Abstract
The majority of peripheral blood γδ T cells in healthy adult humans express the Vγ2/Vδ2 T-cell receptor (TCR) and generate TCR-mediated, major histocompatibility complex (MHC)-unrestricted proliferative responses to low molecular weight alkylphosphates. Vγ2/Vδ2 populations after antigen proliferation maintained diversity in the CDR3s of Vγ2 mRNA, indicating that the response was polyclonal or oligoclonal, and were enriched for Vγ2 TCR chains containing the Jγ1.2 segment. Alkylphosphate stimulation further skewed an already biased peripheral blood γδ T-cell population and increased the abundance of Vγ2-Jγ1.2/Vδ2 T cell receptors, suggesting similarities between the alkylphosphate response and peripheral selection mechanisms shaping this repertoire in human beings.
Introduction
The population of CD3+ T cells recognize antigen through either αβ or γδ T-cell receptors (TCR). The mature γδ T-cell population in primates includes a large proportion of circulating cells that respond to alkylphosphates1 without either professional antigen-presenting cells or presentation by major histocompatibility complex glycoproteins.2 These low molecular weight alkylphosphate antigens stimulate cytokine, chemokine,3,4 and proliferative5,6 responses in peripheral blood Vγ2/Vδ2 T cells. These responses are TCR-specific,7 although the TCR structures mediating recognition of these small organic molecules are not known, and we do not yet understand the relationships between alkylphosphate recognition and innate immune responses involving γδ T cells.
The circulating adult γδ T-cell repertoire is highly selected in primates. Out of six functional Vγ segments and eight possible Vδ segments,2 a majority of γδ T cells in peripheral blood from healthy human8–10 and non-human primates11 express only the Vγ2/Vδ2 TCR. A minor circulating γδ T-cell population expresses the Vδ1 chain, and the ratio of Vδ2 to Vδ1 in healthy adults is around 3·5.12 In addition to the positive selection for circulating Vγ2/Vδ2 T cells, it was also noted that the Vγ2-Jγ1.2 rearrangement was most common.13 We sought to study the relationship between selection for Vγ2-Jγ1.2 chains and the response to alkylphosphates.
Antigen stimulation and the resulting proliferation of responding cells cause shifts in the collection or repertoire of TCR within a lymphocyte population. In the case of major histocompatibility complex (MHC)-restricted peptidic antigens, a strong proliferative response expands one or a small number of clones preferentially and increases their abundance within the stimulated culture. The MHC-unrestricted responses to alkylphosphates have not been analysed at the population level, in order to discern whether this recognition is a clonal or polyclonal property of Vγ2/Vδ2 T cells. Our studies demonstrate polyclonal or oligoclonal expansion of Vγ2/Vδ2 T cells in response to alkylphosphate stimulation with a selective increase in Vγ2-Jγ1.2/Vδ2+ cells, and also suggest that expansion of Vγ2-Jγ1.2 T cells seems mainly based on chain length.
Materials and methods
Collection and preparation of lymphocytes
Heparinized blood was collected from six healthy volunteers, with approval from the Human Subjects Committee at the University of Wisconsin-Madison and informed consent of the donors. Peripheral blood mononuclear cells (PBMC) were isolated by centrifugation over Ficoll–Hypaque density gradients as described by the manufacturer (Pharmacia, Uppsaala, Sweden). Approximately 2 × 106 cells were cultured in supplemented RPMI media containing 10% fetal calf serum (FCS) and 50 U/ml recombinant interleukin-2. Isopentenyl pyrophosphate (IPP, Sigma, St Louis, MO) was added to a final concentration of 12·3 µm. Cells were incubated for 13 days at 37° with 5% CO2 and were replenished every 3– 4 days by adding media without IPP.
Flow cytometry
Thirteen days after IPP stimulation, cultures were washed once with RPMI medium, twice with phosphate-buffered saline (PBS) and then viable cells were counted. Samples containing 2 × 106 cells were stained with fluoroscein isothiocyanate (FITC)-conjugated anti-γδ TCR monoclonal antibody (TCRδ1 clone 5A6, kindly provided by Dr Michael Brenner, Harvard University), phycoerythrin (PE)-conjugated anti Vγ2 (clone B3, Pharmingen, San Diego, CA), biotin-conjugated anti Vδ1 (clone R9.12, Immunotech, San Francisco, CA), or biotin-conjugated anti Vδ2 (clone IMMU 389, Immunotech). After 1 hr incubation, cells were washed with PBS containing 2% fetal bovine serum (FBS); antigen-presenting cell-conjugated streptavidin (Caltag, Burlingame, CA) was added for 30 min where appropriate. Cells were again washed three times with PBS + 2% FBS and then resuspended in PBS containing 4% paraformaldehyde. Up to 2 × 105 cells in a lymphocyte gate were sorted on a fluorescence-activated cell sorting (FACS) analyser (Becton-Dickenson, Franklin Lakes, NJ). Sorted populations were analyzed using Flo-jo software (Tree Star, version 2.6.1).
