Abstract
Activation-induced cell death (AICD), a Fas ligand (FasL)-dependent pathway, is important for maintaining T-cell homeostasis. Interleukin-2 (IL-2), an enhancer of AICD, can also enhance FasL expression. However, we show that the level of FasL or FLIP protein did not correlate with the susceptibility to AICD. Some T cells expressed high levels of FasL yet failed to undergo AICD, while others expressed little FasL and were sensitive. AICD susceptibility did not correlate with the kinetics of FasL up-regulation or down-regulation. The down-regulation of FasL can be mediated by a metalloprotease. However, we describe an alternative mechanism for the loss of FasL by endocytosis. Endocytosis inhibitors such as cytochalasins, sodium azide, deoxyglucose, or low temperatures prevented the loss of FasL. KB8301, a metalloprotease inhibitor had no effect on the loss of FasL or AICD in the T cells. Enhancing FasL expression was not crucial for AICD and the down-regulation of FasL proceeded via endocytosis.
Introduction
Activation-induced cell death (AICD) is an apoptotic pathway involved in the control of T-cell homeostasis.1–3 The inability of T cells to undergo AICD can lead to immunopathological diseases such as lymphadenopathy and a lupus–like syndrome. T cells can either be sensitive or resistant to AICD, and interleukin-2 (IL-2) can regulate the susceptibility of T cells to AICD.4,5 The IL-2-deficient mice and IL-2-receptor-α-chain (CD25)-deficient mice develop lymphadenopathy and an autoimmune disease because of a lack of AICD in vivo.6,7 In culture, T cells become susceptible to AICD when maintained in the presence of IL-2 and are resistant in its absence. The activation of T cells induces the surface expression of Fas ligand (FasL) and IL-2 treatment can enhance this expression in primary T cells.8 However, whether the enhancement of FasL expression directly causes an enhancement of the susceptibility to AICD is unclear because IL-2 can enhance AICD in established T-cell lines without significantly changing FasL expression.
FasL was first described as a cytotoxic protein expressed on activated T cells. The primary functions attributed to FasL-mediated cytotoxicity are the ability of T cells to kill target cells in a calcium-independent manner and the ability of T cells to kill themselves during AICD. In addition to its presence on T cells, FasL is expressed on many other cells from different organs, such as the eye, placenta, brain and testis. Expression of FasL in these organs is suggestive of a mechanism for immune privilege.9–11 FasL can induce immune cells to undergo apoptosis and, thereby, limit any immune response in these organs. Likewise, expression of FasL on some cancerous cells provides a mechanism for evasion from the immune system.12 However, other data have demonstrated that expression of FasL may not always establish an immune-privileged state or cancer evasiveness.13–16
Soluble FasL is generated by the cleavage of membrane-bound FasL by a zinc-dependent metalloprotease.17 Matrilysin, a matrix metalloproteinase, can cleave FasL.18 This process is analogous to the cleavage of tumour necrosis factor (TNF) from the cell membrane by a zinc-dependent metalloprotease, tumour necrosis factor-alpha converting enzyme (TACE).19,20 While soluble TNF is primarily an inflammatory cytokine, the role of soluble FasL is less clear. Soluble FasL is functionally lytic in some cases and antagonistic to membrane-bound FasL in other cases depending on the target cell.21 In addition, soluble FasL can act as a chemotactic factor that recruits inflammatory cells such as neutrophils and macrophages.22,23
Because AICD is a cell-autonomous process,24,25 the existence of soluble FasL may explain how FasL can engage the Fas receptor on the same cell surface. We examined the possibility that IL-2 may regulate the activity of the FasL metalloprotease and hoped to define a role for soluble FasL during AICD. We observed that IL-2 enhanced the ability of T cells to undergo AICD and enhanced the expression of FasL. However, the level of FasL expression was not a good indicator for the ability of T cells to undergo AICD. Although expression of FasL is required for AICD, its mere expression does not necessitate T cells to undergo AICD. No evidence of functional, soluble FasL was detected on our T cells. Therefore, AICD was probably mediated by membrane-bound FasL rather than soluble FasL. IL-2 also did not affect the kinetics of the FasL induction or loss. The loss of membrane-bound FasL was primarily mediated by endocytosis rather than proteolytic cleavage as previously described.
Materials and Methods
Cell lines
The 3L2.12 hybridoma was a gift from Dr Paul Allen (Washington University, St Louis, MO). DO10H hybridoma was a gift from Dr Ken Murphy (Washington University, St Louis, MO). Reagents were purchased from Sigma Chemical Co. (St Louis, MO) unless stated otherwise. The 2C2 [T helper type 1 (Th1)] T-cell clone was derived from C57BL/6 (B6) mice and is specific for pigeon cytochrome C. B6 mice were purchased from The Jackson Laboratory (Bar Harbor, ME). The culture conditions for 2C2 have been described previously.5 The 2C2 T cells were maintained by weekly stimulation with irradiated B6 splenic cells with 0·5 µm pigeon cytochrome C and 10 µg/ml recombinant human IL-2. They were harvested after 5–7 days of activation and maintained with 100 U/ml of IL-2 for 1–2 days before use in a functional assay.
Primary CD4+ T cells were obtained by harvesting B6 splenic cells.26 Purified T cells were maintained with 2 µg/ml concanavalin A (Con A) and 50 U/ml of murine IL-2 (mIL-2) for 2 days. Live cells were purified with Histopaque 1.119 and replated in medium containing 100 U/ml of mIL-2 and 50 mm α-methylmannoside for an additional 1–2 days before use.
