Abstract
Expression of E-selectin on activated endothelium is a critical initial step that leads to extravasation of leucocytes during inflammation, yet E-selectin is largely uncharacterized in several animal species including the horse. We have sequenced and compared E-selectin genes derived from activated cultures of purified equine (horse), cervid (black-tailed deer) and ovine (sheep) pulmonary artery endothelial cells (ECs). Phylogenetic and amino acid sequence comparisons indicate that bovine, cervid and ovine E-selectin are similar, whereas human and equine E-selectin are more closely related to each other than to the ruminant molecules. Human E- and P-selectin-specific monoclonal antibodies that also recognize equine E-selectin were identified and used to characterize its expression. Expression of E-selectin was more readily induced by lipopolysaccharide treatment in equine ECs than in human ECs and supported adhesion and activation of neutrophils, consistent with the extreme sensitivity of horses to endotoxaemia and septic shock.
Introduction
Endothelial cell (EC) activation is a reversible process that occurs in response to a variety of stimuli including bacterial lipopolysaccharide (LPS), viral infection and cytokines such as interleukin-1 (IL-1) and tumour necrosis factor (TNF).1 Several proteins that facilitate the extravasation of leucocytes from blood vessels are expressed on activated endothelium.2 Selectins function in the initial steps that lead to leucocyte recruitment to endothelium at sites of inflammation. All three selectins (L, P and E) participate in leucocyte rolling on activated endothelium. L-selectin is expressed on most leucocyte types; P-selectin is expressed on platelets and is present in ECs; E-selectin is expressed on activated ECs.1
ECs are a critical target of many viral and bacterial diseases of animals yet the response of ECs to infection is largely uncharacterized in a number of animal species, including the horse. Endotheliotropic viral diseases of the horse include equine viral arteritis, African horse sickness, Hendra, equine herpesvirus I and equine infectious anaemia.3–7 Horses are also particularly sensitive to bacterial endotoxaemia and septic shock.8,9 ECs from different species and tissues vary greatly in their response to the same stimuli, both in vitro and in vivo.10–13 Thus, pathogenic mechanisms logically should be characterized in relevant host cells. Characterization of equine E-selectin and the response of equine endothelium to infection is clearly a prerequisite to the logical design and implementation of novel strategies for prevention and treatment of infectious diseases affecting the vasculature of horses.
To our knowledge, equine E-selectin has not been characterized nor have the necessary reagents been available for the investigation of equine E-selectin expression in disease processes. We have sequenced the E-selectin gene derived from activated cultures of purified equine, cervid and ovine ECs. We also identified a cross-reactive human selectin-specific monoclonal antibody (mAb) and used it to characterize the expression of E-selectin and its function on equine ECs.
Materials and methods
Cell isolation
Pure cultures of equine, ovine and cervid pulmonary artery ECs were developed following isolation, propagation and purification essentially as we have previously described.10 Briefly, ECs were harvested by enzymatic digestion of sections of pulmonary artery from a neonatal foal, a black-tailed deer and three mixed-breed lambs, immediately after euthanasia. ECs that had been propagated in vitro were purified by fluorescence-activated cell sorting with a MoFlo instrument (Cytomation, Ft. Collins, CO) based on uptake of 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindo-carbocyanine perchlorate conjugated to acetylated low-density lipoprotein (DiI-Ac-LDL; Biomedical Technologies, Stoughton, MA)14 and/or selection of colonies exhibiting characteristic EC cobblestone morphology. The purity of the various cultures was confirmed by immunofluorescent staining with a panel of antibodies (Dako Corporation, Carpinteria, CA) as previously described.10 EC cultures were stained with an antibody specific for von Willebrand factor (Factor VIII), but were not stained with antibodies specific for smooth muscle actin, desmin and pancytokeratin. Human pulmonary artery ECs were obtained from Clonetics (San Diego, CA). All experiments were performed on EC cultures between passages 5 and 15.
Equine neutrophils were purified with Mono-poly™ Resolving media (ICN Biochemicals, Costa Mesa, CA) essentially as previously described.15 Ten millilitres of whole blood was collected from a healthy adult horse, overlaid onto equal volumes of resolving media with 8% sterile water and centrifuged at 400 g for 30 min. The neutrophils were removed with a venous catheter and a syringe, rinsed in HEPES buffer (30 mm HEPES, 110 mm NaCl, 10 mm KCl, 1 mm MgCl2 10 mm glucose, pH 7·4), resuspended, counted and maintained on ice until use.
