Abstract
The neutrophil bactericidal/permeability-increasing protein (BPI) has both bactericidal and lipopolysaccharide-neutralizing activities. The present study suggests that BPI also plays an important role in phagocytosis of Escherichia coli by neutrophils through promotion of complement activation on the bacterial surface. Flow cytometric analysis indicated that fluorescein-labelled E. coli treated with BPI were phagocytosed in the presence of serum at two- to five-fold higher levels than phagocytosis of the bacteria without the treatment. In contrast, phagocytosis of the fluoresceined bacteria with or without treatment by BPI did not occur at all in the absence of serum. The phagocytosis stimulated by BPI and serum was dose-dependent. The effect of BPI on phagocytosis in the presence of serum was not observed on Gram-positive bacteria (Staphylococcus aureus). Interestingly, the complement C3b/iC3b fragments were deposited onto the bacterial surface also as a function of the BPI concentration under conditions similar to those for phagocytosis. Furthermore, the BPI-promoted phagocytosis was blocked completely by anti-C3 F(ab′)2 and partially by anti-complement receptor (CR) type 1 and/or anti-CR type 3. These findings suggest that BPI accelerates complement activation to opsonize bacteria with complement-derived fragments, leading to stimulation of phagocytosis by neutrophils via CR(s).
Introduction
Lipopolysaccharide (LPS), a major constituent of the Gram-negative bacterial cell wall,1 is introduced into the host bloodstream upon infection by bacteria. A variety of host cell types, including macrophages, neutrophils and endothelial cells, respond to LPS and the stimulated cells produce inflammatory mediators such as cytokines and oxidative radicals.2 Excess production of these molecules causes fever, hypotension, metabolic derangements and disseminated intravascular coagulation, known as symptoms of septic shock.2
There are various host defence systems against the bacterial infection. For example, phagocytic cells such as macrophages and neutrophils include a set of bactericidal peptides/proteins in their granules.3–6 Generally these molecules are released by degranulation when the cells are stimulated upon infection. Human bactericidal/permeability-increasing protein (BPI), a single-chain glycoprotein of 55 000 MW, is stored in the azurophilic granules of neutrophils.7,8 This molecule has specific bactericidal activity for Gram-negative bacteria9 and LPS-neutralizing activity.10–12 A cationic stretch of amino acids is located in the N-terminal half of BPI, which binds to the anionic lipid A moiety of LPS.13 Several lines of evidence indicate that the N-terminal fragment is sufficient to exhibit both the bactericidal and LPS-neutralizing activities.13,14
The complement system, consisting of approximately 20 serum proteins, is also involved in the host defence against Gram-negative bacteria.15 There are two major roles of complement in the defence. One is to form a membrane attack complex (MAC) to make a hole in the bacterial envelope, leading to killing of the bacteria. Another is to coat the bacteria with the complement component-derived fragments, so-called opsonization, to stimulate phagocytosis by macrophages and neutrophils. The complement component C3 has a major role in the opsonization. This molecule is a two-chain form consisting of α (115 000 MW) and β (75 000 MW) chains linked by a disulphide bond. During complement activation, C3 is converted to a C3a (10 000 MW, also called anaphylatoxin) and a C3b, composed of α′ (105 000 MW) and β chains. The resulting C3b is further converted to an iC3b, consisting of α′N (68 000 MW), α′C (40 000 MW) and β chains. The C3b/iC3b fragments are covalently attached to the bacterial surface via an ester and/or amide bond(s). The opsonized bacteria with the C3-derived fragments are ingested by phagocytic cells via a variety of complement receptors (CRs).
Previously, Iovine et al. reported that Gram-negative bacteria are ingested by neutrophils/monocytes in a process requiring only preincubation with BPI.16 It was also suggested in their study that BPI binds to the bacteria through its N-terminal domain, while the C-terminal domain of BPI acts for attachment of bacteria to neutrophils. However, certain issues still remain to be clarified such as what kind of molecule(s) on neutrophils/monocytes is involved in the phagocytosis.
In this study, we found another effect of BPI on phagocytosis of Gram-negative bacteria by neutrophils. The findings include that BPI augmented phagocytosis in the presence of low concentrations of serum, the stimulation being parallel to the BPI-promoted deposition of C3b/iC3b fragments on the bacterial surface. Thus BPI could accelerate complement activation to opsonize Gram-negative bacteria, followed by promotion of phagocytosis by neutrophils via CR(s).