RNA extraction, reverse transcription–polymerase chain reaction (RT–PCR), run-off reactions
Approximately 2 × 106 washed cells were treated with Trizol reagent (Life Technologies, Rockville, MD) to extract RNA. The precipitated RNA pellets were washed with 70% ethanol and briefly dried before being resuspended in diethylpyrocarbonate-treated water and quantitated by absorbance at 260 nm. The absorbance 260 nm : 280 nm ratios for these samples were typically 2·0 ± 0·1. One µg of total RNA was converted to cDNA in a reaction containing 50 ng oligonucleotide A (T15V, where V = A, C or G), 2·0 mm deoxynucleotriphosphates (dNTPs) (Promega, Madison, WI), 10 mm MgCl2, 25 mm Tris pH 8·3, 25 mm KCl, and 20 units avian myeloblastosis virus (AMV) reverse transcriptase (Promega). Reactions were incubated for two hours at 42° and then diluted to 500 µl with deionized H2O. Polymerase chain reactions were carried out with 10 µl template (equivalent to cDNA converted from 20 ng total RNA), 500 nm each of the forward and reverse primer, 0·2 mm dNTPs, 2 mm MgCl2, 10 mm Tris pH 8·3, 50 mm KCl, 0·1% Triton-X-100 and 1 unit of Taq DNA polymerase. The following oligonucleotides were used for PCR: oligo Vδ2-1 (5′ CAT CTA TGG CCC TGG TTT ≈ 3′), oligo Vγ2-2 (5′ AGA CCT GGT GAA GTC ATA C 3′), oligo Cδ-1 (5′ TGG CAG TCA AGA GAA AAT TG 3′), and oligo Cγ-1 (5′ GTT GCT CTT CTT TTC TTG CC 3′). PCR products were separated on 1·6% agarose/Tris–acetate–ETDA buffer (TAE) gels containing 0·5 µg/l ethidium bromide and visualized with UV light.
Primer extension reactions were performed according to published protocols.14 Each reaction contained 1–2 µl PCR, 3 mm MgCl2, 10 mm Tris pH 8·3, 50 mm KCl, 0·2 mm dNTPs, 0·1 µm oligo Cγ-4 [6-carboxyfluorescein (6-FAM) labelled CAT CTG CAT CAA GTT GTT TAT C] and approximately 0·2 units Taq DNA polymerase. The run-off products were diluted 1 : 25 in deionized formamide and 1 µl N,N,N′,N′-tetramethyl 6-rhodamine (TAMRA) labelled molecular size standard was added to each sample. Products were separated on an Perkin Elmer-Applied Biosystems genetic analyser (Perkin-Elmer, Foster City, CA) equipped with a 41-cm capillary and performance optimized polymer (POP-4). Run time for resolution of 50–400 nucleotide fragments was 24 min. The molecular size and quantity of extension products were determined using GeneScan software (Perkin-Elmer). In order to standardize the data irrespective of the primer positions we express CDR3 length variation in terms of the total Vγ2 coding region length. This distance was calculated by adding the remainder of the Vγ2 variable region sequence length outside the primer binding sites (GenBank NID g1848089), the γ constant region length (GenBank NID g37017), and the product length between primer binding sites. Calculated this way, the major Vγ2 peak is 993 nucleotides, whereas the run-off product that represented this gene was approximately 254 nucleotides.15
Cloning and sequencing, sequence analysis
RT–PCR products were purified by passage through a PCR clean-up spin column (Promega) and ligated with a pCRII-TOPO vector (from Invitrogen) for 5 min at room temperature. Recombinant plasmids from ampicillin-resistant colonies were purified and sequenced using either the Cγ-1 or the Vγ2-2 oligonucleotide as a primer and BigDye fluorescent sequencing kits (PerkinElmer), and analysed on automated sequencers. Vγ2 sequences were aligned using Seqman (DNAstar, Madison, WI). Vγ2, Jγ1.2 and Jγ1.3 and Jγ2.3 were identified by comparison to GenBank NIDs g1848089, g339142, g339144 and g339146, respectively.