Polymerase chain reaction (PCR) and sequencing
Confluent monolayers of purified equine, ovine and cervid pulmonary artery ECs were stimulated with 10 ng/ml LPS. E-selectin is transcriptionally up-regulated in activated ECs,1 thus, total RNA was isolated at 4 hr post stimulation using RNAzol B (Tel-test, Friendswood, TX) and the RNA was reverse-transcribed with oligo-dT primers and Superscript reverse transcriptase as described previously (Invitrogen Life Technologies, Carlsbad, CA)16. Degenerate primers (Table 1) specific for conserved regions of known E-selectin sequences (GenBank accession numbers: bovine, L12039; canine, L23087; human, NM000450; murine, M87862; porcine, U37521; and rat, L25527) were used to amplify portions of the E-selectin genes by PCR. The amplified portions were sequenced and specific primers were then designed to amplify the E-selectin genes in their entirety (Table 1; cervid and ovine: −116P and B1605N, horse: −44P and B1605N). Sections of the genes amplified with Taq polymerase and the entire genes amplified with Expand High Fidelity enzyme (Invitrogen) were cloned using the TOPO TA cloning kit (Invitrogen) and both cDNA strands of at least four clones of each were sequenced.17 Sequence and phylogenetic analyses were performed as we have previously described.16
Table 1.
Primers used for amplification and sequencing of the E-selectin genes from mRNA derived from activated black-tailed deer, horse and sheep endothelial cells
| Primer* | Sequence | Length | TM | |
|---|---|---|---|---|
| Degenerate primers | -11P | 5′ CTCYWGAAGTCATGAWYGYBTC 3′ | 22 | 62·7 |
| 523P | 5′ GACYRTCAAYARYTMYACYTGC 3′ | 22 | 63·8 | |
| 1365P | 5′ GAAAGATGAACATGAGCTGCAG 3′ | 22 | 60·2 | |
| 416N | 5′ CATTCCACWTGCCBGAGTC 3′ | 19 | 64 | |
| 1640N | 5′ GCTTTGGCAGCTGCTRG 3′ | 17 | 61·1 | |
| Specific primers† | E1484N | 5′ GCCATTGAGCGTCCATC 3′ | 17 | 60·2 |
| OE1483P | 5′ GGAAAGCTCAACATGGACTG 3′ | 20 | 62 | |
| OE848N | 5′ GTCCAGTGAGTTTATATCCTTC 3′ | 22 | 60·7 | |
| OB256N | 5′ CGTTGATCTTTCTGATTCCAATCC 3′ | 24 | 63 | |
| OB172N | 5′ GAGCTGTGTAAGTTTTCTGGC 3′ | 21 | 63·1 | |
| B-44P | 5′ GCATTTTCAAGTCTAATCTTTGGATC 3′ | 26 | 62·5 | |
| B-116P | 5′ CGTCAAACCTGCAGGAGTG 3′ | 19 | 63·3 | |
| B-102P | 5′ GAGTGGCACAACACAGAAG 3′ | 19 | 61·2 | |
| B1605N | 5′ GGGTATTGCTCTAGTTCCC 3′ | 19 | 62·7 |
Primers are named for their approximate binding position (start codon=1) on the gene for which they are specific.
E, O and B indicates primers specific for equine, ovine (and cervid) and bovine E-selectin sequences, respectively.
Antibodies and immunoassays
Three mAbs [EP-5C7; (Protein Design Laboratory, Mountain View, CA)18 and BBIG-E6 and BBIG-E4 (R & D Systems, Minneapolis, MN)] specific for human E- and P-selectin (EP-5C7 and BBIG-E6) and human E-selectin (BBIG-E4) were assayed for reactivity with the various ECs at intervals after their stimulation with LPS (10 or 100 ng/ml) or histamine (100 ng/ml). Binding of each mAb was quantified by flow cytometric analysis of labelled ECs. Single cell suspensions of cells were obtained after cell scraping with a rubber policeman and repeated pipetting. Cell suspensions were washed in phosphate-buffered saline (PBS) and 0·1% sodium azide prior to labelling with the primary antibody for 15 min at 37°. Cells were washed and then fixed with 1% paraformaldehyde with 0·1% goat serum in PBS, washed and labelled with a fluorescein isothiocyanate (FITC) -conjugated secondary antibody for 30 min at room temperature. All washes and antibody dilutions were performed in flow buffer [divalent cation-free PBS, 1% goat serum, 5 mm ethylenediaminetetraacetic acid (EDTA), 0·01% NaN3, pH 7·4]. Data were collected in at least three independent experiments after 1–3 hr of stimulation of equine ECs.
