place in a tissue-like environment – a collagen matrix – were highly dynamic and lasted only for a few minutes.46 The cumulative effect of such short interactions was sufficient to lead to T-cell proliferation. These surprising findings shed a new light on the accepted idea that a T–APC contact must be sustained for several hours in order to fully activate T cells.47 In addition, such fast dynamics are hardly compatible with the idea of an I-synapse progressively evolving towards a stable structure (‘mature immunological synapse’) with a well-defined cSMAC surrounded by a pSMAC. The T–DC synapse is likely to be much more fragmented27 and fluid than indicated in the classical but schematic view of the I-synapse.
Now that some aspects of the I-synapse have been established or questioned, and even though the structure of the T–DC synapse remains to be described, what can we learn from the comparison between I- and N-synapses? First, what are the main features of the N-synapse?
Characteristics of the N-synapse
Two ideas were common a few years ago concerning neuronal synapses: (1) for neurotransmission to occur, the pre- and postsynaptic cells do not (need to) interact directly, since neurons use neurotransmitters for their communication (except at electrical synapses), and (2) N-synapses are long-lived, permanent, fixed structures. From these points of view, N- and I-synapses should be very different. We shall see that these ideas are in need of drastic revision.
Receptor clustering at the neuromuscular junction
At one peculiar synapse, found at the vertebrate neuromuscular junction (NMJ), there is indeed an absence of direct contact between the pre- and postsynaptic cells. At the NMJ, the distance between the two cells is > 40 nm, and a basal lamina is interposed between the two cells (Fig. 1). However, even in this situation, information concerning their positioning needs to be exchanged between the two cells: the postsynaptic concentration of acetylcholine receptors (AChRs) must be exactly opposite the presynaptic terminal with acetylcholine-containing synaptic vesicles. We know now that the motor nerve terminal can release clustering factors such as agrin, which remains trapped in the basal lamina and then interacts with receptors of the muscle plasma membrane such as the dystroglycan complex,48,49 and Musk, a transmembrane receptor with a tyrosine kinase activity.50,51 These molecules appear to be directly involved in the clustering of AChRs below the nerve terminal.
Direct cell-cell contacts in the central nervous system
The situation is somewhat different at all the other N-synapses, in which there is no synaptic basal lamina, and where the synaptic cleft is only 15–30 nm wide.52 The molecular events that initiate the process of synapse formation in the central nervous system have only begun to be elucidated. It appears that the pre- and postsynaptic neurons interact directly with each other through homo- and heterophilic interactions.
The homophilic interactions take place through cadherins. Various classes of cadherins have specific patterns of distribution in the brain, delineating neural pathways.53 Cadherin-dependent synaptic adhesion is dynamically and locally controlled, and modulated by synaptic activity54 (and see below). Heterophilic interactions of several pre- and postsynaptic molecules control neuronal connections. Among them are β-neurexins in the presynaptic element, and neuroligins in the postsynaptic cell. In the human brain, which has approximately 1012 neurons, a daunting question concerns the precise control of the fitness of the connections. At least part of the underlying recognition events takes place through specific β-neurexin–neuroligin interactions.55 Neurexins belong to a family of polymorphic cell-surface proteins. Hundreds of neurexin isoforms are generated from three genes by usage of alternative promoters and alternative splicing. Like neurexins, neuroligins are encoded by three genes and alternatively spliced.
Other pre- and postsynaptic molecules regulate initial steps of synapse formation. Eph B receptor tyrosine kinases form a large family of at least 14 receptors with eight ligands, called ephrins. The binding of presynaptic ephrin to postsynaptic Eph triggers a signalling cascade that can lead to NMDA receptor clustering at the synapse.56
Surprisingly, key molecules of the I-synapse such as MHC class I and CD3ζ are also functionally important in the nervous system. Thus, MHC I and CD3ζ are expressed by neurons, and mice lacking these molecules exhibit abnormalities in the development of certain neuronal connections.57
Finally, the existence of direct cell–cell contact at the I-synapse does not exclude the importance in synapse formation of soluble factors such as Wnt-7a.58
Receptor clustering and specific adaptors
Another important feature of N-synapses is that they are clearly endowed with effective mechanisms for clustering membrane proteins. In the presynaptic nerve terminal, the high density of clustered Ca2+ channels allows them, when opening synchronously, to create transient microdomains of very high intracellular Ca2+ concentrations, required for the exocytosis of neurotransmitter-containing vesicles. In the same microdomain must be concentrated all the machinery for synaptic vesicle fusion and recycling. These various clusterings require the presence of special adaptor proteins, which frequently possess PDZ (PSD-95/Discs Large/ZO-1) domains. Some of these proteins also belong to the MAGUK family (membrane-associated guanylate kinase), which contain a guanylate kinase-like domain devoid of enzymatic activity, SH3 (src-homology 3) and PDZ domains. CASK is a MAGUK with one PDZ domain,59 which interacts with β-neurexins in the presynaptic terminal and contributes to the recruitment of the presynaptic secretory apparatus.55 PSD-95 is present in postsynaptic densities (see Fig. 1), disk-shaped organelles 30 nm thick attached to the postsynaptic membrane (frequently, although not exclusively, at excitatory synapses), providing a structural framework for anchoring functional molecules.60 PSD-95 possesses three PDZ domains and is able to interact with distinct proteins: neuroligins, some neurotransmitter receptors such as NMDA receptors and the associated signalling complex.61,62
Different postsynaptic scaffold proteins are involved in the clustering of neurotransmitter receptors: similarly to PSD-95 for NMDA receptors (but structurally unrelated), gephyrin contributes to the clustering of glycine receptors63,64 and rapsyn to that of AChRs receptors at the NMJ.65 These scaffold proteins also interact with other membrane receptors. For instance, rapsyn interacts also with the dystroglycan membrane complex at the muscle membrane.66,67
Briefly, the specificity of an N-synapse does not depend only upon the specific interaction of postsynaptic receptors with the appropriate neurotransmitter released by the nerve terminal. It also relies upon a first set of molecules (neurexins-neuroligins) which control the appropriate cell–cell adhesion and also couple with a certain degree of specificity to a second set of proteins, which could be called ‘primary adaptors’. Some of these, though not all, contain PDZ domains. These adaptors allow the coclustering of adhesion molecules and the recruitment of the specific synaptic machinery (presynaptic Ca2+ channels plus secretory apparatus or postsynaptic neurotransmitter receptors plus signalling complex). The signalling complex, in turn, includes various enzymes and another set of adaptors, including those allowing anchoring to the cytoskeleton.
