Abstract
In coeliac disease (CD) immunological abnormalities are not confined to the small bowel and it has been suggested that changes in peripheral blood lymphocytes (PBL), such as lymphopenia and increased T-cell activation, may predispose to malignant or autoimmune complications of this condition. In the light of the recent findings about the Fas–Fas ligand (FasL) system in regulating lymphocyte homeostasis, the aim of the present study was to investigate peripheral lymphocyte Fas-mediated apoptosis in CD to establish whether the homeostatic role of apoptosis in peripheral T-cell selection is maintained. Moreover, because a soluble form of Fas has been described to be functionally implicated in the Fas signalling system, suggesting a relationship between some disorders and soluble Fas function, we measured levels of soluble Fas in sera of coeliac patients and analysed the relationship between these levels and the proportions of apoptotic and Fas+ PBL to further explore the function of the Fas–FasL pathway in this condition. Finally, we evaluated whether the increased prevalence of anticardiolipin antibodies, recently described in CD, could be related to PBL apoptosis in this condition. We demonstrated an increased apoptosis and higher levels of Fas and FasL expression in PBL isolated from untreated coeliac patients when compared to treated coeliac patients and controls. In addition, low levels of soluble Fas and a significant positive correlation between anticardiolipin antibodies and PBL apoptosis were found in untreated CD. Then, our results showed an increased susceptibility of PBL to undergo Fas-mediated apoptosis in active CD. This increased apoptosis could be responsible for both lymphopenia and immunogenic exposure of phospholipids with subsequent production of autoantibodies.
Introduction
Coeliac disease (CD) is an immune-mediated enteropathy caused, in genetically susceptible individuals, by a T-cell response to a new epitope generated by the transglutaminase-driven deamidation of dietary gliadin.1,2 In CD, however, immunological abnormalities are not confined only to the small bowel mucosa, and some years ago it was suggested that changes in peripheral blood lymphocytes (PBL) may predispose to the autoimmune and malignant complications of this condition.3–5
In a recent study6 we confirmed the peripheral reduction of both total and T lymphocytes, shown in untreated CD by earlier studies,7,8 and found an increased T-cell activation. Activation-induced lymphocyte apoptosis9 has been proposed as a homeostatic mechanism ensuring the deletion of unwanted T cells.10,11 On this basis, we investigated whether in CD peripheral T lymphocyte depletion, formerly considered secondary to the compartmentalization of gluten-sensitive lymphocytes within the intestinal mucosa12 and/or to their loss into the gut lumen,13 could indeed result from their increased apoptosis. In addition, because Fas–Fas ligand (FasL) system is known to have a crucial role in maintaining apoptosis-mediated lymphocyte homeostasis and T-cell tolerance,14,15 we evaluated the role of this proapoptotic pathway in triggering PBL apoptosis in CD.
Recently, a soluble form of Fas, derived from alternative splicing of the Fas gene, has been described to be functionally implicated in the Fas signalling system by protecting lymphocytes from apoptosis.16 Accordingly, a further aim of our study was to determine whether soluble Fas might control Fas–FasL-induced peripheral apoptosis.
Finally this report focuses on the mechanism of the increased prevalence of autoantibodies, such as anticardiolipin antibodies, in CD.17 Production of autoantibodies against phospholipids of the inner leaflet of the cell membrane may be due to a dysregulation of apoptosis in the peripheral immune system,18,19 and we looked for a relationship between anticardiolipin autoantibody formation and degree of PBL apoptosis.
Materials and Methods
Patients
Peripheral blood and serum samples were obtained from 30 patients with biopsy-proven CD. Fifteen patients (mean age 37·8 years, range 19–66) were untreated, whereas the remaining 15 (mean age 38·1 years, range 21–69) had been on a gluten-free diet for at least 12 months at the time of the study. In all of them a histological improvement of jejunal mucosa following gluten withdrawal was shown. Twenty anti-endomysial antibody-negative healthy volunteers, sex- and age-matched (mean age 36·9 years, range 19–67) with the patients, were also studied. Human leucocyte antigen (HLA) status has been investigated in all the subjects who took part in the study. All coeliac patients were HLA-DQ2+, while only two of 20 healthy volunteers had an HLA-DQ2 aplotype. Serum samples were aliquoted and stored at −80° until use. Informed consent was obtained from all patients and control subjects.