Immunoprecipitation of equine E-selectin was performed essentially as described.19,20 Twenty micrograms of BBIG-E6 and 30 µl protein G-sepharose slurry were added to radiolabelled solubilized equine ECs after 3 hr of LPS stimulation and mixed for 18 hr at 4°. The precipitates were then washed with immunoprecipitation buffer and resuspended in 100 µl endoglycosidase buffer [50 mm sodium phosphate pH 6·8, 20 mm EDTA, 1% nonidet P-40, 0·15% sodium dodecyl sulphate (SDS), 1% β-mercaptoethanol and the protease inhibitors aprotinin, leupeptin and pepstatin A at 1 µg/ml and phenylmethylsulphonyl fluoride (PMSF) at 5 µg/ml]. The precipitates were deglycosylated with 200 mU endoglycosidase F/N-glycosidase F (Glyco-F; F. Hoffmann, La Roche Ltd. Basel, Switzerland) or mock treated with PBS, and incubated at 30° for 16 hr in a shaking incubator. Precipitates were centrifuged and resuspended in sample buffer (including dithiothreitol as a reducing agent) and resolved on 12% SDS–polyacrylamide gel electrophoresis (PAGE) Criterion™ (Bio-rad Laboratories, Hercules, CA) gels. Protein bands were visualized by autoradiography after washing and exposure to Amplify™ as recommended by the manufacturer (Amersham Pharmacia Biotech, Piscataway, NJ).
Functional assay
Adherence of equine neutrophils to equine ECs was measured in static adhesion chambers essentially as previously described.15 ECs were plated onto 25-mm glass coverslips and grown to confluence. Cells were either stimulated with LPS (10 ng/ml) for 2·5 hr, stimulated and treated for 30 min with 20 µg/ml BBIG-E6, or left unstimulated. Coverslips were washed and assembled into Hollers–Smith static adhesion chambers. Equine neutrophils, diluted to 1 × 106 cells per 0·8 ml in HEPES buffer with 1·5 m calcium and 0·1% human serum albumin, were injected and allowed to settle onto the EC monolayer for 8 min. The neutrophils in contact with the EC monolayer were counted. Chambers were then inverted for 8 min to allow non-adherent cells to settle and stick to the upper coverslip. Adherent cells were counted immediately after re-inversion of the chamber. Counts were conducted on six randomly selected fields of a phase-contrast microscope and averaged.
Results
Equine and human E-selectins are closely related
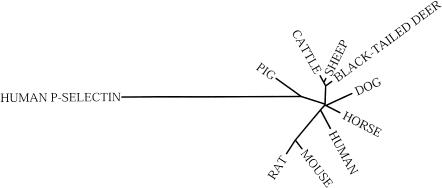
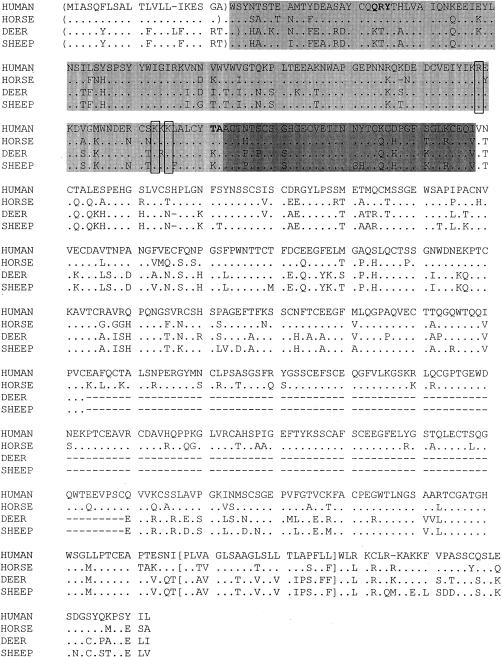
The E-selectin genes in pure cultures of equine, ovine and cervid pulmonary artery ECs were sequenced. Phylogenetic and sequence analyses indicated that the bovine, ovine and cervid E-selectin genes are closely related to each other (Fig. 1), and encode proteins of similar size (Fig. 2; cervid and bovine E-selectins have 486 amino acids, and ovine has 485 amino acids). Equine E-selectin is more closely related to the human form (Fig. 1), and is exactly the same size (Fig. 2; 611 amino acids).21
Figure 1.
Phylogenetic relationships of the E-selectin genes from the various species for which sequence data is available. The human P-selectin gene (NM_003005) is used to root the dendogram.
Figure 2.