Activity-induced synapse remodelling
How stable are N-synapses? These structures are certainly stable to a large extent, with several important exceptions. First, they are highly dynamic during development.68,69 For instance, there is one stage of development of the NMJ where one motoneuron establishes multiple contacts with a given muscle cell. In the following days, all but one of these contacts regress.68 At the end of development, there is less than 1 AChR/µm2 at the muscle surface, except below the nerve terminal, where the AChR density can reach a quasi-crystalline density of 104/µm2! Secondly, video recordings from living neurons expressing GFP-tagged actin revealed spontaneous, rapid (within seconds), and apparently random changes in spine shapes (spines are small and numerous dendritic processes upon which excitatory synapses are formed), which contrast conspicuously with the relative morphological stability of the underlying dendrite.70 Thirdly, there is a relationship between the synaptic structure and its functioning. Neurotransmitter-specific information can only be transmitted once a rudimentary synapse has formed. Thereafter, synaptic activity will result in synaptic plasticity. Thus, the density of neurotransmitter receptors is controlled by activity-dependent mechanisms. For instance, paralysis of the motor neuron results in a redistribution of AChRs at the muscle surface,68 whereas chronic blockade of NMDA receptors in hippocampal neurons increases the synaptic clustering of NMDA receptors.71 But, more subtly and interestingly, as early as 30 min following a specific type of stimulation, synapse remodelling can be observed: when hippocampal dendrites are subjected to a tetanic stimulation known to induce long-term potentiation (a cellular model for the basic mechanisms of memory formation), for several tens of minutes, one can observe an enhanced growth of small filopodia-like protrusions, giving rise to new dendritic spines a few microns long.72,73 Stabilization of the new contacts seems to involve the elevation of protein levels of N-cadherins, a phenomenon depending upon the activation of protein kinase A.74
It is still unclear how synaptic activity can induce synapse remodelling, but it is possible that the ‘primary adaptors’ described above are involved in this phenomenon. Thus, in Drosophila synapses, the postsynaptic distribution and function of the PDZ protein discs large is regulated by calcium/calmodulin-dependent protein kinase II.75
Comparison between N- and I-synapses
Synapse specificity and adaptors
Both I- and N-synapses are structures specialized for the exchange of information between two cells. As summarized in Fig. 1, the specificity of the N-synapse is determined by the combination of at least three sets of molecules: (1) specific adhesion molecules, (2) neurotransmitter-specific molecules, including both the presynaptic apparatus and neurotransmitter receptors, and (3) ‘primary adaptors’, several of which possess PDZ domains. In the I-synapse, (1) adhesion molecules presenting some specificity are also present, and (2) a major part of the specificity is associated with the fit between MHC–peptide complexes and specific TCRs. Finally, very little is known about the equivalent of ‘primary adaptors’, a set of molecules that would be able both to cluster TCRs and to link directly TCR and adhesion molecules. Either these links are only indirect, and are provided by components of the signalling complex already described, or the direct linkers await to be discovered. Vav is a T-cell-specific molecule potentially involved in the link between TCRs and the cytoskeleton. Its influence has been observed on antibody-induced receptor clustering76,77 but more importantly on TCR–MHC accumulation at the T–APC interface.78 However, it should be stressed that Vav is not a ‘primary adaptor’ since it does not bind directly either to the TCR or to the cytoskeleton. Vav is therefore simply an element of the signalling complex necessary for Rho GTPases activation. Interestingly, B and T lymphocytes do possess a MAGUK, PDZ-containing protein, hDLG (human homologue of discs large), which is recruited at the CD2 cap induced by anti-CD2 antibodies.79 It must be stressed that this cap is not a synapse and the role of hDLG in bona fide I-synapses remains to be determined.
Direct cell-cell contacts and soluble messengers
In both I- and N-synapses (except at the NMJ), the size of the synaptic cleft (< 30 nm) allows both direct cell–cell interactions and communication through soluble molecules to take place in parallel. The importance of direct cell–cell interactions is well established in both cases, but the importance of soluble mediators at the I-synapse has not yet been properly examined. More precisely, we do not know how soluble messengers contribute to I-synapse formation, although it is known that, after several hours of contact between a T and a B cell, a significant and polarized secretion of cytokines takes place from the T cell towards the B cell,80 delivering retrograde information to the presynaptic B cell.
