Abstract
We investigated the changes which occur in gene expression in the human macrophage cell line, THP1, at 1, 6 and 12 hr following infection with Mycobacterium tuberculosis. The analysis was carried out at the transcriptome level, using microarrays consisting of 375 human genes generally thought to be involved in immunoregulation, and at the proteomic level, using two-dimensional gel electrophoresis and mass spectrometry. The analysis of the transcriptome using microarrays revealed that many genes were up-regulated at 6 and 12 hr. Most of these genes encoded proteins involved in cell migration and homing, including the chemokines interleukin (IL)-8, osteopontin, monocyte chemotactic protein-1 (MCP-1), macrophage inflammatory protein-1α (MIP-1α), regulated on activation, normal, T-cell expressed and secreted (RANTES), MIP-1β, MIP-3α, myeloid progenitor inhibitory factor-1 (MPIF-1), pulmonary and activation regulated chemokine (PARC), growth regulated gene-β (GRO-β), GRO-γ, MCP-2, I-309, and the T helper 2 (Th2) and eosinophil-attracting chemokine, eotaxin. Other genes involved in cell migration which were up-regulated included the matrix metalloproteinase MMP-9, vascular endothelial growth factor (VEGF) and its receptor Flk-1, the chemokine receptor CCR3, and the cell adhesion molecules vesicular cell adhesion molecule-1 (VCAM-1) and integrin a3. In addition to the chemokine response, genes encoding the proinflammatory cytokines IL-1β (showing a 433-fold induction), IL-2 and tumour necrosis factor-α (TNF-α), were also found to be induced at 6 and/or 12 hr. It was more difficult to detect changes using the proteomic approach. Nevertheless, IL-1β was again shown to be strongly up-regulated. The enzyme manganese superoxide dismutase was also found to be strongly up-regulated; this enzyme was found to be macrophage-, rather than M. tuberculosis, derived. The heat-shock protein hsp27 was found to be down-regulated following infection. We also identified a mycobacterial protein, the product of the atpD gene (thought to be involved in the regulation of cytoplasmic pH) in the infected macrophage extracts.
Introduction
Mycobacterium tuberculosis is one of the most important infectious agents yet identified, being responsible for an estimated annual death rate of 2–3 million humans.1 M. tuberculosis is an intracellular pathogen which survives within macrophages (Mφs); however, only a small percentage of infected individuals will develop the disease. In the majority of cases, the infected individual mounts an effective immune response and successfully controls the infection. The initial interaction between the Mφ and the mycobacterium is thought to play a key role in determining the outcome of infection. Bacterial products are recognized by receptors on the Mφ. These receptors include CD14 and Toll-like receptors (TLRs),2,3 which rapidly signal the presence of the pathogen through the transcription factor, nuclear factor-κB (NF-κB), and result in the transcription of a range of Mφ genes aimed at initiating an effective immune response. In addition to TLRs and CD14, several other Mφ cell-surface molecules, including complement receptors,4–6 mannose receptors,5 scavenger receptors7 and CD43,8 are involved in the adherence and uptake of M. tuberculosis, contributing to the complex signalling and initiation of an immune response.
In order to survive this immune response, mycobacteria have evolved a variety of mechanisms for evading killing by immunologically activated Mφ. For example, phagosomes containing live M. tuberculosis resist fusion with organelles of the endosomal/lysosomal system.9 Mycobacterial phagosomes show signs of maturational arrest in that they express markers associated with the early endocytic pathway, but fail to express late markers.10,11 The proton ATPase, responsible for acidification along the endocytic pathway, is excluded, resulting in a failure to acidify the phagosome.10 Lysosomal degradation of M. tuberculosis is further impeded by a molecule termed TACO, which is recruited to the phagosome in a cholesterol-dependent manner.12,13 M. tuberculosis may also survive an effective immune response by blocking the presentation of its antigens to T cells,14 and by reducing expression of major histocompatibility complex (MHC) class II molecules on the surface of infected Mφ.15
The induction of survival mechanisms, alongside a range of immunological effector molecules, emphasizes the complexity of the cross-talk that occurs between the Mφ and the mycobacterium. In order to further characterize this cross-talk and to detail more fully the changes which occur following the initial interaction between M. tuberculosis and Mφ, we have used a more global approach by combining transcriptomic and proteomic techniques. The analysis of the transcriptome can be automated onto microarrays, which permit the analysis of changes in hundreds or thousands of genes in a single experiment. In this study we have utilized a commercially available ‘immunoregulatory microarray’ consisting of 375 human genes. In parallel, we used a proteomic approach in which the proteins of infected and uninfected Mφ were compared using two-dimensional gels and mass spectrometry. Both of these techniques have their strengths and limitations. For example, the use of microarrays is technically simple and the precise extent of the genome being investigated is known. However, mRNA levels and protein levels do not necessarily correlate because the degradation rates of individual mRNAs are variable. Proteomics, on the other hand, fails to detect proteins of low abundancy, cell membrane-spanning proteins and proteins of high molecular charge or very low molecular mass. By combining these techniques, we analysed changes in gene expression of the human Mφ THP1 cell line 1, 6 and 12 hr following infection with M. tuberculosis. In addition to confirming previously published work on the up-regulation of a number of Mφ genes following phagocytosis of M. tuberculosis, the use of these approaches has enabled us to identify novel changes which emphasize the potential of these global techniques.
Materials and methods
Bacterial cell culture
M. tuberculosis H37Rv was grown in Middlebrook medium at 37° under biosafety level 3 conditions. Bacterial cultures were grown to mid-logarithmic phase (OD620nm = 0·5) and the numbers of colony-forming units (CFU) were determined by growth on Middlebrook agar plates.
Mφ culture and infection
The human monocytic leukaemia cell line THP1 was obtained from the European Collection of Cell Culture (ECACC No: 88081201; Salisbury, Wilts., UK) and cultured in RPMI-1640 (Life Technologies, Paisley, Strathclyde, UK) supplemented with 5% heat-inactivated fetal calf serum (FCS) (Advanced Protein Products, Brierly Hill, UK) and 20 µm mercaptoethanol (ME; Sigma-Aldrich, Poole, UK) without addition of antibiotics. A sample of 5 × 107 cells/20 ml was infected with live M. tuberculosis H37Rv at a multiplicity of infection of 10 bacteria to one cell. After 1, 6 and 12 hr, cells were collected, washed once in phosphate-buffered saline (PBS) and processed for two-dimensional (2-D) gel analysis or total RNA extraction. Independent infections were carried out on four separate occasions. Each of these was subjected to 2-D gel analysis, while three were also analysed using the microarrays. Only changes that were seen consistently in all experiments are reported.