Ovine, cervid (black-tailed deer) and equine (horse) E-selectin proteins aligned with that of human. ( ) indicates the signal sequence of each as predicted by the Signal P program, light shading indicates the C-type lectin binding domain and darker shading indicates the EGF domain based on homology with other selectins. [ ] indicates the transmembrane domain as predicted by the PHDhtm, TMHMM and TopPred G programs. Three conserved positively charged amino acids critical for ligand binding in human E-selectin are boxed. Bold letters indicate the epitope bound by the human E- and P-selectin antibodies EP-5C7 and BBIG-E6.29
The various E-selectin proteins were analysed with a variety of analysis tools (PROSITE,22 PHDhtm,23 SignalP,24 TMHMM,25 TopPred G26). The SignalP program predicted variable signal sequence cleavage sites, although the N-terminal residue was not confirmed by N-terminal protein sequencing. The equine, cervid and ovine E-selectins each have predicted C-type lectin-like, epidermal growth factor (EGF) -like, and transmembrane domains based on comparison to existing selectin sequences and the PROSITE database.27 The PHDhtm, TMHMM and TopPred programs predicted 32 amino acid cytoplasmic tails (Fig. 2). The different lengths of the various E-selectin proteins reflect different numbers of a repetitive domain of approximately 60 amino acids that resembles motifs found in complement regulatory proteins, and are referred to as complement-binding (CB) elements. Although CB motifs were not predicted by the comparison to the PROSITE database, homology to other E-selectin proteins suggests that the ovine and cervid E-selectins have four of these elements whereas equine and human E-selectins have six CB motifs between the EGF and transmembrane domains.1 Three charged amino acids that are highly conserved and important for ligand binding in human E-selectin were also conserved in the other E-selectin molecules (Fig. 2).28 Thus, phylogenetic, sequence and protein comparisons indicate that the E-selectin genes of ruminants cluster into a single group, whereas that of horses is more closely related to human.
Human selectin-specific mAbs cross-react with equine E-selectin
Three mAbs (BBIG-E4, BBIG-E6 and EP-5C7) specific for human E- and P-selectin were assayed by flow cytometry for their reactivity with proteins expressed on LPS- or histamine-stimulated equine, ovine and cervid pulmonary artery ECs. The mAbs EP-5C7 and BBIG-E6 recognized a protein expressed on LPS-stimulated equine ECs, but none of the other EC types. This result is consistent with the fact that amino acids that constitute the E-selectin-binding domain of EP-5C7 and BBIG-E6 (Q21, R22, Y23, T119 and A12029) are conserved in equine but not ovine, cervid, or bovine E-selectin (Fig. 2). The BBIG-E4 mAb did not bind activated equine ECs; it blocks the binding of the EP-5C7 mAb on human E- but not P-selectin18 thus, the failure of the BBIG-E4 mAb to bind activated equine ECs indicates that although the equine and human E-selectin proteins are closely related, they are phenotypically distinct.
Histamine treatment stimulates the immediate redistribution of P-selectin from vesicles in ECs (Weibel–Palade bodies) to the cell surface, whereas histamine does not induce E-selectin expression.1 The BBIG-E6 mAb did not bind equine ECs at 5–60 min after histamine stimulation (data not shown), indicating that BBIG-E6 recognizes E-selectin but probably not P-selectin on the surface of activated equine pulmonary artery ECs.
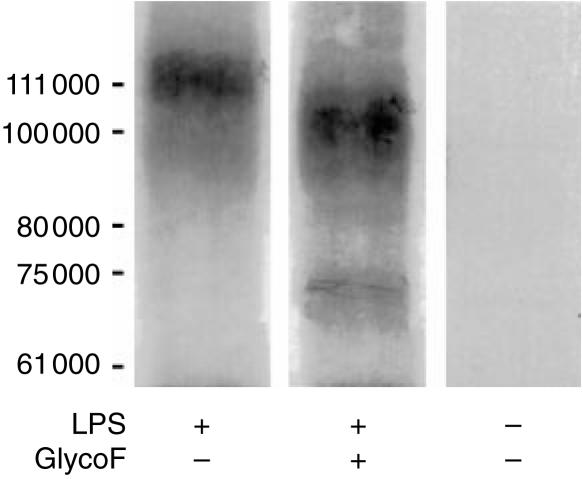
Immunoprecipitation confirmed that the molecule recognized by mAb BBIG-E6 in equine pulmonary artery ECs is E-selectin. BBIG-E6 immunoprecipitated a 115 000 MW protein from solubilized radiolabelled horse ECs after 3 hr of stimulation with LPS, but not from unstimulated cells. Incomplete Endoglycosidase F digestion of the immunoprecipitate produced a protein of approximately 66 000 MW (Fig. 3). These data are consistent with the predicted sizes of the glycosylated and core proteins, respectively, of equine E-selectin, and are similar to the sizes of human E-selectin molecules that are immunoprecipitated with the same antibody (R & D Systems). Together these results indicate that the mAb BBIG-E6 is a useful reagent for detection of E-selectin expression in activated equine ECs.
