Abstract
The fusion protein of canine distemper virus (CDV-F), a 662 amino-acid envelope protein, was used as the target molecule for identification of canine T helper (Th) epitopes. A library of 94 peptides, each 17 residues in length overlapping by 10 residues and covering the entire sequence of CDV-F, was screened using a lymphocyte proliferation assay with peripheral blood mononuclear cells (PBMC) obtained from dogs inoculated with canine distemper virus (CDV) vaccine. Initially we observed low and inconsistent proliferation of PBMC in response to these peptides, even when using cells obtained from dogs that had received multiple doses of CDV. Subsequently, the use of expanded cell populations derived by in vitro stimulation of canine PBMC with pools of peptides allowed the identification of a number of putative canine Th-epitopes within the protein sequence of CDV-F. There were two major clusters of Th-epitopes identified close to the cleavage site of the F0 fusion protein, while some others were scattered in both the F1 and F2 fragments of the protein. Some of these peptides, in particular peptide 35 (p35), were stimulatory in dogs of different breeds and ages. The identification of such promiscuous canine Th-epitopes encouraged us to assemble p35 in tandem with luteinising hormone releasing hormone (LHRH) a 10 amino-acid residue synthetic peptide representing a B-cell epitope which alone induces no antibody in dogs. The totally synthetic immunogen was able to induce the production of very high titres of antibodies against LHRH in all dogs tested. These results indicate that p35 could be an ideal candidate for use as a Th-epitope for use in outbred dogs.
Introduction
Among the class of subunit vaccines, synthetic peptide immunogens display great potential, not only in terms of safety, relative ease of production and quality control, stability in storage and transport, but also because by careful design of the peptide it is possible to manipulate the immune responses.1–4 In subjects where the aim of vaccination is to generate antibodies, peptides representing B-cell epitopes have traditionally been coupled to carrier proteins which act as a source of T helper (Th) cell epitopes to provide T-cell help. In the case of totally synthetic peptide vaccines, the carrier proteins are replaced by peptide sequences that represent defined Th-epitopes. Apart from acting as a source of stimulus for helper T cells, synthetic peptide Th-epitopes may avoid the problems of protein carrier availability and also carrier protein-induced epitope suppression.5,6 For totally synthetic peptide-based vaccines to be successful, however, they have to be able to evoke a strong immune response and for that, incorporation of highly stimulatory Th-epitopes is required.
A number of studies have reported the identification of Th-epitopes in the mouse7,8 and in humans9,10 and some of these have been reported to be active in more than one species,11 although their activities in multiple species are not always particularly significant (S. Ghosh & D. C. Jackson, unpublished). Moreover, Th-epitopes reported to be stimulatory in a particular species are usually major histocompatiblity complex (MHC) restricted and hence of limited use for outbred populations of that species. It seemed to us therefore that identification of helper T-cell epitopes in the species of interest was a more useful way of progressing the design of peptide-based vaccines. Our aim in the present study was therefore to identify new Th-epitopes recognized by the dog. Only a few such Th-epitopes have been reported in the literature.12
Young dogs are commonly immunized against canine distemper virus (CDV), which causes a demyelinating disease.13 CDV is a negative-strand RNA virus belonging to the morbillivirus group of the paramyxovirus family and is closely related to measles virus (MV). CDV possesses two envelope glycoproteins, the attachment (H) and fusion (F) proteins. The F protein of CDV shares extensive sequence homology with the F protein of MV.14 Fusion proteins of morbilliviruses are essential for virus penetration and cell-to-cell spread.15 The inactive fusion protein, F0, of the virus is cleaved into two subunits, F1 and F2, which are linked by disulphide bonds to form the mature protein.16 It has been demonstrated that dogs can be protected against CDV by immunization with isolated CDV-F.17 The fact that dogs can mount an effective immune response against this protein led us to investigate this molecule with a view to defining canine T-cell epitopes within its sequence. The rationale of the study then was to identify T-cell epitopes that are present in CDV-F, which when included as part of a synthetic vaccine will make use of pre-exisiting helper T cells, induced by the routine vaccination of dogs with Canvac 3 in 1.
Algorithm-based prediction of peptide sequences that may posses helper T-cell activity is a popular initial approach for determination of epitopes, but the algorithms used for this purpose are based on data obtained from mouse and humans and even then many of the predicted sequences have not exhibited strong T-cell activity, even in the murine system.18,19 As canine immune responses have not been sufficiently well characterized, we decided to construct a peptide library and examine the ability of individual peptides to stimulate peripheral blood mononuclear cells (PBMC) obtained from dogs.
Materials and methods
Animals
Beagle/foxhound and kelpie/foxhound cross dogs, bred and maintained at CSL Ltd (Parkville, Victoria, Australia), were used for the study. Dogs of other breeds were obtained commercially and subsequently maintained at CSL Ltd. All experimental work on dogs was carried out in accordance with the CSL Animal Ethics and Experimentation Committee (approval no. 493).
CDV antigens
Commercially available canine vaccine Canvac 3 in 1 (CSL Ltd), which contains live CDV, canine parvovirus and canine adenovirus, was used to vaccinate dogs. CDV was also obtained as Vero cell-infected culture supernatants (CSL Ltd). The virus was concentrated from the supernatant by ultracentrifugation and the sedimented material was then heat inactivated by incubation in a boiling water bath for 30 min. This heat-inactivated CDV was used as the source of antigen for in vitro proliferation assays and, in some cases, for immunizing dogs. The protein content of the preparation was estimated using the bicinchoninic acid (BCA) Protein Assay Kit (Pierce, Progen Industries Ltd; Richlands, Qld, Australia).