Results
Vγ2 CDR3 length variation after stimulation with isopentenyl pyrophosphate (IPP)
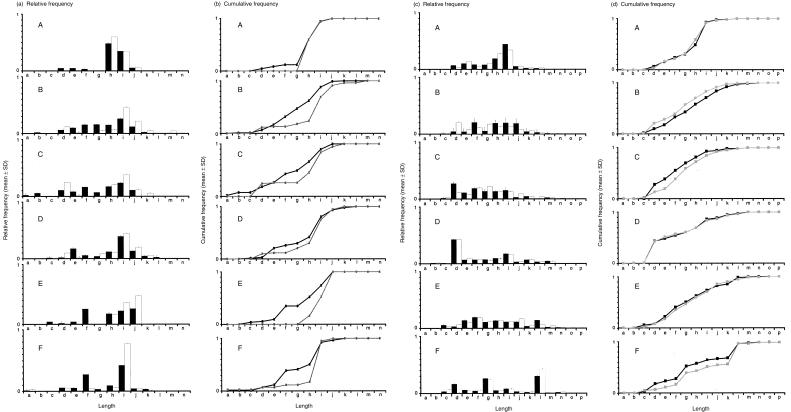
PBMC were collected from six human volunteers. The cells were stimulated in vitro with isopentenyl pyrophosphate (IPP), an alkylphosphate that elicits Vγ2/Vδ2+ T-cell proliferation, as demonstrated by flow cytometry (Table 1). We used immunoscope analysis14 (also called spectratyping) for an initial measurement of diversity among the TCR chains. Immunoscope analysis characterizes a population of cells expressing a single V region. A DNA amplification scheme is used to measure length variation within the complementarity determining region 3 (CDR3), a region that includes (for Vγ2) the carboxy terminus proximal end of the V segment, the N region, and the J region. In the Vγ2+ population from peripheral blood from six healthy individuals, the distribution of CDR3 lengths was strongly biased in the absence of IPP stimulation, a result consistent with previous studies.9,10,16,17 The bias was evident because the profile of CDR3 lengths was not normally distributed, instead being shifted to one or two dominant CDR3 lengths.
Table 1.
Vγ2/Vδ2+ T-cell proliferation after IPP stimulation in vitro
| % Lymphocytes in each subset | ||||
|---|---|---|---|---|
| Donor | IPP | Vδ1 | Vγ2/Vδ2 | Stimulation index |
| A | – | 0·12 | 2·53 | |
| A | + | 0·04 | 39·63 | 13·4 |
| B | – | 0·46 | 0·95 | |
| B | + | 1·50 | 20·81 | 21·9 |
| C | – | 0·48 | 0·19 | |
| C | + | 0·41 | 4·75 | 25·0 |
| D | – | 1.29 | 1·85 | |
| D | + | 0·04 | 20·49 | 11·1 |
| E | – | 3·29 | 12·63 | |
| E | + | 0·25 | 21·75 | 1·7 |
| F | – | 1·88 | 1·12 | |
| F | + | 2·81 | 10·50 | 9·4 |
PBMC cultured in the absence or presence of IPP were stained with fluorescently labelled monoclonal antibodies and analysed by flow cytometry. The proportion of cells staining with a combination of the TCRδ1 and Vδ1 monoclonal antibodies, or a combination of Vγ2 and Vδ2 monoclonal antibodies were compared to the total cells in a lymphocyte gate, which was determined by foward and side scatter (approximately 2 × 105 cells/sample). Stimulation index is calculated as the ratio of percentage Vγ2/Vδ2+ cells with and without IPP stimulation.
The bias to one or two Vγ2 lengths was accentuated after in vitro isopentenyl pyrophosphate (IPP) stimulation (Fig. 1a), resulting in a right-ward shift in cumulative frequency plots (Fig. 1b). In some individuals (e.g. subject A in Fig. 1), the unstimulated Vγ2 population showed a strong bias towards longer chain length. Even in these cases, a further rightward shift could be detected in the IPP stimulated Vγ2 population. Samples that were less biased (e.g. E and F), showed the most pronounced rightward cumulative frequency shift after IPP stimulation. We also performed these studies for Vδ2. In contrast to the distribution of Vγ2 lengths, the patterns of Vδ2 were more normally distributed, indicating that the control peripheral blood population was not biased on the basis of length and did not show consistent changes in the cumulative or relative frequency plots after IPP stimulation (Fig. 1c, d).
Figure 1.
Analysis of CDR3 lengths in the population of Vγ2 or Vδ2 chains from control (black) or IPP stimulated (white) cultures. The Vγ2 and Vδ2 CDR3s were amplified and separated as described in Materials and methods. The pattern of peak heights for each PCR product was compiled using GeneScan software and the distributions of CDR3 lengths are represented here in the form of relative frequency plots for samples from six individuals (a and c). We identified a maximum of 13 peaks for the Vγ2 CDR3s and 16 peaks for Vδ2 CDR3s; the PCR products were all multiples of three bases in length and length is represented by letters in increasing order. The critical peaks for Vγ2 are g (987 bases for the Vγ2 coding region), h (990 bases), i (993 bases), and j (996 bases). The area under each peak was determined and the relative frequency of each peak was calculated as a portion of the total area under all peaks. Additionally, the data was plotted in the form of cumulative frequency plots (b and d). Cumulative frequency was determined by sequentially adding relative frequencies from the smallest to the largest peak as described.11 The results are shown for control (black lines) or IPP stimulated (grey lines) PBMC cultures.