PBL isolation
PBL were isolated from heparinized peripheral blood by Lymphoprep gradient centrifugation (Nicamed, Oslo, Norway), and further purified by plastic adherence to remove monocytes. Cell recovery was routinely 85–95% and viability exceeded 95%, as detected by trypan blue exclusion assay. The resulting PBL population was more than 80% CD3+, as assessed by flow-cytometric analysis on a FACScan II analyser (Becton Dickinson Co., San Jose, CA).
Apoptosis evaluation by propidium iodide solution
Apoptosis was measured by flow cytometry as described elsewhere.20 After culturing, cells were centrifuged, and the pellets were gently resuspended in 1·5 ml hypotonic propidium iodide solution (PI; 50 µg/ml in 0·1% sodium citrate plus 0·1% Triton-X-100; Sigma Chemical Co., St Louis, MO). The tubes were kept at 4° in the dark overnight. The PI-fluorescence of individual nuclei was measured by flow cytometry with standard FACScan equipment (Becton Dickinson). The nuclei traversed the light beam of a 488-nm argon laser. A 560-nm dichroid mirror (DM 570) and a 600-nm band pass filter (band width 35 nm) were used to collect the red fluorescence caused by PI DNA staining, and the data were recorded in logarithmic scale in a Hewlett Packard (HP 900D, model 310) computer. The percentage of apoptotic cell nuclei (subdiploid DNA peak in the DNA fluorescence histogram) was calculated with specific FACScan research software (Lysis II).
Immunofluorescence and flow cytometry
Freshly isolated PBL were washed twice in phosphate-buffered saline (PBS) containing 2% fetal calf serum (FCS) and incubated at 4° for 30 min with phycoerythrin-labelled anti-Fas mouse monoclonal antibody (mAb) immunoglobulin G1 (IgG1; Coulter/Immunotech, Inc., Westbrook, ME) and fluoroscein isothiocyanate-labelled anti-FasL mouse mAb IgG2 (Upstate Biotechnology, Lake Placid, NY). Based on the forward- and side-scatter, cells were gated on the area corresponding to lymphocytes and analysed by FACScan flow cytometer, using the Lysis II software (Becton Dickinson). The proportions of Fas- and FasL-expressing lymphocyte subpopulations were determined by dual-colour analysis using fluoroscein isothiocyanate- or phycoerythrin-conjugated mAb directed against CD4 (anti-human Leu-3a, mouse IgG1, clone SK3; Becton Dickinson) and CD8 (anti-human Leu-2a, mouse IgG1, clone SK1; Becton Dickinson).
Lymphocyte culture with ZB4 anti-Fas blocking antibody
To determine whether PBL apoptosis in CD could involve Fas–FasL interaction, the effect of an antagonistic ZB4 anti-Fas antibody (Ancell Corporation, Bayport, MN) was analysed. Freshly isolated coeliac and control PBL were incubated at 37° in a 5% CO2 atmosphere for 24 hr in the absence or presence of 2 µg/ml ZB4 anti-Fas blocking antibody added to the culture medium. Quantification of apoptotic cells in lymphocyte cultures was performed by flow cytometry with PI staining as decribed above.
Soluble Fas detection
Serum soluble Fas levels were detected by enzyme-linked immunosorbent assay (ELISA; sAPO-1/Fas ELISA Kit, Alexis, San Diego, CA). According to the manufacturer's instructions serum levels were expressed as pg/ml. Sera obtained from 15 anti-endomysial antibody-negative patients with autoimmune diseases, such as systemic lupus erythematosus (n = 4), rheumatoid arthritis (n = 4), systemic sclerosis (n = 2), Sjögren's syndrome (n = 2), polymyositis/dermatomyositis (n = 2), and mixed connective tissue disease (n = 1), were used as positive controls as it is known that most of these patients have elevated levels of soluble Fas.21
Anticardiolipin antibody assay
Serum anticardiolipin auto-antibodies were determined by ELISA assay (VARELISA Cardiolipin Antibodies Enzyme Immunoassay, Pharmacia & Upjohn Diagnostics, Freiburg, Germany). According to the manufacturer's instructions, serum levels were calculated from the absorbance measurement of the sample, and expressed as percentages with respect to a reference serum sample supplied by the manufacturer.
Statistical analysis
Data are expressed as mean percentage. Statistical comparisons between mean values were carried out using the Mann–Whitney U-test for non-parametric data. Correlations were studied by Spearman's rank correlation test. A level of P < 0·05 was considered statistically significant.
Results
PBL apoptosis
In untreated CD, the percentages of apoptotic PBL (mean 16·6%, range 8·7–40·6) were significantly higher than in treated patients (mean 6·1%, range 0·6–12·3, P < 0·01) and controls (mean 1·9%, range 0·32–4·99, P < 0·001). No significant difference was found between treated coeliac patients and controls.
