Abstract
In addition to macromolecular interactions that provide co-stimulation during antigen-presenting cell (APC) and CD4+ T-cell conjugation, covalent chemical events between specialized ligands have been implicated in T-cell co-stimulation. These take the form of transient Schiff base formation between carbonyls and amines expressed on APC and T-cell surfaces. Small Schiff base-forming molecules, such as tucaresol, can substitute for the physiological donor of carbonyl groups and provide co-stimulation to T cells, thereby functioning as orally active immunopotentiatory drugs. The Schiff base co-stimulatory pathway in T cells has been partially characterized in terms of changes in Na+ and K+ transport, and activation of the mitogen activated protein kinase (MAPK) ERK2. In the present study, the effects of Schiff base co-stimulation by tucaresol on the T-cell receptor (TCR)-dependent pathway leading to Ca2+ release were investigated. Schiff base co-stimulation by tucaresol was found to prime for enhanced TCR-dependent phospholipase C-γ phosphorylation, inositol 1,4,5-triphosphate production, and Ca2+ mobilization that correlated with functional enhancement of interleukin-2 production in primary T cells. The effects on Ca2+ occurred comparably in Jurkat and primary CD4+ T cells responding to anti-CD3 monoclonal antibody. Enhancement of the Ca2+ response required a 10-min priming period and was prevented by prior covalent ligation of cell-surface free amino groups by sulpho-N-hydroxy succinimido-biotin; clofilium-mediated inhibition of tucaresol-induced changes in intracellular K+; and selective inhibition of the MAPK pathway. The data are consistent with a priming mechanism in which late co-stimulation-triggered events exert a positive influence on early TCR-triggered events. In additional studies of murine T cells expressing trans-gene TCRs, tucaresol was likewise shown to prime for enhanced Ca2+ mobilization in response to physiological TCR-engagement by MHC–peptide complexes.
Introduction
The response of CD4+ T helper (Th) lymphocytes to ligation of the clonally distributed T-cell receptor (TCR) is pivotally regulated by co-stimulatory signals, many of which are provided by macromolecular interactions during antigen-presenting cell (APC) to T-cell conjugation.1,2 In addition, transient covalent chemical events in the form of Schiff base formation between carbonyls and amines expressed on APC and T-cell surfaces have been implicated in the co-stimulatory mechanism.3–6 Small exogenous Schiff base-forming molecules such as tucaresol can substitute for the natural donor of carbonyl groups to provide a co-stimulatory signal to T cells.7 This form of co-stimulation activates Na+ and K+ transport7 and converges with TCR-dependent signalling at the level of the mitogen activated protein kinase (MAPK) ERK2.8 Previous studies have shown that simultaneous Schiff base-mediated co-stimulation does not affect the TCR-dependent Ca2+ signal. Schiff base-mediated co-stimulation by tucaresol, in protocols where the co-stimulatory signal precedes the TCR-dependent signal, substantially enhances TCR-dependent interleukin-2 (IL-2) production, favouring a Th1 profile of cytokine production.7 Because it is orally bioavailable and systemically active, tucaresol provides a unique mechanism-based therapeutic strategy to potentiate the immune system.9–12
Intracellular free Ca2+ is a principal second messenger in mammalian cells generated in response to a wide range of receptor-mediated events initiated at the plasma membrane.13 TCR signal transduction events involving Ca2+ share many of the general characteristics of such cascades.14–16 As a result of TCR activation, phospholipase C-γ1 (PLCγ1) is recruited to the plasma membrane through the action of p36 LAT, an adaptor protein, where it is activated by tyrosine phosphorylation. This leads to the hydrolysis of membrane lipids and release of the second messengers inositol 1,4,5, triphosphate (IP3) and diacylglycerol (DAG). IP3 binds to p59fyn phosphorylated receptors on the membrane of the endoplasmic reticulum to stimulate the mobilization of Ca2+ from intracellular stores. This early increase in intracellular free Ca2+ is followed by a sustained phase of mobilization dependent on an influx of extracellular Ca2+ through channels not yet precisely characterized. A principal downstream target of cytoplasmic Ca2+ is the Ca2+/calmodulin-dependent serine phosphatase, calcineurin.17 A constitutively active form of calcineurin mimics the effects of Ca2+ ionophore in promoting T-cell activation, and in conjunction with activated Ras, is sufficient to induce IL-2 transcription.18 Calcineurin acts on nuclear factor of activated T cells (NFAT) transcription factors that translocate to the nucleus and interact with activator protein-1 (AP-1) binding co-operatively to the NFAT/AP-1 site in the IL-2 gene, providing one positive pathway to the induction of IL-2.19 However, other Ca2+/calmodulin-dependent pathways are also likely to be important in regulating IL-2 production and the recently identified CaM-kinase has also been shown to function in this role.20 Recent evidence indicates a role for the intensity of calcium signalling in diverting T-cell responses towards Th1, providing an intracellular signalling mechanism by which immune deviation may occur.21 Because intracellular free Ca2+ is central to the transmission of TCR-dependent signals, and may influence Th1 polarization, this aspect of T-cell activation could provide a pivotal target for co-stimulatory signals regulating the outcome of TCR ligation. In the present study, the effects of prior Schiff base-mediated co-stimulation of T cells on the subsequent triggering of the TCR-dependent Ca2+ signalling pathway was investigated.
Materials and methods
Preparation of human T cells and T-cell lines
Blood was obtained from healthy human volunteers and peripheral blood mononuclear cells (PBMCs) were isolated using Lymphoprep (Life Technologies, Paisley, UK). CD4+ T cells were prepared by immunomagnetic negative selection. Cells were incubated with saturating concentrations of anti-CD14, anti-DR, anti-CD20, anti-CD8 and anti-CD56 (Becton Dickinson, San Jose, CA) for 30 min at room temperature. Cells were washed twice and Dynal beads (Dynal, Oslo, Norway) coated with sheep anti-mouse immunoglobulin G (IgG) and mouse anti-human CD8 were added, mixed and incubated for 15 min at room temperature. Cells were then incubated with a Dynal magnet and non-magnetic cells were harvested. The separation process was repeated before the cells were washed twice and resuspended in 30 mm HEPES-buffered RPMI-1640 (Life Technologies, UK). The resultant population was 90% or greater CD4+ cells with the remaining contaminating cells (null cells) negative for the depletion markers. Jurkat J6 cells were maintained in continuous culture in RPMI-1640 medium containing penicillin and streptomycin supplemented with 10% fetal bovine serum (Flow Laboratories, Calne, UK).