Materials and Methods
Materials
The sources of materials used for this study were as follows: fluorescein isothiocyanate (FITC) and bovine serum albumin (BSA) from Sigma-Aldrich (St Louis, MO); Ham's F-12, Opti-MEM I and neomycin (G418) from Gibco BRL (Grand Island, NY); fetal bovine serum (FBS) from Irvine Scientific (Santa Ana, CA); Ficoll-Paque and Mono S HR5/5 from Amersham-Pharmacia (Uppsala, Sweden); Bio-Rex 70 resin from Bio-Rad (Hercules, CA); Chinese hamster ovarian tumor (CHO)-K1 cells and Staphylococcus aureus ATCC 25923 from the American Type Culture Collection (Rockville, MD); and Escherichia coli DH5α from Toyobo (Tokyo, Japan). Escherichia coli ATCC 25922 and a pCXN2 expression vector17 were kind gifts from Drs K. Takeshi (Hokkaido Institute of Public Health) and J. Miyazaki (Osaka University), respectively.
Antibodies
Goat anti-human C3 antiserum and F(ab′)2 fragment of goat immunoglobulin (IgG) to human C3 were purchased from ICN Pharmaceuticals (Irvine, CA). Anti-CD11b (CR type 3, CR3) monoclonal antibody (IgG1, clone 44) was obtained from Leinco Technologies (St. Louis, MO). Mouse anti-guinea-pig Fc receptor monoclonal antibody (IgG1, clone 6A2)18 was a kind gift from Dr T. Yamashita (Hokkaido University). Rabbit anti-human CR type 1 (CR1) polyclonal antibody and control IgG were provided by Dr T. Seya (Osaka Medical Centre for Cancer and Cardiovascular Disease).
Establishment of CHO-K1 cell line producing human BPI
Human BPI cDNA19 was amplified from poly(A)+ RNA of HL-60 cells by reverse transcription-polymerase chain reaction (RT-PCR), using an RNA LA PCR Kit (AMV) Ver. 1·1 (Takara Biomedicals, Kyoto, Japan). All procedures were carried out according to the manufacturer's protocol. The PCR primer set used was 5′-AGTCTAGAATGAGAGAGAACATG-3′ (forward) and 5′-AGTCTAGATCATTTATAGACAACGTC-3′ (reverse). After the first-strand cDNA was synthesized, PCR (30 cycles) was carried out with denaturation at 94° for 1 min, annealing at 65° for 2 min and extension at 72° for 3 min. The PCR product obtained was subcloned into a pCR II vector (Invitrogen-Novex, Carlsbad, CA). BPI cDNA was excised by XbaI digestion followed by incubation with Klenow fragment to create blunt ends. After a pCXN2 expression vector was digested with XhoI followed by Klenow fragment-treatment, the cDNA was subcloned into the vector.
CHO-K1 cells were transfected with 20 µg of the constructed vector by a calcium phosphate precipitation method and the transfected cells were cultured in Ham's F-12/10% heat-inactivated FBS in the presence of 500 µg/ml G418 at 37° in 5% CO2/95% air. Sixteen neomycin-resistant clones obtained were cultured in Opti-MEM I, used as serum-free medium, for 3 days at 37° in 5% CO2/95% air. To screen positive clones, the culture medium was tested for LPS-binding activity by addition of the medium to a reaction mixture for the LPS-binding protein (LBP) assay.20,21 There was no significant LPS-binding activity in the culture medium of untransfected CHO-K1 cells on the LBP assay (data not shown). This assay enabled us to screen positive clones more rapidly and easily than conventional bactericidal assay. One of the positive clones (named CHO-BPI) was used for further experiments.
Purification of recombinant human BPI
CHO-BPI were cultured (70–80% confluency in a 10-cm dish) in 7 ml of Opti-MEM I at 37° for 3 days in 5% CO2/95% air. After dialysis against 20 mm Tris–HCl (pH 7·4)/40 mm NaCl/2 mm ethylenediaminetetraacetic acid (EDTA), the culture medium (total 200 ml) was applied to a Bio-Rex 70 column (1 ml), equilibrated with the same buffer. Unbound materials were removed by extensive washing and BPI was eluted with 2 m NaCl in the buffer. The elution profile was monitored at 280 nm and BPI (LPS-binding) activity in each fraction was tested as described above.
BPI-positive fractions were pooled, then diluted 10-fold with 20 mm Tris–HCl (pH 7·4)/10 mm EDTA. The sample was subjected to Mono S column chromatography using a fast protein liguid chromatography (FPLC) system (Amersham-Pharmacia) as described previously.20 The elution profile was monitored at 280 nm and the BPI-positive fraction was detected as described above. The purified protein was dialysed against 20 mm HEPES-NaOH (pH 7·4), and molar concentration and purity of the samples were verified by amino acid composition analysis and 12·5% sodium dodecyl sulphate–polyacrylamide gel electrophoresis (SDS–PAGE) under reducing conditions followed by silver staining (data not shown).
Isolation of neutrophils
Neutrophils were isolated from human peripheral blood by means of dextran and Ficoll-Paque sedimentations followed by hypotonic lysis of contaminating erythrocytes.22 The cells were suspended in phosphate-buffered saline (PBS) and kept on ice until use.