Fas and FasL espression by PBL
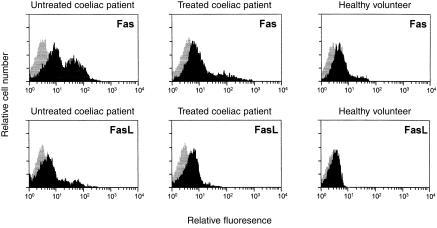
Figure 1 shows flow cytometric analysis of Fas and FasL expression by PBL isolated from an untreated coeliac patient, a treated coeliac patient, and a healthy volunteer. In untreated CD, the percentages of Fas+ PBL (mean 52·6%, range 38·3–66·7) were significantly higher than in treated patients (mean 37·0%, range 23·3–61·1, P < 0·05) and controls (mean 27·0%, range 20·9–41·6, P < 0·001). No significant difference was found between treated coeliac patients and controls. In untreated CD, the percentages of FasL+ PBL (mean 14·1%, range 5·3–36·6) were significantly higher than in treated patients (mean 6·8%, range 0·8–14·5, P < 0·05) and controls (mean 2·3%, range 0·3–6·4, P < 0·001). In treated CD, FasL+ PBL were significantly higher than in controls (P < 0·05).
Figure 1.
Flow cytometric histogram analysis of Fas and FasL expression by PBL of an untreated coeliac patient, a treated coeliac patient, and a healthy volunteer.
Data about Fas and FasL expression in peripheral lymphocyte subsets from the three groups studied are summarized in Table 1. In untreated CD, a significantly higher percentage of FasL+ cells in both CD4+ and CD8+ subsets when compared with treated CD and controls was found. In treated CD, the percentages of FasL+ CD4+ and CD8+ lymphocytes were significantly higher than in controls. Fas expression was found significantly higher in CD8+ lymphocytes when compared to treated coeliac patients and controls, but did not differ in CD4+ lymphocytes between the three groups studied. In treated CD, the percentages of Fas+ CD8+ lymphocytes were significantly higher than in controls.
Table 1.
Expression of Fas and FasL in PBL subsets
| Mean percentage of positive cells (range) | ||||
|---|---|---|---|---|
| CD4+ | CD8+ | |||
| Fas+ | FasL+ | Fas+ | FasL+ | |
| Untreated coeliacs | 18.3 | 5.7†§ | 24.1†§ | 8.3†§ |
| (12.2–20.1) | (2.1–18.0) | (18.2–36.3) | (7.1–21.7) | |
| Treated coeliacs | 15.6 | 2.1* | 11.2‡ | 4.2‡ |
| (10.3–18.6) | (0.7–4.5) | (8.4–19.7) | (0.9–14.6) | |
| Healthy volunteers | 15.1 | 0.9 | 6.2 | 1.3 |
| (8.0–17.5) | (0.4–2.1) | (9.3–4.4) | (0.5–3.6) | |
P < 0.05 versus healthy volunteers;
P < 0.01 versus treated coeliacs;
P < 0.01 versus healthy volunteers;
P < 0.001 versus healthy volunteers.
PBL culture with ZB4 anti-Fas blocking antibody
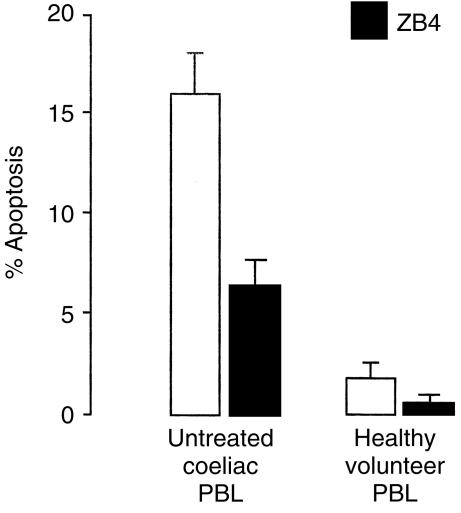
To investigate whether the PBL apoptosis is mediated by a Fas-dependent mechanism, we added the antagonistic ZB4 anti-Fas antibody, which blocks the cognate interaction between Fas and FasL, to the culture of both coeliac and control PBL. After 4 hr culture, a moderate number of coeliac PBL were apoptotic (mean 16·1%). On the contrary, when ZB4 anti-Fas blocking was added to the culture, the percentages (mean 6·4%) significantly decreased (P < 0·001) as shown in Fig. 2. As regards PBL isolated from healthy volunteers, after 4 hr culture lymphocyte apoptosis was slight (mean 2·1%), but decreased significantly (P < 0·05) in the presence of ZB4 anti-Fas blocking (mean 0·9%).