2-D gel electrophoresis
Infected and uninfected cells were lysed for 30 min on ice with 0·2 ml of buffer containing 0·2% sodium dodecyl sulphate (SDS) (Genomic Solutions, Cambridge, UK), 40 mm Tris, pH 7·0, 65 mm dithiothreitol (DTT; Sigma-Aldrich), 2 mm magnesium chloride (Sigma-Aldrich), 2·5 µl of 2 mg/ml leupeptin (Roche, Lewes, UK), 5 µl of 1 mg/ml pepstatin (Roche) and 10 µl/ml of AEBSF hydrochloride (Melford Laboratories Ltd, Ipswich, UK). DNA was digested with 40–50 U of Benzonase (Merck, Poole, UK) for 15 min on ice. The samples were solubilized for 15 min on ice with 0·8 ml of a buffer containing 7·7 m urea (Genomic Solutions), 2·2 m thiourea (Merck), 5% CHAPS (Genomic Solutions), 81 mm DTT and 50 mm Tris, pH 7·0. The lysates were spun at 15 000 g and supernatants were sterilized by filtering through a low-protein-binding 0·22-µm Durapore filter (Millipore, Watford, UK). Protein concentrations were determined by using the Coomassie dye binding assay (Pierce, Chester, UK). Two-hundred micrograms of protein in 350 µl of urea/thiourea/2% IPG buffer (Pharmacia Biotech, Little Chalfont, UK) were adsorbed onto either pH 3–10 non-linear or pH 4–7 linear immobilized gradient 18 cm Immobiline DryStrips (Pharmacia) overnight at room temperature.16
The samples were focused using a seven-steps programme (150 V for 30 min; 300 V for 2·5 hr; 700 V for 30 min; 1500 V for 30 min; 2000 V for 30 min; 3000 V for 30 min; 3500 V for 40 hr) in a Multiphor II apparatus (Pharmacia). A minimum of 65 kVh was reached before loading onto the 2-D gels.
The 2-D gels were 7·5% or 12% polyacrylamide (Duracryl; Genomic Solutions) linear gels (size 20 cm × 16 cm × 1·5 mm). Before loading, the samples were equilibrated as described previously.16 The gels were stained with silver as described by Rabilloud et al.,17 and quantitative analysis was carried out using Melanie II 2D PAGE software (BioRad Laboratories, Hemel Hempstead, UK).
In-gel digestion with trypsin, and MALDI-TOF mass spectrometry
Protein spots of interest were cut out of the gel and diced into small pieces in microcentrifuge tubes. The gels were destained by washing twice with 200 µl of 25 mm ammonium bicarbonate (AB; Sigma), three times with 50 : 50 acetonitrile/50 mm AB and twice with 50 mm AB. The proteins were reduced for 1 hr at 37° with 10 mm DTT (Sigma) in 50 mm AB, washed twice with 50 mm AB, alkylated with 10 mm iodoacetamide (Sigma) and washed twice with 50 mm AB.
The gels were dehydrated with two washes of 200 µl of acetonitrile (Rathburn Chemicals, Walkerburn, UK) and dried at room temperature. Digestion was carried out by adding 10 µl of a 2-µg/ml solution of trypsin (Promega, Southampton, UK) to each sample; the samples were incubated overnight at 32° before MALDI-TOF analysis.
A Reflex III MALDI time-of-flight mass spectrometer (Brukner Daltonik GmbH, Bremen, Germany), equipped with a Scout-384 probe, was used to obtain positive ion mass spectra of the digested peptides. The peptide samples were acidified by the addition of a 1 : 10 volume of 2% trifluoroacetic acid prior to the MALDI analysis. Thin-layer matrix surfaces of α-ciano-4-hydroxy cinnamic acid mixed with nitrocellulose were prepared as described by Shevchenko et al.18 The acidified digestion was deposited as a thin layer and allowed to dry prior to rinsing with water. Peptide mass fingerprints were searched against the non-redundant database (available from the National Centre for Biotechnology Information; http://http://www.ncbi.nlm.nih.gov/). Partial enzyme cleavages leaving two cleavage sites, oxidation of methionine, pyroglutamic acid formation at the N-terminal glutamine and modification of cysteine by acrylamide were considered in these searches.
RNA isolation
Total RNA was isolated from 5 × 106 THP1 cells after 1, 6 or 12 hr of infection with M. tuberculosis using RNAzol B (AMS Biotech, Abingdon, Oxfordshire, UK) in accordance with the manufacturer's instructions. A DNA digestion step was included to avoid any genomic contamination. In brief, the RNA was dissolved in 60 µl of diethyl pyrocarbonate (DEPC)-treated water (Sigma) and a mixture comprising 5 mm MgSO4 (Sigma), 0·1 m sodium acetate (Sigma), 40 U of RNAse inhibitor (Promega), 10 U of DNAse I (Roche Diagnostic) and DEPC water was added to a final volume of 75 µl. The RNA was incubated at 37° for 15 min, extracted with an equal volume of choloroform : isoamylalcohol (24 : 1; ICN, Basingstoke, UK) and precipitated at −70° by adding 0·1 vol of 3 m sodium acetate and 2 vol of absolute ethanol (ICN). In a typical experiment, 5 × 106 THP1 cells yielded 15–20 µg of RNA.
Preparation of 33P-radiolabelled probes
33P-radiolabelled cDNA probes for array hybridization were prepared by reverse transcription. To a solution of 2 µg of RNA in 11 µl of DEPC-treated water, 4 µl of human cytokine cDNA labelling primers (Sigma-Genosys, Cambridge, UK) was added. After incubation for 2 min at 90° in a heating block, the solution was cooled to 42° and then added to 6 µl of 5 × reverse transcription buffer (Sigma-Genosys), 3 µl of dNTP mix (10 nm each of dATP, dGTP and dTTP), 0·5 µl of dCTP (100 µm), 2 µl of 10 µCi/µl [α-33P]dCTP (1000–3000 Ci/mmol; Amersham Pharmacia Biotech Inc., Little Chalfont, Bucks., UK), 0·5 µl of 40 U/µl ribonuclease inhibitor (Promega) and 2 µl of 25 U/µl reverse transcriptase (Sigma-Genosys). Following 2–3 hr of incubation at 42°, the probes were purified by passage through MicroSpin Sephadex G-50 Columns (Amersham Pharmacia Biotech Inc.).