Expansion of the Jγ1.2 segment during IPP stimulation
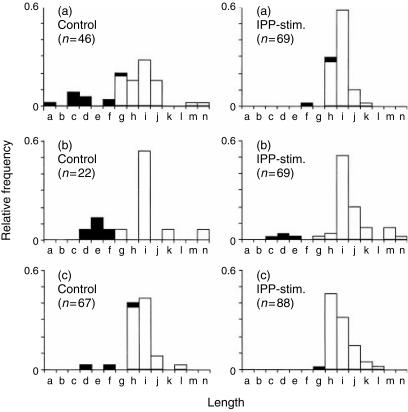
We next analysed DNA sequences to explain the shift to longer CDR3s during IPP stimulation. As Jγ1.2 is 11 nucleotides longer than either the Jγ1.3 or Jγ2.3 segments, an increased abundance of Jγ1.2 after IPP stimulation might account for the rightward shift to longer CDR3 lengths. Based on the sequence data, we calculated the length for each Vγ2 sequence and identified the proportion of Jγ1.3, Jγ2.3, or Jγ1.2 segments in control and IPP-stimulated samples (Fig. 2). In all cases, the longer CDR3s are overwhelmingly comprised of Vγ2-Jγ1.2 chains. The Vγ2 chains shorter than 987 nucleotides contained Jγ1.2 only 3% of the time; this J segment was present in 71% of all Vγ2 that were 987 bases long (size g in Fig. 2), and comprised 98% of all Vγ2 sequences with a length of 990 bases or longer (in our database with more than 380 total sequences). The IPP-stimulated cells had a higher proportion of Vγ2-Jγ1.2 chains and this was consistent with the shift to longer CDR3s (Fig. 2). In general, histograms of CDR3 length distribution that were calculated from DNA sequences were similar to the data obtained after capillary electrophoresis of run-off amplification products.
Figure 2.
Proportion of Jγ1.3, Jγ2.3, or Jγ1.2 usage according to CDR3 length in control and IPP-stimulated PBMC cultures. Samples A, B, and C represent PBMC from the individual donors identified by the same letters in Fig. 1. Sequence analysis was performed on cDNA samples from control or IPP stimulated samples and the number of individual sequences are shown by the n-values in parentheses. Sequences that contained either Jγ1.3 or Jγ2.3 segments are identified by black bars and sequences containing the Jγ1.2 segment are identified by white bars. The total length (in bases) for the Vγ2 coding region is represented by letters (as described for Fig. 1) and the critical peaks are g (987 bases), h (990 bases), i (993 bases), and j (996 bases).
We performed statistical analyses on the frequency of Vγ2-Jγ1.2 chains after IPP stimulation, using the DNA sequences (Table 2). Using Fisher's exact test, the probability values for increased Jγ1.2 usage following IPP stimulation ranged from 0·09 to 0·01, indicating that, for one individual, the increase in Vγ2-Jγ1.2 abundance was statistically significant. When data were combined for all three samples, the increased Vγ2-Jγ1.2 abundance after stimulation was highly significant with P = 0·0004. The cumulative frequency measurements and DNA sequence analysis both imply that Vγ2-Jγ1.2 chains are responsible for the proliferative response to IPP. Therefore, we sought to evaluate the complexity of Vγ2-Jγ1.2 populations to determine whether these responses represented clonal or polyclonal responses to alkylphosphate.
Table 2.
IPP stimulation positively selects for cells expressing the Vγ2-Jγ1.2 chains
| Jγ1.2 | Jγ1.3 or 2.3 | Fisher's exact test | |||
|---|---|---|---|---|---|
| Donor | IPP | (number of sequences) | (number of sequences) | Chi-squared value | (P-value) |
| A | – | 42 | 4 | ||
| A | + | 69 | 1 | 2·01 | 0·0797 |
| B | – | 18 | 4 | ||
| B | + | 65 | 4 | 3·19 | 0·0928 |
| C | – | 54 | 13 | ||
| C | + | 84 | 5 | 6·31 | 0·0120 |
| A, B, C | – | 113 | 20 | ||
| A, B, C | + | 218 | 10 | 12·51 | 0·0004 |
Analysis of Vγ2 sequences. cDNA libraries prepared from PBMC cultured in the presence or absence of IPP were amplified with Vγ2 and Cγ-specific primers and sequenced as described. Vγ2, Jγ, and CDR3 sequences were identified. Jγ1.3 and Jγ2.3 segments are very similar, and are referred to here as Jγ1.3 or 2.3. The proportion of clones having inserts with Jγ1.2 and Jγ1.3 or 2.3 segments were compared for control and IPP-stimulated cultures. The Chi-squared value and probability based on Fisher's Exact test were calculated using software (EpiInfo) to determine if these changes were statistically significant.