Synthetic peptides and peptide immunogens
Overlapping peptides covering the entire sequence of CDV-F (CDV strain onderstepoort, Barrett et al.14) (Fig. 1) were obtained from Mimotopes Pty Ltd. (Clayton, Victoria, Australia). Each peptide was 17 amino acids in length and overlapped previous neighbours by 10 amino-acid residues; peptide 1 (p1) encompasses amino-acids 1–17, p2 encompasses amino-acids 8–24, etc. The purity of these peptides was greater than 80%, as judged by reverse-phase HPLC. Sequences of randomly selected peptides in the pepset were confirmed by amino acid analyses.
Figure 1.
Sequence, in single-letter code, of the fusion protein from canine distemper virus (CDV) (onderstepoort strain). The amino acid residues displayed in bold indicate the start of each of the 17-mer peptides that were used in this study. Peptides overlapped by 10 residues and covered the whole sequence. The last peptide in the series overlapped the penultimate member by 16 residues.
The peptides, p35, luteinising hormone releasing hormone (LHRH) and the immunogen, p35-LHRH, were synthesized in-house. The sequence corresponding to p35 (TAAQITAGIALHQSNLN) was assembled at the N-terminus of LHRH, which has the sequence EHWSYGLRPG. This yields the 27 amino-acid residue synthetic immunogen TAAQITAGIALHQSNLNEHWSYGLRPG. The synthesis was carried out manually in the solid phase using Fmoc chemistry, essentially as described by Fitzmaurice et al.20
All peptides synthesized in this laboratory were greater than 90% pure (as judged by HPLC) and had the expected mass when analysed by MALDI-TOF mass spectrometry. None of the peptides used in this study were toxic for cells.
In vitro T-cell proliferation assays
Heparinized blood obtained from dogs inoculated with Canvac 3 in 1 vaccine was used as a source of PBMC which were isolated on Ficoll–Paque (Amersham Pharmacia Biotech Aus., NSW, Australia). An aliquot of these cells from each individual dog was frozen in autologous plasma (containing 10% dimethylsulphoxide) for subsequent use as antigen-presenting cells (APC). PBMC were tested for proliferation in response to heat-killed CDV or to the individual peptides; phytohaemagglutinin was used as a positive control for proliferation. PBMC were cultured in round-bottom 96-well microtitre plates (Nunc, Medos, Vic., Australia) at a concentration of 105 cells per well in canine TCM: 150 µl of RPMI supplemented with 10% (v/v) pooled heat-inactivated canine serum, 2 mm glutamine, 2 mm sodium pyruvate, 0·1 mm 2-mercaptoethanol, 30 µg/ml of gentamicin, 100 IU/ml of penicillin and 100 µg/ml of streptomycin. Heat-killed CDV was added at doses ranging from 0·012 µg to 12 µg of protein per well and synthetic peptides were added at concentrations of 2–8 nmol per well. PBMC were cultured at 37° in an atmosphere containing 5% in CO2 for 7 days or as indicated. [3H]Thymidine ([3H]TdR) (1 µCi per well), was added during the last 18 hr of the culture period. At culture completion, cells were harvested onto glass-fibre filters and the level of cellular thymidine incorporation was measured using a Hewlett Packard Matrix 9600 direct β-counter. The results of proliferation assays were expressed as the stimulatory index (SI), calculated as the ratio of the counts of the antigen-containing culture to medium control.
Expansion of peptide-specific cells from PBMC and their use in proliferation assays
Fresh PBMC obtained by sedimentation through Ficoll–Paque were incubated with respective pools of peptides, containing 2 nmol of each peptide, for 30 min at 37°. Peptide-pulsed PBMC were then washed and cultured at a density of 1 × 107/ml in canine TCM for 7 days. Following this culture period, viable cells at a density of 2 × 104 were incubated in the presence of γ-irradiated (2200 rads, 60Co source) autologous PBMC (at a concentration of 105) as a source of APC, together with antigen, in a total volume of 150 µl of canine TCM for 6 days. PBMC frozen and stored in liquid nitrogen were thawed and used as APC. [3H]TdR was then added and incubation continued for a further 20 hr. At completion of culture, cells were then harvested onto glass-fibre filters and the level of cellular thymidine incorporation measured as described above.
Enzyme linked immunosorbent assays (ELISA)
Flat-bottom polyvinyl microtitre plates (Dynex Technologies Inc., Trace Scientific, Vic., Australia) were coated with 50 µl/well of LHRH (5 µg/ml) in phosphate-buffered saline (PBS), pH 7·3, for 18–20 hr at room temperature in a humidified atmosphere. The antigen solution was then removed from wells by aspiration and 100 µl of a solution (10 mg/ml) of bovine serum albumin (BSA) in PBS was added for 1 hr. Plates were then washed with PBS containing 0·05% Tween-20 (PBST) and serial dilutions (50 µl) of individual canine sera in PBST containing 5 mg/ml of BSA were added to the wells. Following overnight incubation at room temperature in a humidified atmosphere, the sera were removed and the plates washed with PBST. Fifty microlitres of a 1 : 50 000 dilution (made in PBST containing 5 mg/ml of BSA) of horseradish peroxidase-conjugated goat antibody directed against canine IgG (heavy chain and light chain) (Kirk-Gaard & Perry Laboratories, Biomediq, Vic., Australia), was then added and the plates incubated at room temperature for 1 hr. The plates were washed and 100 µl of enzyme substrate (0·2 mm 2,2′-azino-bis 3-ethylbenzthiazoline-sulphonic acid in 50 mm citric acid, pH 4·0, containing 0·004% v/v hydrogen peroxide) was added. The colour reaction was stopped by the addition of 50 µl/well of 45 mm sodium fluoride. The absorbances of the resulting solutions were determined at a wavelength of 405 nm using a Labsystems Multiscan Multisoft microplate reader (Pathtech Diagnostics Pty. Ltd, Melbourne, Australia). Antibody titres were expressed as the reciprocal of the serum dilution which gave an absorbance three times higher than that obtained in wells lacking canine antisera but containing all other components of the assay system.