Human cDNA expression arrays
Panorama human cytokine gene arrays (Sigma-Genosys) consist of a matched set of charged nylon membranes containing polymerase chain reaction (PCR) products spotted in duplicate. Each array contains 375 different human cytokine-related genes. Hybridization was carried out according to the manufacturer's instructions. Briefly, 33P-radiolabelled first-strand cDNA probes were prepared from 2 µg of total RNA obtained as described above. The arrays were prehybridized for 1 hr at 65° with hybridization solution (Sigma-Genosys). The filters were then incubated with the denatured, labelled cDNAs for 12–18 hr at 65° in a hybridization oven (Mini 10; Qiagen, Crawley, UK). The filters were washed extensively at low- and high-stringency conditions in hybridization bottles and exposed to a phosphor-imager screen (Molecular Dynamics, Sunnyvale, CA) for 24 hr at 4° and the resulting hybridization signals were quantified using a Phosphor-imager Storm 860 (Molecular Dynamics) and ImageQuant, version 5·0, software (Molecular Dynamics). The intensity of each spot was corrected for background levels and normalized for differences in probe labelling using the average values for housekeeping genes (β-actin, β2-microglobulin, cyclophilin A, HLA-A 0201 heavy chain, glyceraldehyde-3-phosphate dehydrogenase [GAPDH], hypoxanthine phosphoribosyl/transferase [HPRT], L19, transferrin receptor and α-tubulin). Genes showing a change of four-fold or more in intensity were considered to be up- or down-regulated following infection.
Results
Microarray analysis of the effect of M. tuberculosis infection on THP1 cell gene expression
33P-labelled cDNA was prepared from uninfected THP1 cells and from cells which had been infected for 1, 6 and 12 hr with M. tuberculosis. The cDNA was used to probe microarrays consisting of 375 genes belonging to the following families: adhesion molecules, angiogenic factors, cell-surface proteins, chemokines and their receptors, cytokines and their receptors, epidermal growth factor family genes, ephrins and ephrin receptors, fibroblast growth factor family genes, integrins, interleukins and their receptors, neurotrophic factors, genes involved in nitric oxide metabolism, proteases, orphan receptors and members of the transforming growth factor-β (TGF-β) and tumour necrosis factor-α (TNF-α) superfamilies (a full list of genes is available on the website: http://http://www.resgen.com.
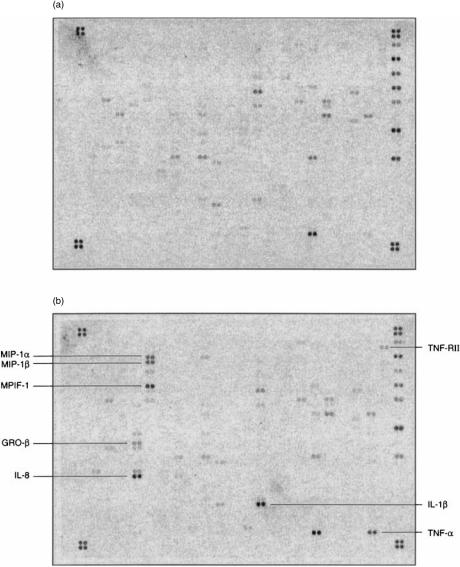
Figure 1 shows an example of a microarray hybridized with cDNA taken from THP1 cells infected for 6 hr, compared to uninfected cells. In the experimental design used it was not possible to differentiate between gene expression changes which occurred as a result of contact between the cells and the mycobacteria and those which occurred as a result of phagocytosis. In fact, although we did not see significant changes in gene expression at the 1-hr time-point, we were able to detect activation of signalling pathways much earlier than this (data not shown). Therefore, the changes probably result from a combination of mycobacterial–Mφ contact and mycobacterial phagocytosis. Those genes which showed changes in levels of expression following infection at any of the three time-points are summarized in Table 1. There was no change in the level of gene expression of any of the genes within the first hour following infection. However, by 6 hr several genes had become significantly induced. Many of these were chemokines, with macrophage inflammatory protein (MIP)-1α, MIP-1β, myeloid progenitor inhibitory factor-1 (MPIF-1), growth regulated gene-β (GRO-β) and interleukin (IL)-8 showing induction ratios of 40-fold or more at either 6 or 12 hr. Other chemokines which were induced at lower levels included MIP-3α, pulmonary and activation regulated chemokine (PARC), regulated on activation, normal, T-cell expressed and secreted (RANTES), eotaxin, GRO-α, GRO-γ, I-309, osteopontin (eta-1), monocyte chemotactic protein (MCP)-1 and MCP-2. Of the cytokines and interleukins, IL-1β showed the highest induction ratio of all, with a 433-fold induction by 12 hr. TNF-α was transiently induced, with an 18-fold induction at 6 hr, and we were unable to detect any increase in transcription of IL-12. Of note among other induced genes was the 14-fold induction of the matrix metalloproteinase, MMP-9. Matrix metalloproteinases play an important role in the remodelling of the extracellular matrix; MMP-9 is a gelatinase which is thought to play a role in the migration of immature dendritic cells, particularly Langerhans' cells.19,20 The up-regulation of vascular endothelial growth factor (VEGF) would also be compatible with a general role in the migration of cells to the site of infection. Using the criterion of a four-fold change seen at any time-point, we were unable to detect any genes on the microarray which were consistently down-regulated. Thus, by far the most dominant picture which emerges at these early time-points is the wide-scale switching on of genes encoding proteins involved in cell migration and homing. One of the key roles of the Mφ immediately following infection is to attract cells involved in the innate and acquired immune response to the site of infection.
Figure 1.
Microarray analysis of Mycobacterium tuberculosis-infected THP1 cells. Gene expression 6 hr following infection (b) was compared with that in uninfected cells (a). The most strongly up-regulated genes are labelled.
Table 1.