Figure 2.
Effect of ZB4 anti-Fas antibody on PBL apoptosis in untreated coeliac disease. Antagonistic ZB4 reduces Fas-dependent PBL apoptosis. PBL from active coeliac disease and healthy volunteers were incubated for 24 hr in the absence (open bars) or presence (black bars) of 2 µg/ml ZB4 antagonistic antibody. The percentages of apoptotic cells were determined by PI staining by flow cytometry. Results are expressed as the means±SD of three independent experiments.
Serum levels of soluble Fas
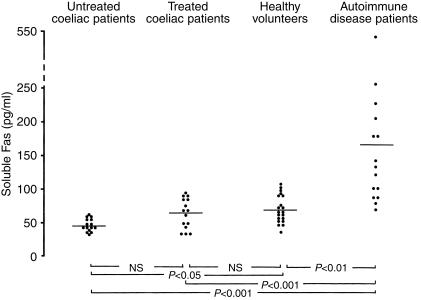
Figure 3 shows levels of soluble Fas in sera of untreated coeliac patients, treated patients, healthy volunteers and autoimmune disease patients as positive controls. In untreated CD, serum soluble Fas levels were significantly lower (mean 45·1 pg/ml, range 33–60) than in healthy volunteers (mean 69·5 pg/ml, range 36–108, P < 0.05). No significant difference was found between untreated and treated patients (mean 63·9 pg/ml, range 34–93) and between treated patients and healthy volunteers. In all the three groups studied the soluble Fas serum levels were significantly lower compared to autoimmune disease patients (mean 167·1 pg/ml, range 69–539, P < 0.001).
Figure 3.
ELISA detection of soluble Fas levels in sera of 15 untreated coeliac patients, 15 treated coeliac patients, 20 healthy volunteers and 15 autoimmune disease patients.
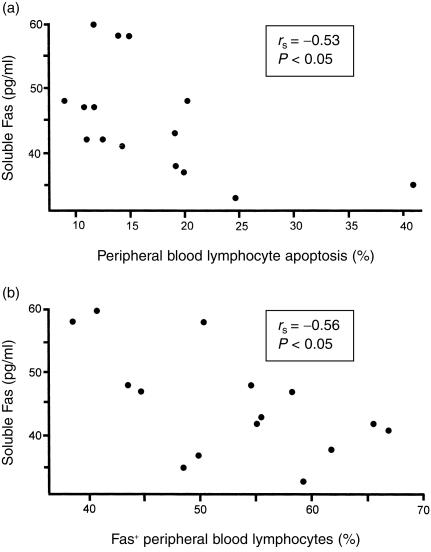
In untreated CD, significant inverse correlations were found between soluble Fas levels and PBL apoptosis (Fig. 4a), and between soluble Fas levels and Fas expression on PBL (Fig. 4b).
Figure 4.
Significant inverse correlations in untreated CD between the percentage of apoptotic PBL and the serum soluble Fas levels (a), and between the percentage of Fas+ PBL and the serum soluble Fas levels (b).
Anticardiolipin antibodies
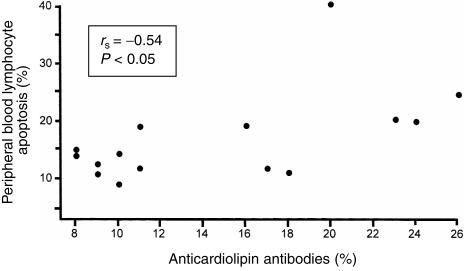
In untreated CD, the percentages of anticardiolipin antibodies (mean 14·6%, range 8–26) were significantly higher than in treated patients (mean 8·2%, range 3–18, P < 0·05) and controls (mean 6·9%, range 0–19, P < 0·01). No significant difference was found between treated coeliac patients and controls. In the group of untreated patients, a significant positive correlation between anticardiolipin antibodies and PBL apoptosis was found (Fig. 5).
Figure 5.
Significant positive correlation in untreated CD between PBL apoptosis and the serum levels of anticardiolipin antibodies.
Discussion
The mechanisms responsible for peripheral lymphopenia of untreated CD6–8 are still unclear. Because we had previously demonstrated that the phenotypic changes found in this condition indicate peripheral T-cell activation,6 it was therefore of interest to determine whether such cells were characterized by an abnormal pattern of apoptosis.