Preparation of murine T cells
Mice transgenic for the DO11.10 αβTCR22 specific for the ovalbumin peptide (OVA323–339) were maintained on a BALB/c genetic background. Heterozygous mice more than 75% positive for Vβ expression by flow cytometric (FCM) analysis were maintained in isolators. BALB/c mice were obtained from Charles River Breeding Laboratories (Margate, UK). DO11.10 spleens were collected, and pooled cell suspensions were subjected to red blood cell lysis. CD4+ T cells were purified using a CD4 enrichment column (R + D Systems, Abingdon, UK) according to the manufacturer's instructions. This yielded a population that was 90% or greater CD4+ murine T cells.
T-cell stimulation
TCR-CD3 stimulation was carried out by first adding cross-linking goat anti-mouse IgG antibody (Sigma, Poole, UK) followed 1 min later by mouse anti-CD3 monoclonal antibody (mAb; OKT3, American Type Culture Collection, Rockville, MD) at the specified concentration. This provided a precise zero time-point for anti-CD3 ligation. Tucaresol co-stimulation was performed by preincubating cells for 10 min at 37°. Simultaneous co-stimulation was achieved by adding tucaresol at the same time as the cross-linking antibody.
Stimulation of naïve murine T cells was performed using T-depleted BALB/c splenocytes (APC) pulsed for 1 hr with 20 µm OVA peptide (323ISQAVHAAHAEINEAGR339) at 37°. These were pelleted with T cells at a 1 : 1 ratio using brief centrifugation at 300 g. Following a 3-min incubation period, the cells were analysed for Ca2+ mobilization as described below. Control T cells were activated using non-peptide-pulsed APC.
The selective inhibitor of MEK, 2-(2′-amino-3′methoxyphenyl)-oxanaphthalen-4-one (provided by David Miller, GlaxoSmithKline, Stevenage, UK) was added 30 min prior to tucaresol at a concentration of 25 µm. Sulpho-N-hydroxy succinimido-biotin (S-NHS biotin) (Pierce and Warriner, Tattenhall, UK) was added at a concentration of 100 µm for 1 hr to ligate reactive cell surface amino groups before washing the cells and subjecting them to tucaresol treatment. Clofilium tosylate (Research Biochemicals International, Natick, MA), a selective inhibitor of clofilium-sensitive K+ channels, was added at a concentration of 30 µm 1 min prior to tucaresol treatment.
Measurement of TCR-dependent phosphorylation of PLCγ
Jurkat cells were suspended in 30 mm HEPES-buffered RPMI and prewarmed at 37° for 10 min. Tucaresol was added at the concentrations shown with phosphate-buffered saline (PBS) as a vehicle control. Anti-mouse IgG (Sigma) was added at a final concentration of 20 µg/ml and incubated with the cells for 2 min. The anti-CD3 antibody (Clone OKT3, ATCC) was added at a final concentration of 20 µg/ml for 1 min. Cells were snap frozen in liquid nitrogen and then lysed in 1% n-octyl glucoside, 10 mm Tris pH 8·0, 50 mm NaCl, 10 mm iodacetamide, 10 mm sodium orthovanadate, 2 mm ethylenediaminetetraacetic acid (EDTA), 1 mm phenylmethylsulphonyl fluoride (PMSF), 0·076 TIU/ml aprotinin, 10 µg/ml chymostatin, 10 µg/ml leupeptin and 10 µg/ml pepstatin (Sigma). The lysate was separated from insoluble material by centrifugation for 5 min at 11 000 g. Following the addition of sample loading buffer (125 mm Tris pH 6·8, 4% (w/v) sodium dodecyl sulphate (SDS), 20% (w/v) glycerol, 0·2 m dithiothreitol (DTT), 0·013% (w/v) bromophenol blue (Sigma), samples were boiled for 10 min at 100°, prior to loading the equivalent of 4 × 105 cells/track onto 4–20% gradient SDS–polyacrylamide gels. Proteins were transferred to polyvinyl difluoride membrane (Millipore, Watford, UK) by semi-dry electroblotting; membranes were blocked to prevent non-specific binding of detecting reagents using 5% bovine serum albumin (Sigma) in TBS. Phosphorylated PLCγ was detected using the pY783 polyclonal antibody (Biosource International, Nixelles, Belgium) followed by goat anti-rabbit horseradish peroxidase-conjugated antibody (Sigma) and then exposure to electrochemiluminescence (ECL) reagents (Amersham, UK). PLCγ protein was detected using mixed mAb (Upstate Biotechnology, Lake Placid, NY) followed by goat anti-mouse horse radish peroxidase-conjugated antibody (Sigma) and then exposure to ECL reagents (Amersham).
Measurement of IP3
Jurkat J6 cells (106) were incubated in 100 µl of 30 mm HEPES-buffered RPMI-1640 at 37° before the addition of 15 µg/ml goat anti-mouse IgG followed 1 min later by 15 µg/ml OKT3 for 1 min. Tucaresol prestimulation (50 µm and 300 µm) was for 10 min. Extraction was performed by the addition of 27 µl of 10% perchloric acid with incubation at 4° for 10 min. The samples were centrifuged at 12 000 g for 2 min and the supernatant was neutralized before measurement of IP3 content using a radioligand binding assay.23
Measurement of Ca2+ signalling
Jurkat J6, and CD4+ T cells were suspended in 30 mm HEPES-buffered RPMI-1640 and loaded with 3 µm Fluo-3 AM and 8 µm Fura-red AM (Molecular Probes Europe, Leiden, the Netherlands) for 30 min at 37° in the presence of pluronic F127 detergent (Sigma) at a final concentration of 0·05%. Cells were then washed and suspended at 5 × 105/ml and warmed to 37° for 10 min prior to use. The concentration of intracellular free Ca2+ was measured ratiometrically as a function of time using a Coulter XL-MCL flow cytometer (Coulter, Miami, FL). The mean ratio of Fluo-3/Fura-red fluorescence was recorded during the acquisition time–course and expressed graphically to indicate Ca2+ flux.