Labelling of E. coli with FITC
Escherichia coli ATCC 25922 were used on all the experiments in this study except for Table 1. Bacteria tested were cultured in Trypticase Soy medium (Becton-Dickinson, San Jose, CA) at 37° for 12–16 hr with constant agitation. Cells were collected by centrifugation (2300 g for 5 min at 4°) and washed three times with 50 mm HEPES-NaOH (pH 8·5)/0·15 m NaCl. After being suspended in the same buffer, 1·0 × 109 colony forming units (CFU)/ml E. coli were incubated with 5 µg/ml FITC at 37° for 30 min. The fluoresceined cells (FITC-E. coli) were washed three times with 20 mm HEPES-NaOH (pH 7·4)/150 mm NaCl/metal ion- and NaCl-free Hanks' balanced salt solution [HBSS(–)] to remove free FITC molecules, followed by suspension in the buffer.
Table 1.
Phagocytosis of Gram-negative and -positive bacteria induced by BPI and serum
| Bacteria tested | |||
|---|---|---|---|
| Gram-negative | Gram-positive | ||
| Condition of phagocytosis | E. coli (ATCC 25922)* | E. coli (DH5α) | S. aureus (ATCC 25923) |
| 50 nm BPI | ND† | ND | ND |
| 200 nm BPI | ND | ND | ND |
| 10% serum | 22.3 ± 1.7 (1.0) | 22.8 ± 1.4 (1.0) | 8.8 ± 1.4 (1.0) |
| 10% serum+50 nm BPI | 50.8 ± 5.4 (2.3) | 40.6 ± 4.0 (1.8) | 10.2 ± 1.7 (1.2) |
| 10% serum+200 nm BPI | 106.8 ± 7.7 (4.8) | 67.5 ± 7.2 (3.0) | 12.2 ± 2.2 (1.4) |
Data are expressed as average ΔMFI ± SD (n = 3) of phagocytosis under various conditions. Each number in parentheses is a relative phagocytic level assuming that the level of phagocytosis in the presence of 10% serum alone is 1.0.
Data on E. coli ATCC 25922 were taken from Fig. 2(b).
ND, not quantitatively determined.
Phagocytosis assay
Basically, phagocytosis of FITC-E. coli by neutrophils was measured by procedures as described below.
Step 1: 2·0 × 109 CFU/ml FITC-E. coli were incubated with 75 nm BPI at 37° for 5 min in 100 µl of 20 mm HEPES-NaOH (pH 7·4)/300 mm NaCl/HBSS(–).
Step 2: 45 µl of 34% human serum/4·2 mm CaCl2/1·4 mm MgSO4/1·6 mm MgCl2 was then put into the reaction mixture followed by incubation at 37° for 5 min.
Step 3: 5 µl of 6·0 × 107/ml neutrophils were added and allowed to stand at 37° for 1 hr. The cells were washed three times with PBS/0·5 mm EDTA/0·1% BSA and suspended in 20 mm sodium acetate (pH 4·5)/150 mm NaCl/0·05% trypan blue to quench external fluorescence. Fluorescence derived from FITC-E. coli ingested by neutrophils was measured with a FACSort (Becton-Dickinson). The relative amount of ingested FITC-E. coli was calculated by subtraction of the mean fluorescence intensity (MFI) of neutrophils alone from that of each tested sample (shown as ΔMFI). When adhesion of bacteria to neutrophils was examined, neutrophils were washed and suspended with PBS/0·1% BSA at step 3. The relative amount of associated FITC-E. coli was calculated by subtraction of ΔMFI of each tested sample in trypan blue from that of the corresponding sample in PBS/0·1% BSA.
Phagocytosis was also observed under the fluorescence microscope (Olympus model BHS) with 200× magnification. Images of neutrophils suspended in the trypan blue solution at step 3 were captured by a model PM-10AD recorder (Olympus) attached to the microscope. In this case, the final concentration of serum was 2%. Phagocytosis in the presence of 10% serum alone yielded a strong signal under the microscope. Thus use of 2% serum enabled us to more easily obtain suitable contrast of signals of phagocytosis between with and without the BPI treatment than 10% serum.
Inhibition of phagocytosis by antibodies to human C3,CR1 and CR3
When the effect of anti-human C3 was examined, procedures for the phagocytosis assay were changed as follows: at step 2, the reaction mixture of step 1 was incubated with 30 µl of 50% human serum/6·3 mm CaCl2/2·1 mm MgSO4/2·5 mm MgCl2 at 37° for 5 min. Fifteen microlitres of various concentrations of F(ab′)2 fragment of anti-human C3 or of anti-mouse IgG (control) were then added and allowed to stand at 37° for 15 min. The subsequent procedures were the same as step 3.