Vγ2 responses to IPP stimulation
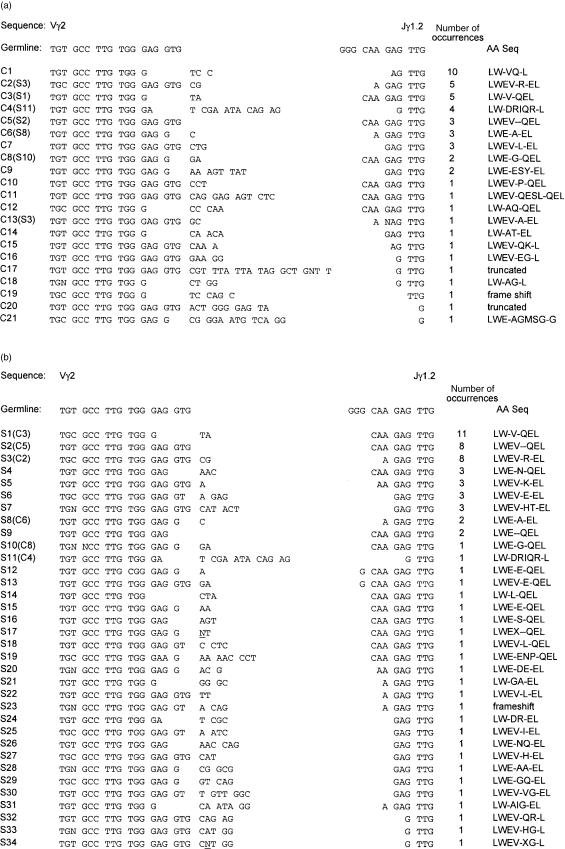
We envisioned two possible effects of IPP stimulation on the γδ TCR repertoire. A clonal response to IPP would decrease the diversity of Vγ2 CDR3 sequences, because a single (or small number) of sequences would have increased abundance after proliferation. In this case, we would conclude that individual CDR3 sequences mediated alkylphosphate recognition and that this portion of the γδ TCR plays a role similar to αβ TCR recognition of peptide–MHC complexes. Alternately, a polyclonal response to IPP would have little effect on complexity in the γδ TCR repertoire, as was postulated previously.2 In order to discriminate these possibilities, we examined the Vγ2-Jγ1.2 CDR3 sequences from control and IPP stimulated cultures; an example of these data are shown (Fig. 3). The control sample (individual C, Fig. 1) had 49 Vγ2 sequence entries consisting of 21 different sequences (average of 2·3 entries per sequence). The IPP stimulated sample contained 68 entries consisting of 34 different sequences (average of 2·0 entries per sequence). For sequences that arose more than once in a set of individual sequences, we found three (C1, C7, and C9) that were present in control and absent in stimulated samples. We also found five sequences (S4, S5, S6, S7, and S9) that were present in stimulated but not control samples. For sequences found in both samples and present more than once in each set, there were no statistically significant differences in their proportional representation. Two additional sample sets (samples A and B from Fig. 1) showed similar levels of sequence repetition and similar differences among control and stimulated cultures, and did not reveal any evidence of clonal responses to IPP stimulation. On the basis of nucleotide sequence data, we concluded that alkylphosphate stimulation induced a polyclonal or oligoclonal expansion of Vγ2-Jγ1.2-positive cells.
Figure 3.
Vγ2 cDNA sequences from control and IPP stimulated PBMC cultures. Sequences were aligned in the V and J regions, and the CDR3s are displayed between the aligned regions, and include additional spaces as necessary. Data from control (a) and IPP stimulated (b) are shown. The individual sequences are labelled with C (for control) or S (for IPP-stimulated), along with the clone number. At the end of each sequence, the number in parentheses indicates how many times this sequence was observed in the sample set. Nucleotides that were not distinct in the automated sequencing reactions are indicated by the letter N.
Structural requirements for alkylphosphate recognition
The CDR3 nucleotide sequences for Vγ2-Jγ1.2 chains from control and IPP stimulated cultures were translated into amino acid sequences and aligned with a hypothetical sequence representing germ line segment recombination in the absence of deletion or non-templated nucleotide additions (Fig. 3a, b). In all cases, the amino terminal Gly of the Jγ1.2 segment was deleted. In sequence C8 and S10 (Fig. 3a) a Gly residue was found in the position corresponding to the amino terminus of Jγ1.2, but the germline codon was not used, indicating that this was a non-templated addition. In 49 sequences from the control culture, we observed one frame-shifted and two truncated sequences and there were nine sequences present two or more times in this sample (Fig. 3a). In a collection of 68 sequences from an IPP-stimulated culture we identified one frame-shifted sequence and no truncated sequences and there were nine sequences present two or more times (Fig. 3b).