Vaccination of dogs with peptide immunogens
Beagle/foxhound dogs, previously vaccinated with Canvac 3 in 1, were divided into two groups. One group of four dogs was inoculated with 40 nmol of the peptide antigen p35-LHRH in the presence of 150 µg Iscomatrix® as adjuvant. A similar dose of peptide in Iscomatrix® was administered 28 days following the first inoculation. A second group consisting of two dogs did not receive peptides and served as controls.
Results
The proliferative response of canine PBMC to CDV and CDV peptides
The possibility of using the F protein of CDV as a source of helper T-cell epitopes was first investigated using PBMC from beagle/foxhound dogs that had been vaccinated with live CDV vaccine (Canvac 3 in 1). Initial proliferation studies using PBMC obtained from animals vaccinated once with Canvac 3 in 1 proliferated poorly in response to CDV. The in vitro conditions used for these preliminary experiments with canine PBMC were similar to those used for our previous experiments with mice21 in which animals were inoculated in the footpad with synthetic peptide emulsified in complete Freund's adjuvant (CFA) and the draining lymph node cells then used as a source of lymphocytes in proliferation assays.
In light of the poor SI achieved with canine PBMC using conditions established for mice, we reasoned that different assay conditions for determining canine T-cell activity may be required because of: (i) the different species and source of lymphocytes; (ii) the different inoculation regimes used; and (iii) the fact that the dogs received a vaccine containing three live viruses compared with the situation in mice where we used a single peptide as inoculating antigen.
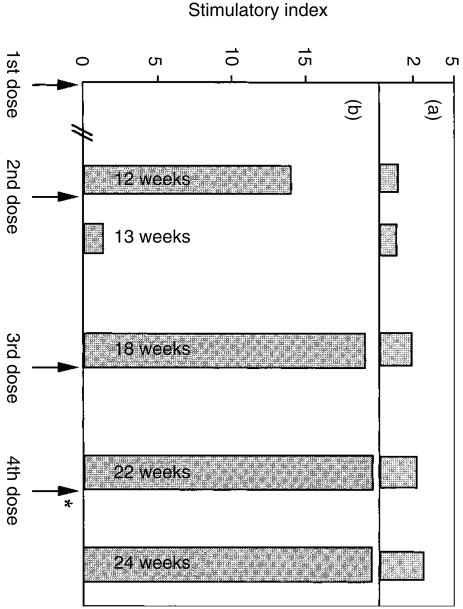
In an attempt to optimize the proliferation assay, we investigated the effect of time elapsed between vaccination with Canvac 3 in 1 and the isolation of PBMC for in vitro proliferation assays. It was found (Fig. 2) that PBMC obtained from dogs between 4 and 6 weeks following inoculation with Canvac 3 in 1 vaccine responded well in vitro to killed CDV. PBMC obtained 1 week following adminstration of the second dose of Canvac 3 in 1 (i.e. at the 13-week time-point) did not respond well. These results suggested that reactive T cells are not present in sufficiently large numbers in the peripheral blood shortly after vaccination. For this reason, PBMC for further experiments were not obtained until at least 4 weeks after vaccination with live CDV.
Figure 2.
Proliferative response of canine peripheral blood mononuclear cells (PBMC) obtained from one representative dog primed in vivo with Canvac 3 in 1 vaccine and restimulated in vitro with either canine distemper virus (CDV) or with 94 individual CDV–fusion protein (CDV-F) peptides. In total, four beagle/foxhound dogs vaccinated with Canvac 3 in 1 were bled at various time-points following vaccination, and their PBMC were assessed for proliferation when restimulated with each of 94 overlapping peptide (panel a) or with heat-killed CDV (panel b). PBMC (105 per well) were cultured in 96-well round-bottom plates for 7 days in the presence of different concentrations of heat-killed CDV (0·012 µg−12 µg) or peptide (2–8 nmol). The arrows indicate the time-points at which the dogs received Canvac 3 in 1 and the asterisk indicates vaccination with heat-killed CDV instead of with Canvac 3 in 1. The bars represent the proliferative response at the time-point indicated in the respective bars. The time-points shown in the bars indicate the period elapsing between the first dose of vaccine and obtaining PBMC. Proliferative responses from a representative dog (dog no. 18) are presented here, other dogs had very similar patterns. The stimulatory index (SI) values indicated in panel (a) represent the highest SI obtained with any particular peptide, e.g. p2 and p8 out of the 94 that were tested; most peptides exhibited no stimulatory activity.