Which soluble factors could participate in the initial T–DC interaction? Chemokines are particularly important in the direction of T cells to sites of inflammation. They also play an important role in promoting the adhesion of T cells to endothelial cells.81 DCs are known to secrete several chemokines that have the ability to attract T lymphocytes.82 In addition, chemokines have been shown to trigger LFA-1 activation.83 Therefore, a possible role of chemokines in T–DC synapse formation would be worth examining more closely, even though negative effects of chemokines on TCR-induced signalling pathways have been reported.84
Synapse formation/stabilization and functioning: bidirectional influences
In the nervous system, a presynapse allows the transmission of initial synaptic activity, which influences the final maturation and stabilization of the synapse. The molecules participating in the presynapse and in the mature synapse are not necessarily identical. For instance, it seems that N-cadherin adhesion may stabilize early synapses that can then be remodelled to express different cadherins.85 In our opinion, similar principles may apply to the I-synapse: a presynapse, which can form and start to function even in the absence of any antigen-specific information, probably undergoes further maturation and stabilization to become an I-synapse.
Can one determine the order of the different protein–protein interactions which allow the formation of the presynapse, then of the synapse? A strict predefined order seems unlikely; we favour the idea that the final assembly may be initiated in several different ways by transient, random interactions which are progressively stabilized by additional interactions. Nevertheless, the restricted expression of adhesion molecules at specific locations (microvilli) is very likely to influence the order of interactions.
At T–DC synapses, everything happens as if ‘a DC delivers a signal promoting T cell membrane ruffling, in addition to triggering a Ca2+ signal which tends to round up and immobilize the T cell’45. Similarly, at the N-synapse, according to a hypothetical scheme for the effect of glutamate receptors on dendritic spine development, glutamate might first promote the outgrowth of motile filopodia through the stimulation of NMDA receptors and, after some growth, acquisition of AMPA receptors provides a mechanism that may suppress actin-based spine motility.69 In addition to these postsynaptic mechanisms influencing synapse formation and plasticity, retrograde mechanisms must also be taken into account. Thus, at the T–DC synapse, Ca2+ responses have been observed in DCs following their interaction with T cells,45 and dynamic actin polarization in DCs has been described at the T–DC interface,86 showing that DCs receive early signals from the T cells, which could influence the formation of a mature T–DC synapse.
Receptor clustering
Does receptor clustering precede or follow synapse formation? It probably does both. Thus, it appears that NMDA receptors may initially aggregate in the absence of PSD-95, and their association with PSD-95 may function to retain preformed receptor clusters at synapses.71 Similarly, gephyrin- & independent formation of glycine receptor clusters precedes the gephyrin-mediated postsynaptic accumulation of clusters.64 At the T–DC synapse, TCR clustering may precede full TCR signalling, since TCR clustering occurs even in the absence of antigen (Revy et al., submitted). In addition, a number of results indicate that TCR signalling extends and stabilizes the I-synapse. The existence of TCR/CD3 clusters in the absence of stimulation helps to explain why, after activation, both stimulated and unstimulated TCRs are found colocalized in the same patches.87 Similarly, it appears that the number of MHC molecules which accumulate at the I-synapse is much larger than that of specific MHC-peptide complexes,78 which implies that only a minority of the clustered MHC molecules have interacted with TCRs in a specific way. Whether these clusters result from direct, homophilic interactions (e.g. between MHC molecules) or require the presence of ‘primary adaptors’ remains to be established.
Conclusion
In the past few years, our knowledge of the molecular dynamics taking place at the contact zone between an APC and a T cell has advanced rapidly. The validity of the schematic view of the so-called immunological synapse remains to be tested in physiological models, in particular at the interface between DCs and naive T cells. Calling this structure a synapse may be justified, due to a number of similarities between I- and N-synapses. However, one should not forget that, in addition to the T–APC interaction, other well-structured cell–cell contacts can be observed in the immune system, for instance in NK cell–target cell conjugates.88 More importantly, the connotations of the word ‘synapse’ may have biased our thinking towards considering I-synapses as stable structures. This was partly due to a certain ignorance of N-synapses, since we know now that there is more movement and remodelling in N-synapses than previously thought. But, in addition, N- and I-synapses do differ, and physiological I-synapses are likely to be much more fragmented and fluid than currently thought. The comparison between N- and I-synapses proves to be quite stimulating, raising a number of questions, in particular concerning the mechanisms of receptor clustering and the role of soluble messengers in the formation and functioning of the I-synapse.
Acknowledgments
We thank Dr Antoine Triller for very helpful suggestions and for electron microscopy of a N-synapse, Dr Graça Raposo for electron microscopy of a T–DC synapse, Dr Jean Cartaud for useful discussions and Dr Boris Barbour for his comments on the manuscript. PR was supported by a fellowship from Association pour la recherche sur le Cancer. The laboratory is supported by grants from CNRS and Ligue Nationale contre le Cancer.