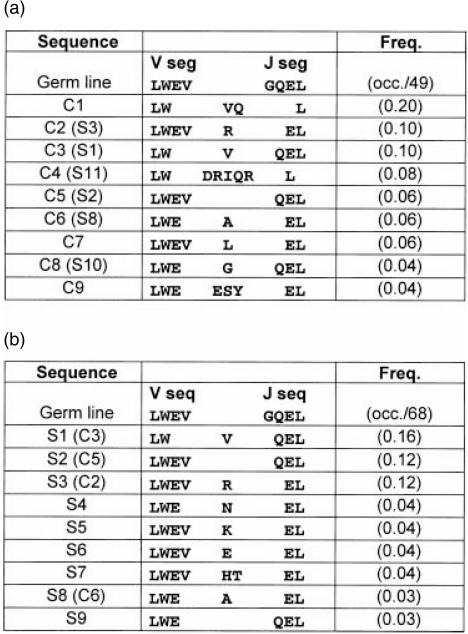
We compared the most common sequences from control and IPP stimulated cultures (Fig. 4a, b). Both control and stimulated samples retained the Leu–Trp residues from the V segment and all sequences retained the Leu from the J segment. Val appeared often in the CDR3, and this residue was frequently (C1, C3, S1) encoded by a non-templated codon. Most sequences included a charged residue in the CDR3, either a non-templated codon or Glu from the amino-terminus of the J segment. Aside from these observations, it was difficult to discern a strongly preferred CDR3 motif in the IPP-stimulated samples. The most common sequence from the control culture (C1) was not present in the stimulated sample set despite having the Jγ1.2 segment. Importantly, C1 was shorter than every Jγ1.2-containing chain found in the IPP stimulated set (Fig. 3).
Figure 4.
CDR3 amino acid sequences for Vγ2 + clones in control (a) and IPP stimulated (b) samples sets. The clone numbers (e.g. C1 or S5) refer to nucleotide sequences displayed in Fig. 3. The predicted germline sequences of complete Vγ2 and Jγ1.2 segments are shown in bold type. The predicted amino acid sequences for clones present more than once in the sample set, are aligned with dashes included for alignment purposes. The frequency (Freq.) for each sequence in the sample set is calculated by the number of occurrences divided by the total number of sequences in the set (49 total for control and 68 total for stimulated in this example).
Discussion
The γδ T-cell response to alkylphosphate stimulation is mediated primarily by cells expressing the Vγ2-Jγ1.2/Vδ2 TCR. Receptors with this structure are already present at high levels in peripheral blood as a result of natural selection mechanisms, but their abundance is increased further during the proliferative response to alkylphosphate stimulation. The polyclonal or oligoclonal expansion of Vγ2-Jγ1.2/Vδ2 T cells suggests similarities between these natural selection mechanisms and the response in vitro, and shows that the capacity to recognize alkylphosphate is likely to be important for innate immune responses mediated by peripheral blood γδ T cells. Examination of Vγ2-Jγ1.2 sequences in control and IPP-stimulated cells strongly suggested an IPP specific expansion based on Vγ2 chain length.
Bukowski et al. used a directed mutagenesis approach to explore the structural requirements for Vγ2-mediated recognition of IPP and the closely related ethyl pyrophosphate. They started with an unusual Vγ2 chain that had the CDR3 sequence Glu-Trp–Glu and lacked the conserved Val.18 When the Trp was changed to Gly–Asn (reinstating the Gly encoded at the amino terminus proximal position of Jγ1.2), IPP recognition was abolished but the mutant Vγ2 chain still allowed recognition of other stimulating compounds found in mycobacterial extracts. We have not found this Gly residue in naturally occurring Vγ2 chains based on more than 600 molecular clones from multiple donors. We often find the sequence Leu–Trp–Glu but never have observed the Glu–Trp sequence (where Trp would be a non-templated codon). When Bukowski and colleagues combined the Gly–Asn mutant with a shorter J segment that lacked the amino terminus proximal Glu residue, all recognition of alkylphosphates was abolished.18
The mechanism for Vγ2/Vδ2 TCR recognition of alkylphosphate may be similar to that for conventional superantigens that elicit polyclonal expansion of αβT cells in a Vβ-specific manner. For example, the Staphylococcus enterotoxin B is a macromolecular superantigen that binds directly to the TCR Vβ region19 and induces polyclonal proliferation of T cells. Within the population of responding cells, individual J segments were positively selected during the response to either Staphylococcus enterotoxin B or Urtica dioica superantigens. The selected J segments were thought to alter Vβ region conformations through long-range interactions, and these conformational changes favored superantigen binding.20 Alkylphosphate stimulation of PBMC expanded the Vγ2/Vδ2+ T cells, and positively selected for the Vγ2-Jγ1.2 expressing subset, a result similar to what was described above for conventional superantigen stimulation of Vβ-expressing cells.