AICD assay
The 2C2 T cells (1×105−2×105 cells/well) were added to a 96-well plate coated with 10 µg/ml of anti-CD3 antibody (2C11) for 16–24 hr. T-cell hybridomas (1×105−2×105 cells/well) were added to a 96-well plate with or without 50 nm phorbol 12-myristate 13-acetate (PMA) and 670 nm ionomycin. Cell death was measured by trypan-blue exclusion assay; each data point was performed in triplicate. The % specific death was calculated as [(% death with activation − % death without activation)/(100 − % death without activation)].
Flow cytometry
Anti-FasL antibody (MFL3-biotin) and anti-CD8-fluorescein isothiocyanate (FITC) antibody were purchased from Pharmingen (San Diego, CA). Secondary reagent, R-phycoerythrin-labelled streptavidin, was purchased from Southern Biotechnology Associates, Inc. (Birmingham, AL) and used at a 1 : 300 dilution. Cells, 1×105, were stained with 100 µl of primary antibody for 20 min at 4°, washed twice with phosphate-buffered saline (PBS), stained with 100 µl of secondary antibody for 20 min, washed twice and fixed with 0·66% paraformaldehyde in a 150-mm NaCl solution, pH 7. Analyses were performed on a fluorescence-activated cell sorter (FACScan) with Lysis software (Becton Dickinson, Mountain View, CA).
Chromium release assay
EL4 or EL4 transfected with Fas (EL4-Fas) were used as target cells to assess the FasL-dependent lytic potential of activated T cells. Target cells were labelled with 250 µCi/ml of Na251CrO4 for 1 hr at 37°, washed twice with PBS, and plated at 1×104 cells/well in a 96-well, round-bottom plate. Effector T cells (1×105 cells/well) were added in triplicate reactions to the target cells for 4–6 hr. 51Cr released in the supernatant was measured and the results were expressed as % specific bystander lysis, calculated as [100 × (% lysisexp − % lysiscontrol)/(100 − % lysiscontrol)].
TNF enzyme-linked immunosorbent assay (ELISA)
RAW264.7 were kindly provided by Dr Robert Schreiber (Washington University, St Louis, MO). RAW cells were plated at 2×106 cells/well in 24-well plates and activated with 10 µg/ml lipopolysaccharide (LPS) and 10 U/ml interferon-γ. The supernatant was harvested at the indicated time. Soluble TNF production was measured by a standard ELISA assay for TNF.27
Western blot analysis
Cytokine-treated T cells were harvested, washed and resuspended in 100 µl extraction buffer [1×PBS, 0·5 mm ethylenediaminetetraacetic acid (EDTA), 0·5 mm dithiothreitol, 50 mm NaCl, 0·5% Triton X-100, 1 mg/ml Leupeptin, 1 mg/ml Aprotinin, 0·5 mm phenylmethylsulphonyl fluoride, 1 nm Okadaic acid, 30 mm β glycerol phosphate, 0·1 mm sodium vanadate, 0·1% sodium dodecyl sulphate (SDS)]. After extraction and measuring the concentration of proteins, equal amounts (6–8 µg) of cellular protein extracts were fractionated by denaturing SDS–polyacrylamide gel electrophoresis (SDS–PAGE), then electro-transferred to polyvinyldifluoride membrane (Tropix, Bedford, MA). Actin proteins were detected by using an anti-mouse actin antibody (Calbiochem, Cambridge, MA). Flice-inhibitory protein (FLIP) protein was detected with a rabbit anti-human FLIP antibody.28
Results
Susceptibility to AICD does not correlate with the level of FasL surface expression
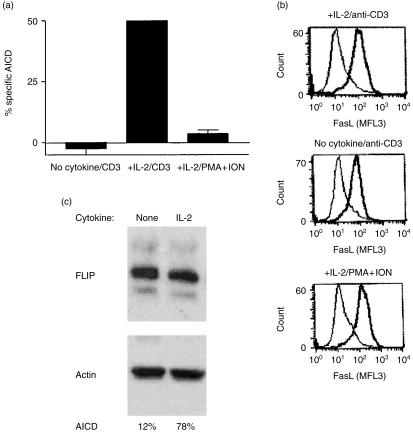
Pretreatment with IL-2 is required for T cells to become sensitive to AICD, while lack of IL-2 pretreatment causes resistance. IL-2 significantly enhances the expression of FasL in primary T cells,8 and a higher level of FasL expression correlates with the ability of Th1 T cells, but not Th2 T cells, to undergo AICD.29 Therefore, we examined the effects of IL-2 on the expression of FasL and AICD in our T cells. We found no correlation between the level of FasL expression and the degree of cell death. Figure 1 shows that T cells deprived of IL-2 are resistant to AICD even though they can be induced to express FasL. Pretreatment with IL-2 sensitized the same T cells to AICD when activated with an anti-CD3 antibody but not with PMA and ionomycin. FACS analysis of the expression of FasL after anti-CD3 stimulation showed that T cells treated with IL-2 expressed 1·5 times the level of FasL as T cells deprived of IL-2 (Fig. 1b). However, activating IL-2-treated T cells with PMA and ionomycin also induced a maximal level of FasL expression but failed to induce AICD. Thus the mere expression of FasL was not sufficient to induce T cells to undergo AICD; therefore, susceptibility to AICD must be regulated by other factors besides the level of FasL expression. Fas expression was equivalent in both IL-2-treated and untreated T cells (data not shown).
Figure 1.