References
- 1.Paul WE, Seder RA. Lymphocyte responses and cytokines. Cell. 1994;76:241–51. doi: 10.1016/0092-8674(94)90332-8. [DOI] [PubMed] [Google Scholar]
- 2.Grakoui A, Bromley SK, Sumen C, Davis MM, Shaw AS, Allen PM, Dustin ML. The immunological synapse: a molecular machine controlling T cell activation. Science. 1999;285:221–7. doi: 10.1126/science.285.5425.221. 10.1126/science.285.5425.221. [DOI] [PubMed] [Google Scholar]
- 3.Dustin ML, Cooper JA. The immunological synapse and the actin cytoskeleton: molecular hardware for T cell signaling. Nature Immunol. 2000;1:23–9. doi: 10.1038/76877. [DOI] [PubMed] [Google Scholar]
- 4.Delon J, Germain RN. Information transfer at the immunological synapse. Curr Biol. 2000;10:R923–R33. doi: 10.1016/s0960-9822(00)00870-8. 10.1016/s0960-9822(00)00870-8. [DOI] [PubMed] [Google Scholar]
- 5.van der Merwe AP, Davis SJ, Shaw AS, Dustin ML. Cytoskeletal polarization and redistribution of cell-surface molecules during T cell antigen recognition. Semin Immunol. 2000;12:5–21. doi: 10.1006/smim.2000.0203. [DOI] [PubMed] [Google Scholar]
- 6.Kupfer A, Dennert G. Reorientation of the microtubule-organizing center and the Golgi apparatus in cloned cytotoxic lymphocytes triggered by binding to lysable target cells. J Immunol. 1984;133:2762–6. [PubMed] [Google Scholar]
- 7.Kupfer A, Swain SL, Singer SJ. The specific direct interaction of helper T cells and antigen-presenting B cells. II. Reorientation of the microtubule organizing center and reorganization of the membrane-associated cytoskeleton inside the bound helper T cells. J Exp Med. 1987;165:1565–80. doi: 10.1084/jem.165.6.1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kupfer A, Swain SL, Janeway CA, Jr, Singer SJ. The specific direct interaction of helper T cells and antigen- presenting B cells. Proc Natl Acad Sci USA. 1986;83:6080–3. doi: 10.1073/pnas.83.16.6080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sedwick CE, Morgan MM, Jusino L, Cannon JL, Miller J, Burkhardt JK. TCR, LFA-1, and CD28 play unique and complementary roles in signaling T cell cytoskeletal reorganization. J Immunol. 1999;162:1367–75. [PubMed] [Google Scholar]
- 10.Kupfer A, Singer SJ, Janeway CA, Jr, Swain SL. Coclustering of CD4 (L3T4) molecule with the T-cell receptor is induced by specific direct interaction of helper T cells and antigen-presenting cells. Proc Natl Acad Sci USA. 1987;84:5888–92. doi: 10.1073/pnas.84.16.5888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kupfer A, Singer SJ. The specific interaction of helper T cells and antigen-presenting B cells. IV. Membrane and cytoskeletal reorganizations in the bound T cell as a function of antigen dose. J Exp Med. 1989;170:1697–713. doi: 10.1084/jem.170.5.1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Monks CR, Kupfer H, Tamir I, Barlow A, Kupfer A. Selective modulation of protein kinase C-theta during T-cell activation. Nature. 1997;385:83–6. doi: 10.1038/385083a0. [DOI] [PubMed] [Google Scholar]
- 13.Sun Z, Arendt CW, Ellmeier W, Schaeffer EM, Sunshine MJ, Gandhi L, Annes J, Petrzilka D, Kupfer A, Schwartzberg PL, Littman DR. PKC-theta is required for TCR-induced NF-kappaB activation in mature but not immature T lymphocytes. Nature. 2000;404:402–7. doi: 10.1038/35006090. 10.1038/35006090. [DOI] [PubMed] [Google Scholar]
- 14.Monks CR, Freiberg BA, Kupfer H, Sciaky N, Kupfer A. Three-dimensional segregation of supramolecular activation clusters in T cells. Nature. 1998;395:82–6. doi: 10.1038/25764. 10.1038/25764. [DOI] [PubMed] [Google Scholar]
- 15.Donnadieu E, Lang V, Bismuth G, Ellmeier W, Acuto O, Michel F, Trantmann A. Differential roles of Lck and Itk in T cell response to antigen recognition revealed by calcium imaging and electron microscopy. J Immunol. 2001. pp. 5540–9. [DOI] [PubMed]
- 16.Sperling AI, Sedy JR, Manjunath N, Kupfer A, Ardman B, Burkhardt JK. TCR signaling induces selective exclusion of CD43 from the T cell-antigen-presenting cell contact site. J Immunol. 1998;161:6459–62. [PubMed] [Google Scholar]
- 17.Leupin O, Zaru R, Laroche T, Muller S, Valitutti S. Exclusion of CD45 from the T-cell receptor signaling area in antigen-stimulated T lymphocytes. Curr Biol. 2000;10:277–80. doi: 10.1016/s0960-9822(00)00362-6. 10.1016/s0960-9822(00)00362-6. [DOI] [PubMed] [Google Scholar]