The Vδ2+ T-cell subset is both a diverse and a minor population in the infant thymus, but this subset becomes less diverse and eventually dominates the peripheral blood in adults.21 Thus, extrathymic selection and amplification of Vγ2/Vδ2+ T cells results in a biased repertoire that favors Vγ2-Jγ1.2 chains and promotes alkylphosphate responses while maintaining polyclonal diversity. Our results draw a parallel between in vitro responses to IPP and the endogenous mechanisms shaping the natural γδ T-cell repertoire, because both mechanisms amplify the proportion of Vγ2-Jγ1.2+ cells, select for Vγ2 chains of a preferred length, and produce a population with increased responses to alkylphosphates. Parker et al. showed that Vγ2 chains from infant thymus were highly diverse and length restriction occurred only after peripheral selection and amplification.21 In Vγ2/Vδ2+ T-cell clones from human thymocytes, there were no apparent length restrictions for either γ or δ chains,22 further supporting the view that restricted Vγ2 chain lengths in peripheral blood lymphocytes are due to extrathymic selection and amplification. We observed no length-dependent selection of the Vδ2 chains after alkylphosphate stimulation in vitro, and the collection of Vδ2 chains from blood did not show specific length biases. The preferential utilization of Jγ1.2 segments tends to argue that Vγ2 chain length is the more important criterion for both peripheral selection and alkylphosphate responsiveness, because this J segment is distinguished from others mainly by its length.
We studied the responses to IPP, a compound used extensively to model the γδ T-cell responses to alkylphosphates.1,5,7 This compound is highly abundant in mycobacterial supernatant, and is an intermediate in the synthesis of polyisoprenoid compounds in microbial and mammalian cells.6 Other naturally occurring alkylphosphates including formyl-butyl pyrophosphate,23 are more potent than IPP and might be more important in vivo for selecting γδ T cells. Alkylphosphates are released from many microbes24 that might also be present during normal development. Thus, these compounds might positively select the peripheral blood γδ T-cell repertoire during development, to increase the proportion of Vγ2/Vδ2+ cells and to further bias the Vγ2 repertoire by expanding the Jγ1.2+ subset. This may be important for promoting innate γδ T-cell responses at sites of infection or pathology where stimulatory concentrations of alkylphosphates signal a danger to the host.
Acknowledgments
Studies were supported by PHS grants AI38491 and AI41977 (C.D.P.) and AI42716 (M.M.). P.S.E. and P.J.E. were supported by the PHS Viral Oncology Training Grant (5T32 CA09075).
Abbreviations
- PBMC
peripheral blood mononuclear cells
- IPP
isopentenyl pyrophosphate
- TCR
T-cell receptor
- CDR3
complementarity determining region-3
- MHC
major histocompatibility complex
- PBS
phosphate-buffered saline
- FCS
fetal calf serum
- FBS
fetal bovine serum
- RT–PCR
reverse transcription–polymerase chain reaction
References
- 1.Morita CT, Beckman EM, Bukowski JF, Tanaka Y, Band H, Bloom BR, Golan DE, Brenner MB. Direct presentation of nonpeptide prenyl pyrosphate antigens to human γδ T cells. Immunity. 1995;3:496–507. doi: 10.1016/1074-7613(95)90178-7. [DOI] [PubMed] [Google Scholar]
- 2.McVay LD, Carding SR. Generation of human γδ T cell repertoires. Crit Rev Immunol. 1999;19:431–60. [PubMed] [Google Scholar]
- 3.Poccia F, Wallace M, Colizzi V, Malkovsky M. Possible protective and pathogenic roles of γδ T lymphocytes in HIV-infections (review) Int J Mol Med. 1998;1:409–13. doi: 10.3892/ijmm.1.2.409. [DOI] [PubMed] [Google Scholar]
- 4.Wallace M, Malkovsky M, Carding SR. Gamma/delta T lymphocytes in viral infections. J Leukocyte Biol. 1995;58:277–83. doi: 10.1002/jlb.58.3.277. [DOI] [PubMed] [Google Scholar]
- 5.Tanaka Y, Sano S, De Nieves E, et al. Nonpeptide ligands for human gamma delta T cells. Proc Natl Acad Sci USA. 1994;91:8175–9. doi: 10.1073/pnas.91.17.8175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tanaka Y, Morita CT, Tanaka Y, Nieves E, Brenner MB, Bloom BR. Natural and synthetic non-peptide antigens recognized by human γδ T cells. Nature. 1995;375:155–8. doi: 10.1038/375155a0. [DOI] [PubMed] [Google Scholar]
- 7.Bukowski JF, Morita CT, Tanaka Y, Bloom BR, Brenner MB, Band H. Vγ2Vδ2 TCR-dependent recognition of non-peptide antigens and Daudi cells analyzed by TCR gene transfer. J Immunol. 1995;154:998–1006. [PubMed] [Google Scholar]