Figure 3.
Immunoprecipitation of a 115 000 MW protein from horse ECs 3 hr after stimulation with LPS, but not from unstimulated cells. Partial digestion with GlycoF resulted in a core protein of MW 66 000.
E-selectin expression is more readily induced by LPS stimulation of equine than human ECs
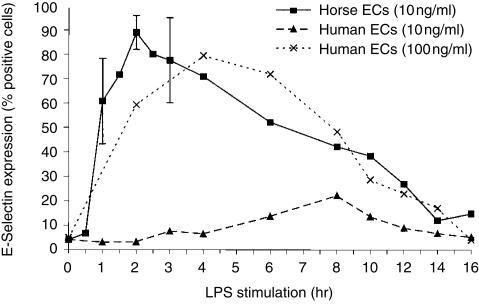
The temporal expression of E-selectin on equine and human pulmonary artery ECs was compared by flow cytometric analysis of ECs after intervals of activation with LPS. It has previously been reported that maximal expression of E-selectin on LPS-activated human umbilical vein ECs occurs after 4–6 hr of stimulation and declines to baseline levels by 24 hr.1,30,31 The maximal expression of E-selectin in equine ECs occurred after 2 hr of stimulation with 10 ng/ml LPS, whereas the same stimulation had minimal effect on human ECs (Fig. 4). Maximal expression of E-selectin occurred after 4 hr of stimulation of human ECs with a 10-fold higher dose of LPS (100 ng/ml; Fig. 4), and further increases in LPS concentration (200 and 400 ng/ml) did not increase E-selectin expression in the cultures. Recombinant human TNF-α and IL-1 also up-regulated E-selectin in equine and human ECs (data not shown), however, the equivalent recombinant equine molecules are not available, which precludes further comparisons. These data indicate that equine pulmonary artery ECs are significantly more sensitive to LPS stimulation and appear to up-regulate E-selectin more rapidly than do the equivalent human ECs.
Figure 4.
Up-regulation of E-selectin in activated (10 or 100 ng/ml LPS-stimulated) horse and human endothelial cells (ECs).
E-selectin expression on equine ECs promotes neutrophil adherence and activation
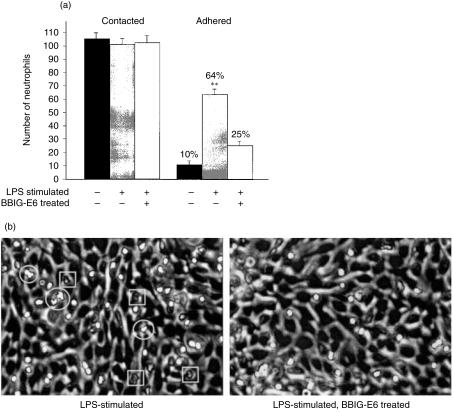
To determine the functional significance of E-selectin expression on equine ECs, adherence of equine neutrophils to EC monolayers was measured in static adhesion chambers (Fig. 5). Sixty-four per cent of contact neutrophils adhered to the LPS-stimulated EC monolayer, whereas only 10% adhered to the unstimulated monolayer. The mAb BBIG-E6 reduced the adherence of neutrophils to 25% indicating that adherence was in part dependent on expression of functional E-selectin (P < 0·001). Neutrophils that adhered to LPS-stimulated ECs underwent shape change and transmigration more frequently than on stimulated and mAb-treated ECs (Fig. 5b). These results indicate that E-selectin expression on equine ECs functions both in promoting neutrophil adhesion and activation, as previously demonstrated for human E-selectin.32
Figure 5.
(a) Static adhesion chambers were used to measure neutrophils that passively contacted and firmly adhered to LPS-stimulated and unstimulated ECs. Adhesion is partially blocked by pretreatment of the EC monolayer with the mAb specific to equine E-selectin (BBIG-E6). (b) Neutrophil (refractile under phase contrast optics) shape changes (circled) and transmigration (squared) were more prominent on LPS-stimulated ECs than on those treated with BBIG-E6.
Discussion
E-selectin mediates the adhesion of most leucocytes to activated vascular endothelium, an early process in inflammation. We have characterized equine E-selectin to facilitate future studies of inflammation and EC activation in horses. Specifically, we have sequenced the E-selectin genes from equine, ovine and cervid pulmonary artery ECs and used a human selectin-specific mAb to show that E-selectin expression was readily induced by LPS in equine ECs and that equine E-selectin functions in both neutrophil adherence and activation. This result is consistent with the extreme sensitivity of horses to endotoxaemia and septic shock following Gram-negative bacterial infection.8 The equine gastrointestinal tract is a vast reservoir of Gram-negative bacteria and free endotoxin that may gain access to the systemic circulation if the mucosal barrier is breached. Inflammatory mediators such as TNF-α and IL-1 are released from macrophages in the course of septic shock in horses, leading to global activation of pro-inflammatory pathways and EC injury with resultant disseminated intravascular coagulation and cardiovascular collapse that culminates in multi-organ failure and death.33,34 Activation and injury of ECs is clearly central to the pathogenesis of septic shock in horses, thus the availability of pure cultures of primary equine ECs and mAbs that detect equine E-selectin will facilitate further investigation of the role of ECs in this process.