In the case of anti-human CR1 and CR3 antibodies, procedures were changed as follow: at step 2, 30 µl of 50% human serum/6·3 mm CaCl2/2·1 mm MgSO4/2·5 mm MgCl2 was added to the reaction mixture of step 1 followed by incubation at 37° for 5 min. At step 3, 5 µl of 6·0 × 107/ml neutrophils were preincubated with 15 µl of 20 µg/ml anti-human CR1 or anti-human CR3. Neutrophils were also preincubated with 20 µg/ml rabbit IgG and mouse IgG1 (6A2), respectively, as control experiments. For co-inhibition with both the antibodies, cells were incubated with 15 µl of PBS including 20 µg/ml anti-human CR1 and 20 µg/ml anti-human CR3 or 20 µg/ml rabbit IgG and 20 µg/ml mouse IgG1. The cell suspension was then added to the mixture obtained by the modified step 2 and the subsequent procedures for flow cytometric analysis were the same as step 3.
Detection of C3 fragments deposited on bacterial surface
BPI was added to an E. coli suspension as described above, and 50 µl of 30% human serum/3·8 mm CaCl2/1·2 mm MgSO4/1·5 mm MgCl2 were added to the reaction mixture (total 150 µl) followed by incubation at 37° for 1 hr. Cells were washed three times with PBS and were then suspended in 200 µl of 0·1 m Na2CO3 (pH 10·0)/1·0 m NH2OH to release proteins covalently attached to the bacterial surface through an ester bond. After incubation at 37° for 1·5 hr, a supernatant was harvested by centrifugation at 2300 g for 5 min at 4°. Two hundred microlitres of 1·0 m Tris–HCl (pH 7·4) were added to the supernatant and proteins were extracted from the neutralized sample with methanol/chloroform.23 The samples were resolved by 10% SDS–PAGE under reducing conditions followed by transfer to Immobilon P membranes (Millipore, Bedford, MA). The blots were stained with goat anti-human C3 antiserum and signals were detected by an enhanced chemiluminescence system (Amersham-Pharmacia). Each band was identified by using a Broad Range Prestained Protein Marker (New England Biolabs, Beverly, MA) and purified human C3, C3b, and iC3b.
Results
BPI promotes phagocytosis of FITC-E. coli by neutrophils in the presence of serum
Phagocytosis of FITC-E. coli by neutrophils was examined under various conditions (Fig. 1). When FITC-E. coli were preincubated with BPI or buffer, bacteria were barely ingested by neutrophils in the absence of serum. In contrast, the bacteria preincubated with either BPI or buffer could be phagocytosed in the presence of 10% serum. The phagocytic level of the BPI-primed bacteria was two-fold higher than that of the non-primed bacteria. Moreover, preincubation of the bacteria with BPI for 5 min was sufficient to reach the maximum level of phagocytosis. As for the BPI-induced increase of phagocytosis, we found sample to sample variation (two- to five-fold) in some different E. coli and neutrophil preparations (e.g. Fig. 2 and Fig. 5), although the phagocytic level of the BPI-primed bacteria was consistently higher than that of the non-primed bacteria.
Figure 1.
Time course of phagocytosis of FITC-E. coli by neutrophils stimulated by BPI. FITC-E. coli were preincubated with buffer (○, □) or BPI (final 50 nm) (•, ▪) at 37° for various periods. The bacteria were then subjected to the phagocytosis assay in the absence (□, ▪) or presence of serum (final 10%) (○, •). Horizontal axis, incubation time of the bacteria with BPI; and vertical axis, relative amount of ingested bacteria. This result is representative of three independent experiments. For details, see the Materials and methods.
Figure 2.
Dose-dependence of phagocytosis on serum (a) and BPI (b). (a) FITC-E. coli were preincubated with buffer (○) or BPI (final 50 nm) (•) at 37° for 5 min. The bacteria were then subjected to the phagocytosis assay in the presence of various concentrations of serum. Horizontal axis, the final concentration of serum added and vertical axis, relative amount of ingested bacteria. Results are expressed as the mean of three independent experiments ±SD. (b) FITC-E. coli were incubated with various concentrations of BPI at 37° for 5 min. The bacteria were then subjected to the phagocytosis assay in the presence of serum (final 10%). Horizontal axis, the final concentration of BPI added and vertical axis, relative amount of ingested bacteria. Results are expressed as the mean of three independent experiments ±SD. For details, see the Materials and methods.
Figure 5.
Effect of anti-human C3 antibody on phagocytosis. FITC-E. coli were preincubated with buffer (□, ▪) or BPI (final 50 nm) (○, •) at 37° for 5 min. Serum and divalent cations were added to each bacterial suspension followed by incubation at 37°. After 5 min, the reaction mixtures were inseminated with various concentrations of F(ab′)2 to mouse IgG (□, ○) or to human C3 (▪, •), and allowed to stand at 37° for 15 min. Neutrophils were then added and incubated at 37° for additional 1 hr. Horizontal axis, the final concentration of antibody added; and vertical axis, relative amount of ingested bacteria. Results are expressed as the mean of three independent experiments ±SD. For details, see the Materials and methods.