Measurement of p42/p44 MAPK activity
Following the experimental treatments, cells were lysed in 20 µl of extraction buffer containing glycerophosphate (50 mm), EGTA (1·5 mm), benzamidine (1 mm), DTT (1 mm), Na3VO4 (0·5 mm), PMSF (0·1 mm), pepstatin (1 µg/ml), aprotinin (10 µg/ml) and leupeptin (20 µm). After 15 min on ice, lysed cells were centrifuged at 14 000 g for 10 min at 4° and the supernatant was harvested. MAPK activity was measured using the Biotrak p42/p44 MAP Kinase Enzyme System (Amersham Life Science) as previously described.8 Briefly, 15 µl of lysate was used to determine [γ-33P]ATP phosphorylation of a specific substrate peptide under standard conditions for 30 min at 30° according to the manufacturer's instructions. Results were determined by liquid scintillation spectrometry and expressed as pmol of phosphate transferred per minute per 106 cells.
Measurement of IL-2 generation
PBMCs (1×106 in 200 µl) were activated by anti-CD3 coated onto tissue culture plastic at 10 µg/ml following pretreatment for 10 min in the absence or presence of tucaresol, ranging in concentration from 50 µm to 600 µm. After 24 hr, cell-free supernatants were collected and assayed for IL-2 content using commercial kits (Quantikine, R & D Systems). The amount of cytokine was calculated by reference to a simultaneously prepared standard curve.
Results
Schiff base co-stimulation primes for enhanced TCR-induced PLCγ phosphorylation
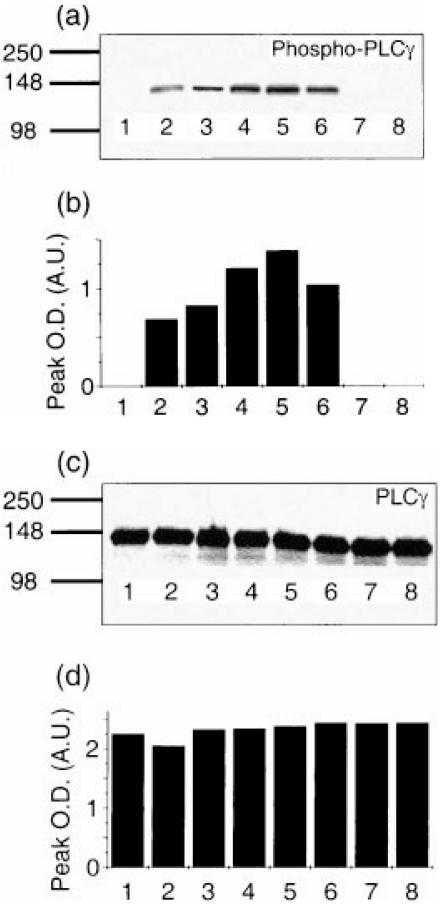
PLCγ phosphorylation is among the principal TCR-proximal signalling events essential in T-cell activation. Schiff base co-stimulation by tucaresol for a 10-min period prior to TCR stimulation by cross-linked anti-CD3 significantly enhanced TCR-dependent PLCγ phosphorylation in a dose-dependent manner in Jurkat cells (Fig. 1). The effect of tucaresol exhibiting a charactersitic bell-shaped dose–response with maximal enhancement at 100 µm. No phosphorylated PLCγ was present in unstimulated cells and tucaresol alone did not induce PLCγ phosphorylation.
Figure 1.
Differences in the phosphorylation of PLCγ following tucaresol treatment and anti-CD3 activation in Jurkat cells. Proteins from cell equivalents corresponding to 4 × 106 cells were resolved by 4–20% gradient SDS–PAGE, transferred to PVDF membrane and then probed with anti-phospho-specific PLCγ (a). Intensity of the bands was measured by densitometry and is shown in (b). The filter was stripped and re-probed with anti-PLCγ (c) to demonstrate that the differences were not due to different protein loading in the tracks (d). Unstimulated cells (lane 1) have no phosphorylated PLCγ, anti-CD3 activation induces PLCγ phosphorylation (lane 2), which is enhanced by 10 µm (lane 3), 50 µm (lane 4), 100 µm (lane 5) and 300 µm (lane 6) tucaresol with a bell-shaped dose–response profile. Tucaresol alone at 10 µm (lane 7) and 300 µm (lane 8) does not induce PLCγ phosphorylation. The data are representative of three separate experiments.
Schiff base co-stimulation by tucaresol primes for enhanced IP3 generation
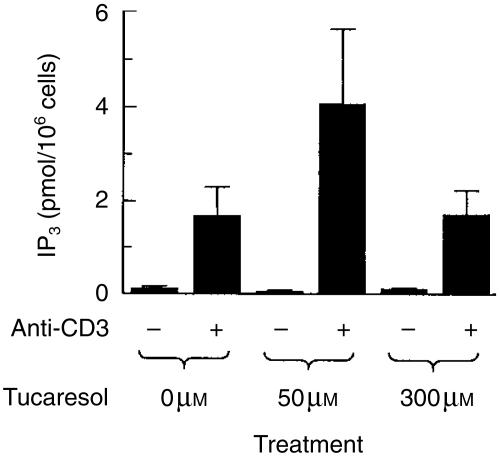
Activated PLCγ leads to the hydrolysis of membrane lipids and release of the second messengers IP3 and DAG. We therefore next investigated the effect of tucaresol priming on the production of IP3 as measured using a radioligand-binding assay. The generation of IP3 measured in response to stimulation with cross-linked anti-CD3 was substantially increased by a 10-min priming exposure to tucaresol (50 µm). At a higher tucaresol concentration (300 µm) no enhancement was observed, demonstrating the characteristic bell-shaped dose–response in IP3 priming (Fig. 2). Previously published negative results on IP3 were also obtained at the high (300 µm) dose.8
Figure 2.