Primary EC cultures also provide a highly convenient and relevant in vitro model in which to study the pathogenesis of endotheliotropic viral infections of animals and humans. For example, cultured ECs have recently been used to determine the differential tropism and cytopathogenicity of strains of cytomegalovirus,35,36 a basis for virulence in Hantaan virus,37 a major determinant of cell injury in Ebola virus,38 and modulation of the outcome of bluetongue virus infection of ruminants.10 Similarly, the cultured equine ECs and E-selectin-specific mAbs that we have characterized will facilitate in vitro studies to define the pathogenesis of endotheliotropic viral diseases of horses.3–7,39
In summary, activation of ECs is central to the inflammatory response of horses, as well as to the pathogenesis of important diseases such as septic shock and endotheliotropic viral infections. We have shown that equine ECs are extremely sensitive to endotoxin, as indicated by rapid expression of E-selectin. In addition, LPS-induced up-regulation of E-selectin on equine ECs promoted neutrophil adhesion and activation. Future analyses of cultured equine ECs will facilitate in vitro characterization of inflammation and various infectious diseases of horses.
Acknowledgments
These studies were supported by the Bernice Barbour Foundation and the Center for Equine Health at University of California, Davis with funds provided by the Oak Tree Racing Association, the State of California pari-mutual fund and contributions by private donors. S. I. Simon is an established investigator of the American Heart Association and is funded by NIH AI47294. We thank Dr Nick Landolfi for generous provision of the mAb EP-5C7. The full-length sequences of the equine, cervid and ovine E-selectin genes have been deposited into GenBank (accession numbers AF307970-AF307972).
Abbreviations
- CB
complement binding
- EC
endothelial cells
- EGF
epidermal growth factor
- Glyco-F
Endoglycosidase F/N-glycosidase F
- IL-1
interleukin-1
- LPS
lipopolysaccharide
- mAb
monoclonal antibody
- TNF
tumour necrosis factor
References
- 1.Vestweber D, Blanks JE. Mechanisms that regulate the function of the selectins and their ligands. Physiol Rev. 1999;70:181–213. doi: 10.1152/physrev.1999.79.1.181. [DOI] [PubMed] [Google Scholar]
- 2.Kevil CG, Bullard DC. Roles of leukocyte/endothelial cell adhesion molecules in pathogenesis of vasculitis. Am J Med. 1999;106:677–87. doi: 10.1016/s0002-9343(99)00132-1. 10.1016/s0002-9343(99)00132-1. [DOI] [PubMed] [Google Scholar]
- 3.Timoney PJ, McCollum WH. Equine viral arteritis. Vet Clin N Am: Eq Pract. 1993;9:295–309. doi: 10.1016/S0749-0739(17)30397-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gomez-Villamandos JC, Sanchez C, Carrasco L, et al. Pathogenesis of African horse sickness: Ultrastructural study of the capillaries in experimental infection. J Comp Pathol. 1999;121:101–16. doi: 10.1053/jcpa.1999.0305. 10.1053/jcpa.1999.0305. [DOI] [PubMed] [Google Scholar]
- 5.Oaks JL, Ulibarri C, Crawford TB. Endothelial cell infection in vivo by equine infectious anaemia virus. J Gen Virol. 2000;80:2393–7. doi: 10.1099/0022-1317-80-9-2393. [DOI] [PubMed] [Google Scholar]
- 6.Kohn CW, Fenner WR. Equine herpes myeloenchephalopathy. Vet Clin N Am: Eq Pract. 1987;3:405–19. doi: 10.1016/s0749-0739(17)30683-1. [DOI] [PubMed] [Google Scholar]
- 7.Hooper PT, Ketterer PJ, Hyatt AD, Russell GM. Lesions of experimental equine morbillivirus pneumonia in horses. Vet Pathol. 1997;34:312–22. doi: 10.1177/030098589703400407. [DOI] [PubMed] [Google Scholar]
- 8.Burrows GE. Endotoxaemia in the horse. Equine Vet J. 1981;13:89–94. doi: 10.1111/j.2042-3306.1981.tb04120.x. [DOI] [PubMed] [Google Scholar]