Both BPI and serum promote phagocytosis in a dose-dependent manner
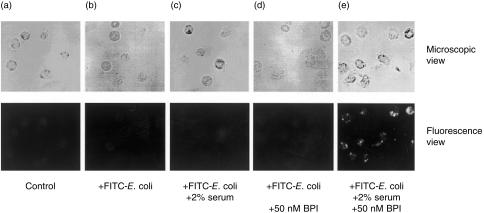
Phagocytosis of FITC-E. coli by neutrophils was also examined in the presence of various concentrations of BPI and serum. As shown in Fig. 2(a), phagocytosis of the non-primed or primed bacteria with BPI was promoted by serum in a dose-dependent manner and 10% serum was sufficient to reach the maximum level of phagocytosis. The phagocytic level for the BPI-primed bacteria was consistently higher than that for the non-primed bacteria at every serum concentration tested. Figure 2(b) shows a dose-dependence of phagocytosis on BPI in the presence of 10% serum. Ingestion of FITC-E. coli was dependent on BPI, although a clear plateau was not observed within the range of BPI concentrations tested. Another E. coli strain (DH5α) and Gram-positive bacteria (S. aureus) were also subjected to phagocytosis assay to examine the specificity of bacteria for phagocytosis in the presence of BPI and serum (Table 1). As expected, there was no significant effect of BPI on Gram-positive bacteria even in the presence of 10% serum, while BPI promoted phagocytosis of E. coli DH5α in the presence of 10% serum, as well as E. coli ATCC 25922. When adhesion of FITC-E. coli to neutrophils was also examined under the same conditions as those on Fig. 2(b), its pattern was very similar to the profile of ingestion on Fig. 2(b) (data not shown). As shown in Fig. 3, the remarkably enhanced phagocytosis of FITC-E. coli by neutrophils was also observed [Fig. 3(e)], while there was not a clear signal in the absence of BPI and/or serum [Fig. 3(b–d)].
Figure 3.
Fluorescence microscopy of neutrophils after ingestion of FITC-E. coli under various conditions. FITC-E. coli were preincubated with buffer (b, c) or BPI (final 50 nm) (d, e) at 37° for 5 min. The bacteria were then subjected to the phagocytosis assay in the absence (b, d) or presence of serum (final 2%) (c, e). (a) A view of neutrophils alone in the same buffer. Upper panel, phase contrast (200× magnification); and lower panel, fluorescence image of the same area as that of the upper image.
We postulated from these findings that BPI accelerates complement activation to opsonize bacteria at a higher level, leading to promotion of phagocytosis by neutrophils via CR(s).
BPI promotes opsonization of E. coli with the C3b/iC3b fragments
To assess this hypothesis, we tested if BPI promotes complement activation on the bacterial surface. As shown in Fig. 4, BPI definitely enhanced deposition of C3b/iC3b fragments onto the bacterial surface in a dose-dependent manner. These results suggest that BPI-mediated promotion of phagocytosis of E. coli parallels that of opsonization of the bacteria with C3b/iC3b fragments. Signals of α-related fragments (α′ and α′N) on the blot were for some reasons lower than that of β. This problem seems to be independent of the BPI-promoted opsonization of bacteria, since similar results were observed in Western blotting using purified human C3, C3b and iC3b (data not shown). The reason for the weaker signals of α-related fragments might be due to a low efficiency of transfer of the α-related fragments to the membrane and/or a low titre of antibody specific to the fragments in the polyclonal antibody used.
Figure 4.
Western blot analysis to detect deposition of C3 fragments on the bacterial surface. Escherichia coli were preincubated with various concentrations of BPI at 37° for 5 min. Serum (final 10%) and divalent cations were then added to the bacterial suspension followed by additional incubation at 37° for 1 hr. After release of proteins deposited onto the bacteria by treatment with hydroxylamine, they were resolved by 12·5% SDS–PAGE under reducing conditions. C3-derived fragments in the sample were then detected by Western blotting using goat anti-human C3 antiserum. Molecular weights indicated at the left side are based on a pattern of the prestained protein ladder. Arrows at the right side indicate migrating positions of C3b- and iC3b-derived polypeptide chains, which were judged by those of the authentic C3b and iC3b. For details, see the Materials and methods.
Antibodies to human C3, CR1 and CR3 inhibit BPI-promoted phagocytosis by neutrophils
The result in Fig. 4 led us to examine if BPI-promoted opsonization of bacteria contributes to phagocytosis as observed in Figs 1, 2 and 3. To address this issue, the effect of antibody to human C3, CR1 and CR3 on phagocytosis was investigated. When the phagocytosis assay was carried out in the presence of the F(ab′)2 fragment of anti-human C3 (Fig. 5), phagocytosis of the non-primed or primed bacteria with BPI in the presence of serum was completely blocked.