Susceptibility to AICD does not correlate with the level of FasL surface expression. (a) Day-5-activated-2C2 T cells were maintained with 100 U/ml recombinant human IL-2 or no cytokine for 24 hr. Their susceptibility to AICD was tested by activation with addition of PMA and ionomycin, or by plating on anti-CD3-antibody-coated plates overnight. Spontaneous death was less than 5% with all cytokine treatments. (b) IL-2-treated 2C2 T cells were activated for 4 hr as described above, harvested, and used to measure the level of FasL induction by FACS analysis. The thin lines denote the staining of unactivated T cells with primary and secondary antibodies. Thick lines represent staining of activated T cells with the primary and secondary antibodies. (c) FLIP protein level was measured in IL-2-deprived or IL-2-treated T cells by Western blot analysis. Actin expression served as a loading control. Sensitivity to AICD was also measured and showed no correlation to the expression of FLIP.
IL-2 has been shown to induce the loss of FLIP, a cellular inhibitor of the Fas signalling pathway, in primary T cells.8 However, pretreating 2C2 T cells with IL-2 did not result in a loss of FLIP expression (Fig. 1c). Densitometric scan of the Western blots showed that the average ratios of FLIP to actin of four independent experiments were 1·57 and 1·58 for IL-2-deprived and IL-2-treated T cells, respectively. Activation of the T cells also did not affect the expression of FLIP as suggested by others (data not shown).30
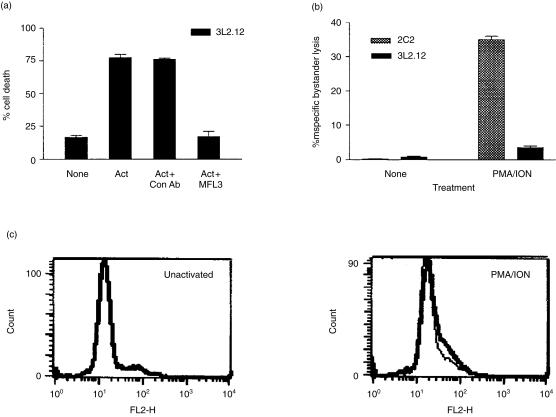
The hybridoma, 3L2.12, underwent FasL-mediated AICD while expressing a low level of FasL. 3L2.12 expressed enough FasL to undergo AICD but did not express enough FasL to be measured by FACS analysis (Fig. 2c). Death of 3L2.12 was dependent on the expression of FasL because an anti-mouse FasL antibody (MFL3), but not a control antibody, inhibited AICD (Fig. 2a). Using a functional assay, we observed a small amount of FasL expression on activated 3L2.12 cells (Fig. 2b). This observation was also made in other non-transformed T cell lines. Our Th2 T-cell clones also underwent AICD without detectable FasL (data not shown). Therefore, very little expression of FasL is required to induce Fas-mediated death of some T cells.
Figure 2.
Very little FasL is needed to induce AICD. (a) 3L2.12 T-cell hybridoma underwent AICD when activated (‘Act’) for 24 hr with 50 nm PMA and 670 nm ionomycin. This death was inhibited by 10 µg/ml of anti-FasL antibody (MFL3) but not by a control antibody. (b) The expression of FasL on activated 3L2.12 hybridoma or 2C2 T cells was measured by a standard chromium-release assay as described in the Materials and methods. Less than 10% killing of EL4 was observed; only the data for FasL-mediated killing of EL4-Fas are shown for simplicity. 2C2 T cells were used as controls for the induction of FasL. (c) The surface expression of FasL on 3L2.12 was measured by FACS analysis. The thin lines represent unactivated cells and thick lines represent activated cells.
The kinetics of FasL induction
We tested the possible correlation between the effect of IL-2 on AICD and the kinetics of FasL induction. Expression of FasL may be required during a crucial period after activation in order for T cells to commit to AICD. The lack of IL-2 possibly delayed the expression of FasL so that it was not expressed during this crucial period.
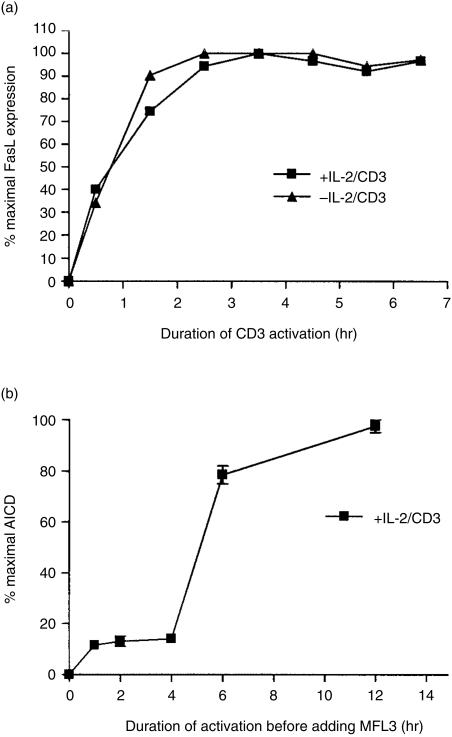
Figure 3(a) shows that T cells expressed FasL as early as 30 min after activation. Expression of FasL reached a maximal level by 1·5–2 hr after activation with an anti-CD3 antibody. Maximal expression of FasL was achieved with PMA and ionomycin within 30 min of activation (data not shown). In some human cells, the induction of FasL on the cell surface is derived from preformed FasL contained in intracellular vesicles.31,32 Induction of FasL in our cells was not from preformed FasL because induction was inhibited by emetine, a protein synthesis inhibitor (data not shown). No major difference occurred in the kinetics of FasL induction between IL-2-treated T cells or untreated T cells. Therefore, the increase in susceptibility to AICD cannot be correlated with the kinetics of FasL induction.
Figure 3.