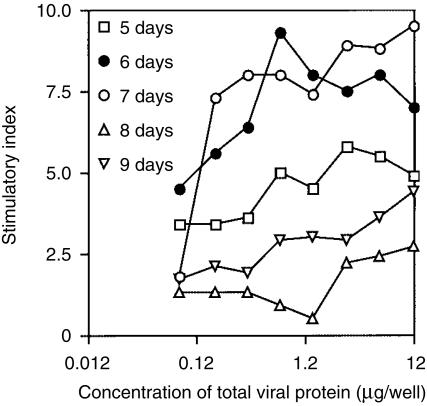
The optimum time required for culturing PBMC in the presence of antigen was also determined, and the results of this experiment (Fig. 3) demonstrated that a 6- or 7-day culture period gave the best results.
Figure 3.
Effect of culture period on the stimulatory indices (SI) obtained with canine peripheral blood mononuclear cells (PBMC). Duplicate cultures of PBMC (105 per well), obtained from a beagle/foxhound dog that had been vaccinated twice with Canvac 3 in 1, were restimulated with heat-killed canine distemper virus (CDV) (0·012 µg-12 µg) for different periods of time: (□) 5 days, (•) 6 days, (○) 7 days, (▵) 8 days or (▿) 9 days. [3H]Thymidine ([3H]TdR) was added to wells 20 hr prior to the end of the culture period, cells were then harvested and the [3H]TdR content determined. The results are expressed as SI.
Having determined optimal conditions, PBMC were obtained from four beagle/foxhound dogs 6 weeks following a second dose of Canvac 3 in 1 and tested against either CDV or each of the 94 overlapping peptides representing the complete sequence of CDV-F protein. The results of this experiment (Fig. 4) demonstrated that the PBMC proliferated in response to CDV, but the SI values varied from dog to dog. In contrast, poor and inconsistent proliferation was observed to only a few of the 94 peptides tested with PBMC obtained at any of the time-points following vaccination with Canvac 3 in 1 (Fig. 2). Low stimulatory responses observed with these peptides did not improve when different doses of the peptides were used for in vitro stimulation. Peptides that were most frequently observed to be stimulatory were p2, p4 and p8–12.
Figure 4.
Variation in immune responses to canine distemper virus (CDV) from CDV-primed dogs. Peripheral blood mononuclear cells (PBMC) were isolated from beagle/foxhound dogs that had been vaccinated twice with Canvac 3 in 1. PBMC (105) were restimulated with different concentrations of heat-killed CDV and cultured for 7 days. [3H]Thymidine ([3H]TdR) was added to wells 20 hr prior to the end of the culture period. Cells were then harvested and the proliferative responses expressed as stimulatory indices (SI).
A third dose of Canvac 3 in 1 was then administered to these animals in an attempt to increase the number of circulating Th cells specific for CDV. PBMC were isolated 4 weeks later and their ability to proliferate in response to the peptides was again assessed. Although some stimulatory activity was observed with a few of the peptides, the results were not consistent (data not shown). These results were surprising because it is known that CDV-F provides protective immunity against CDV17 and it is assumed that it must therefore contain epitopes recognized by Th cells.
CDV is known to be lymphotropic22 and it is therefore possible that by vaccinating animals with live virus, insufficient numbers of CDV-specific T cells were detectable in the peripheral blood. To circumvent this potential problem, the same dogs received a dose of heat-killed CDV and 2 weeks later PBMC were examined for proliferation. Again, no significant proliferation to any of the peptides was observed.
Expansion of peptide-specific helper T cells by in vitro stimulation of PBMC with peptides
Because repeated vaccinations did not increase the numbers of T cells specific for CDV-F peptides in peripheral blood, an attempt was made to expand this population of T cells by in vitro stimulation. PBMC obtained 2 weeks after inoculation with heat-killed CDV were pulsed in vitro with a mixture of all 94 peptides and then cultured for 7 days in the absence of peptide. At the end of this culture period the cells were tested in a proliferation assay against each of the 94 individual peptides. Using these experimental conditions, high levels of proliferation were observed with many of the peptides and each of the animals responded to a number of common peptides (Fig. 5). Most of the peptides recognized by dogs 18, 19, 20 and 21 appeared to occur in a cluster consisting of peptides 21–40. The experiment was repeated by obtaining blood from the same dogs 3 weeks later. This time, PBMC obtained from the dogs were pulsed in vitro with either a pool containing all 94 peptides or a pool containing peptides 21–40 only. The results of this experiment (Table 1) confirmed that the pool of peptides 21–40 contained the majority of peptides within the sequence of CDV-F that were stimulatory. In three out of four dogs, in vitro stimulation with the pool of peptides 21–40 resulted in higher proliferative responses to the individual peptides.
Figure 5.
Stimulatory indices (SI) of an expanded population of cells derived from peripheral blood mononuclear cells (PBMC). Beagle/foxhound dogs primed in vivo three times with Canvac 3 in 1 and finally with heat-killed canine distemper virus (CDV) were bled 2 weeks after the last inoculation. PBMC were isolated and pulsed in vitro with all 94 peptides and cultured for 7 days. Viable cells (2 × 104) were then restimulated with 4 nmol of each of the 94 overlapping peptides in the presence of γ-irradiated autologous PBMC (105) as a source of antigen-presenting cells (APC) in a total volume of 150 µl of canine TCM (150 µl of RPMI supplemented with 10% (v/v) pooled heat-inactivated canine serum, 2 mm glutamine, 2 mm sodium pyruvate, 0·1 mm 2-mercaptoethanol, 30 µg/ml of gentamicin, 100 IU/ml of penicillin and 100 µg/ml of streptomycin) and incubated for 7 days. Proliferative responses from duplicate wells, and in some instances duplicate plates, were measured and expressed as SI. SI values of ≥ 2 have been plotted. The x-axis denotes the peptide numbers, p1 corresponds to the peptide representing the first 17 amino acids of the sequence of CDV-F, p2 corresponds to the sequence 8–22, etc. The arrow indicates the cleavage site between F2 and F1.