CR1 is a receptor for the C3b fragment, present on neutrophils.15 This receptor does not act as a phagocytic receptor in resting conditions, but is active in phagocytosis in the presence of tumour necrosis factor-α and C5a, an anaphylatoxin released from the complement protein C5.15 CR3 is the most abundunt molecule among the CRs present on the neutrophil surface and the opsonized bacteria with the iC3b fragment are phagocytosed via this receptor.
As shown in Table 2, inhibitory levels of phagocytosis of non-primed bacteria by anti-CR1, -CR3, and -CR1/-CR3 were 70·9%, 13·2%, and 57·8%, respectively. Phagocytosis of the primed bacteria with BPI was also inhibited by anti-CR1 (51·4%), -CR3 (31·8%), and -CR1/-CR3 (57·9%).
Table 2.
Effect of anti-CR1 and -CR3 on phagocytosis in the presence of BPI and serum
| Inhibition (%) by | |||
|---|---|---|---|
| Condition of phagocytosis | anti-CR1 | anti-CR3 | anti-CR1/-CR3 |
| 10% serum | 70.9 ± 2.8 | 13.2 ± 1.6 | 57.8 ± 1.6 |
| 10% serum+50 nm BPI | 51.4 ± 10.0 | 31.8 ± 12.3 | 57.9 ± 7.5 |
Data are expressed as mean percentage ± SD (n = 3) of inhibition of phagocytosis by anti-CR1 and/or anti-CR3. Each value was calculated by assuming that the inhibitory level of phagocytosis for control rabbit IgG and/or mouse IgG was 0%.
Discussion
BPI is a potent antibacterial protein of neutrophils with bactericidal and LPS-neutralizing activities. The findings in the present study support an additional function of BPI, which is stimulation of phagocytosis of Gram-negative bacteria by neutrophils by means of promotion of complement activation. The earlier work of Iovine et al.16 suggested another pathway for the BPI-mediated phagocytosis of Gram-negative bacteria, which needs only preincubation of the bacteria with BPI. The report might conflict with our results, since we did not observe a significant level of phagocytosis in the presence of BPI alone (Figs 1 and 2a and Table 1). Although we cannot exclude the possibility that BPI alone promotes phagocytosis if a high concentration of BPI is used, this discrepancy can be explained in two ways. First, the Gram-negative bacteria used here were E. coli ATCC 25922 and DH5α, with a smooth form of LPS, while the previous study was based on E. coli K1/r, with a rough form of LPS. Since an E. coli strain with the rough form of LPS is more sensitive to BPI,9,24 the higher sensitivity of E. coli K1/r to BPI could cause the ingestion of the bacteria in the presence of BPI alone. As a second possibility, we employed flow cytometric analysis to evaluate phagocytosis while Iovine and co-workers used techniques of electron microscopy and chemiluminescence. The discrepancy could be due to different detection limits of the individual methods.
The BPI action on Gram-negative bacteria is proposed to consist of two major steps.25 The initial (reversible) step has sublethal effects and occurs immediately after binding to the bacteria. On the other hand, the later (irreversible) step has lethal effects. The immediate action of BPI is probably to promote complement activation, since phagocytosis in the presence of serum reached a maximum level at 5 min after incubation of the bacteria with BPI (Fig. 1).
As shown in Fig. 5, the BPI-induced promotion of phagocytosis completely depended on that of opsonization of bacteria with the C3 fragments. When the effect of anti-CR1 and -CR3 was examined, 51·4% and 31·8% of phagocytosis in the presence of BPI and serum were blocked, respectively (Table 2). The inhibitory activities of anti-CR1 and -CR3 are reasonable since they were approximately parallel to each ratio of the C3b and iC3b fragments among the C3 fragments deposited onto the bacterial surface (Fig. 4). Thus these data suggest that both CR1 and CR3, at least partially, contribute to phagocytosis in the presence of BPI and serum. Co-incubation of neutrophils with anti-CR1 and -CR3 was not so effective to reduce the phagocytic level of the non-primed (57·8%) or primed (57·9%) bacteria with BPI, when compared with those in the presence of anti-CR1 or anti-CR3 alone (Table 2). The reason(s) is unclear, but co-incubation of neutrophils with anti-CR1 and -CR3 might stimulate a signalling pathway(s) required for phagocytosis.
As the next step of the present study, it should be clarified how BPI promotes complement activation on the bacterial surface. There seem to be two hypotheses about the mechanism by which BPI accelerates opsonization. First, BPI could bind to the bacterial surface via its N-terminal half while the C-terminal half of BPI, which is of unknown function, acts as a powerful opsonin for complement activation. For example, the complement protein C1q could bind to the C-terminal region of BPI, resulting in initiation of the complement cascade. A second hypothesis is that BPI alters properties of the bacterial envelope leading to an increased sensitivity of bacteria to complement. Currently in vitro and in vivo experiments to address these possibilities are ongoing in our laboratories.