- 8.Panchamoorthy G, McLean J, Modlin RL, Morita CT, Ishikawa S, Brenner MB, Band H. A predominance of the T cell receptor Vγ2/Vδ2 subset in human mycobacteria-responsive T cells suggests germline gene encoded recognition. J Immunol. 1991;147:3360–9. [PubMed] [Google Scholar]
- 9.DeLibero G, Casorati G, Giachino C, Carbonara C, Migone N, Matzinger P, Lanzavecchia A. Selection by two powerful antigens may account for the presence of the major population of human peripheral γ/δ T cells. J Exp Med. 1991;173:1311–22. doi: 10.1084/jem.173.6.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Casorati G, DeLibero G, Lanzavecchia A, Mingone N. Molecular analysis of human γ/δ+ clones from thymus and peripheral blood. J Exp Med. 1989;170:1521–35. doi: 10.1084/jem.170.5.1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rakasz E, MacDougall AV, Zayas MT, et al. Gammadelta T cell receptor repertoire in blood and colonic mucosa of rhesus macaques. J Med Primatol. 2000;6:387–96. doi: 10.1111/j.1600-0684.2000.290602.x. [DOI] [PubMed] [Google Scholar]
- 12.Boullier S, Cochet M, Poccia F, Gougeon M. CDR3-independent T cell expansion in the peripheral blood of HIV-infected persons. J Immunity. 1995;154:1418–31. [PubMed] [Google Scholar]
- 13.Davodeau F, Peyrat M-A, Hallet M-M, Gaschet J, Houde I, Viven R, Vie H, Bonneville M. Close correlation between Daudi and mycobacterial antigen recognition by human γδ T cells and expression of V9JPC1γ/V2DJCδ-encoded T cell receptors. J Immunol. 1993;151:1214–23. [PubMed] [Google Scholar]
- 14.Pannetier C, Levraud J-P, Lim A, Even J, Kourilsky P. The immunoscopic approach for the analysis of T cell repetoires. In: Oskenberg JR, editor. The Antigen T Cell Receptor: Selected Protocols and Applications. Detroit: Chapman & Hall; 1997. pp. 287–385. [Google Scholar]
- 15.MacDougall AV, Enders P, Hatfield G, Pauza DC, Rakasz E. Vgamma2 TCR repertoire overlap in different anatomical compartments of healthy, unrelated Rhesus macaques. J Immunol. 2000;4:2296–302. doi: 10.4049/jimmunol.166.4.2296. [DOI] [PubMed] [Google Scholar]
- 16.Delfau M-H, Hance AJ, Lecossier D, Vilmer E, Gradchamp B. Restricted diversity of Vγ9-JP rearrangement in unstimulated human γ/δ lymphocytes. Eur J Immunol. 1992;22:2437–43. doi: 10.1002/eji.1830220937. [DOI] [PubMed] [Google Scholar]
- 17.Tamura N, Holroyd KJ, Banks T, Kirby M, Okayama H, Crystal RG. Diversity in junctional sequences associated with the common human Vγ9 and Vδ2 Gene segments in normal blood and lung compared with the limited diversity in granulomatous disease. J Exp Med. 1990;172:169–81. doi: 10.1084/jem.172.1.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bukowski JF, Morita CT, Band H, Brenner MB. Crucial role of TCRγ chain junctional region in prenyl pyrophosphate antigen recognition by γδ T cells. J Immunol. 1998;154:998–1006. [PubMed] [Google Scholar]
- 19.Li H, Llera A, Tsuchiya D, Leder L, Ysern X, Schlievert PM, Karjalainen K, Mariuzza RA. Three-dimensional structure of the complex between a T cell receptor β chain and the superantigen staphylococcal enterotoxin B. Immunity. 1998;9:807–16. doi: 10.1016/s1074-7613(00)80646-9. [DOI] [PubMed] [Google Scholar]
- 20.Musette P, Galelli A, Truffa-Bachi P, Peumans W, Kourilsky P, Gachelin G. The Jβ segment of the T cell receptor contributes to the Vβ-specific T cell expansion caused by staphylococcal enterotoxin B and Urtica dioica superantigens. Eur J Immunol. 1996;26:618–22. doi: 10.1002/eji.1830260317. [DOI] [PubMed] [Google Scholar]
- 21.Parker CM, Groh V, Band H, et al. Evidence for extrathymic changes in the T cell receptor gamma/delta repertoire. J Exp Med. 1990;171:1597–612. doi: 10.1084/jem.171.5.1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Davodeau F, Peyrat MA, Hallet MM, Houde I, Vie H, Bonneville M. Peripheral selection of antigen receptor junctional sequences in a major human γδ subset. Eur J Immunol. 1993;23:804–8. doi: 10.1002/eji.1830230405. [DOI] [PubMed] [Google Scholar]
- 23.Belmant C, Esponisa E, Poupot R, Peyrat MA, Guiraud M, Poquet Y, Bonneville M, Fournie JJ. 3-formyl-1-butyl pyrophosphate: a novel mycobacterial metabolite-activating human gammadelta T cells. J Biol Chem. 1999;45:32079–84. doi: 10.1074/jbc.274.45.32079. [DOI] [PubMed] [Google Scholar]
- 24.Bukowski JF, Morita CT, Brenner MB. Human gamma delta T cells recognize alkylamines derived from microbes, edible plants, and tea: implications for innate immunity. Immunity. 1999;11:57–65. doi: 10.1016/s1074-7613(00)80081-3. [DOI] [PubMed] [Google Scholar]