- 18.Wulfing C, Sjaastad MD, Davis MM. Visualizing the dynamics of T cell activation: intracellular adhesion molecule 1 migrates rapidly to the T cell/B cell interface and acts to sustain calcium levels. Proc Natl Acad Sci USA. 1998;95:6302–7. doi: 10.1073/pnas.95.11.6302. 10.1073/pnas.95.11.6302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schaefer BC, Ware MF, Marrack P, Fanger GR, Kappler JW, Johnson GL, Monks CR. Live cell fluorescence imaging of T cell MEKK2: redistribution and activation in response to antigen stimulation of the T cell receptor. Immunity. 1999;11:411–21. doi: 10.1016/s1074-7613(00)80116-8. [DOI] [PubMed] [Google Scholar]
- 20.Krummel MF, Sjaastad MD, Wulfing C, Davis MM. Differential clustering of CD4 and CD3zeta during T cell recognition. Science. 2000;289:1349–52. doi: 10.1126/science.289.5483.1349. 10.1126/science.289.5483.1349. [DOI] [PubMed] [Google Scholar]
- 21.Wulfing C, Davis MM. A receptor/cytoskeletal movement triggered by costimulation during T cell activation. Science. 1998;282:2266–9. doi: 10.1126/science.282.5397.2266. [DOI] [PubMed] [Google Scholar]
- 22.Johnson KG, Bromley SK, Dustin ML, Thomas ML. A supramolecular basis for CD45 tyrosine phosphatase regulation in sustained T cell activation. Proc Natl Acad Sci USA. 2000;97:10138–43. doi: 10.1073/pnas.97.18.10138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wild MK, Cambiaggi A, Brown MH, Davies EA, Ohno H, Saito T, van der Merwe PA. Dependence of T cell antigen recognition on the dimensions of an accessory receptor-ligand complex. J Exp Med. 1999;190:31–41. doi: 10.1084/jem.190.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shaw AS, Dustin ML. Making the T cell receptor go the distance: a topological view of T cell activation. Immunity. 1997;6:361–9. doi: 10.1016/s1074-7613(00)80279-4. [DOI] [PubMed] [Google Scholar]
- 25.Dustin ML, Golan DE, Zhu D-M, Miller JM, Meier W, Davies EA, Anton van der Merwe P. Low affinity interaction of human or rat T cell adhesion molecule CD2 with its ligand aligns adhering membranes to achieve high physiological affinity. J Biol Chem. 1997;272:30889–98. doi: 10.1074/jbc.272.49.30889. 10.1074/jbc.272.49.30889. [DOI] [PubMed] [Google Scholar]
- 26.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–52. doi: 10.1038/32588. 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 27.Delon J, Bercovici N, Raposo G, Liblau R, Trautmann A. Antigen-dependent and -independent Ca2+ responses triggered in T cells by dendritic cells compared with B cells. J Exp Med. 1998;188:1473–84. doi: 10.1084/jem.188.8.1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Inaba K, Romani N, Steinman RM. An antigen-independent contact mechanism as an early step in T cell- proliferative responses to dendritic cells. J Exp Med. 1989;170:527–42. doi: 10.1084/jem.170.2.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Berlin C, Bargatze RF, Campbell JJ, von Andrian UH, Szabo MC, Hasslen SR, Nelson RD, Berg EL, Erlandsen SL, Butcher EC. Alpha 4 integrins mediate lymphocyte attachment and rolling under physiologic flow. Cell. 1995;80:413–22. doi: 10.1016/0092-8674(95)90491-3. [DOI] [PubMed] [Google Scholar]
- 30.von Andrian UH, Hasslen SR, Nelson RD, Erlandsen SL, Butcher EC. A central role for microvillous receptor presentation in leukocyte adhesion under flow. Cell. 1995;82:989–99. doi: 10.1016/0092-8674(95)90278-3. [DOI] [PubMed] [Google Scholar]
- 31.Stein JV, Cheng G, Stockton BM, Fors BP, Butcher EC, von Andrian UH. l-selectin-mediated leukocyte adhesion in vivo: microvillous distribution determines tethering efficiency, but not rolling velocity. J Exp Med. 1999;189:37–50. doi: 10.1084/jem.189.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Demotz S, Grey HM, Sette A. The minimal number of class II MHC-antigen complexes needed for T cell activation. Science. 1990;249:1028–30. doi: 10.1126/science.2118680. [DOI] [PubMed] [Google Scholar]
- 33.Harding CV, Unanue ER. Quantitation of antigen-presenting cell MHC class II/peptide complexes necessary for T-cell stimulation. Nature. 1990;346:574–6. doi: 10.1038/346574a0. [DOI] [PubMed] [Google Scholar]
- 34.Bachmann MF, McKall-Faienza K, Schmits R, Bouchard D, Beach J, Speiser DE, Mak TW, Ohashi PS. Distinct roles for LFA-1 and CD28 during activation of naive T cells: adhesion versus costimulation. Immunity. 1997;7:549–57. doi: 10.1016/s1074-7613(00)80376-3. [DOI] [PubMed] [Google Scholar]
- 35.Lollo BA, Chan KW, Hanson EM, Moy VT, Brian AA. Direct evidence for two affinity states for lymphocyte function-associated antigen 1 on activated T cells. J Biol Chem. 1993;268:1693–70. [PubMed] [Google Scholar]