- 9.Benbarek H, Deby-Dupont G, Caudron I, Grülke S, Deby C, Lamy M, Serteyn D. Interactions between lipopolysaccharides and blood factors on the stimulation of equine polymorphonuclear neutrophils. Vet Immunol Immunopathol. 1998;64:313–22. doi: 10.1016/s0165-2427(98)00142-1. 10.1016/s0165-2427(98)00142-1. [DOI] [PubMed] [Google Scholar]
- 10.DeMaula CD, Jutila MA, Wilson DW, MacLachlan NJ. Infection kinetics, prostacyclin release, and cytokine-mediated modulation of the mechanism of cell death during bluetongue virus infection of cultured ovine and bovine pulmonary artery and lung microvascular endothelial cells. J Gen Virol. 2001. pp. 787–94. [DOI] [PubMed]
- 11.Hickey MJ, Kanwar S, McCafferty DM, Granger DN, Eppihimer MJ, Kubes P. Varying roles of E-selectin and P-selectin in different microvascular beds in response to antigen. J Immunol. 1999;162:1137–43. [PubMed] [Google Scholar]
- 12.Craig LE, Spelman JP, Strandberg JD, Zink MC. Endothelial cells from diverse tissues exhibit differences in growth and morphology. Microvas Res. 1998;55:65–76. doi: 10.1006/mvre.1997.2045. 10.1006/mvre.1997.2045. [DOI] [PubMed] [Google Scholar]
- 13.Page C, Rose M, Yacoub M, Pigott R. Antigenic heterogeneity of vascular endothelium. Am J Pathol. 1992;141:673–83. [PMC free article] [PubMed] [Google Scholar]
- 14.Voyta JC, Via DP, Butterfield CE, Zetter BR. Identification and isolation of endothelial cells based on their increased uptake of acetylated-low density lipoprotein. J Cell Biol. 1984;99:2034–40. doi: 10.1083/jcb.99.6.2034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tsang YTM, Neelamegham S, Hu Y, Berg EL, Burns AR, Smith CW, Simon SI. Synergy between l-selectin signaling and chemotactic activation during neutrophil adhesion and transmigration. J Immunol. 1997;159:4566–77. [PubMed] [Google Scholar]
- 16.Hedges JF, Balasuriya UBR, Timoney PJ, McCollum WH, MacLachlan NJ. Genetic divergence with emergence of phenotypic variants of equine arteritis virus during persistent infection of stallions. J Virol. 1999;73:3672–81. doi: 10.1128/jvi.73.5.3672-3681.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hedges JF, Balasuriya UBR, Timoney PJ, McCollum WH, MacLachlan NJ. Genetic variation in open reading frame 2 of field isolates and laboratory strains of equine arteritis virus. Virus Res. 1996;42:41–52. doi: 10.1016/0168-1702(96)01294-4. 10.1016/0168-1702(96)01294-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Berg EL, Fromm C, Melrose J, Tsurushita N. Antibodies cross-reactive with E- and P-selectin block both E- and P-selectin functions. Blood. 1995;85:31–7. [PubMed] [Google Scholar]
- 19.Lawson C, Ainsworth M, Yacoub M, Rose M. Ligation of ICAM-1on endothelial cells leads to expression of VCAM-1 via a nuclear factor-κB-independent mechanism. J Immunol. 1999;162:2990–6. [PubMed] [Google Scholar]
- 20.Balasuriya UBR, MacLachlan NJ, de Vries AAF, Rossitto PV, Rottier PJM. Identification of a neutralization site in the major envelope glycoprotein (GL) of equine arteritis virus. Virology. 1995;207:518–27. doi: 10.1006/viro.1995.1112. 10.1006/viro.1995.1112. [DOI] [PubMed] [Google Scholar]
- 21.Bevilacqua MP, Stenglin S, Gimbrone MA, Jr, Seed B. Endothelial leukocyte adhesion molecule 1: an inducible receptor for neutrophils related to complement regulatory proteins and lectins. Science. 1989;243:1160–5. doi: 10.1126/science.2466335. [DOI] [PubMed] [Google Scholar]
- 22.Bairoch A, Bucher P, Hofmann K. The PROSITE database, its status In 1997. Nucleic Acids Res. 1997;25:217–21. doi: 10.1093/nar/25.1.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rost B, Casadio R, Fariselli P, Sander C. Prediction of helical transmembrane segments at 95% accuracy. Protein Sci. 1995;4:521–33. doi: 10.1002/pro.5560040318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nielsen H, Engelbrecht J, Brunak S, von Heijne G. Identification of prokaryotic and eukaryotic signal peptides and prediction of their cleavage sites. Protein Engineering. 1997;10:1–6. doi: 10.1093/protein/10.1.1. 10.1093/protein/10.1.1. [DOI] [PubMed] [Google Scholar]