Enhancement of anti-CD3-induced IP3 generation by tucaresol. Jurkat cells were treated with cross-linked anti-CD3 at a concentration of 5 µg/ml for 1 min. Pre-treatment with tucaresol at 50 µm, but not 300 µm, significantly increases the generation of IP3 in response to the activation stimulus. The basal IP3 level measured after 1 min of activation with the cross-linking antibody alone is unaffected by tucaresol treatment at either 50 µm or 300 µm. Data shown are means± SEM of three separate experiments.
Effect of Schiff base co-stimulation by tucaresol on the TCR-CD3-induced Ca2+ response in T lymphocytes
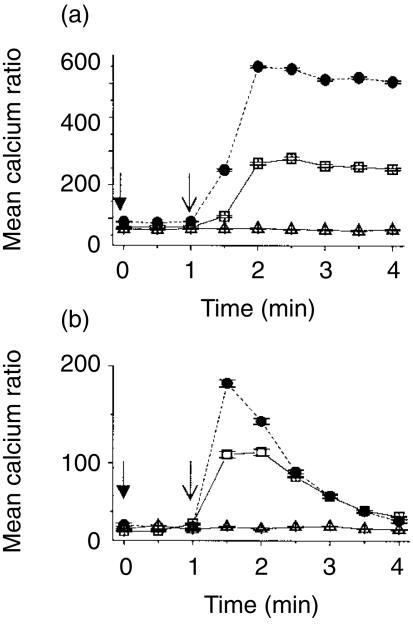
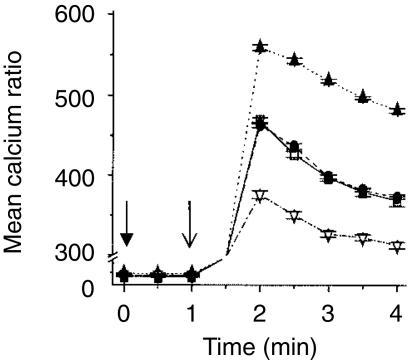
IP3 binds to p59fyn phosphorylated receptors on the membrane of the endoplasmic reticulum to stimulate the mobilization of Ca2+ from intracellular stores. The effect of tucaresol priming on TCR-dependent calcium release was therefore investigated next. Stimulation of the TCR complex with cross-linked anti-CD3 induced a rapid rise in intracellular free Ca2+ that peaked within 2 min and was sustained unchanged over the subsequent 2 min in Jurkat cells (Fig. 3a). Priming with tucaresol (50 µm) for 10 min prior to the TCR-CD3 directed stimulus resulted in a more rapid Ca2+ response to cross-linked anti-CD3. Co-stimulatory priming by tucaresol also elevated the TCR-CD3-dependent Ca2+ response to approximately twice the level of non-co-stimulated cells, and this enhancement was sustained throughout the 4-min period of observation (Fig. 3a). Tucaresol treatment given simultaneously with cross-linked anti-CD3 stimulation has been shown not to increase the TCR-dependent rise in intracellular free Ca2+ whether at high (300 µm)8 or lower (unpublished data) doses. In primary CD4+ T cells TCR stimulation by anti-CD3 produced an initial rapid rise in intracellular free Ca2+ followed by a gradual decline over the 4-min time–course. Tucaresol (50 µm) added 10 min before the TCR-directed stimulus induced a more rapid Ca2+ response that also peaked at a higher level (Fig. 3b). This enhanced signal declined over the 4-min time–course.
Figure 3.
The enhancement of calcium flux in T cells following tucaresol treatment. Cross-linked anti-CD3 induced elevation in intracellular calcium in (a) Jurkat cells and (b) human CD4+ T cells. IgG was added at the time indicated by (↓) and anti-CD3 at (↓). PBS control treatment (▵) shows no change in calcium concentration when compared to basal levels. There is a sustained rise with anti-CD3 in Jurkat cells and a more transient rise and gradual decline in CD4+ cells (□). 50 µm tucaresol added 10 min before the antibody treatments induces a more rapid and greater calcium mobilization that in Jurkat cells is maintained for the 4-min time–course (•). The data shown are the mean of three observations from one donor or batch of cells and are representative of three separate assessments.
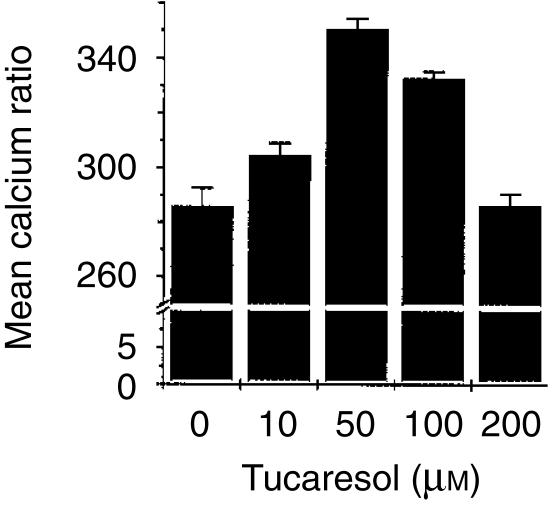
The data in Fig. 4 show that the effect of tucaresol on the TCR-dependent Ca2+ response in CD4+ primary T cells exhibits a bell-shaped curve. The data shown are maximum Ca2+ responses induced by the standard TCR-CD3 stimulus following priming by tucaresol at the concentrations indicated. A bell-shaped dose–response curve is highly characteristic of the immunopotentiatory effects of tucaresol.7
Figure 4.
Tucaresol has a bell-shaped dose–response profile of calcium flux enhancement in CD4+ T cells. A 10-min pretreatment with tucaresol at the concentration indicated results in an enhanced mean maximum intracellular calcium level with a bell-shaped dose–response curve. CD4+ cells were activated with cross-linked anti-CD3 at 15 µg per ml. The time taken to achieve the maximal response was 1 min and did not differ significantly between tucaresol-treated samples and controls.