- 36.Kucik DF, Dustin ML, Miller JM, Brown EJ. Adhesion-activating phorbol ester increases the mobility of leukocyte integrin LFA-1 in cultured lymphocytes. J Clin Invest. 1996;97:2139–44. doi: 10.1172/JCI118651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stewart MP, McDowall A, Hogg N. LFA-1-mediated adhesion is regulated by cytoskeletal restraint and by a Ca2+-dependent protease, calpain. J Cell Biol. 1998;140:699–707. doi: 10.1083/jcb.140.3.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.van Kooyk Y, van Vliet SJ, Figdor CG. The actin cytoskeleton regulates LFA-1 ligand binding through avidity rather than affinity changes. J Biol Chem. 1999;274:26869–77. doi: 10.1074/jbc.274.38.26869. 10.1074/jbc.274.38.26869. [DOI] [PubMed] [Google Scholar]
- 39.van Kooyk Y, van de Wiel-van Kemenade P, Weder P, Kuijpers TW, Figdor CG. Enhancement of LFA-1-mediated cell adhesion by triggering through CD2 or CD3 on T lymphocytes. Nature. 1989;342:811–3. doi: 10.1038/342811a0. [DOI] [PubMed] [Google Scholar]
- 40.Dustin ML, Springer TA. T-cell receptor cross-linking transiently stimulates adhesiveness through LFA-1. Nature. 1989;341:619–24. doi: 10.1038/341619a0. [DOI] [PubMed] [Google Scholar]
- 41.Zell T, Hunt SW, III, Mobley JL, Finkelstein LD, Shimizu Y. CD28-mediated up-regulation of beta 1-integrin adhesion involves phosphatidylinositol 3-kinase. J Immunol. 1996;156:883–6. [PubMed] [Google Scholar]
- 42.Geijtenbeek TB, Torensma R, van Vliet SJ, van Duijnhoven GC, Adema GJ, van Kooyk Y, Figdor CG. Identification of DC-SIGN, a novel dendritic cell-specific ICAM-3 receptor that supports primary immune responses. Cell. 2000;100:575–85. doi: 10.1016/s0092-8674(00)80693-5. [DOI] [PubMed] [Google Scholar]
- 43.Campanero MR, Sanchez-Mateos P, del Pozo MA, Sanchez-Madrid F. ICAM-3 regulates lymphocyte morphology and integrin-mediated T cell interaction with endothelial cell and extracellular matrix ligands. J Cell Biol. 1994;127:867–78. doi: 10.1083/jcb.127.3.867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hauss P, Selz F, Cavazzana-Calvo M, Fischer A. Characteristics of antigen-independent and antigen-dependent interaction of dendritic cells with CD4+ T cells. Eur J Immunol. 1995;25:2285–94. doi: 10.1002/eji.1830250826. [DOI] [PubMed] [Google Scholar]
- 45.Montes M, McIlroy D, Hosmalin A, Trautmann A. Calcium responses elicited in human T cells and dendritic cells by cell–cell interaction and soluble ligands [published erratum appears in Int Immunol 1999 11:1275–6] Int Immunol. 1999;11:561–8. doi: 10.1093/intimm/11.4.561. 10.1093/intimm/11.4.561. [DOI] [PubMed] [Google Scholar]
- 46.Gunzer M, Schafer A, Borgmann S, Grabbe S, Zanker KS, Brocker EB, Kampgen E, Friedl P. Antigen presentation in extracellular matrix: interactions of T cells with dendritic cells are dynamic, short lived, and sequential. Immunity. 2000;13:323–32. doi: 10.1016/s1074-7613(00)00032-7. [DOI] [PubMed] [Google Scholar]
- 47.Iezzi G, Karjalainen K, Lanzavecchia A. The duration of antigenic stimulation determines the fate of naive and effector T cells. Immunity. 1998;8:89–95. doi: 10.1016/s1074-7613(00)80461-6. [DOI] [PubMed] [Google Scholar]
- 48.Campanelli JT, Roberds SL, Campbell KP, Scheller RH. A role for dystrophin-associated glycoproteins and utrophin in agrin-induced AChR clustering. Cell. 1994;77:663–74. doi: 10.1016/0092-8674(94)90051-5. [DOI] [PubMed] [Google Scholar]
- 49.Gee SH, Montanaro F, Lindenbaum MH, Carbonetto S. Dystroglycan-alpha, a dystrophin-associated glycoprotein, is a functional agrin receptor. Cell. 1994;77:675–86. doi: 10.1016/0092-8674(94)90052-3. [DOI] [PubMed] [Google Scholar]
- 50.Glass DJ, Bowen DC, Stitt TN, Radziejewski C, Bruno J, Ryan TE, Gies DR, Shah S, Mattsson K, Burden SJ, DiStefano PS, Valenzuela DM, DeChiara TM, Yancopoulos GD. Agrin acts via a MuSK receptor complex. Cell. 1996;85:513–23. doi: 10.1016/s0092-8674(00)81252-0. [DOI] [PubMed] [Google Scholar]
- 51.Borges LS, Ferns M. Agrin-induced phosphorylation of the acetylcholine receptor regulates cytoskeletal anchoring and clustering. J Cell Biol. 2001;153:1–12. doi: 10.1083/jcb.153.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Peters A, Palay SL, Webster H. Neurons and Their Supporting Cells. New York: Oxford University Press; 1991. The Fine Structure of the Nervous System. [Google Scholar]
- 53.Suzuki SC, Inoue T, Kimura Y, Tanaka T, Takeichi M. Neuronal circuits are subdivided by differential expression of type-II classic cadherins in postnatal mouse brains. Mol Cell Neurosci. 1997;9:433–47. doi: 10.1006/mcne.1997.0626. 10.1006/mcne.1997.0626. [DOI] [PubMed] [Google Scholar]