- 25.Sonnhammer ELL, von Heijne G, Krogh A. Proc Of Sixth International Conference on on Intelligent Systems for Molecular Biology. Menlo Park: AAAI Press; 1998. A hidden Markov model for predicting transmembrane helices in protein sequences. [PubMed] [Google Scholar]
- 26.von Heijne G. Membrane protein structure prediction, hydrophobicity analysis and the positive inside rule. J Mol Biol. 1992;225:487–94. doi: 10.1016/0022-2836(92)90934-c. [DOI] [PubMed] [Google Scholar]
- 27.Huang K, Graves BJ, Wolitsky BA. Functional analysis of selectin structure. In: Vestweber D, editor. The Selectins: Initiators of Leukocyte Endothelial Adhesion. Amsterdam: Harwood Academic Publishers; 1997. pp. 1–29. [Google Scholar]
- 28.Carlos TM, Harlan JM. Leukocyte-endothelial adhesion molecules. Blood. 1994;84:2068–101. [PubMed] [Google Scholar]
- 29.Goda K, Tanaka T, Monden M, Miyasaka M. Characterization of an apparently conserved epitope in E- and P-selectin identified by dual-specific monoclonal antibodies. Eur J Immunol. 1999;29:1551–60. doi: 10.1002/(SICI)1521-4141(199905)29:05<1551::AID-IMMU1551>3.0.CO;2-0. 10.1002/(sici)1521-4141(199905)29:05<1551::aid-immu1551>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 30.Wellicome SM, Thornhill MH, Pitzalis C, Thomas DS, Lanchbury JSS, Panayi GS, Haskard DO. A monoclonal antibody that detects a novel antigen on endothelial cells that is induced by tumor necrosis factor, IL-1 or lipopolysaccharide. J Immunol. 1990;144:2558–65. [PubMed] [Google Scholar]
- 31.Tsang YTM, Stephens PE, Licence ST, Haskard DO, Binns RM, Robinson MK. Porcine E-selectin: cloning and functional characterization. Immunology. 1995;85:140–5. [PMC free article] [PubMed] [Google Scholar]
- 32.Simon SI, Hu Y, Vestweber D, Smith CW. Neutrophil tethering on E-selectin activates β2 integrin binding to ICAM-1 through a mitogen-activated protein kinase signal transduction pathway. J Immunol. 2000;164:4348–58. doi: 10.4049/jimmunol.164.8.4348. [DOI] [PubMed] [Google Scholar]
- 33.Holbrook TC, Moore JN. Anti-inflammatory and immune support in endotoxemia and septicemia. Vet Clin N Am: Eq Pract. 1994;10:535–47. doi: 10.1016/s0749-0739(17)30345-0. [DOI] [PubMed] [Google Scholar]
- 34.MacKay RJ. Inflammation in horses. Vet Clin N Am: Eq Pract. 2000;16:15–27. doi: 10.1016/s0749-0739(17)30116-5. [DOI] [PubMed] [Google Scholar]
- 35.Kahl M, Seigel-Axel D, Stenglein S, Jahn G, Sinzger C. Efficient lytic infection of human arterial endothelial cells by human cytomegalovirus strains. J Virol. 2000;74:7628–35. doi: 10.1128/jvi.74.16.7628-7635.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sinzger C, Kahl M, Laib K, Kingel K, Reiger P, Plachter B, Jahn G. Tropism of human cytomegalovirus for endothelial cells is determined by a post-entry step dependent on efficient translocation to the nucleus. J Gen Virol. 2000;81:3021–35. doi: 10.1099/0022-1317-81-12-3021. [DOI] [PubMed] [Google Scholar]
- 37.Ebihara H, Yoshimatsu K, Ogino M, et al. Pathogenicity of hantaan virus in newborn mice: Genetic reassortant study demonstrating that a single amino acid change in glycoprotein G1 is related to virulence. J Virol. 2000;74:9245–55. doi: 10.1128/jvi.74.19.9245-9255.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yang Z, Drukers HJ, Sullivan NJ, Sanchez A, Nabel EG, Nabel GJ. Identification of the ebola virus glycoprotein as the main viral determinant of vascular cell cytotoxicity and injury. Nature Med. 2000;6:886–9. doi: 10.1038/78645. [DOI] [PubMed] [Google Scholar]
- 39.Del Piero F. Equine viral arteritis. Vet Pathol. 2000;37:287–96. doi: 10.1354/vp.37-4-287. [DOI] [PubMed] [Google Scholar]