Differential gene expression of Mycobacterium tuberculosis-infected THP1 cells
| Fold induction at: | ||||
|---|---|---|---|---|
| Gene name | Gene group | 1 hr | 6 hr | 12 hr |
| MIP-1α | Chemokine | – | 28 | 60 |
| MIP-1β | Chemokine | – | 98 | 33 |
| MIP-3α | Chemokine | – | 10 | – |
| MPIF-1 | Chemokine | – | 45 | 20 |
| PARC | Chemokine | – | – | 4 |
| RANTES | Chemokine | – | 5 | 9 |
| Eotaxin | Chemokine | – | – | 10 |
| GRO-α | Chemokine | – | 8 | 3 |
| GRO-β | Chemokine | – | 54 | 16 |
| GRO-γ | Chemokine | – | 6 | 3 |
| IL-8 | Chemokine | – | 74 | 100 |
| MCP-1 | Chemokine | – | – | 10 |
| MCP-2 | Chemokine | – | 1 | 13 |
| Osteopontin | Chemokine | – | – | 4 |
| I-309 | Chemokine | – | 19 | 8 |
| CCR3 | Chemokine receptor | – | 6 | 1 |
| IGF.BP8 | Binding protein | – | 8 | – |
| l-Selectin | Adhesion/migration | – | 5 | – |
| VCAM-1 | Adhesion/migration | – | 5 | – |
| Integrin a3 | Adhesion | – | 5 | |
| IL-1β | Interleukin | – | 97 | 433 |
| IL-2 | Interleukin | – | 7 | – |
| IL-11 | Interleukin | – | 7 | – |
| TNF-α | Cytokine/TNF superfamily | – | 18 | – |
| CD30L | TNF superfamily | – | 6 | – |
| TNF RII | Cytokine receptor | – | 5 | – |
| TRANCE | Cytokine receptor family | – | 6 | – |
| VEGF | Angiogenic factor | – | 3 | 6 |
| Flk-1 | VEGF receptor/signalling | – | 6 | – |
| PDGF Ra | Receptor/signalling | – | 6 | 3 |
| Eph A1 | Receptor/signalling | – | 1 | 5 |
| MMP-9 | Metalloproteinase | – | – | 14 |
| CD21 | Cell surface protein | – | 5 | – |
| CD28 | Cell surface protein | – | 6 | – |
The results shown are representative of three experiments. Only genes showing a four-fold or greater induction for at least one time-point in all three experiments are included. No genes showed a four-fold or more down-regulation.
GRO, xxxxxxxxxxxxx; IGF, insulin-like growth factor; IL, interleukin; MCP, monocyte chemotactic protein; MIP, macrophage inflammatory protein; MPIF, xxxxxxxxxxxxx; PARC, xxxxxxxx xxxxxxxxx; RANTES, regulated on activation, normal, T-cell expressed, and secreted; TNF, tumour necrosis factor; TRANCE, xxxxxxxx; VCAM, vascular cell adhesion molecule; VEGF, vascular endothelial growth factor.
Changes in protein profiles of THP1 cells infected with M. tuberculosis
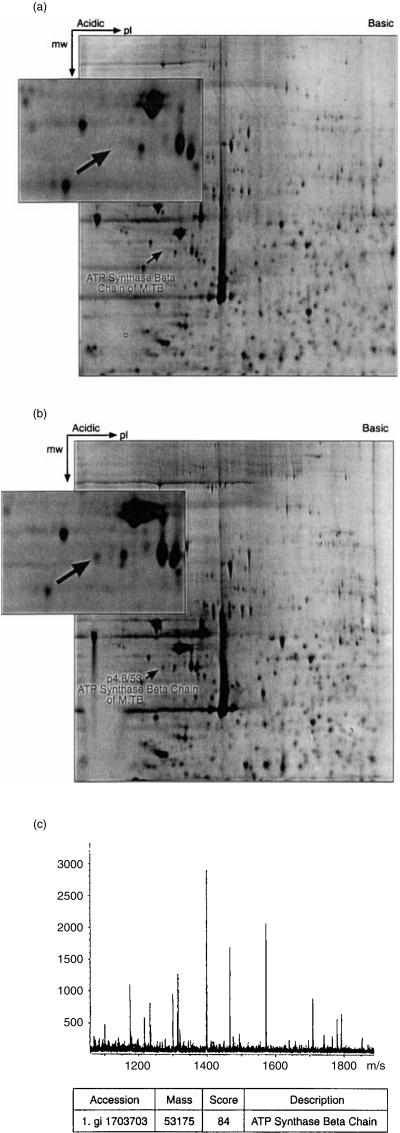
Protein extracts of THP1 cells were prepared after 12 hr of infection with M. tuberculosis and compared to uninfected cells using 2-D polyacrylamide gel electrophoresis (PAGE). Four separate infection experiments were carried out and the 2-D PAGE profiles were very reproducible. An example is shown in Fig. 2. In these initial experiments, the pH range was 3–10 (non-linear). Approximately 1000 spots were visible on the gels; this is a very small fraction of the protein content of the THP1 cells and represents the most abundant, non-membrane proteins which are separable within this pI/molecular mass (Mr) range. Loading of a greater quantity of protein in a attempt to detect less abundant proteins resulted in masking by the most abundant proteins, making resolution of individual spots more difficult. Only a small number of proteins could be readily identified as being consistently up- or down-regulated under these conditions. Using Melanie II 2-D PAGE software, three proteins were identified as being either down-or up-regulated following infection (Fig. 2). The MALDI-TOF analysis revealed one of the up-regulated proteins to be IL-1β (pI 4·8, Mr 30·7 kDa), thus supporting the microarray analysis described above. A second strongly up-regulated protein was found to be manganese superoxide dismutase (MnSOD, pI 8·3, Mr 21·9 kDa), which was not present on the microarray. This MnSOD was derived from the THP1 cells, rather than from the bacteria. Both IL-1β and MnSOD were also strongly up-regulated in bacille Calmette–Guérin (BCG)-infected and heat-killed M. tuberculosis-infected THP1 cells, but not in latex bead-loaded THP1 cells (data not shown). A third protein, revealed by MALDI-TOF to be heat-shock protein 27 (hsp27), was found to be significantly down-regulated following infection.
Figure 2.
Analysis by two-dimensional gel electrophoresis of Mycobacterium tuberculosis-infected THP1 cells. Protein extracts of THP1 cells infected with M. tuberculosis (b) were compared with those of uninfected THP1 cells (a). A non-linear, pH 3–10 IEF first-dimension and 12% second-dimension gel was used. Two up-regulated proteins and one down-regulated protein were identified (inserts) and analysed by MALDI-TOF.
In an attempt to increase the resolution of proteins, we next concentrated on the pI range of 4–7. We were able to identify an additional protein which was repeatedly seen in the 2-D gels of infected cells but was absent from uninfected cells. This protein (Fig. 3) was found to be the product of the atpD gene of M. tuberculosis, which encodes the ATP synthase β chain (Fig. 3c). This is the catalytic unit of ATP synthase which plays an important role in energy conversion using a proton-motive force to drive ATP synthesis from ADP and inorganic phosphates.21,22
Figure 3.