- 54.Tanaka H, Shan W, Phillips GR, Arndt K, Bozdagi O, Shapiro L, Huntley GW, Benson DL, Colman DR. Molecular modification of N-cadherin in response to synaptic activity. Neuron. 2000;25:93–107. doi: 10.1016/s0896-6273(00)80874-0. [DOI] [PubMed] [Google Scholar]
- 55.Missler M, Sudhof TC. Neurexins: three genes and 1001 products. Trends Genet. 1998;14:20–6. doi: 10.1016/S0168-9525(97)01324-3. 10.1016/s0168-9525(97)01324-3. [DOI] [PubMed] [Google Scholar]
- 56.Dalva MB, Takasu MA, Lin MZ, Shamah SM, Hu L, Gale NW, Greenberg ME. EphB receptors interact with NMDA receptors and regulate excitatory synapse formation. Cell. 2000;103:945–56. doi: 10.1016/s0092-8674(00)00197-5. [DOI] [PubMed] [Google Scholar]
- 57.Huh GS, Boulanger LM, Du H, Riquelme PA, Brotz TM, Shatz CJ. Functional requirement for class I MHC in CNS development and plasticity. Science. 2000;290:2155–9. doi: 10.1126/science.290.5499.2155. 10.1126/science.290.5499.2155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hall AC, Lucas FR, Salinas PC. Axonal remodeling and synaptic differentiation in the cerebellum is regulated by WNT-7a signaling. Cell. 2000;100:525–35. doi: 10.1016/s0092-8674(00)80689-3. [DOI] [PubMed] [Google Scholar]
- 59.Hata Y, Butz S, Sudhof TC. CASK: a novel dlg/PSD95 homolog with an N-terminal calmodulin-dependent protein kinase domain identified by interaction with neurexins. J Neurosci. 1996;16:2488–94. doi: 10.1523/JNEUROSCI.16-08-02488.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Craven SE, Bredt DS. PDZ proteins organize synaptic signaling pathways. Cell. 1998;93:495–8. doi: 10.1016/s0092-8674(00)81179-4. [DOI] [PubMed] [Google Scholar]
- 61.Kornau HC, Schenker LT, Kennedy MB, Seeburg PH. Domain interaction between NMDA receptor subunits and the postsynaptic density protein PSD-95. Science. 1995;269:1737–40. doi: 10.1126/science.7569905. [DOI] [PubMed] [Google Scholar]
- 62.Migaud M, Charlesworth P, Dempster M, Webster LC, Watabe AM, Makhinson M, He Y, Ramsay MF, Morris RG, Morrison JH, O'Dell TJ, Grant SG. Enhanced long-term potentiation and impaired learning in mice with mutant postsynaptic density-95 protein. Nature. 1998;396:433–9. doi: 10.1038/24790. 10.1038/24790. [DOI] [PubMed] [Google Scholar]
- 63.Feng G, Tintrup H, Kirsch J, Nichol MC, Kuhse J, Betz H, Sanes JR. Dual requirement for gephyrin in glycine receptor clustering and molybdoenzyme activity. Science. 1998;282:1321–4. doi: 10.1126/science.282.5392.1321. [DOI] [PubMed] [Google Scholar]
- 64.Meier J, Meunier-Durmort C, Forest C, Triller A, Vannier C. Formation of glycine receptor clusters and their accumulation at synapses. J Cell Sci. 2000;113:2783–95. doi: 10.1242/jcs.113.15.2783. [DOI] [PubMed] [Google Scholar]
- 65.Apel ED, Merlie JP. Assembly of the postsynaptic apparatus. Curr Opin Neurobiol. 1995;5:62–7. doi: 10.1016/0959-4388(95)80088-3. [DOI] [PubMed] [Google Scholar]
- 66.Apel ED, Roberds SL, Campbell KP, Merlie JP. Rapsyn may function as a link between the acetylcholine receptor and the agrin-binding dystrophin-associated glycoprotein complex. Neuron. 1995;15:115–26. doi: 10.1016/0896-6273(95)90069-1. [DOI] [PubMed] [Google Scholar]
- 67.Cartaud A, Coutant S, Petrucci TC, Cartaud J. Evidence for in situ and in vitro association between beta-dystroglycan and the subsynaptic 43K rapsyn protein. Consequence for acetylcholine receptor clustering at the synapse. J Biol Chem. 1998;273:11321–6. doi: 10.1074/jbc.273.18.11321. 10.1074/jbc.273.18.11321. [DOI] [PubMed] [Google Scholar]
- 68.Hall ZW, Sanes JR. Synaptic structure and development: the neuromuscular junction. Cell. 1993;72:99–121. doi: 10.1016/s0092-8674(05)80031-5. [DOI] [PubMed] [Google Scholar]
- 69.Matus A. Actin-based plasticity in dendritic spines. Science. 2000;290:754–8. doi: 10.1126/science.290.5492.754. 10.1126/science.290.5492.754. [DOI] [PubMed] [Google Scholar]
- 70.Fischer M, Kaech S, Knutti D, Matus A. Rapid actin-based plasticity in dendritic spines. Neuron. 1998;20:847–54. doi: 10.1016/s0896-6273(00)80467-5. [DOI] [PubMed] [Google Scholar]
- 71.Rao A, Craig AM. Activity regulates the synaptic localization of the NMDA receptor in hippocampal neurons. Neuron. 1997;19:801–12. doi: 10.1016/s0896-6273(00)80962-9. [DOI] [PubMed] [Google Scholar]
- 72.Engert F, Bonhoeffer T. Dendritic spine changes associated with hippocampal long-term synaptic plasticity. Nature. 1999;399:66–70. doi: 10.1038/19978. 10.1038/19978. [DOI] [PubMed] [Google Scholar]