Kinetics of FasL induction. (a). Day-5-activated-2C2 T cells were harvested and cultured with or without 100 U/ml human IL-2 for 24 hr in the presence of 10% S4B6-culture supernatant. The T cells were activated on an anti-CD3-antibody-coated plate for the indicated time before being harvested and used to measure the expression of FasL by FACS analysis. The mean fluorescence channel was used to calculate the percentage of maximal expression. (b) IL-2-treated 2C2 T cells were activated on an anti-CD3-coated plate for the indicated time before being harvested, washed, and replated in medium with 10 µg/ml anti-FasL-blocking antibody (MFL3). AICD was assessed 24 hr later. Spontaneous death was 15% and maximal death was 64%.
Even after 24 hr (data not shown), expression of FasL continued as long as the T cells were activated by an anti-CD3 antibody. Because expression of FasL was maintained by the activated T cells, we wanted to determine the period during which FasL expression was crucial. To determine the minimum amount of time required before T cells irreversibly committed to AICD, T cells were activated on an anti-CD3-antibody-coated plate for a given time, harvested, washed and replated in medium containing an anti-FasL antibody (MFL3) to block subsequent Fas signalling. The percentage of cell death after 24 hr of total incubation was measured by trypan-blue exclusion. The data show that AICD induction was inhibited with an anti-FasL antibody even after 4 hr of activation although maximal FasL expression occurred by 2 hr of activation (Fig. 3b). T cells did not become irreversibly committed to AICD until FasL was expressed on the cell surface for several hours.
The kinetics of FasL loss is unaffected by IL-2
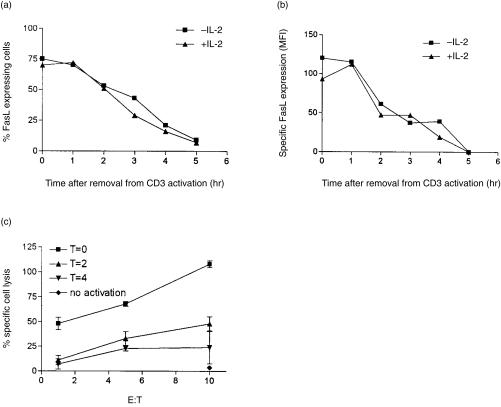
After we established the crucial time for FasL expression to be 4–6 hr after activation, we asked whether IL-2 could affect the down-regulation of FasL during this period. T cells were activated for 4 hr on anti-CD3-antibody-coated plates, harvested, washed twice with medium, and replated in medium without any activation stimulus. The expression of FasL was measured at various times after replating the T cells. Upon removal of the T cells from activation, the percentage of FasL-expressing cells (Fig. 4a), as well as the absolute level of FasL (Fig. 4b), decreased with time.
Figure 4.
IL-2 does not affect the kinetics of FasL down-regulation. 2C2 T cells that were treated or untreated with IL-2 were activated for 4 hr on plate-bound anti-CD3 antibody. The cells were harvested, washed, and replated in medium alone. At the indicated times, the cells were harvested and FasL expression was measured by FACS analysis. (a) The percentage of T cells expressing FasL decreased with time after removal from the activation stimulus. (b) The level of FasL expression also decreased with time after removal from the activation stimulus. Specific mean-fluorescence intensity (MFI) designates the difference between MFI from activated versus unactivated T cells. (c) The loss of FasL correlates with the loss of Fas-dependent lytic potential. T cells were activated as above and removed from the activating agent at the indicated time. T cells were then incubated with 51Cr-labelled-target cells expressing Fas. The ability of T cells to lyse Fas-expressing target cells was measured in a 4-hr lytic assay. The spontaneous release for T = 0, T = 2, T = 4, and no activation was 20%, 30%, 38% and 20%, respectively.
The reduction in the expression of FasL was not observed until 1 hr after removal from activation. This is attributed to a residual activation signal and continued export of FasL. Notably, the loss of FasL expression was only observed once the activation stimulus had been removed. After 2 hr of resting, the level of FasL expression dropped by half and was undetectable by 5 hr (Fig. 4b). Treatment of T cells with IL-2 did not affect the kinetics of FasL down-regulation. The decrease in the expression of FasL correlated with the decreased lytic potential as measured by a functional assay (Fig. 4c). The kinetics of FasL down-regulation is in agreement with a previous report in which the half-life of FasL down-regulation was observed to be 90 min.33 However, the previous report used a functional assay to measure the level of FasL on a population level, as was performed in Fig. 4(c). This method of quantification is less accurate and cannot distinguish between the percentage of cells expressing FasL and the level of FasL expression on individual cells as we have done by using FACS analysis (Fig. 4a,b).
Classical endocytosis inhibitors prevent the loss of FasL
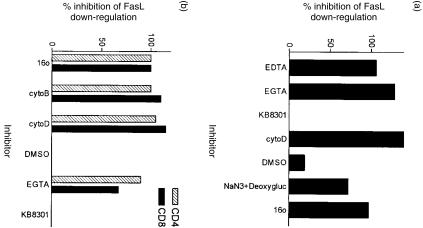
Loss of FasL from the cell surface can be mediated by a zinc-dependent metalloprotease.17 We employed an established protocol for studying the proteolysis of FasL by using EDTA to chelate zinc, thus, inhibiting FasL metalloprotease. Ethyleneglycoltetraacetic acid (EGTA) served as a negative control because it does not chelate zinc. Unexpectedly, we found that both EDTA and EGTA inhibited the loss of FasL (Fig. 5a). EGTA blocked the loss of FasL in both IL-2 treated and untreated T cells (data not shown). We also tested whether a known inhibitor of the TNF and FasL metalloproteases, KB8301, would inhibit the loss of FasL expression.34 Contrary to published reports, KB8301 did not inhibit the loss of FasL (Fig. 5), although KB8301 was effective in blocking the cleavage of TNF (Fig. 6b).