The present study shows that PBL shared increased apoptosis and higher levels of Fas and its ligand in untreated CD. Because Fas–FasL interaction is closely related to the process of lymphocyte activation, and is crucial for its control and termination,22 it is likely that in this condition the increased propensity of PBL to undergo apoptosis is a consequence of their greater activation.15 The reduction of apoptosis observed after culturing coeliac lymphocytes in the presence of ZB4 anti-Fas antibody supports the involvement of the Fas–FasL system in CD-associated peripheral lymphocyte apoptosis.
After gluten-free diet, while PBL apoptosis and expression of Fas turned out to be normal, lymphocyte FasL expression remained significantly higher than in controls. The lack of complete normalization of cytotoxic activity by PBL after treatment might be related to the persistent ingestion of minimal amounts of gliadin, or to the capacity of dendritic cells to retain gliadin in its native form for a long time,23 or, finally, to the maintenance of an immunological memory towards gliadin. Evidence supporting this latter hypothesis comes from the study by Kerttula et al.24 which shows an enhanced T-cell ‘memory activity’ in the peripheral blood of treated coeliac patients in the form of an increased percentage of peripheral CD45RO+ T cells.
As regards lymphocyte subsets, Fas expression was significantly increased in CD8+, but not in CD4+ cells. Although the single proportions of CD4+ and CD8+ subsets undergoing apoptosis have not been quantified in this study, it is conceivable that in active CD the PBL apoptotic population is mainly represented by CD8+ cells. In all the three groups studied the much higher proportion of FasL+ cells in CD8+ compared to CD4+ subset fits with the cytolytic role of CD8+ lymphocytes.
Human activated PBL were recently shown to express not only the full-length Fas mRNA but also an alternatively spliced mRNA variant encoding a soluble Fas protein lacking the transmembrane domain because of the deletion of an exon encoding this region.16 This truncated Fas molecule has been proposed to prevent FasL-induced apoptosis, thus providing a mechanism of escape from immunosurveillance for both tumour cells and potentially autoreactive lymphocytes.25 In an attempt to elucidate the role of soluble Fas in mediating Fas–FasL interaction in CD, the concentration of this protein was measured in the serum of patients and controls. Our finding of low levels of soluble Fas in untreated CD is consistent with the demonstration that Fas–FasL system regulates increased PBL apoptosis in this condition, as confirmed by the inverse correlation between soluble and membrane-bound form of Fas, and between soluble Fas and PBL apoptosis. These results are in agreement with those of Liu et al.26 who found that activation of PBL yielded a concomitant increase in cell-surface expressed Fas and a decrease in soluble Fas mRNA transcripts, thus suggesting that the expression of these molecules is differentially regulated. In this respect, CD differs from other autoimmune diseases,16,21,27–29 which are characterized by increased levels of soluble Fas. It may be possible that, unlike autoimmune diseases, where raised levels of soluble Fas protect autoreactive cells from apoptosis so leading to autoimmune phenomena, its low levels in CD reflect the preservation of the homeostatic and protective role of apoptosis in the deletion of autoreactive T-cell clones.
Nevertheless, CD has many features in common with classic autoimmune diseases, including the identification of a specific auto-antigen,1 the association with other autoimmune-type disorders,30,31 and the occurrence of several organ-specific autoantibodies.32,33 In particular, an increased prevalence of antibodies directed against intracellular antigens, such as anticardiolipin antibodies, has been recently shown in CD.17 A novel result of this study consists of the positive correlation of anticardiolipin antibodies with increased levels of PBL apoptosis in active CD. Anionic phospholipids are regular constituents of the inner leaflet of the cell membrane and, during the early stages of apoptosis, their exposure34 could induce humoral immune response.19 Our observation that anticardiolipin antibodies are related to peripheral lymphocyte apoptosis may help to explain their occurrence in active CD. The role of anticardiolipin antibodies in CD, however, is still obscure. It may be speculated that, similarly to what happens in human immunodeficiency virus-1 infection,18 they could co-operate with macrophages in the clearance of peripheral dead lymphocytes by an enhanced antibody-dependent cellular cytotoxicity and this engagement could, in turn, justify why no association between CD and antiphospholipid syndrome has been reported so far.