- 73.Maletic-Savatic M, Malinow R, Svoboda K. Rapid dendritic morphogenesis in CA1 hippocampal dendrites induced by synaptic activity. Science. 1999;283:1923–7. doi: 10.1126/science.283.5409.1923. 10.1126/science.283.5409.1923. [DOI] [PubMed] [Google Scholar]
- 74.Bozdagi O, Shan W, Tanaka H, Benson DL, Huntley GW. Increasing numbers of synaptic puncta during late-phase LTP. N-cadherin is synthesized, recruited to synaptic sites, and required for potentiation. Neuron. 2000;28:245–59. doi: 10.1016/s0896-6273(00)00100-8. [DOI] [PubMed] [Google Scholar]
- 75.Koh YH, Popova E, Thomas U, Griffith LC, Budnik V. Regulation of DLG localization at synapses by CaMKII-dependent phosphorylation. Cell. 1999;98:353–63. doi: 10.1016/s0092-8674(00)81964-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Holsinger LJ, Graef IA, Swat W, Chi T, Bautista DM, Davidson L, Lewis RS, Alt FW, Crabtree GR. Defects in actin-cap formation in Vav-deficient mice implicate an actin requirement for lymphocyte signal transduction. Current Biol. 1998;8:563–72. doi: 10.1016/s0960-9822(98)70225-8. [DOI] [PubMed] [Google Scholar]
- 77.Fischer KD, Kong YY, Nishina H, Tedford K, Marengere LE, Kozieradzki I, Sasaki T, Starr M, Chan G, Gardener S, Nghiem MP, Bouchard D, Barbacid M, Bernstein A, Penninger JM. Vav is a regulator of cytoskeletal reorganization mediated by the T-cell receptor. Current Biol. 1998;8:554–62. doi: 10.1016/s0960-9822(98)70224-6. [DOI] [PubMed] [Google Scholar]
- 78.Wulfing C, Bauch A, Crabtree GR, Davis MM. The vav exchange factor is an essential regulator in actin-dependent receptor translocation to the lymphocyte-antigen–presenting cell interface. Proc Natl Acad Sci USA. 2000;97:10150–5. doi: 10.1073/pnas.97.18.10150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hanada T, Lin L, Tibaldi EV, Reinherz EL, Chishti AH. GAKIN, a novel kinesin-like protein associates with the human homologue of the Drosophila discs large tumor suppressor in T lymphocytes. J Biol Chem. 2000;275:28774–84. doi: 10.1074/jbc.M000715200. [DOI] [PubMed] [Google Scholar]
- 80.Kupfer H, Monks CR, Kupfer A. Small splenic B cells that bind to antigen-specific T helper (Th) cells and face the site of cytokine production in the Th cells selectively proliferate: immunofluorescence microscopic studies of Th-B antigen-presenting cell interactions. J Exp Med. 1994;179:1507–15. doi: 10.1084/jem.179.5.1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Campbell JJ, Butcher EC. Chemokines in tissue-specific and microenvironment-specific lymphocyte homing. Curr Opin Immunol. 2000;12:336–41. doi: 10.1016/s0952-7915(00)00096-0. 10.1016/s0952-7915(00)00096-0. [DOI] [PubMed] [Google Scholar]
- 82.Adema GJ, Hartgers F, Verstraten R, de Vries E, Marland G, Menon S, Foster J, Xu Y, Nooyen P, McClanahan T, Bacon KB, Figdor CG. A dendritic-cell-derived C-C chemokine that preferentially attracts naive T cells. Nature. 1997;387:713–7. 10.1038/42716. [Google Scholar]
- 83.Constantin G, Majeed M, Giagulli C, Piccio L, Kim JY, Butcher EC, Laudanna C. Chemokines trigger immediate beta2 integrin affinity and mobility changes. Differential regulation and roles in lymphocyte arrest under flow. Immunity. 2000;13:759–69. doi: 10.1016/s1074-7613(00)00074-1. [DOI] [PubMed] [Google Scholar]
- 84.Peacock JW, Jirik FR. TCR activation inhibits chemotaxis toward stromal cell-derived factor-1: evidence for reciprocal regulation between CXCR4 and the TCR. J Immunol. 1999;162:215–23. [PubMed] [Google Scholar]
- 85.Benson DL, Tanaka H. N-cadherin redistribution during synaptogenesis in hippocampal neurons. J Neurosci. 1998;18:6892–904. doi: 10.1523/JNEUROSCI.18-17-06892.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Al-Alwan MM, Rowden G, Lee TD, West KA. Fascin is involved in the antigen presentation activity of mature dendritic cells. J. Immunol. 2001;166:338–45. doi: 10.4049/jimmunol.166.1.338. [DOI] [PubMed] [Google Scholar]
- 87.Niedergang F, Dautry-Varsat A, Alcover A. Peptide antigen or superantigen-induced down-regulation of TCRs involves both stimulated and unstimulated receptors. J Immunol. 1997;159:1703–10. [PubMed] [Google Scholar]
- 88.Davis DM, Chiu I, Fassett M, Cohen GB, Mandelboim O, Strominger JL. The human natural killer cell immune synapse. Proc Natl Acad Sci USA. 1999;96:15062–7. doi: 10.1073/pnas.96.26.15062. 10.1073/pnas.96.26.15062. [DOI] [PMC free article] [PubMed] [Google Scholar]