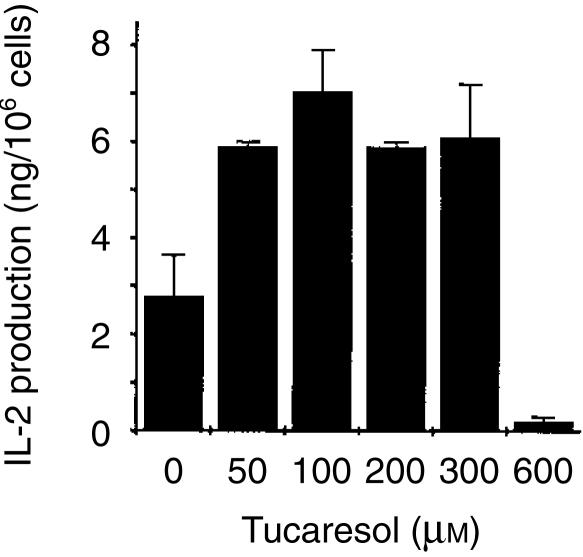
Effect of Schiff base co-stimulation by tucaresol on TCR-CD3 induced IL-2 generation in T lymphocytes
In order to establish the functional significance of the enhanced calcium signal, the effects of Schiff base co-stimulation by tucaresol on IL-2 production were examined over the same time–course and under the same conditions. A 10-min priming period by tucaresol was sufficient to enhance IL-2 production in response to CD3 ligation in PBMC. Enhancement was seen at 50, 100, 200 and 300 µm and inhibition at 600 µm (Fig. 5). This is a somewhat broader dose–response than seen in the calcium response and is consistent with the the following general features invariably observed for the tucaresol dose–response. In the absence of accessory cells the tucaresol bell-shaped curve is narrow and peaks at a lower concentration. In the presence of accessory cells the bell-shaped curve is broader and peaks at a higher concentration. Effects on MAPK in the absence of accessory cells (below and in previous studies8) exhibit a broader dose–response curve.
Figure 5.
Tucaresol has a bell-shaped dose–response profile of IL-2 enhancement in T cells. A 10-min pretreatment with tucaresol at the concentrations indicated results in an enhanced mean maximum IL-2 generation compared to cells not pretreated with tucaresol. This enhancement was evident at 50, 100, 200 and 300 µm concentrations of tucaresol. PBMC were activated with plastic coated anti-CD3 at 10 µg/ml. Cell-free supernatants were collected after 24 hr and subjected to an IL-2 ELISA. Data shown depict mean± SEM of triplicate samples and are representative of two experiments. Control samples not exposed to activating antibodies had no detectable IL-2 generation (data not shown).
Ligation of cell surface amines inhibits the tucaresol-induced enhancement of Ca2+ mobilization
Tucaresol is believed to act through Schiff base-formation on cell surface amines, mimicking events that occur physiologically. However, tucaresol can readily enter cells where it could exert effects at other loci. It is therefore important to know if the effects of co-stimulation by tucaresol on the TCR-CD3-induced Ca2+ pathway are dependent on covalent reactions with cell surface amino groups. This can be addressed by blockade of cell-surface Schiff base-forming amines through irreversible covalent ligation with S-NHS-biotin.7 The data in Fig. 6 show that this pretreatment prevented subsequent tucaresol priming for an enhanced TCR-CD3-induced Ca2+ signal. Interestingly, while S-NHS-biotin treatment alone had no effect on the TCR-CD3 induced Ca2+ signal, tucaresol priming of S-NHS-biotin-treated cells resulted in an inhibition of the TCR-CD3-dependent Ca2+ response. Conceivably, this could be due to diversion of tucaresol to another locus of action in cells with blocked cell-surface Schiff base-forming groups where its effects are inhibitory rather than potentiatory. Such inhibitory effects are known to occur with other Schiff base-forming aldehydes acting at the level of NFAT.24
Figure 6.
Ligation of cell surface amines with S-NHS biotin completely inhibits the tucaresol-induced enhancement of calcium flux in Jurkat cells. The enhancement of the anti-CD3-induced calcium response in Jurkat cells is completely inhibited by prior treatment for 1 hr with S-NHS biotin at a concentration of 100 µm. 5 µg/ml IgG (↓) and anti-CD3 (↓) were added at the times indicated and the treatment groups were as follows: Anti-CD3 alone (□), S-NHS Biotin/anti-CD3 (•), 50 µm tucaresol/anti-CD3 (▴) and S-NHS Biotin/50 µm tucaresol/anti-CD3 (▿). S-NHS biotin alone has no effect on the control anti-CD3 response, but in tucaresol-treated samples the calcium flux is less than in the controls, suggesting that there are additional effects with the combined stimulus.
Blocking the activity of clofilium-sensitive K+ channels inhibits the tucaresol-induced enhancement of Ca2+ mobilization
Tucaresol has been shown to produce a rapid and substantial fall in the level of intracellular K+ in T cells and this effect is inhibited by the ion-channel antagonist clofilium.7 This K+ channel antagonist also prevents tucaresol-induced enhancement of TCR-dependent IL-2 production. The data in Fig. 7 show that pretreatment of cells for 1 min with clofilium tosylate prevented the priming effects of tucaresol on the TCR-CD3-dependent Ca2+ signal. Clofilium tosylate also reduced the Ca2+ signal to anti-CD3 stimulation alone indicating that K+ fluxes are involved in the TCR-dependent Ca2+ signal independently of the effects of tucaresol. The initial rapid rise in response to CD3 cross-linking was less affected by clofilium than the subsequent sustained elevation, suggesting that clofilium-sensitive export of K+ may be more important in driving the influx of extracellular Ca2+ rather than early release of Ca2+ from intracellular stores.
Figure 7.
The enhanced calcium flux induced by tucaresol in Jurkat cells is inhibited by prior treatment with clofilium tosylate. Tucaresol-mediated enhancement of anti-CD3 induced calcium flux in Jurkat cells (□), is largely inhibited by pretreatment of cells with 30 µm clofilium tosylate for 1 min (▿). The initial sharp rise in calcium is less affected than the subsequent sustained phase, and this suggests that clofilium-sensitive K+ channels are implicated in the process whereby an influx of extracellular calcium follows the emptying of intracellular stores. The sustained calcium signal induced by anti-CD3 without tucaresol (▪) is also inhibited by clofilium tosylate pretreatment (▴), indicating that K+ flux is also important in TCR-mediated signalling in the absence of tucaresol.