In conclusion, our results show an increased susceptibility of PBL from untreated CD patients to undergo Fas-mediated apoptosis. If on the one hand this increased apoptosis acts as a protective mechanism to limit the expansion of unwanted T cells, on the other it is responsible for both lymphopenia and immunogenic exposure of phospholipids with subsequent production of autoantibodies. Moreover, increased PBL apoptosis and the consequent T-cell functional deficiency may render coeliac patients more susceptible to the occurrence of fatal infections,35,36 malignancy,3,37 and splenic atrophy.8,38 As for the latter, it has been shown that chronic drainage of the thoracic duct in animals induces generalized reticuloendothelial atrophy,39 probably caused by the depletion of T lymphocytes.40 On the basis of these findings, it would thus be interesting to verify whether the increased PBL apoptosis occurring in CD could be in any way involved in the generation of splenic atrophy in this condition. Interestingly, hyposplenism has also been reported in acquired immune deficiency syndrome,41 which, similarly to what we have shown here in active CD, is characterized by lymphopenia resulting from peripheral Fas-mediated apoptosis.42
Acknowledgments
The Authors wish to acknowledge the Associazione Italiana Celiachia, the Istituto Superiore di Sanità (research project ‘Prevention of risk factors of maternal and child health’) and the Ministero dell'Università e della Ricerca Scientifica e Tecnologica (PRIN ‘Autoimmunity and coeliac disease: gluten, induction of autoimmune phenomena and effector mechanisms of intestinal damage’), for financial support.
Abbreviations
- CD
coeliac disease
- FasL
Fas ligand
- PBL
peripheral blood lymphocyte
- PI
propidium iodide
References
- 1.Dieterich W, Ehnis T, Bauer M, Donner P, Volta U, Riecken EO, Schuppan D. Identification of tissue transglutaminase as the autoantigen of celiac disease. Nat Med. 1997;3:797–801. doi: 10.1038/nm0797-797. [DOI] [PubMed] [Google Scholar]
- 2.Arentz-Hansen H, Körner R, Molberg Ø, et al. The intestinal T cell responce to α-gliadin in adult celiac disease is focused on a single deamidated glutamine targeted by tissue transglutaminase. J Exp Med. 2000;191:603–12. doi: 10.1084/jem.191.4.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Scott BB, Losowsky MS. Depressed cell-mediated immunity in coeliac disease. Gut. 1976;17:900–5. doi: 10.1136/gut.17.11.900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sollid LM, Brusend O, Gauderneck G, Thorsby E. The role of CD8-positive subset of T cells in proliferative responces to soluble antigens. Scand J Immunol. 1986;23:461–7. doi: 10.1111/j.1365-3083.1986.tb03077.x. [DOI] [PubMed] [Google Scholar]
- 5.Verkasalo MA, Aratò A, Savilahti E, Tainio VM. Effect of diet and age on jejunal and circulating lymphocyte subsets in children with coeliac disease: persistence of CD4–8– intraepithelial T cells through treatment. Gut. 1990;31:422–5. doi: 10.1136/gut.31.4.422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Di Sabatino A, Bertrandi E, Casadei Maldini M, Pennese F, Proietti F, Corazza GR. Phenotyping of peripheral blood lymphocytes in adult coeliac disease. Immunology. 1998;95:572–6. doi: 10.1046/j.1365-2567.1998.00651.x. 10.1046/j.1365-2567.1998.00651.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.O'Donoghue DP, Lancaster-Smith M, Laviniere P, Kumar PJ. T cell depletion in untreated adult coeliac disease. Gut. 1976;17:328–31. doi: 10.1136/gut.17.5.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bullen AW, Losowsky MS. Lymphocyte subpopulations in adult coeliac disease. Gut. 1978;19:892–7. doi: 10.1136/gut.19.10.892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kabelitz D, Pohl T, Pechhold K. Activation induced cell death (apoptosis) of mature peripheral T lymphocytes. Immunol Today. 1993;14:338–9. doi: 10.1016/0167-5699(93)90231-9. [DOI] [PubMed] [Google Scholar]