Weiss et al. reported before that BPI participates in the killing of serum-resistant Gram-negative bacteria in the presence of a non-lethal dose of serum.26 This effect disappeared when the complement component C7-deficient serum was used, while it was restored by the addition of purified C7 to the aberrant serum. Thus BPI could also act in synergy with late complement components (ex. MAC), although how this might happen is not yet understood at the molecular level. In this case, the region of BPI required for this action is likely to be located within the N-terminal half, since the synergistic effect with MAC was also observed by using a recombinant N-terminal fragment of BPI instead of the whole protein.26
When invading bacteria are killed by the host defence system, the next important action should be clearance of the bacteria from the host body fluid. In this respect, the present findings propose an additional and interesting role of BPI that this molecule is involved not only in killing but also in clearance of bacteria by stimulation of phagocytosis through promotion of comlement activation.
Acknowledgments
We thank Drs K. Takeshi (Hokkaido Institute of Public Health), J. Miyazaki (Osaka University), T. Yamashita (Hokkaido University) and T. Seya (Osaka Medical Centre for Cancer and Cardiovascular Disease) for providing E. coli ATCC 25922, pCXN2, anti-guinea-pig Fc receptor monoclonal antibody, and rabbit anti-human CR1 antibody and control IgG, respectively. Critical reading and helpful comments by Drs P. Primakoff (University of California Davis) and T. Seya are also acknowledged. Drs M. Fukuoka and T. Hashimoto (University of Tsukuba) helped us with isolation of neutrophils. This work was supported in part by a grant-in-aid for Scientific Research from the Ministry of Education, Science, Sports, and Culture of Japan.
Abbreviations
- BPI
bactericidal/permeability-increasing protein
- CHO
Chinese hamster ovarian tumour
- CHO-BPI
CHO-K1 cells stably producing human BPI
- CR
complement receptor
- CR1
CR type 1
- CR3
CR type 3
- FBS
fetal bovine serum
- FITC-E. coli
fluorescein isothiocyanate-labelled Escherichia coli
- CFU
colony forming unit
- HBSS(–)
metal ion- and NaCl-free Hanks' balanced salt solution
- LBP
lipopolysaccharide-binding protein
- LPS
lipopolysaccharide
- MAC
membrane attack complex
- MFI
mean fluorescence intensity
- RT-PCR
reverse transcription-PCR
References
- 1.Raetz CRH. Biochemistry of endotoxin. Annu Rev Biochem. 1990;59:129–70. doi: 10.1146/annurev.bi.59.070190.001021. [DOI] [PubMed] [Google Scholar]
- 2.Ulevitch RJ. Recognition of bacterial endotoxins by receptor-dependent mechanisms. Adv Immunol. 1993;53:267–89. doi: 10.1016/s0065-2776(08)60502-7. [DOI] [PubMed] [Google Scholar]
- 3.Lehrer RI, Lichtenstein AK, Ganz T. Defensins: antimicrobial and cytotoxic peptides of mammalian cells. Annu Rev Immunol. 1993;11:105–28. doi: 10.1146/annurev.iy.11.040193.000541. [DOI] [PubMed] [Google Scholar]
- 4.Pereira HA, Erdem I, Pohl J, Spitznagel JK. Synthetic bactericidal peptide based on CAP37: a 37-kDa human neutrophil granule-associated cationic antimicrobial protein chemotactic for monocytes. Proc Natl Acad Sci USA. 1993;90:4733–7. doi: 10.1073/pnas.90.10.4733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hirata M, Shimomura Y, Yoshida M, et al. Characterization of a rabbit cationic protein (CAP18) with lipopolysaccharide-inhibitory activity. Infect Immun. 1994;62:1421–6. doi: 10.1128/iai.62.4.1421-1426.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Spitznagel JK. Antibiotic proteins of human neutrophils. J Clin Invest. 1990;86:1381–6. doi: 10.1172/JCI114851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Elsbach P, Weiss J. Bactericidal/permeability increasing protein and host defense against gram-negative bacteria and endotoxin. Curr Opin Immunol. 1993;5:103–7. doi: 10.1016/0952-7915(93)90088-a. [DOI] [PubMed] [Google Scholar]
- 8.Elsbach P. The bactericidal/permeability-increasing protein (BPI) in antibacterial host defense. J Leukoc Biol. 1998;64:14–8. doi: 10.1002/jlb.64.1.14. [DOI] [PubMed] [Google Scholar]
- 9.Weiss J, Elsbach P, Olsson I, Odeberg H. Purification and characterization of a potent bactericidal and membrane active protein from the granules of human polymorphonuclear leukocytes. J Biol Chem. 1978;253:2664–72. [PubMed] [Google Scholar]