Figure 5.
Inhibitors of endocytosis block the loss of FasL. (a) 2C2 T cells were harvested and assayed for the loss of FasL as described in Fig. 4 except protease inhibitors or endocytosis inhibitors were added to the media after replating the cells. EGTA and EDTA were used at 5 µm. Cytochalasin D was used at 12·5 µg/ml, and dimethylsulphoxide (DMSO) was used as a solvent control for cytochalasin D at 0·25%. Sodium azide (0·1% NaN3) and 10 mm deoxyglucose were used to inhibit the energy requirement of endocytosis. KB8301, a metalloprotease-specific inhibitor, was used at 10 µm. A 16°-water bath was used to maintain the T cells at the lower temperature. The level of FasL expression after 4 hr of activation was used as the maximal level of FasL expression. The level of FasL expression after 5 hr of resting was used as a measure for the loss of FasL. (c) Primary T cells were obtained as described in the Materials and methods and assayed for FasL down-regulation as described above. Inhibitors were used at the same concentration as above. Cytochalasin B was used at 5 µm. CD4 T cells were distinguished from CD8 T cells in the FACS analysis by an anti-CD8-FITC antibody.
Figure 6.
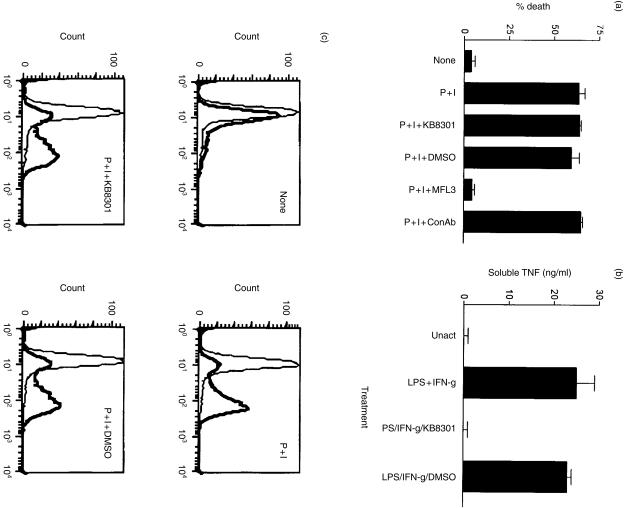
KB8301 has no effect on FasL expression or FasL-dependent AICD. (a) DO10 T-cell hybridoma (DO10H) underwent AICD when activated for 24 hr with 50 nm PMA and 670 nm ionomycin. This death was inhibited by 10 µg/ml of anti-FasL antibody (MFL3) but not by a control antibody. AICD was not inhibited by the zinc-metalloprotease inhibitor, 10 µm KB8301, or by 0·1% dimethylsulphoxide (DMSO) solvent control. (b) KB8301 was functional and inhibited the TNF metalloprotease. KB8301 (10 µm) or DMSO (0·1%) was used to inhibit the cleavage of TNF from the cell surface as described in the Materials and methods. (c) The expression of FasL on DO10H was measured by FACS analysis. PMA/ionomycin induction of FasL was not affected by KB8301.
Although the effect of EGTA on the loss of FasL was unexpected, chelation of calcium can inhibit endocytosis.35 We tested whether endocytosis rather than proteolytic cleavage might account for the loss of FasL from the cell surface. Further examination showed that classic inhibitors of endocytosis blocked the loss of FasL (Fig. 5). Cytochalasin D and cytochalasin B, which inhibit actin depolymerization and vesicle redistribution, completely inhibited the loss of FasL. The loss of FasL was also energy dependent because low temperatures, sodium azide, or deoxyglucose inhibited the loss of FasL. Down-regulation of FasL by endocytosis was not a unique feature of our T-cell clone. The endocytosis of FasL was also observed in primary T cells (Fig. 5b). The inhibitor of endocytosis sometimes enhanced the expression of FasL, possibly because of the continued export of FasL to the cell surface after cessation of the activation signal. Our data demonstrate that the loss of FasL was primarily dependent on an endocytic pathway rather than a proteolytic pathway.
FasL metalloprotease inhibitor, KB8301, does not inhibit AICD
The data in Fig. 2 support prior observations that AICD is mediated via a cell-autonomous pathway, whereby one T cell kills itself by using the Fas and FasL proteins expressed on its cell surface.24,25 These T cells do not express enough FasL to kill each other in trans; therefore, AICD must occur in cis. How Fas and FasL engage each other on the same cell membrane is not clear. One can imagine a form of membrane invagination that allows Fas and FasL to engage each other in the proper orientation. Conversely, membrane-bound FasL may be cleaved to generate a soluble and labile FasL that can mediate AICD in a cell-autonomous manner. Figure 2 shows that we were not always able to quantify the level of FasL that was capable of inducing AICD. Given that very little FasL is needed to induce AICD, a small amount of soluble FasL might have induced AICD but was undetectable by FACS analysis. Therefore, we used a potent metalloprotease inhibitor, KB8301, to attempt to inhibit AICD to test this theory.
KB8301 inhibits both FasL and TNF cleavage.34 KB8301 effectively inhibited TNF cleavage but had no effect on the expression of FasL (Fig. 6b,c). KB8301 also had no effect on the ability of T cells to undergo AICD (Fig. 6a). We also found no evidence that our T cells produced soluble FasL in a functional assay.36 Other T cells, such as 3L2.12 hybridoma, primary T cells, and the established T cell line 2C2, show similar results (data not shown). Therefore, membrane-bound FasL was probably the mediator of AICD rather than soluble FasL.