- 10.Golstein P, Ojcius DM, Young JD. Cell death mechanism and the immune system. Immunol Rev. 1991;121:29–65. doi: 10.1111/j.1600-065x.1991.tb00822.x. [DOI] [PubMed] [Google Scholar]
- 11.Osborne BA, Smith SW, Liu ZG, McLaughlin KA, Grimm L, Schwartz LM. Identification of genes induced during apoptosis in T lymphocytes. Immunol Rev. 1994;142:301–20. doi: 10.1111/j.1600-065x.1994.tb00894.x. [DOI] [PubMed] [Google Scholar]
- 12.Howdle PD, Simpson FG, Losowsky MS. The distribution of gluten-sensitive lymphocytes in coeliac patients – is it related to dietary gluten? Clin Exp Immunol. 1986;66:393–8. [PMC free article] [PubMed] [Google Scholar]
- 13.Douglas AP, Weetman AP, Haggit JW. The distribution and enteric loss of 51Cr-labelled lymphocytes in normal subjects and in patients with coeliac disease and other disorders of the small intestine. Digestion. 1976;14:29–43. doi: 10.1159/000197797. [DOI] [PubMed] [Google Scholar]
- 14.Lynch DH, Ramsdell F, Alderson MR. Fas and FasL in the homeostatic regulation of immune responses. Immunol Today. 1995;16:569–74. doi: 10.1016/0167-5699(95)80079-4. [DOI] [PubMed] [Google Scholar]
- 15.Ju ST, Panka DJ, Cui H, Ettinger R, el-Khatib M, Sherr DH, Stanger BZ, Marshak-RothStein A. Fas (CD95) /FasL interactions required for programmed cell death after T-cell activation. Nature. 1995;373:444–8. doi: 10.1038/373444a0. [DOI] [PubMed] [Google Scholar]
- 16.Cheng J, Zhou T, Liu C, Shapiro JP, Brauer MJ, Kiefer MC, Barr PJ, Mountz JD. Protection from Fas-mediated apoptosis by a soluble form of the Fas molecule. Science. 1994;263:1759–62. doi: 10.1126/science.7510905. [DOI] [PubMed] [Google Scholar]
- 17.Lerner A, Blank M, Lahat N, Shoenfeld Y. Increased prevalence of autoantibodies in coeliac disease. Dig Dis Sci. 1998;43:723–6. doi: 10.1023/a:1018801711413. [DOI] [PubMed] [Google Scholar]
- 18.Silvestris F, Frassanito MA, Cafforio P, et al. Antiphosphatidylserine antibodies in human immunodeficiency virus-1+ patients correlate with evidence of T-cell apoptosis and mediate antibody-dependent cellular cytotoxicity. Blood. 1996;87:5185–95. [PubMed] [Google Scholar]
- 19.Mevorach D, Zhou JL, Song X, Elkon KB. Systemic exposure to irradiated apoptotic cells induces autoantibody production. J Exp Med. 1998;188:387–92. doi: 10.1084/jem.188.2.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nicoletti I, Migliorati G, Pagliacci MC, Grignani F, Riccardi C. A rapid and simple method for measuring thymocyte apoptosis by propidium iodide staining and flow cytometry. J Immunol Methods. 1991;139:271–9. doi: 10.1016/0022-1759(91)90198-o. [DOI] [PubMed] [Google Scholar]
- 21.Nozawa K, Kayagaki N, Tokano Y, Yagita H, Okomura K, Hasimoto H. Soluble Fas (APO-1, CD95) and soluble Fas ligand in rheumatic diseases. Arthritis Rheum. 1997;40:1126–9. doi: 10.1002/art.1780400617. [DOI] [PubMed] [Google Scholar]
- 22.Dhein J, Walczak H, Baumler C, Debatin KM, Krammer PH. Autocrine T-cell suicide mediated by APO-1 (Fas/CD95) Nature. 1995;373:438–41. doi: 10.1038/373438a0. [DOI] [PubMed] [Google Scholar]
- 23.Tew JG, Phipps RP, Mandel TE. The maintenance and regulation of the humoral immune response: persisting antigen and the role of follicular antigen-binding dendritic cells as accessory cells. Immunol Rev. 1980;53:175–9. doi: 10.1111/j.1600-065x.1980.tb01044.x. [DOI] [PubMed] [Google Scholar]
- 24.Kerttula TO, Hällström O, Mäki M. Phenotypical characterization of peripheral blood T cells in patients with coeliac disease: elevation of antigen-primed CD45RO+ T lymphocytes. Immunology. 1995;86:104–9. [PMC free article] [PubMed] [Google Scholar]
- 25.Knipping E, Debatin KM, Stricker K, Heilig B, Eder A, Krammer PH. Identification of soluble APO-1 in supernatants of human B- and T-cell lines and increased serum levels in B- and T-cell leukemias. Blood. 1995;85:1562–9. [PubMed] [Google Scholar]