Table 1.
Stimulatory indices of expanded population of cells to individual canine distemper virus fusion protein (CDV-F) peptides
| Peptide pool used to select PBMCs from vaccinated dogs | ||||||||
|---|---|---|---|---|---|---|---|---|
| Dog 18 | Dog 19 | Dog 20 | Dog 21 | |||||
| Peptide used in proliferation assay | 1–94 | 21–40 | 1–94 | 21–40 | 1–94 | 21–40 | 1–94 | 21–40 |
| p8* | 3† | 1·8 | ||||||
| p10 | 2 | 1·5 | ||||||
| p12 | 1·8 | |||||||
| p22 | 4·8 | 5·7 | ||||||
| p24 | 4·5 | 2·3 | 4·7 | |||||
| p25 | 2·3 | 4·2 | 5·4 | 6 | 15·2 | 2·4 | 6·3 | 7 |
| p27 | 6·9 | 7·8 | 15·7 | 11·8 | 3 | 3·6 | 7·8 | |
| p29 | 3·6 | 6·3 | 7·9 | 5·6 | 3·5 | 7·1 | 13·8 | |
| p33 | 2·8 | 4·1 | 1·9 | 3·4 | 13·1 | |||
| p35 | 4 | 3·8 | 2 | 6·7 | 1·8 | 3·9 | 5·9 | |
| p36 | 3 | 7·5 | 2·7 | 3 | 5 | 7·6 | 13·6 | |
| p37 | 1·7 | 2·7 | 1·5 | 4·1 | 1·5 | 3·2 | ||
| p38 | 2·2 | 4·4 | 15 | |||||
| p39 | 3·2 | |||||||
| p47 | 5·2 | 4·8 | ||||||
| p62 | 8·8 | 4·6 | 4·3 | |||||
| p64 | 2·5 | 2·5 | ||||||
| p68 | 9 | |||||||
| p75 | 2·4 | 4·8 | ||||||
Beagle/foxhound dogs primed in vivo three times with Canvac 3 in 1 vaccine and once with heat-killed canine distemper virus (CDV) were bled 5 weeks after the last inoculation. Peripheral blood mononuclear cells (PBMC) were isolated and pulsed with a pool of peptides containing all 94 peptides, or with a pool containing peptides 21–40, and then cultured for 7 days to obtain an expanded population of peptide-specific cells. After the expansion step, viable cells were restimulated in vitro with individual peptides from the respective peptide pools in the presence of γ-irradiated autologous PBMC (105) as a source of antigen-presenting cells (APC) and incubated for 7 days.
Peptides, 17 amino acids in length and overlapping by 10 residues, were used for the in vitro proliferation assay. Peptide 8 represents sequence 50–66 of CDV-F, peptide 10 sequence 64–80, etc. Only peptides to which the cells responded are tabulated here.
The results of proliferation assays are expressed as the stimulatory index (SI) and were calculated as the ratio of the counts of the antigen containing culture to medium control. SI values of ≥ 1·5 are shown.
Proliferation assays carried out in different breeds of dogs with CDV-F peptides
Having optimized the proliferation assay to a point where significant T-cell proliferation was observed, we determined whether the CDV-F peptides stimulated T cells in other breeds of dogs. Dogs were vaccinated only once with Canvac 3 in 1 vaccine and PBMC were isolated 4–8 weeks later. The cells were pulsed in vitro with a pool of all those peptides that were stimulatory in beagle/foxhound dogs and cultured for 7 days after which they were assessed for proliferation in response to the individual peptides. The results of this experiment (Fig. 6) demonstrated that each of the dogs responded to at least two of the peptides previously identified as being antigenic for PBMC obtained from beagle/foxhound dogs. In addition, it was observed that most of these dogs responded to a cluster of peptides at the N-terminus of CDV-F.
Figure 6.
Stimulation indices of an expanded population of cells derived from peripheral blood mononuclear cells (PBMC) of different breeds of dogs. An expanded population of peptide-specific cells were derived from PBMC of different breeds that had been primed in vivo once with Canvac 3 in 1. These cells were pulsed in vitro with a pool of peptides stimulatory for beagle/foxhound dogs. Cells (2 × 104) were then restimulated with 4 nmol of each of the 94 overlapping peptides of canine distemper virus fusion protein (CDV-F) in the presence of γ-irradiated autologous PBMC (105) as a source of antigen-presenting cells (APC). Following incubation in a total volume of canine TCM (150 µl of RPMI supplemented with 10% (v/v) pooled heat-inactivated canine serum, 2 mm glutamine, 2 mm sodium pyruvate, 0·1 mm 2-mercaptoethanol, 30 µg/ml of gentamicin, 100 IU/ml of penicillin and 100 µg/ml of streptomycin) for 7 days, proliferative responses from duplicate wells were measured and expressed as stimulatory index (SI). SI values of ≥ 2 were plotted. The peptide numbers are indicated on the x-axis.