Discussion
We have studied the regulation of FasL expression during activation-induced cell death to determine whether changes in the expression of FasL might account for the differences in the sensitivity to AICD. Our data in Figs 1 and 2 show that the level of FasL expression cannot be used to predict the ability of T cells to undergo AICD. T cells expressed a high level of FasL yet failed to undergo Fas-mediated death. Other T cells expressed little FasL and readily underwent AICD.
Not all T cells that express the Fas receptor were sensitive to Fas-mediated death; the conversion of a T cell from a resistant phenotype towards a sensitive phenotype required at least two factors: IL-2 pretreatment and a proper activation stimulus. For 2C2 T cells, the proper activation signal required to induce AICD was observed only with anti-CD3-antibody stimulation and not with PMA/ionomycin stimulation (Fig. 1). Although T-cell receptor and co-stimulatory signals enhance the sensitivity of T cells to Fas-mediated killing, what is engendered by the proper activation stimulus is unclear.37–39 Two separate CD3 activation factors are important for T cells to undergo AICD. One factor is the up-regulation of FasL. The other factor remains undefined at this point.
The expression of FLIP, an inhibitor of Fas signalling, is down-regulated by IL-2 in primary T cells.8 The ability of IL-2 to down-regulate FLIP is a plausible mechanism for enhancing the susceptibility to AICD. However, our data suggest that the mechanism of IL-2 was not solely the result of down-regulating FLIP expression. First, Fig. 1 shows that IL-2-treated T cells, which express Fas and FasL, were not sensitive to AICD when activated with PMA and ionomycin. These same T cells, expressing the same level of FLIP, were sensitive to AICD when activated with an anti-CD3 antibody (Fig. 1a). Second, Western-blot analysis of FLIP expression did not show any preferential loss caused by IL-2 treatment in 2C2 T cells (Fig. 1c). FLIP expression also was unchanged after anti-CD3 antibody activation (data not shown). Our data are consistent with studies of human T cells in which FLIP expression is similar in AICD-resistant and AICD-sensitive T cells.40
The observation that FasL expression did not necessitate the death of T cells may explain why the expression of FasL on non-lymphoid cells does not necessarily establish an immunologically privileged state.13 Likewise, cancerous cells may not be able to escape the immune system by merely expressing FasL.15,16 Ironically, expression of FasL on tumour cells may lead to their demise if the tumour cells produce soluble FasL because soluble FasL can act as a chemotactic agent.14 The inflammatory cells that are recruited by soluble FasL can lead to the destruction of the tumour.
Our data (Fig. 4) are in agreement with the reported kinetics of FasL down-regulation.33 However, the earlier report does not address the mechanism of FasL down-regulation. In certain cell types, FasL can be down-regulated by a zinc-dependent metalloprotease, but we were unable to reproduce this effect in our murine T cells. Prior characterization of the FasL metalloprotease includes human T-cell lines, FasL-transfected cells, and transformed cell lines.17,34,41 The loss of FasL in our T cells was mediated by an endocytic pathway since classic inhibitors of endocytosis blocked the loss of FasL. The endocytosis of FasL was observed in both a T-cell clone and primary T cells. Of note, the net loss of FasL expression was only observed once T cells were no longer activated. In our T cells, the major pathway for reducing the expression of FasL proceeded by endocytosis rather than proteolysis.
Loss of FasL by endocytosis may be preferable over that of proteolytic cleavage because soluble FasL has multiple functions that can be potentially harmful. Soluble FasL may act as a lytic agent and kill Fas-expressing cells non-specifically. Conversely, soluble FasL may compete with membrane-bound FasL and inhibit Fas-mediated killing under a different condition.42 Whether soluble FasL acts as an agonist or an antagonist may depend on the concentration of soluble FasL and the target cell. Soluble FasL acts as a chemotactic molecule that recruits neutrophils and macrophages. The elicitation of an inflammatory response may not always be desirable; thus, loss of FasL by endocytosis could avoid this dilemma.
Acknowledgments
We thank Dr Paul Allen and Dr Ken Murphy for the 3L2.12 and DO11.10 hybridomas, respectively. We also thank Dr Robert Schreiber for help with the TNF assay.