Analysis by two-dimensional gel electrophoresis of Mycobacterium tuberculosis-infected THP1 cells. The experiment is identical to that described in Figure 2, except that a linear pH 4–7 IEF first-dimension and 7·5% second-dimension gel was used. The up-regulated protein shown in the insert was found to be the ATP synthase β chain, the product of the M. tuberculosis atpD gene (c).
Discussion
The outcome of infection with M. tuberculosis depends on a highly complex chain of interactions, involving the altered transcription and expression of both bacterial and host cell genes. In this study, we investigated the early change in expression of Mφ genes during and immediately following phagocytosis of M. tuberculosis. This interaction between the Mφ and M. tuberculosis represents the earliest events in the infection process and probably plays a key role in determining the outcome of infection. In order to broaden the range of genes/proteins which we were able to investigate, we utilized two complementary techniques to investigate the transcriptome and proteome of infected cells. The use of gene microarrays to investigate changes at the transcriptional level provides a rapid, technically straightforward approach, and enables the simultaneous analysis of hundreds or thousands of genes. It also has the advantage that the precise extent of the genome being surveyed is known. In the example here, we investigated changes in the transcription of 375 genes, most of which are thought to play some role in immunoregulation. The main disadvantage of this approach is that interpretation of the data relies on the assumption that changes in steady-state mRNA levels correlate with changes in protein levels and with biological activity. Several of the genes which we identified as being strongly up-regulated, encode proteins which have previously been reported to increase in activity following M. tuberculosis infection. It is also reassuring that the product of the most highly induced gene, IL-1β, was also detected (using the proteomic approach) as being the most highly up-regulated protein. However, it is also probable that the regulation of other molecules will be at the translational and/or post-translational level. It is clear from our proteomic studies that global analysis of changes in protein levels is much less developed; while our proteomic results provided some interesting and novel information, it was clear that only a very small fraction of the total proteome can be investigated and, most importantly, one does not know the extent of the proteome which is being analysed.
The results of our gene microarray analysis show that within 6 hr of infection with M. tuberculosis, Mφ gene expression undergoes significant changes indicative of a strong proinflamatory response and, particularly, the requirement to recruit a wide range of other immunoregulatory and effector cells to the site of infection. In our study, the most strongly up-regulated chemokine-encoding gene was IL-8. IL-8 has previously been reported to be secreted from the pulmonary epithelial cell line A54923 and human bronchial epithelial cells following exposure to M. tuberculosis.24 M. tuberculosis-stimulated release of IL-8 has also been reported in aveolar Mφ,25 human polymorphonuclear granulocytes26 and previously in THP1 cells.27 A variety of other chemokines which we found (using the microarray analysis) to be up-regulated, have previously been shown to be stimulated following exposure of a variety of cell types to M. tuberculosis. These include osteopontin or eta-1,28 MCP-1,23,29,30 MIP-1α29,31 and RANTES.23,29 Our study suggests that an even broader range of chemokines is stimulated, at least in THP1 cells, following phagocytosis of M. tuberculosis. Of particular note was the range of chemokines which are up-regulated and particularly that some of these are selective for T helper 2 (Th2) T cells. For example, the receptor for eotaxin and RANTES, two of the up-regulated chemokines which we identified, is selectively expressed on human Th2 cells,32,33 in addition to eosinophils and basophils.34,35 While the involvement of RANTES in Th2 rather than Th1 type responses is controversial,36 there is extensive literature on the role of eotaxin in promoting atopic, Th2 responses37–40 and in the selective recruitment of Th2 cells to the lung in a model of allergic airway disease.41 As a Th2 cell response is generally thought to be disadvantageous to the control of M. tuberculosis infection, this would suggest that the Mφ response could result in the recruitment of a response which does not favour bacterial elimination. It seems probable that subtle, individual differences in the balance of chemokine production could profoundly affect the quality of the ensuing acquired immune response. Other chemokine-encoding genes, or genes encoding proteins which are probably involved in cell recruitment, and which have not previously been reported to be up-regulated in Mφ following exposure of M. tuberculosis, include the chemokine I-309, the matrix metalloprotein MMP-9 and the angiogenic factor VEGF.
Of the other genes probed, IL-1β was dramatically up-regulated, with ≈ 100-fold induction by 6 hr and 400-fold by 12 hr. TNF-α was transiently up-regulated with 18-fold induction by 6 hr, returning to baseline levels by 12 hr. Again, these results are supported by previous findings and help to validate the microarray approach as a means of screening for changes in gene expression. IL-1β has been previously demonstrated in the supernatants of monocytes stimulated with M. tuberculosis.42 TNF-α is also known to be stimulated in Mφ infected with M. tuberculosis.43,44 Surprisingly, IL-12 was not found to be up-regulated according to the criteria used here. However, a closer examination of the results demonstrated that the inducible IL-12 p40 gene was up-regulated 3·4-fold at 6 hr, falling below our four-fold cut-off criterion.
The analysis of changes at the proteome level was much more difficult, probably because highly regulated proteins are not major components of the cell compared to structural proteins. It is significant that only one of the cytokine/chemokine genes identified by microarray analysis was identified by proteomic analysis, and this was the product of the massively up-regulated (433-fold) IL-1β gene. The fact that we did not detect IL-8 expression following infection, despite the fact that it was induced 100-fold at the transcriptional level, illustrates one of the limitations of the approach. IL-8 has a low molecular weight (11.1 kDa) and a high pI (calculated to be 9·1); such proteins are poorly resolved using standard 2-D PAGE. Nevertheless, we made three interesting findings.
First, in addition to the induction of IL-1β, the proteomic analysis also revealed a significant up-regulation of Mφ-derived MnSOD. Stimulation of Mφs triggers an oxidative burst and the generation of superoxide anions (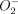 ) and other reactive oxygen species.45,46 Superoxide dismutases scavenge increased
) and other reactive oxygen species.45,46 Superoxide dismutases scavenge increased 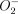 . MnSOD is usually inactive in resting cells but is stimulated by phagocytosis,47 IL-1β and TNF-α.48 In Mφ infected with human immunodeficiency virus-1 (HIV-1), MnSOD has been shown to be significantly up-regulated, probably through a TNF-α-mediated pathway.49 Phagocytosis, together with the presence of TNF-α and particularly large amounts of IL-1β in the Mφ cultures, would be expected to stimulate high levels of
. MnSOD is usually inactive in resting cells but is stimulated by phagocytosis,47 IL-1β and TNF-α.48 In Mφ infected with human immunodeficiency virus-1 (HIV-1), MnSOD has been shown to be significantly up-regulated, probably through a TNF-α-mediated pathway.49 Phagocytosis, together with the presence of TNF-α and particularly large amounts of IL-1β in the Mφ cultures, would be expected to stimulate high levels of 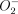 . The induction of MnSOD probably plays an important role in protecting the cell against
. The induction of MnSOD probably plays an important role in protecting the cell against 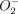 -mediated cytotoxicity, but could also help to protect the M. tuberculosis from
-mediated cytotoxicity, but could also help to protect the M. tuberculosis from 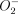 killing and hence promote the Mφ as a reservoir for viable M. tuberculosis.
killing and hence promote the Mφ as a reservoir for viable M. tuberculosis.