Immunogenicity of a putative Th-epitope in a peptide vaccine
One of the stimulatory peptides – p35 – which induced proliferation of PBMC in most dogs, was selected for examination of its ability to provide T-cell help for antibody production against LHRH. The peptide vaccine was assembled as the sequence TAAQITAGIALHQSNLNEHWSYGLRPG in which the Th-epitope is placed at the N-terminus. We have shown in a previous study that this configuration was very effective in eliciting anti-LHRH antibodies in mice.21
Beagle/foxhound dogs used for the study had been previously inoculated with Canvac 3 in 1 vaccine. Each of the four dogs inoculated with two doses of the peptide vaccine, p35-LHRH, elicited high titres of anti-LHRH antibodies (Fig. 7). The two dogs that did not receive any vaccine had no detectable anti-LHRH antibody at any time. The proliferative responses of PBMC obtained from peptide-inoculated dogs (Fig. 7), demonstrate that the peptide vaccine containing p35 activates T cells, which then provide help for the generation of antibodies to LHRH. Significantly, PBMC from peptide-inoculated dogs did not require the cell-expansion step that was necessary for detection of proliferative responses to the peptide antigens following inoculation with Canvac 3 in 1 vaccine.
Figure 7.
Immune responses induced by a synthetic peptide-based vaccine, p35-luteinising hormone releasing hormone (LHRH), in dogs. The peptide was formulated in Iscomatrix® and four dogs of beagle/foxhound background (dog nos: 187, 196, 198 and 217) were inoculated twice with 40 nmol of the peptide, with 28 days between doses. Two dogs, no. 159 and no. 190, were not inoculated and served as controls. (a) Anti-LHRH antibody titres of sera obtained 4 weeks following the first (solid bars) and 2 weeks following the second (shaded bars) dose of peptide vaccine. (b) Proliferative responses of PBMC when restimulated in vitro with p35-LHRH (dark bars), p35 (shaded bars) or LHRH (open bars), 2 weeks following the second dose of peptide vaccine.
Discussion
The incorporation of defined synthetic Th-cell epitopes into vaccines has a number of advantages over the use of carrier proteins, not the least of which could be an avoidance of carrier-induced epitope suppression, especially if no antibodies are elicited to the synthetic Th-epitope itself.21,23 A second rationale for this study is that if Th-epitopes are identified in proteins with which animals are already inoculated, then there is the opportunity to make use of an existing reservoir of primed and/or memory helper T cells using a separate vaccine containing one or more of those Th-epitopes.
Initial optimization of the in vitro proliferation assays using canine PBMC was essential for identification of putative Th-epitopes. The time interval between vaccinations and obtaining peripheral blood as a source of PBMC for in vitro proliferation assays was found to be critical for detecting T-cell activity, possibly because very small quantities of live virus present in the vaccine requires this time to replicate to levels sufficient to stimulate an immune response. It is also possible that the insignificant levels of in vitro proliferation of PBMC observed when they were exposed to peptides may be a result of the lymphotropic property of CDV and as a consequence CDV-specific T cells may not be located in the periphery. The fact that immunization with heat-killed CDV did not improve responsiveness, however, tends to argue against this possibility. By inoculating dogs with three different viruses, the resulting Th-cell responses will presumably be directed against a large number of different viral proteins and consequently the frequency of any particular CDV-F peptide-specific Th cells in the periphery could be reasoned to be low.
The introduction of an in vitro stimulation step allowed the expansion of the peptide-specific Th-cell population, and measurable levels of proliferation with individual peptides were then observed. Other investigators19,24 have made similar observations in their efforts to identify murine Th-epitopes using peptide libraries. Muller et al.24 reported that mice immunized with MV proliferated in response to only one peptide from a library consisting of 108 overlapping peptides that encompassed the whole sequence of measles virus fusion protein (MV-F). In contrast, when mice were inoculated with individual peptides, 20 of these were identified as immunogenic by in vitro T-cell proliferation assays. In the current study, PBMC from dogs inoculated with peptide p35-LHRH did not require the expansion step for detection of proliferative responses to the immunizing peptide. This finding is in agreement with the above observations of Muller et al.,24 that inoculation with individual peptide antigens results in generation of higher frequencies of T cells with that specificity.
PBMC from outbred dogs that were restimulated in vitro with a pool of all 94 peptides responded predominantly to peptides 21–40, which cluster around the cleavage site of F1 and F2. One of these clusters, encompassing amino acids 148–213, is located at the C-terminus of F2 and the second cluster is located early in the N-terminal sequence of F1. The occurrence of Th epitopes around the cleavage site may be because these sequences are more exposed, allowing processing and presentation of the peptides by canine APCs. Muller et al.24 have described murine Th-epitopes occurring within MV-F but, except for one short sequence (amino acids 91–105), none were reported at the cleavage site. The difference in location of Th-epitopes within MV-F and CDV-F, proteins that share significant (67%) sequence homology,14 highlights the difficulties in extrapolating data from laboratory animals to the relevant target species. Partidos et al.25 have predicted the existence of a Th-epitope within the sequence of MV-F (amino acids 288–302) based on predictive algorithms. The peptide was demonstrated to be stimulatory in different strains of mice as well as in humans and corresponds to one of those described by Muller et al.24 Because MV-F and CDV-F share significant sequence homology it is not surprising that this particular sequence is represented in CDV-F by p58. This peptide has the sequence LSEVKGVIVHRLEAVSY, which compares very closely with the Th-epitope sequence (LSEIKGVIVHRLEGV) described by Partidos et al.25 and Muller et al.24 Nevertheless, we could detect no stimulatory effect of peptide 58 (LSEVKGVIVHRLEAVSY) using canine PBMC. We do not know whether this lack of recognition of a sequence that is recognized in mice and humans is: (i) caused by an absence of the appropriate processing machinery in dogs to generate this epitope; or (ii) whether the sequence is not bound by canine MHC class II molecules; or (iii) whether there is no appropriate canine T-cell receptor capable of recognizing the peptide–canine class II MHC complex.