- 10.Marra MN, Wilde CG, Griffith JE, Snable JL, Scott RW. Bactericidal/permeability-increasing protein has endotoxin-neutralizing activity. J Immunol. 1990;144:662–6. [PubMed] [Google Scholar]
- 11.Ooi CE, Weiss J, Doerfler ME, Elsbach P. Endotoxin-neutralizing properties of the 25 kD N-terminal fragment and a newly isolated 30 kD C-terminal fragment of the 55–60 kD bactericidal/permeability-increasing protein of human neutrophils. J Exp Med. 1991;174:649–55. doi: 10.1084/jem.174.3.649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marra MN, Wilde CG, Collins MS, Snable JL, Thornton MB, Scott RW. The role of bactericidal/permeability-increasing protein as a natural inhibitor of bacterial endotoxin. J Immunol. 1992;148:532–7. [PubMed] [Google Scholar]
- 13.Gazzano-Santoro H, Parent JB, Grinna L, et al. High-affinity binding of the bactericidal/permeability-increasing protein and a recombinant amino-terminal fragment to the lipid A region of lipopolysaccharide. Infect Immun. 1992;60:4754–61. doi: 10.1128/iai.60.11.4754-4761.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ooi CE, Weiss J, Elsbach P, Frangione B, Mannion B. A 25-kDa NH2-terminal fragment carries all the antibacterial activities of the human neutrophil 60-kDa bactericidal/permeability-increasing protein. J Biol Chem. 1987;262:14891–4. [PubMed] [Google Scholar]
- 15.Rother K, Till GO, Hansch GM. The Complement System, Heidelberg. Berlin: Spring-Verlag; 1998. [Google Scholar]
- 16.Iovine NM, Elsbach P, Weiss J. An opsonic function of the neutrophil bactericidal/permeability-increasing protein depends on both its N- and C-terminal domains. Proc Natl Acad Sci USA. 1997;94:10973–8. doi: 10.1073/pnas.94.20.10973. 10.1073/pnas.94.20.10973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Niwa H, Yamamura K, Miyazaki J. Efficient selection for high-expression transfectants with a novel eukaryotic vector. Gene. 1991;108:193–9. doi: 10.1016/0378-1119(91)90434-d. [DOI] [PubMed] [Google Scholar]
- 18.Yamashita T, Koyama J. Further evidence for heterogeneity of Fc gamma-receptors on guinea pig splenic B and T lymphocytes: analysis using monoclonal antibodies to two distinct types of Fc gamma-receptor. Microbiol Immunol. 1988;32:199–210. doi: 10.1111/j.1348-0421.1988.tb01378.x. [DOI] [PubMed] [Google Scholar]
- 19.Gray PW, Flaggs G, Leong SR, Gumina RJ, Weiss J, Ooi CE, Elsbach P. Cloning of the cDNA of a human neutrophil bactericidal protein. Structural and functional correlations. J Biol Chem. 1989;264:9505–9. [PubMed] [Google Scholar]
- 20.Nanbo A, Nishimura H, Nagasawa S. Lipopolysaccharide binding protein from normal human plasma purified with high efficiency. Protein Expr Purif. 1997;10:55–60. doi: 10.1006/prep.1996.0712. 10.1006/prep.1996.0712. [DOI] [PubMed] [Google Scholar]
- 21.Nanbo A, Nishimura H, Muta T, Nagasawa S. Lipopolysaccharide stimulates HepG2 human hepatoma cells in the presence of lipopolysaccharide-binding protein via CD14. Eur J Biochem. 1999;260:183–91. doi: 10.1046/j.1432-1327.1999.00141.x. 10.1046/j.1432-1327.1999.00141.x. [DOI] [PubMed] [Google Scholar]
- 22.Cohen HJ, Chovaniec ME, Takahashi K, Whitin JC. Activation of human granulocytes by arachidonic acid: its use and limitations for investigating granulocyte functions. Blood. 1986;67:1103–9. [PubMed] [Google Scholar]
- 23.Wessel D, Flugge UI. A method for the quantitative recovery of protein in dilute solution in the presence of detergents and lipids. Anal Biochem. 1984;138:141–3. doi: 10.1016/0003-2697(84)90782-6. [DOI] [PubMed] [Google Scholar]
- 24.Weiss J, Beckerdite-Quagliata S, Elsbach P. Resistance of gram-negative bacteria to purified bactericidal leukocyte proteins: relation to binding and bacterial lipopolysaccharide structure. J Clin Invest. 1980;65:619–28. doi: 10.1172/JCI109707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mannion BA, Weiss J, Elsbach P. Separation of sublethal and lethal effects of polymorphonuclear leukocytes on Escherichia coli. J Clin Invest. 1990;86:631–41. doi: 10.1172/JCI114755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Weiss J, Elsbach P, Shu C, Castillo J, Grinna L, Horwitz A, Theofan G. Human bactericidal/permeability-increasing protein and a recombinant NH2-terminal fragment cause killing of serum-resistant gram-negative bacteria in whole blood and inhibit tumor necrosis factor release induced by the bacteria. J Clin Invest. 1992;90:1122–30. doi: 10.1172/JCI115930. [DOI] [PMC free article] [PubMed] [Google Scholar]