- 26.Liu C, Cheng J, Mountz JD. Differential expression of human Fas mRNA species upon peripheral blood mononuclear cell activation. Biochem J. 1995;310:957–63. doi: 10.1042/bj3100957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fujihara T, Takeuchi T, Tsubota K, Kayagaki N, Yagita H, Okumura K, Abe T. Serum soluble Fas/APO-1 is increased in patients with primary Sjögren's syndrome. Clin Rheumatol. 1998;17:496–9. doi: 10.1007/BF01451286. [DOI] [PubMed] [Google Scholar]
- 28.Zipp F, Weller M, Calabresi PA, Frank JA, Bash CN, Dichgans J, McFarland HF, Martin R. Increased serum levels of soluble CD95 (APO-1/Fas) in relapsing-remitting multiple sclerosis. Ann Neurol. 1998;43:116–9. doi: 10.1002/ana.410430120. [DOI] [PubMed] [Google Scholar]
- 29.Hiromatsu Y, Bednarczuk T, Soyejima E, Miyake I, Yang D, Fukazawa H, Nonaka K. Increased serum soluble Fas in patients with Grave's disease. Thyroid. 1999;9:341–5. doi: 10.1089/thy.1999.9.341. [DOI] [PubMed] [Google Scholar]
- 30.Collin P, Reunala T, Pukkala E, Laippala P, Keyrilainen O, Pasternack A. Coeliac disease-associated disorders and survival. Gut. 1994;35:1215–8. doi: 10.1136/gut.35.9.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ventura A, Magazzù G, Greco L. Duration of exposure to gluten and risk for autoimmune disorders in patients with celiac disease. Gastroenterology. 1999;117:297–303. doi: 10.1053/gast.1999.0029900297. [DOI] [PubMed] [Google Scholar]
- 32.Counsell CE, Taha A, Ruddel WSJ. Coeliac disease and autoimmune thyroid disease. Gut. 1994;35:844–6. doi: 10.1136/gut.35.6.844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rapoport MJ, Bistritzer T, Vardi O, Broide E, Azizi A, Vardi P. Increased prevalence of diabetes-related autoantibodies in coeliac disease. J Pediatr Gastroenterol Nutr. 1996;23:524–7. doi: 10.1097/00005176-199612000-00002. [DOI] [PubMed] [Google Scholar]
- 34.Mower DA, Peckham DW, Illera VA, Fishbaugh JK, Stunz LL, Ashman RF. Decreased membrane phospolipid packing and decreased cell size precede DNA cleavage in mature mouse B cell apoptosis. J Immunol. 1994;152:4832–42. [PubMed] [Google Scholar]
- 35.O'Donoghue DJ. Fatal pneumococcal septicaemia in coeliac disease. Postgrad Med J. 1986;62:229–30. doi: 10.1136/pgmj.62.725.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Matuchansky C, Colin R, Hemet J, et al. Cavitation of mesenteric lymph nodes, splenic atrophy, and a flat small intestinal mucosa. Report of six cases. Gastroenterology. 1984;87:606–14. [PubMed] [Google Scholar]
- 37.Holmes GKT, Prior P, Lane MR, Pope D, Allan RN. Malignancy in coeliac disease – effect of a gluten free diet. Gut. 1989;30:333–8. doi: 10.1136/gut.30.3.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Corazza GR, Zoli G, Di Sabatino A, Ciccocioppo R, Gasbarrini G. A reassessment of splenic hypofunction in celiac disease. Am J Gastroenterol. 1999;94:391–7. doi: 10.1111/j.1572-0241.1999.00865.x. [DOI] [PubMed] [Google Scholar]
- 39.Fish JC, Beathard G, Sarles HE, Remmers AR, Ritzmann SE. Circulating lymphocyte depletion: effect on lymphoid tissue. Surgery. 1970;67:658–66. [PubMed] [Google Scholar]
- 40.Bohs CT, Harris NS, Thomson PD, Fish JC, Traber DL. T lymphocyte depletion in peripheral blood sheep undergoing chronic thoracic duct drainage. J Reticuloendothel Soc. 1976;19:383–8. [PubMed] [Google Scholar]
- 41.Corazza GR, Zoli G, Ginaldi L, Cancellieri C, Profeta V, Gasbarrini G, Quaglino D. Tuftsin deficiency in AIDS. Lancet. 1991;337:12–3. doi: 10.1016/0140-6736(91)93331-3. [DOI] [PubMed] [Google Scholar]
- 42.Gougeon ML, Lecoeur H, Dulioust A, Enouf MG, Crouvoisier M, Goujard C, Debord T, Montagnier L. Programmed cell death in peripheral lymphocytes from HIV-infected persons: increased susceptibility to apoptosis of CD4 and CD8 T cells correlates with lymphocyte activation and with disease progression. J Immunol. 1996;156:3509–20. [PubMed] [Google Scholar]