Selective inhibition of MEK inhibits the tucaresol-induced enhancement of Ca2+ mobilization
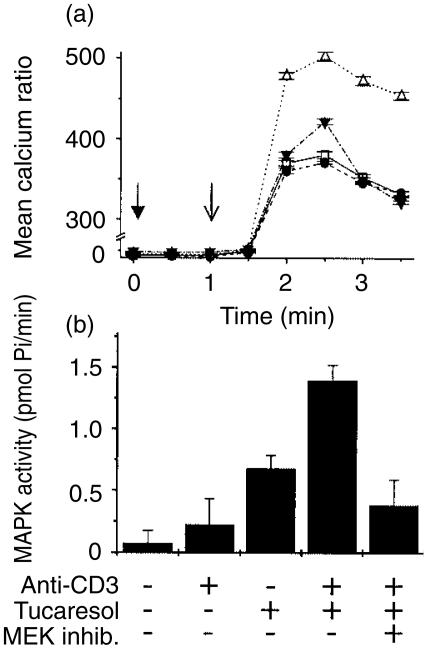
To determine further the dependency of tucaresol priming on intracellular events, the effects of selective inhibition of the MAPK pathway were investigated on priming of the TCR-CD3-dependent Ca2+ signal. This study employed the well-characterized selective inhibitor 2-(2′-amino-3′-methoxyphenyl)-oxanaphthalen-4-one.25,26 The data in Fig. 8(a)) show that inhibition of MEK partially prevented the priming effect of tucaresol on the TCR-CD3 Ca2+ response. The profile of inhibition indicates a partial inhibition of the enhanced initial rapid rise in Ca2+ and a more complete inhibition of the tucaresol-dependent sustained elevation of Ca2+. As expected, MEK inhibition produced no effect on the TCR-CD3-induced Ca2+ signal in the absence of priming by tucaresol. Inhibition of tucaresol-induced MAPK activation by the MEK inhibitor was also assessed in a functional assay of MAPK activation, as previous studies of this inhibitor were confined to band-shift assays.8 Prior incubation for 30 min with the MEK inhibitor (25 µm), before stimulation with cross-linked anti-CD3, produced substantial inhibition of MAPK activity in response to combined tucaresol and TCR-CD3 stimulation (Fig. 8b).
Figure 8.
The selective inhibitor of MEK partially prevents the enhancement of calcium mobilization induced by tucaresol. Inhibition of the tucaresol's enhancement of the anti-CD3-induced calcium response by the MEK inhibitor is shown in (a). Cells treated with anti-CD3 alone (□) or with anti-CD3 and 25 µm MEK inhibitor pretreatment (•) show an equivalent calcium rise. Tucaresol priming significantly enhances the anti-CD3 response (▵). The MEK inhibitor produces a partial inhibition of the initial rapid calcium rise induced by tucaresol/anti-CD3 and the sustained component of tucaresol-induced enhancement is completely inhibited (▾). Data in (b) demonstrate a synergistic increase in MAPK activity of Jurkat cells activated with combined tucaresol (300 µm) and anti-CD3. This enhancement is inhibited by pretreatment with the MEK inhibitor.
Schiff base co-stimulation by tucaresol primes for enhanced Ca2+ mobilization during physiological TCR ligation
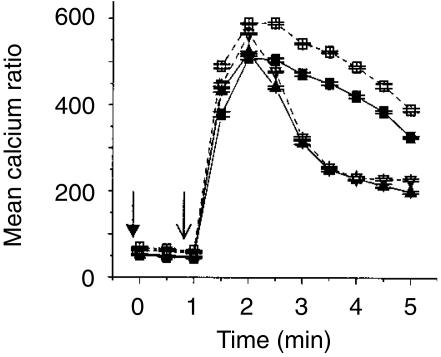
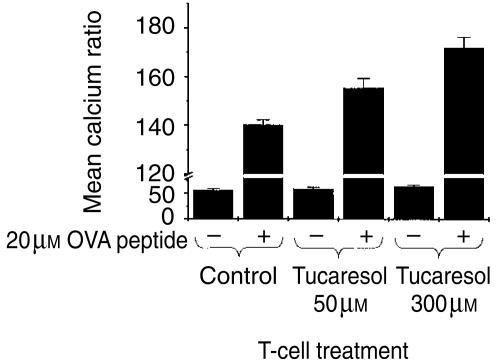
In order to confirm that priming effects of tucaresol on Ca2+ mobilization are not confined to mAb-induced activation, we examined the response of DO11.10 murine T cells, expressing OVA peptide-specific trans-gene TCRs, to peptide pulsed APC. The data in Fig. 9 show that stimulation of CD4+ naïve T cells by peptide-pulsed APC produced a Ca2+ response significantly greater than the background response seen in the absence of peptide. Priming the T cells with tucaresol at 50 µm and 300 µm for 10 min before stimulation produced a significant elevation in the antigen-induced Ca2+ response compared to the control. The enhanced signal was maintained for the entire 10-min time–course. Tucaresol enhancement occurred across a broader dose-range than for anti-CD3 induced respones – a consistent feature seen in the presence of accessory cells/APC. Presumably the additional co-stimulation provided by the latter may alter the balance between TCR and co-stimulatory signals and thereby shift the tucaresol dose–response relationship.
Figure 9.
Tucaresol primes for enhanced calcium responses in CD4+ T cells activated physiologically. An enhancement of calcium mobilization is seen in CD4+ naïve T cells derived from the OVA transgenic mouse. Stimulation of T cells by OVA-peptide-pulsed APC produces a calcium response significantly higher than the background level obtained from activation with unpulsed APC. Priming of the T cells with tucaresol at 50 µm and 300 µm generated a calcium signal significantly higher than that seen in the control, and this was maintained for the duration of the time course. Data shown here are representative of three separate experiments.