The observation that Th-cell epitopes are clustered around the cleavage site of CDV-F was confirmed by carrying out the in vitro expansion step with a peptide pool containing only peptides 21–40. We observed that PBMC obtained from all four dogs could be expanded in culture with this peptide pool Furthermore, the level of proliferation in response to individual peptides within the pool of peptides 21–40 did not change significantly, whether the in vitro expansion was carried out using the pool containing peptides 21–40 or all 94 peptides. For practical purposes this is an important result because there is no evidence for competition with the 21–40 peptide pool by other peptides present in the larger pool. This property could be of use in the event that a pool of Th-epitopes is to be used for development of synthetic vaccines. Also of practical importance was the finding that the same peptides were similarly active in other breeds of dogs. This could mean that a mixture of two to three Th-epitopes in a vaccine may be able to cover most of the MHC background of the dog population. With a view to development of peptide-based vaccines, it is perhaps also important that the two main clusters of canine Th-epitopes – peptides 22–29 (amino acids 148–213) and peptides 32–39 (amino acids 212–283) – occur within sites of CDV-F that are conserved between the wild-type and the vaccine strain of CDV, as well as among most paramyxoviruses.14,26
We have demonstrated in this study that by combining in vitro stimulation with in vivo inoculations it is possible to identify Th-epitopes in large animals where it is normally not possible to obtain the lymphoid organs for in vitro proliferation assays nor to immunize them with a panel of peptides. We have also demonstrated that putative Th-epitopes identified by this method when incorporated into synthetic vaccines provide help for potent antibody responses.
Acknowledgments
This work was supported by the Cooperative Research Centre for Vaccine Technology and grant 980664 from the National Health & Medical Research Council of Australia. We wish to thank Ms Caterina Colantini and Ms Andrea Cirnigliaro for their assistance in bleeding and inoculating the dogs.
Abbreviations
- APC
antigen-presenting cells
- BSA
bovine serum albumin
- CDV
canine distemper virus
- CDV-F
canine distemper virus fusion protein
- ELISA
enzyme-linked immunosorbent assay
- LHRH
luteinising hormone releasing hormone
- MV
measles virus
- MV-F
measles virus fusion protein
- PBMC
peripheral blood mononuclear cells
- PBS
phosphate-buffered saline
- PBST
phosphate-buffered saline containing 0·05% Tween-20
- Th
T helper
References
- 1.Meloen RH, Turkstra JA, Lankhof H, Puijk WC, Schaaper WM, Dijkstra G, Wensing CJ, Oonk RB. Efficient immunocastration of male piglets by immunoneutralization of GnRH using a new GnRH-like peptide [published erratum appears in Vaccine 1994; 12:1152] Vaccine. 1994;12:741–6. doi: 10.1016/0264-410x(94)90226-7. [DOI] [PubMed] [Google Scholar]
- 2.Beekman NJ, Schaaper WM, Turkstra JA, Meloen RH. Highly immunogenic and fully synthetic peptide-carrier constructs targetting GnRH. Vaccine. 1999;17:2043–50. doi: 10.1016/s0264-410x(98)00407-1. 10.1016/S0264-410X(98)00407-1. [DOI] [PubMed] [Google Scholar]
- 3.Zeng W, Jackson DC, Murray J, Rose K, Brown LE. Totally synthetic lipid-containing polyoxime peptide constructs are potent immunogens. Vaccine. 2000;18:1031–9. doi: 10.1016/s0264-410x(99)00346-1. 10.1016/S0264-410X(99)00346-1. [DOI] [PubMed] [Google Scholar]
- 4.Brandt ER, Sriprakash KS, Hobb RI, Hayman WA, Zeng W, Batzloff MR, Jackson DC, Good MF. New multi-determinant strategy for a group A streptococcal vaccine designed for the Australian Aboriginal population. Nat Med. 2000;6:455–9. doi: 10.1038/74719. 10.1038/74719. [DOI] [PubMed] [Google Scholar]
- 5.Herzenberg LA, Tokuhisa T. Carrier-priming leads to hapten-specific suppression. Nature. 1980;285:664–7. doi: 10.1038/285664a0. [DOI] [PubMed] [Google Scholar]
- 6.Schutze MP, Leclerc C, Jolivet M, Audibert F, Chedid L. Carrier-induced epitopic suppression, a major issue for future synthetic vaccines. J Immunol. 1985;135:2319–22. [PubMed] [Google Scholar]