Second, we also found that the heat-shock protein hsp27 was down-regulated in infected Mφ. hsp27 has recently been shown to interfere negatively with apoptosis,50 and hence its down-regulation could indicate a very early trend towards the mitochondrial pathway of caspase-dependent cell death.50
Third, the most surprising finding to emerge from the proteomic studies was the fact that we were able to detect the presence of a mycobacterial protein, the ATP synthase β chain, in the Mφ extracts. Although we took no precautions to eliminate the possibility of identifying proteins derived from M. tuberculosis, the low multiplicity of infection used in this study, the short incubation period and the low sensitivity of the detection system meant that we considered it unlikely that mycobacterial proteins would be detected. Using 2-D gels on the same number of M. tuberculosis bacteria used for these experiments, but in the absence of Mφ, we were unable to do so (data not shown). The product of the atpD gene is thought to be important for the survival of a number of organisms that inhabit the gastrointestinal tract, because of the importance of the proton-translocating F1Fo-ATPase in tolerance to low pH.51–53 Indeed the F1Fo-ATPase is thought to function as a regulator of cytoplasmic pH.52,54 The most probable explanation of our finding is that atpD expression is induced following phagocytosis in response to a small downwards shift in pH. Alternatively, its presence in high amounts in M. tuberculosis-infected Mφ could play a role in inhibiting the acidification of the phagosome and hence promote bacterial survival.
The results of this study, using both transcriptomic and proteomic approaches, complements the recent reports of the use of microarrays to investigate gene regulation in Mφ infected with Listeria monocytogenes55 and Salmonella typhimurium.56 Such approaches will enable us to extend our understanding of the cross-talk between intracellular pathogens and their host cells, and identify novel mechanisms of bacterial evasion or immunological elimination.
Acknowledgments
M. Romano was supported by The National Research Council of Argentina (CONICET).
References
- 1.Kochi A. The global tuberculosis situation and the new control strategy of the World Health Organization. Bull World Health Organ. 2001;79:71–5. [PMC free article] [PubMed] [Google Scholar]
- 2.Brightbill HD, Libraty DH, Krutzik SR, et al. Host defense mechanisms triggered by microbial lipoproteins through toll-like receptors. Science. 1999;285:732–6. doi: 10.1126/science.285.5428.732. 10.1126/science.285.5428.732. [DOI] [PubMed] [Google Scholar]
- 3.Means TK, Wang S, Lien E, Yoshimura A, Golenbock DT, Fenton MJ. Human toll-like receptors mediate cellular activation by Mycobacterium tuberculosis. J Immunol. 1999;163:3920–7. [PubMed] [Google Scholar]
- 4.Hirsch CS, Ellner JJ, Russell DG, Rich EA. Complement receptor-mediated uptake and tumor necrosis factor-alpha-mediated growth inhibition of Mycobacterium tuberculosis by human alveolar macrophages. J Immunol. 1994;152:743–53. [PubMed] [Google Scholar]
- 5.Schlesinger LS. Macrophage phagocytosis of virulent but not attenuated strains of Mycobacterium tuberculosis is mediated by mannose receptors in addition to complement receptors. J Immunol. 1993;150:2920–30. [PubMed] [Google Scholar]
- 6.Stokes RW, Haidi ID, Jeffries WA, Speert DP. Mycobacteria macrophage interactions: macrophage phenotype determines the nonopsonic binding of Mycobacterium tuberculosis to murine macrophages. J Immunol. 1993;151:7067–76. [PubMed] [Google Scholar]
- 7.Zimmerli S, Edwards S, Ernst JD. Selective receptor blockade during phagocytosis does not alter the survival and growth of Mycobacterium tuberculosis in human macrophages. Am J Respir Cell Mol Biol. 1996;15:760–70. doi: 10.1165/ajrcmb.15.6.8969271. [DOI] [PubMed] [Google Scholar]
- 8.Fratazzi C, Manjunath N, Arbeit RD, Carini C, Gerken TA, Ardman B, Eremold-O'Donnell E, Remold HG. A macrophage invasion mechanism for mycobacteria implicating the extracellular domain of CD43. J Exp Med. 2000;192:183–92. doi: 10.1084/jem.192.2.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Armstrong JA, Hart PD. Phagosome–lysosome interactions in cultured macrophages infected with virulent tubercle bacilli. Reversal of the usual nonfusion pattern and observations on bacterial survival. J Exp Med. 1975;142:1–16. doi: 10.1084/jem.142.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sturgill-Koszycki S, Schlesinger PH, Chakraborty P, et al. Lack of acidification in Mycobacterium phagosomes produced by exclusion of the vesicular proton-ATPase. Science. 1994;263:678–81. doi: 10.1126/science.8303277. [DOI] [PubMed] [Google Scholar]
- 11.Sturgill-Koszycki S, Schaible UE, Russell DG. Mycobacterium-containing phagosomes are accessible to early endosomes and reflect a transitional state in normal phagosome biogenesis. EMBO J. 1996;15:6960–8. [PMC free article] [PubMed] [Google Scholar]
- 12.Gatfield J, Pieters J. Essential role for cholesterol in entry of mycobacteria into macrophages. Science. 2000;288:1647–50. doi: 10.1126/science.288.5471.1647. 10.1126/science.288.5471.1647. [DOI] [PubMed] [Google Scholar]
- 13.Ferrari G, Langen H, Naito M, Pieters J. A coat protein on phagosomes involved in the intracellular survival of mycobacteria. Cell. 1999;97:435–47. doi: 10.1016/s0092-8674(00)80754-0. [DOI] [PubMed] [Google Scholar]
- 14.Pancholi P, Mirza A, Bhardwaj N, Steinman RM. Sequestration from immune CD4 T cells of mycobacteria growing in human macrophages. Science. 1993;260:984–6. doi: 10.1126/science.8098550. [DOI] [PubMed] [Google Scholar]