References
- 1.Van Parijs L, Abbas AK. Role of Fas-mediated cell death in the regulation of immune responses. Curr Opin Immunol. 1996;8:355–61. doi: 10.1016/s0952-7915(96)80125-7. [DOI] [PubMed] [Google Scholar]
- 2.Osborne BA. Apoptosis and the Maintenance of Homeostasis in the Immune System. Curr Opin Immunol. 1996;8:245–54. doi: 10.1016/s0952-7915(96)80063-x. [DOI] [PubMed] [Google Scholar]
- 3.Lenardo MJ. The molecular regulation of lymphocyte apoptosis. Seminars Immunol. 1997;9:1–5. doi: 10.1006/smim.1996.0050. [DOI] [PubMed] [Google Scholar]
- 4.Boehme SA, Lenardo MJ. Propriocidal apoptosis of mature T lymphocytes occurs at S phase of the cell cycle. Eur J Immunol. 1993;23:1552–60. doi: 10.1002/eji.1830230724. [DOI] [PubMed] [Google Scholar]
- 5.Wang R, Ciardelli TL, Russell JH. Partial signaling by cytokines: cytokine regulation of cell cycle and Fas-dependent, activation-induced death in CD4+ subsets. Cell Immunol. 1997;182:152–60. doi: 10.1006/cimm.1997.1220. 10.1006/cimm.1997.1220. [DOI] [PubMed] [Google Scholar]
- 6.Sadlack B, Lohler J, Schorle H, et al. Generalized autoimmune disease in interleukin-2-deficient mice is triggered by an uncontrolled activation and proliferation of CD4+ T cells. Eur J Immunol. 1995;25:3053–9. doi: 10.1002/eji.1830251111. [DOI] [PubMed] [Google Scholar]
- 7.Willerford DM, Chen J, Ferry JA, Davidson L, Ma A, Alt FW. Interleukin-2 receptor alpha chain regulates the size and content of the peripheral lymphoid compartment. Immunity. 1995;3:521–30. doi: 10.1016/1074-7613(95)90180-9. [DOI] [PubMed] [Google Scholar]
- 8.Rafaeli Y, Parijis L, London C, Tschopp J, Abbas A. Biochemical mechanisms of IL-2 regulated Fas mediated T cell apoptosis. Immunity. 1998;8:615–23. doi: 10.1016/s1074-7613(00)80566-x. [DOI] [PubMed] [Google Scholar]
- 9.Griffith TS, Brunner T, Fletcher SM, Green DR, Ferguson TA. Fas ligand-induced apoptosis as a mechanism of immune privilege [see comments] Science. 1995;270:1189–92. doi: 10.1126/science.270.5239.1189. [DOI] [PubMed] [Google Scholar]
- 10.Bellgrau D, Duke RC. Apoptosis and CD95 ligand in immune privileged sites. Int Rev Immunol. 1999;18:547–62. doi: 10.3109/08830189909088498. [DOI] [PubMed] [Google Scholar]
- 11.Guller S, Lachapelle L. The role of placental Fas ligand in maintaining immune privilege at maternal–fetal interfaces. Semin Reprod Endocrinol. 1999;17:39–44. doi: 10.1055/s-2007-1016210. [DOI] [PubMed] [Google Scholar]
- 12.O'connell J, Bennett MW, O'Sullivan GC, Collins JK, Shanahan F. The Fas counterattack: cancer as a site of immune privilege. Immunol Today. 1999;20:46–52. doi: 10.1016/s0167-5699(98)01382-6. 10.1016/s0167-5699(98)01382-6. [DOI] [PubMed] [Google Scholar]
- 13.Okamoto S, Takamizawa S, Bishop W, Wen J, Kimura K, Sandler A. Overexpression of Fas ligand does not confer immune privilege to a pancreatic beta tumor cell line (betaTC-3) J Surg Res. 1999;84:77–81. doi: 10.1006/jsre.1999.5613. 10.1006/jsre.1999.5613. [DOI] [PubMed] [Google Scholar]
- 14.O'connell J. Immune privilege or inflammation? The paradoxical effects of Fas ligand. Arch Immunol Ther Exp (Warsz) 2000;48:73–9. [PubMed] [Google Scholar]
- 15.Restifo NP. Not so Fas: Re-evaluating the mechanisms of immune privilege and tumor escape. Nat Med. 2000;6:493–5. doi: 10.1038/74955. 10.1038/74955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Favre-Felix N, Fromentin A, Hammann A, Solary E, Martin F, Bonnotte B. Cutting edge: the tumor counterattack hypothesis revisited: colon cancer cells do not induce T cell apoptosis via the Fas (CD95, APO-1) pathway. J Immunol. 2000;15:5023–7. doi: 10.4049/jimmunol.164.10.5023. [DOI] [PubMed] [Google Scholar]
- 17.Mariani SM, Matiba B, Baumler C, Krammer PH. Regulation of cell surface APO-1/Fas (CD95) ligand expression by metalloproteases. Eur J Immunol. 1995;25:2303–7. doi: 10.1002/eji.1830250828. [DOI] [PubMed] [Google Scholar]
- 18.Powell WC, Fingleton B, Wilson CL, Boothby M, Matrisian LM. The metalloproteinase matrilysin proteolytically generates active soluble Fas ligand and potentiates epithelial cell apoptosis. Curr Biol. 1999;9:1441–7. doi: 10.1016/s0960-9822(00)80113-x. [DOI] [PubMed] [Google Scholar]
- 19.Black RA, Rauch CT, Kozlosky CJ, et al. A metalloproteinase disintegrin that releases tumour-necrosis factor-alpha from cells. Nature. 1997;385:729–33. doi: 10.1038/385729a0. [DOI] [PubMed] [Google Scholar]
- 20.Moss ML, Jin SL, Milla ME, et al. Cloning of a disintegrin metalloproteinase that processes precursor tumour-necrosis factor-alpha [published erratum appears in Nature. April 17; 386 (6626): 738] Nature. 1997;385:733–6. doi: 10.1038/385733a0. [DOI] [PubMed] [Google Scholar]