Discussion
The importance of Ca2+ in T-cell activation is evident from the fact that combining Ca2+ ionophore with a phorbol ester that bypasses the TCR to activate directly protein kinase C (PKC) and Ras, provides a sufficient signal for IL-2 production.16 This importance is reinforced by the observation that a principal site of action of the immunosuppressive drugs cyclosporin and FK506 is cytosolic calcineurin, a Ca2+/calmodulin-dependent serine phosphatase that acts on NFAT transcription factors.17 The latter translocate to the nucleus where they interact with AP-1 to bind co-operatively to the NFAT/AP-1 site in the IL-2 gene.19 Downstream of PLCγ phosphorylation and IP3 generation, the mechanisms of calcium release and the sustained elevation of intracellular free calcium remain relatively poorly understood.15 Favoured models have postulated an important role for an IP3 receptor cloned from Jurkat cells which appears to mediate IP3-dependent release of Ca2+ from stores in the endoplasmic reticulum. These stores are replenished by sarcoplasmic/endoplasmic reticulum Ca2+ ATPases (SERCA). The type I IP3 receptor is phosphorylated on tyrosine by p59fyn providing an additional TCR-dependent positive element for increased [Ca2+]i. A plasmalemmal IP3-gated channel and capacitative entry through calcium-activated plasma membrane calcium channels have also been discussed. A recent study has implicated an additional principal mediator of the TCR-dependent Ca2+ signal in the form of a cytosolic ADP-ribosyl cyclase that generates cADP ribose, a natural compound that mobilizes Ca2+ ions in several types of eukaryotic cell.26 The target for this mediator appears to be the type-3 ryanodine receptor–Ca2+ channel through which the sustained increase in intracellular Ca2+ is achieved. This second messenger may act in temporal sequence with the initial IP3 mediated events to produce the sustained phase of Ca2+ elevation.
We have previously shown that simultaneous Schiff base-mediated co-stimulation by tucaresol has no effect on TCR-CD3-dependent calcium signalling (ref. 8 and unpublished observations). The chief significance of the present study is that it shows a priming effect of Schiff base co-stimulation by tucaresol on this pivotal pathway, and this is shown to correlate with enhanced IL-2 production by primary T cells. Thus, PLCγ phosphorylation, IP3 generation and the release of intracellular free calcium in response to TCR stimulation were substantially enhanced by a 10-min priming exposure to tucaresol. This enhancement was prevented by inhibition of the tucaresol-induced signalling events characterized in previous studies, namely, the rapid formation of Schiff bases on cell surface amines (inhibited by S-NHS-biotin blockade), the subsequent activation of monovalent cation transport (inhibited by clofilium), and the activation of MAPK (prevented by inhibition of MEK activity). Importantly, the simultaneous stimulation with tucaresol that fails to amplify the TCR-dependent calcium signalling pathway also fails to enhance IL-2 production which invariably requires a co-stimulatory priming period.7,8 The co-stimulatory priming observed here, and its prevention by inhibitors of downstream events, is therefore consistent with a mechanism in which late co-stimulatory events exert a positive influence on early TCR-mediated signalling events. This differs from the conventional picture of co-stimulation as an exclusively downstream integration of contemporaneous, convergent events and provides a mechanism for amplification of the TCR-dependent signal at a number of levels.
Given the importance of TCR-dependent elevation of [Ca2+]i in the activation of IL-2 transcription, enhancement of this signal by tucaresol is likely to contribute substantially to the enhanced production of IL-2 seen with this form of co-stimulation. In naïve T-cells, tucaresol favours a Th1 profile of cytokine production in that TCR-induced IL-2 and interferon-γ production are enhanced while the Th2 cytokines IL-4 and IL-6 are unaffected7 or down-regulated at the level of mRNA (unpublished observations), although the mechanistic basis for this has remained unknown. The present study suggests a potential mechanism whereby tucaresol may favour Th1 responses. Recent studies of the differentiation of T cells towards Th1 or Th2 suggest that the intensity of Ca2+ signalling may be a principal intracellular determinant in the immune deviation mechanism, with increased Ca2+ signalling associated with differentiation to the Th1 phenotype.21
The mechanism of TCR ligation by physiological ligand provides for subtle regulation of T-cell responses at a number of levels upstream of CD3-mediated activation. It is therefore important to know if calcium signalling induced by physiological ligation of the TCR is susceptible to enhancement by Schiff base-mediated co-stimulation in the same way as anti-CD3 initiated signalling. The data obtained using trans-gene TCR ligation by peptide–major histocompatibility complex, confirm the potential of tucaresol to enhance physiological responses to antigen at the level of Ca2+ and thus contribute to our understanding of how tucaresol mediates antigen-specific immunopotentiatory effects in vitro and in vivo.