- 7.Su H, Morrison RP, Watkins NG, Caldwell HD. Identification and characterization of T helper cell epitopes of the major outer membrane protein of Chlamydia trachomatis. J Exp Med. 1990;172:203–12. doi: 10.1084/jem.172.1.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Williams KM, Bigley EC, III, Raybourne RB. Identification of murine B-cell and T-cell epitopes of Escherichia coli outer membrane protein F with synthetic polypeptides. Infect Immun. 2000;68:2535–45. doi: 10.1128/iai.68.5.2535-2545.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Levely ME, Bannow CA, Smith CW, Nicholas JA. Immunodominant T-cell epitope on the F protein of respiratory syncytial virus recognized by human lymphocytes. J Virol. 1991;65:3789–96. doi: 10.1128/jvi.65.7.3789-3796.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mustafa AS, Shaban FA, Abal AT, Al-Attiyah R, Wiker HG, Lundin KE, Oftung F, Huygen K. Identification and HLA restriction of naturally derived Th1-cell epitopes from the secreted Mycobacterium tuberculosis antigen 85B recognized by antigen-specific human CD4(+) T-cell lines. Infect Immun. 2000;68:3933–40. doi: 10.1128/iai.68.7.3933-3940.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Russell-Jones GJ, Sullivan JS, Geczy AF. Peptide sequences with strong stimulatory activity for lymphoid cells: implications for vaccine development. Vaccine. 1993;11:1310–5. doi: 10.1016/0264-410x(93)90100-c. [DOI] [PubMed] [Google Scholar]
- 12.Rimmelzwaan GF, Poelen MC, Meloen RH, Carlson J, UytdeHaag FG, Osterhaus AD. Delineation of canine parvovirus T cell epitopes with peripheral blood mononuclear cells and T cell clones from immunized dogs. J Gen Virol. 1990;71:2321–9. doi: 10.1099/0022-1317-71-10-2321. [DOI] [PubMed] [Google Scholar]
- 13.Vandevelde M, Zurbriggen A. The neurobiology of canine distemper virus infection. Vet Microbiol. 1995;44:271–80. doi: 10.1016/0378-1135(95)00021-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barrett T, Clarke DK, Evans SA, Rima BK. The nucleotide sequence of the gene encoding the F protein of canine distemper virus: a comparison of the deduced amino acid sequence with other paramyxoviruses. Virus Res. 1987;8:373–86. doi: 10.1016/0168-1702(87)90009-8. [DOI] [PubMed] [Google Scholar]
- 15.Merz DC, Scheid A, Choppin PW. Importance of antibodies to the fusion glycoprotein of paramyxoviruses in the prevention of spread of infection. J Exp Med. 1980;151:275–88. doi: 10.1084/jem.151.2.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Graves MC, Silver SM, Choppin PW. Measles virus polypeptides synthesis in infected cells. Virology. 1978;86:254–63. doi: 10.1016/0042-6822(78)90025-9. [DOI] [PubMed] [Google Scholar]
- 17.Norrby E, Utter G, Orvell C, Appel MJ. Protection against canine distemper virus in dogs after immunization with isolated fusion protein. J Virol. 1986;58:536–41. doi: 10.1128/jvi.58.2.536-541.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sad S, Chauhan VS, Arunan K, Raghupathy R. Synthetic gonadotrophin-releasing hormone (GnRH) vaccines incorporating GnRH and synthetic T-helper epitopes. Vaccine. 1993;11:1145–50. doi: 10.1016/0264-410x(93)90077-b. [DOI] [PubMed] [Google Scholar]
- 19.Obeid OE, Partidos CD, Steward MW. Identification of helper T cell antigenic sites in mice from the haemagglutinin glycoprotein of measles virus. J Gen Virol. 1993;74:2549–57. doi: 10.1099/0022-1317-74-12-2549. [DOI] [PubMed] [Google Scholar]
- 20.Fitzmaurice CJ, Brown LE, McInerney TL, Jackson DC. The assembly and immunological properties of non-linear synthetic immunogens containing T-cell and B-cell determinants. Vaccine. 1996;14:553–60. doi: 10.1016/0264-410x(95)00217-o. 10.1016/0264-410X(95)00217-O. [DOI] [PubMed] [Google Scholar]
- 21.Ghosh S, Jackson DC. Antigenic and immunogenic properties of totally synthetic peptide-based anti-fertility vaccines. Int Immunol. 1999;11:1103–10. doi: 10.1093/intimm/11.7.1103. [DOI] [PubMed] [Google Scholar]
- 22.Loffler S, Lottspeich F, Lanza F, Azorsa DO, ter Meulen V, Schneider-Schaulies J. CD9, a tetraspan transmembrane protein, renders cells susceptible to canine distemper virus. J Virol. 1997;71:42–9. doi: 10.1128/jvi.71.1.42-49.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brons NH, Blaich A, Wiesmuller KH, Schneider F, Jung G, Muller CP. Hierarchic T-cell help to non-linked B-cell epitopes. Scand J Immunol. 1996;44:478–84. doi: 10.1046/j.1365-3083.1996.d01-336.x. [DOI] [PubMed] [Google Scholar]
- 24.Muller CP, Bunder R, Mayser H, et al. Intramolecular immunodominance and intermolecular selection of H2d-restricted peptides define the same immunodominant region of the measles virus fusion protein. Mol Immunol. 1995;32:37–47. doi: 10.1016/0161-5890(94)00132-k. [DOI] [PubMed] [Google Scholar]
- 25.Partidos CD, Steward MW. Prediction and identification of a T cell epitope in the fusion protein of measles virus immunodominant in mice and humans. J Gen Virol. 1990;71:2099–105. doi: 10.1099/0022-1317-71-9-2099. [DOI] [PubMed] [Google Scholar]
- 26.Cherpillod P, Beck K, Zurbriggen A, Wittek R. Sequence analysis and expression of the attachment and fusion proteins of canine distemper virus wild-type strain A75/17. J Virol. 1999;73:2263–9. doi: 10.1128/jvi.73.3.2263-2269.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]