- 21.Suda T, Hashimoto H, Tanaka M, Ochi T, Nagata S. Membrane Fas ligand kills human peripheral blood T lymphocytes, and soluble Fas ligand blocks the killing. J Exp Med. 1997;186:2045–50. doi: 10.1084/jem.186.12.2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ottonello L, Tortolina G, Amelotti M, Dallegri F. Soluble Fas ligand is chemotactic for human neutrophilic polymorphonuclear leukocytes. J Immunol. 1999;162:3601–6. [PubMed] [Google Scholar]
- 23.Seino K, Iwabuchi K, Kayagaki N, et al. Chemotactic activity of soluble Fas ligand against phagocytes. J Immunol. 1998;161:4484–8. [PubMed] [Google Scholar]
- 24.Brunner T, Mogil R, Laface D, et al. Cell-autonomous Fas (CD95)/Fas–ligand interaction mediates activation-induced apoptosis in T-cell hybridomas. Nature. 1995;373:441–4. doi: 10.1038/373441a0. [DOI] [PubMed] [Google Scholar]
- 25.Dhein J, Walczak H, Baumler C, Debatin KM, Krammer PH. Autocrine T-cell suicide mediated by APO-1/ (Fas/CD95) Nature. 1995;373:438–41. doi: 10.1038/373438a0. [DOI] [PubMed] [Google Scholar]
- 26.Abrams SI, Russell JH. CD4+ T lymphocyte-induced target cell detachment. A model for T cell-mediated lytic and nonlytic inflammatory processes. J Immunol. 1991;146:405–13. [PubMed] [Google Scholar]
- 27.Sheehan KC, Pinckard JK, Arthur CD, Dehner LP, Goeddel DV, Schreiber RD. Monoclonal antibodies specific for murine p55 and p75 tumor necrosis factor receptors: identification of a novel in vivo role for p75. J Exp Med. 1995;181:607–17. doi: 10.1084/jem.181.2.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Irmler M, Thome M, Hahne M, et al. Inhibition of death receptor signals by cellular FLIP. Nature. 1997;388:190–5. doi: 10.1038/40657. 10.1038/40657. [DOI] [PubMed] [Google Scholar]
- 29.Ramsdell F, Seaman MS, Miller RE, Picha KS, Kennedy MK, Lynch DH. Differential ability of Th1 and Th2 T cells to express Fas ligand and to undergo activation-induced cell death. Int Immunol. 1994;6:1545–53. doi: 10.1093/intimm/6.10.1545. [DOI] [PubMed] [Google Scholar]
- 30.Algeciras-Schimnich A, Griffith TS, Lynch DH, Paya CV. Cell cycle-dependent regulation of FLIP levels and susceptibility to Fas-mediated apoptosis. J Immunol. 1999;162:5205–11. [PubMed] [Google Scholar]
- 31.Martinez-Lorenzo MJ, Alava MA, Anel A, Pineiro A, Naval J. Release of preformed Fas ligand in soluble form is the major factor for activation-induced death of Jurkat T cells. Immunology. 1996;89:511–17. doi: 10.1046/j.1365-2567.1996.d01-782.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kiener PA, Davis PM, Rankin BM, Klebanoff SJ, Ledbetter JA, Starling GC, Liles WC. Human Monocytic Cells Contain High Levels of Intracellular Fas Ligand – Rapid Release Following Cellular Activation. J Immunol. 1997;159:1594–8. [PubMed] [Google Scholar]
- 33.Glass A, Walsh CM, Lynch DH, Clark WR. Regulation of the Fas lytic pathway in cloned CTL. J Immunol. 1996;156:3638–44. [PubMed] [Google Scholar]
- 34.Kayagaki N, Kawasaki A, Ebata T, et al. Metalloproteinase-Mediated Release of Human Fas Ligand. J Exp Med. 1995;182:1777–83. doi: 10.1084/jem.182.6.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ribeiro F, Coelho PM, Vieira LQ, Powell K, Kusel JR. Membrane internalization processes in different stages of Schistosoma mansoni as shown by a styryl dye (Frei Mao 1–43) Parasitology. 1998;116:51–9. doi: 10.1017/s0031182097001960. 10.1017/s0031182097001960. [DOI] [PubMed] [Google Scholar]
- 36.Wang R, Rogers AM, Ratliff TL, Russell JH. CD95-dependent bystander lysis caused by CD4+ T helper 1 effectors. J Immunol. 1996;157:2961–8. [PubMed] [Google Scholar]
- 37.Boussiotis VA, Lee BJ, Freeman GJ, Gribben JG, Nadler LM. Induction of T Cell Clonal Anergy Results in Resistance, Whereas Cd28-Mediated Costimulation Primes For Susceptibility to Fas- and Bax-Mediated Programmed Cell Death. J Immunol. 1997;159:3156–67. [PubMed] [Google Scholar]
- 38.Daniel Pt, Kroidl A, Cayeux S, Bargou R, Blankenstein T, Dorken B. Costimulatory Signals Through B7.1/Cd28 Prevent T Cell Apoptosis During Target Cell Lysis. J Immunol. 1997;159:3808–15. [PubMed] [Google Scholar]
- 39.Fisher GH, Lenardo MJ, Zuniga-Pflucker JC. Synergy between T cell Receptor and Fas (CD95/APO-1) signaling in mouse thymocyte death. Cell Immunol. 1996;169:99–106. doi: 10.1006/cimm.1996.0096. 10.1006/cimm.1996.0096. [DOI] [PubMed] [Google Scholar]
- 40.Scaffidi C, Schmitz I, Krammer PH, Peter ME. The role of c-FLIP in modulation of CD95-induced apoptosis. J Biol Chem. 1999;274:1541–8. doi: 10.1074/jbc.274.3.1541. 10.1074/jbc.274.3.1541. [DOI] [PubMed] [Google Scholar]
- 41.Tanaka M, Suda T, Takahashi T, Nagata S. Expression of the functional soluble form of human fas ligand in activated lymphocytes. EMBO J. 1995;14:1129–35. doi: 10.1002/j.1460-2075.1995.tb07096.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Suda T, Tanaka M, Miwa K, Nagata S. Apoptosis of mouse naive T cells induced by recombinant soluble Fas ligand and activation-induced resistance to Fas ligand. J Immunol. 1996;157:3918–24. [PubMed] [Google Scholar]