- 15.Gercken J, Pryjma J, Ernst M, Flad HD. Defective antigen presentation by Mycobacterium tuberculosis-infected monocytes. Infect Immun. 1994;62:3472–8. doi: 10.1128/iai.62.8.3472-3478.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sanchez JC, Rouge V, Pisteur M, Ravier F, Tonella L, Moosmayer M, Wilkins MR, Hochstrasser DF. Improved and simplified in-gel sample application using reswelling of dry immobilized pH gradients. Electrophoresis. 1997;18:324–7. doi: 10.1002/elps.1150180305. [DOI] [PubMed] [Google Scholar]
- 17.Rabilloud T, Carpentier G, Tarroux P. Improvement and simplification of low-background silver staining of proteins by using sodium dithionite. Electrophoresis. 1988;9:288–91. doi: 10.1002/elps.1150090608. [DOI] [PubMed] [Google Scholar]
- 18.Shevchenko A, Wilm M, Vorm O, Mann M. Mass spectrometric sequencing of proteins from silver-stained polyacrylamide gels. Anal Chem. 1996;68:850–8. doi: 10.1021/ac950914h. 10.1021/ac950914h. [DOI] [PubMed] [Google Scholar]
- 19.Kobayashi Y. Langerhans' cells produce type IV collagenase (MMP-9) following epicutaneous stimulation with haptens. Immunology. 1997;90:496–501. doi: 10.1046/j.1365-2567.1997.00212.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kobayashi Y, Matsumoto MV, Kotani M, Makino T. Possible involvement of matrix metalloproteinase-9 in Langerhans' cell migration and maturation. J Immunol. 1999;163:5989–93. [PubMed] [Google Scholar]
- 21.Kanazawa H, Futai M. Structure and function of H+-ATPase: what we have learned from Escherichia coli H+-ATPase. Ann N Y Acad Sci. 1982;402:45–64. doi: 10.1111/j.1749-6632.1982.tb25731.x. [DOI] [PubMed] [Google Scholar]
- 22.Senior AE, Wise JG. The proton-ATPase of bacteria and mitochondria. J Membr Biol. 1983;73:105–24. doi: 10.1007/BF01870434. [DOI] [PubMed] [Google Scholar]
- 23.Lin Y, Zhang M, Barnes PF. Chemokine production by a human alveolar epithelial cell line in response to Mycobacterium tuberculosis. Infect Immun. 1998;66:1121–6. doi: 10.1128/iai.66.3.1121-1126.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wickremasinghe MI, Thomas LH, Friedland JS. Pulmonary epithelial cells are a source of IL-8 in the response to Mycobacterium tuberculosis: essential role of IL-1 from infected monocytes in a NF-kappa B-dependent network. J Immunol. 1999;163:3936–47. [PubMed] [Google Scholar]
- 25.Pace E, Gjomarkaj M, Melis M, Profita M, Spatafora M, Vignola AM, Bonsignore G, Mody CH. Interleukin-8 induces lymphocyte chemotaxis into the pleural space. Role of pleural macrophages. Am J Respir Crit Care Med. 1999;159:1592–9. doi: 10.1164/ajrccm.159.5.9806001. [DOI] [PubMed] [Google Scholar]
- 26.Riedel DD, Kaufmann SH. Chemokine secretion by human polymorphonuclear granulocytes after stimulation with Mycobacterium tuberculosis and lipoarabinomannan. Infect Immun. 1997;65:4620–3. doi: 10.1128/iai.65.11.4620-4623.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Friedland JS, Shattock RJ, Johnson JD, Remick DG, Holliman RE, Griffin GE. Differential cytokine gene expression and secretion after phagocytosis by a human monocytic cell line of Toxoplasma gondii compared with Mycobacterium tuberculosis. Clin Exp Immunol. 1993;91:282–6. doi: 10.1111/j.1365-2249.1993.tb05896.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nau GJ, Guilfoile P, Chupp GL, Berman JS, Kim SJ, Kornfeld H, Young RA. A chemoattractant cytokine associated with granulomas in tuberculosis and silicosis. Proc Natl Acad Sci USA. 1997;94:6414–9. doi: 10.1073/pnas.94.12.6414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sadek MI, Sada E, Toossi Z, Schwander SK, Rich EA. Chemokines induced by infection of mononuclear phagocytes with mycobacteria and present in lung alveoli during active pulmonary tuberculosis. Am J Respir Cell Mol Biol. 1998;19:513–21. doi: 10.1165/ajrcmb.19.3.2815. [DOI] [PubMed] [Google Scholar]
- 30.Friedland JS, Shattock RJ, Griffin GE. Phagocytosis of Mycobacterium tuberculosis or particulate stimuli by human monocytic cells induces equivalent monocyte chemotactic protein-1 gene expression. Cytokine. 1993;5:150–6. doi: 10.1016/1043-4666(93)90054-9. [DOI] [PubMed] [Google Scholar]
- 31.Kasahara K, Sato I, Ogura K, Takeuchi H, Kobayashi K, Adachi M. Expression of chemokines and induction of rapid cell death in human blood neutrophils by Mycobacterium tuberculosis. J Infect Dis. 1998;178:127–37. doi: 10.1086/515585. [DOI] [PubMed] [Google Scholar]
- 32.Sallusto F, MacKay CR, Lanzavecchia A. Selective expression of the eotaxin receptor CCR3 by human T helper 2 cells. Science. 1997;277:2005–7. doi: 10.1126/science.277.5334.2005. 10.1126/science.277.5334.2005. [DOI] [PubMed] [Google Scholar]
- 33.Gerber BO, Zanni MP, Uguccioni M, et al. Functional expression of the eotaxin receptor CCR3 in T lymphocytes co-localizing with eosinophils. Curr Biol. 1997;7:836–43. doi: 10.1016/s0960-9822(06)00371-x. [DOI] [PubMed] [Google Scholar]
- 34.Uguccioni M, MacKay CR, Ochensberger B, et al. High expression of the chemokine receptor CCR3 in human blood basophils. Role in activation by eotaxin, MCP-4, and other chemokines. J Clin Invest. 1997;100:1137–43. doi: 10.1172/JCI119624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ponath PD, Qin S, Post TW, et al. Molecular cloning and characterization of a human eotaxin receptor expressed selectively on eosinophils. J Exp Med. 1996;183:2437–48. doi: 10.1084/jem.183.6.2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chensue SW, Warmington KS, Allenspach EJ, Lu B, Gerard C, Kunkel SL, Lukacs NW. Differential expression and cross-regulatory function of RANTES during mycobacterial (type 1) and schistosomal (type 2) antigen-elicited granulomatous inflammation. J Immunol. 1999;163:165–73. [PubMed] [Google Scholar]