References
- 1.Mondino A, Jenkins MK. Surface proteins involved in T cell costimulation. J Leukoc Biol. 1994;55:805–15. doi: 10.1002/jlb.55.6.805. [DOI] [PubMed] [Google Scholar]
- 2.Wingren AG, Parra E, Varga M, Kalland T, Sjogren HO, Hedlund G, Dohlsten MT. Cell activation pathways – B7, LFA-3, and ICAM shape unique T cell profiles. Crit Rev Immunol. 1995;15:235–53. doi: 10.1615/critrevimmunol.v15.i3-4.30. [DOI] [PubMed] [Google Scholar]
- 3.Rhodes J. Evidence for an intercellular covalent reaction essential in antigen specific T cell activation. J Immunol. 1989;143:1482–9. [PubMed] [Google Scholar]
- 4.Gao X-M, Rhodes J. An essential role for constitutive Schiff base-forming ligands in antigen presentation to murine T cell clones. J Immunol. 1990;144:2883–90. [PubMed] [Google Scholar]
- 5.Rhodes J. E-Rosettes provide an analogue for Schiff base formation in specific T cell activation. J Immunol. 1990;145:463–9. [PubMed] [Google Scholar]
- 6.Zheng B, Brett S, Tite JP, Lifely MR, Brodie TA, Rhodes J. Galactose oxidation in the design of immunogenic vaccines. Science. 1992;256:1560–3. doi: 10.1126/science.1598588. [DOI] [PubMed] [Google Scholar]
- 7.Rhodes J, Chen H, Hall SR, Beesley JE, Jenkins DC, Collins P, Zheng B. Therapeutic potentiation of the immune system by costimulatory Schiff base-forming drugs. Nature. 1995;377:71–5. doi: 10.1038/377071a0. [DOI] [PubMed] [Google Scholar]
- 8.Chen H, Hall S, Heffernan B, Thompson NT, Rogers MV, Rhodes J. Convergence of Schiff base costimulatory signaling and T-cell receptor signaling at the level of mitogen activated protein kinase ERK2. J Immunol. 1997;159:2274–81. [PubMed] [Google Scholar]
- 9.Chen H, Rhodes J. Schiff base-forming drugs: mechanisms of immune potentiation and therapeutic potential. J Mol Med. 1996;74:497–504. doi: 10.1007/BF00204975. [DOI] [PubMed] [Google Scholar]
- 10.Kirkwood JM, Schucter LM, Donelly S, et al. A novel immunopotentiatory agent, tucaresol: Results from a multicenter, pilot study in patients with metastatic melanoma. Proc Am Assoc Cancer Res. 1997;38:402. (Abstract) [Google Scholar]
- 11.Smith AC, Yardley V, Rhodes J, Croft SL. Activity of the novel immunomodulatory compound tucaresol against experimental visceral Leishmaniasis. Antimicrob Agents Chemother. 2000;44:1494–8. doi: 10.1128/aac.44.6.1494-1498.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Clerici M, Cogliati M, Rizzardini G, Colombo F, Fossati S, Rhodes J, Bray D, Piconi S. In vitro immunomodulatory properties of tucaresol in HIV infection. Clin Immunol. 2000;97:211–20. doi: 10.1006/clim.2000.4937. 10.1006/clim.2000.4937. [DOI] [PubMed] [Google Scholar]
- 13.Bootman MD, Berridge MJ. The elemental principles of Ca++ signaling. Cell. 1995;83:75–8. doi: 10.1016/0092-8674(95)90179-5. [DOI] [PubMed] [Google Scholar]
- 14.Berridge MJ. Lymphocyte activation in health and disease. Crit Rev Immunol. 1997;17:155–78. doi: 10.1615/critrevimmunol.v17.i2.30. [DOI] [PubMed] [Google Scholar]
- 15.Guse AH. Ca++ signaling in T-lymphocytes. Crit Rev Immunol. 1998;18:419–48. doi: 10.1615/critrevimmunol.v18.i5.20. [DOI] [PubMed] [Google Scholar]
- 16.Cantrell D. T cell antigen receptor signaling pathways. Ann Rev Immunol. 1996;4:259–74. doi: 10.1146/annurev.immunol.14.1.259. [DOI] [PubMed] [Google Scholar]
- 17.Schreiber SL, Crabtree GR. The mechanism of action of cyclosporin A and FK506. Immunol Today. 1992;13:136–42. doi: 10.1016/0167-5699(92)90111-J. [DOI] [PubMed] [Google Scholar]
- 18.Woodrow MA, Clipstone NA, Cantrell D. P21 ras and calcineurin synergise to regulate the nuclear factor of activated T-cells. J Exp Med. 1993;178:1517–22. doi: 10.1084/jem.178.5.1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Masuda ES, Imamura R, Amasaki Y, Arai K, Arai N. Signalling into T cell nucleus – NFAT Regulation. Cell Signalling. 1998;10:599–611. doi: 10.1016/s0898-6568(98)00019-9. [DOI] [PubMed] [Google Scholar]
- 20.Nghiem P, Ollick T, Gardner P, Schulman H. Interleukin-2 transcriptional block by multifunctional Ca++/calmodulin kinase. Nature. 1994;371:347–50. doi: 10.1038/371347a0. [DOI] [PubMed] [Google Scholar]
- 21.Noble A, Truman JP, Vyas B, Vukmanovic-Stejic M, Hirst WJ, Kemeny M. The balance of protein kinase and calcium signalling directs T-cell subset development. J Immunol. 2000;164:1807–13. doi: 10.4049/jimmunol.164.4.1807. [DOI] [PubMed] [Google Scholar]
- 22.Murphy KM, Heimberger AB, Loh DY. Induction by antigen of intrathymic apoptosis of CD4+CD8+TCRlow thymocytes in vivo. Science. 1990;250:1720–3. doi: 10.1126/science.2125367. [DOI] [PubMed] [Google Scholar]
- 23.Palmer S, Hughes KT, Lee DY, Wakelam MJ. Development of a novel Ins(1,4,5) P3-specific binding assay. Its use to determine the intracellular concentration of Ins(1,4,5) P3 in unstimulated and vasopressin stimulated rat hepatocytes. Cell Signal. 1989;1:147–56. doi: 10.1016/0898-6568(89)90004-1. [DOI] [PubMed] [Google Scholar]
- 24.Page S, Fischer C, Baumgartner B, et al. 4-hydroxynonenal prevents NF-kappa B activation and tumor necrosis factor expression by inhibiting I-kappa B phosphorylation and subsequent proteolysis. J Biol Chem. 1999;274:11611–18. doi: 10.1074/jbc.274.17.11611. [DOI] [PubMed] [Google Scholar]
- 25.Dudly DT, Pang L, Decker SJ, Bridges AJ, Salteil AR. A synthetic inhibitor of the mitogen-activated protein kinase cascade. Proc Natl Acad Sci USA. 1995;92:7686–9. doi: 10.1073/pnas.92.17.7686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Alessi DT, Cuendra A, Cohen P, Dudleyand DT, Salteil AR. PD098059 is a specific inhibitor of the action of mitogen activated protein kinase kinase in vitro and in vivo. J Biol Chem. 1995;270:27489–94. doi: 10.1074/jbc.270.46.27489. [DOI] [PubMed] [Google Scholar]
- 27.Guse AH, da Silva CP, Berg I, et al. Regulation of Ca++ signalling in T-lymphocytes by the second messenger cyclic ADP-ribose. Nature. 1999;398:70–3. doi: 10.1038/18024. 10.1038/18024. [DOI] [PubMed] [Google Scholar]