- 37.Fernvik E, Gronneberg R, Lundahl J, Raud J, Zetterstrom O, Van Hage-Hamsten M, Hallden G. Characterization of eosinophils and detection of eotaxin in skin chamber fluid after challenge with relevant allergen in patients with mild asthma. Clin Exp Allergy. 1999;29:1516–25. doi: 10.1046/j.1365-2222.1999.00572.x. 10.1046/j.1365-2222.1999.00572.x. [DOI] [PubMed] [Google Scholar]
- 38.Zeibecoglou K, MacFarlane AJ, Ying S, Meng Q, Pavord I, Barnes NC, Robinson DS, Kay AB. Increases in eotaxin-positive cells in induced sputum from atopic asthmatic subjects after inhalational allergen challenge. Allergy. 1999;54:730–5. doi: 10.1034/j.1398-9995.1999.00058.x. 10.1034/j.1398-9995.1999.00058.x. [DOI] [PubMed] [Google Scholar]
- 39.Yawalkar N, Uguccioni M, Scharer J, Braunwalder J, Karlen S, Dewald B, Braathen LR, Baggiolini M. Enhanced expression of eotaxin and CCR3 in atopic dermatitis. J Invest Dermatol. 1999;113:43–8. doi: 10.1046/j.1523-1747.1999.00619.x. 10.1046/j.1523-1747.1999.00619.x. [DOI] [PubMed] [Google Scholar]
- 40.Gutierrez–Ramos JC, Lloyd C, Gonzalo JA. Eotaxin: from an eosinophilic chemokine to a major regulator of allergic reactions. Immunol Today. 2000;20:500. doi: 10.1016/s0167-5699(99)01522-4. [DOI] [PubMed] [Google Scholar]
- 41.Lloyd CM, Delaney T, Nguyen T, Tian J, Martinez AC, Coyle AJ, Gutierrez-Ramos JC. CC chemokine receptor (CCR) 3/eotaxin is followed by CCR4/monocyte-derived chemokine in mediating pulmonary T helper lymphocyte type 2 recruitment after serial antigen challenge in vivo. J Exp Med. 2000;191:265–74. doi: 10.1084/jem.191.2.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wilkinson RJ, Patel P, Llewelyn M, Hirsch CS, Pasvol G, Snounou G, Davidson RN, Toossi Z. Influence of polymorphism in the genes for the interleukin (IL)-1 receptor antagonist and IL-1beta on tuberculosis. J Exp Med. 1999;189:1863–74. doi: 10.1084/jem.189.12.1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rojas M, Olivier M, Gros P, Barrera LF, Garcia LF. TNF-alpha and IL-10 modulate the induction of apoptosis by virulent Mycobacterium tuberculosis in murine macrophages. J Immunol. 1999;162:6122–31. [PubMed] [Google Scholar]
- 44.Manca C, Tsenova L, Barry CE, III, et al. Mycobacterium tuberculosis CDC1551 induces a more vigorous host response in vivo and in vitro, but is not more virulent than other clinical isolates. J Immunol. 1999;162:6740–6. [PubMed] [Google Scholar]
- 45.Murray HW, Nathan CF, Cohn ZA. Macrophage oxygen-dependent antimicrobial activity I V. Role of endogenous scavengers of oxygen intermediates. J Exp Med. 1980;152:1610–24. doi: 10.1084/jem.152.6.1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yuo A, Kitagawa S, Motoyoshi K, Azuma E, Saito M, Takaku F. Rapid priming of human monocytes by human hematopoietic growth factors: granulocyte–macrophage colony-stimulating factor (CSF), macrophage–CSF, and interleukin-3 selectively enhance superoxide release triggered by receptor-mediated agonists. Blood. 1992;79:1553–7. [PubMed] [Google Scholar]
- 47.Murray HW, Cohn ZA. Macrophage oxygen-dependent antimicrobiol activity III. Enhanced oxidative metabolism as an expression of macrophage. J Exp Med. 1980;152:1596–609. doi: 10.1084/jem.152.6.1596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wong GH, Goeddel DV. Induction of manganese superoxide dismutase by tumor necrosis factor: possible protective mechanism. Science. 1988;242:941–4. doi: 10.1126/science.3263703. [DOI] [PubMed] [Google Scholar]
- 49.Raoul H, Le Naour R, Blond D, Dormont D. HIV type 1 infection of human macrophages induces an upregulation of manganese superoxide dismutase gene that may protect cells from death. AIDS Res Hum Retroviruses. 1998;14:427–34. doi: 10.1089/aid.1998.14.427. [DOI] [PubMed] [Google Scholar]
- 50.Bruey JM, Ducasse C, Bonniaud P, et al. hsp27 negatively regulates cell death by interacting with cytochrome c. Nat Cell Biol. 2000;2:645–52. doi: 10.1038/35023595. 10.1038/35023595. [DOI] [PubMed] [Google Scholar]
- 51.Kobayashi H, Suzuki T, Unemoto T. Streptococcal cytoplasmic pH is regulated by changes in amount and activity of a proton-translocating ATPase. J Biol Chem. 1986;261:627–30. [PubMed] [Google Scholar]
- 52.Shibata C, Ehara T, Tomura K, Igarashi K, Kobayashi H. Gene structure of Enterococcus hirae (Streptococcus faecalis) F1F0-ATPase, which functions as a regulator of cytoplasmic pH. J Bacteriol. 1992;174:6117–24. doi: 10.1128/jb.174.19.6117-6124.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.McGowan CC, Cover TL, Blaser MJ. Analysis of F1F0-ATPase from Helicobacter pylori. Infect Immun. 1997;65:2640–7. doi: 10.1128/iai.65.7.2640-2647.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bender GR, Sutton SV, Marquis RE. Acid tolerance, proton permeabilities, and membrane ATPases of oral streptococci. Infect Immun. 1986;53:331–8. doi: 10.1128/iai.53.2.331-338.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cohen P, Bouaboula M, Bellis M, et al. Monitoring cellular responses to Listeria monocytogenes with oligonucleotide arrays. J Biol Chem. 2000;275:11181–90. doi: 10.1074/jbc.275.15.11181. [DOI] [PubMed] [Google Scholar]
- 56.Rosenberger CM, Scott MG, Gold MR, Hancock REW, Finlay BB. Salmonella typhimurium infection and lipopolysaccharide stimulation induce similar changes in macrophage gene expression. J Immunol. 2000;164:5894–904. doi: 10.4049/jimmunol.164.11.5894. [DOI] [PubMed] [Google Scholar]