Abstract
Interleukin-2-deficient (IL-2−/−) mice develop a spontaneous, progressive, CD4+ T-cell-mediated colitis with an age-related decrease in the number of B lymphocytes. The aim of this study was to determine the mechanisms of B-cell loss in IL-2−/− mice. Serum immunoglobulin G1 (IgG1) levels in 8-week-old IL-2−/− mice were above normal but then decreased dramatically with advancing age. Between 8 and 11 weeks of age, the number of B-cell progenitors (B220+ IgM−) in the bone marrow of IL-2−/− mice was less than half of those in IL-2+/+ littermates. By 22 weeks of age, very few progenitor cells remained in the bone marrow of most mice, and spleens were almost devoid of B cells. Likewise, B1 cells were not present in the peritoneal cavity of aged IL-2−/− mice. Flow cytometry analysis of B-cell differentiation in the bone marrow suggested a progressive loss of B cells from the most mature to the least mature stages, which was not dependent on IL-2 receptor-α (IL-2Rα) expression. B cells transferred from normal animals had similar survival rates in IL-2−/− and wild-type mice. We conclude that conventional B cells in older IL-2−/− mice are lost by attrition owing to a derangement in B-cell development. Because B1 cells are less dependent on the bone marrow, a separate mechanism for their loss is suggested.
Introduction
Interleukin-2 (IL-2) is secreted by a subset of activated CD4+ T lymphocytes and is regarded as a central growth and activation factor for many mononuclear cells, promoting T-cell proliferation and activation of macrophages, natural killer cells and lymphokine-activated killer cells.1,2 The role of IL-2 in early B-cell development is still controversial, but it plays a pivotal role during an immune response by stimulating antigen-activated B lymphocytes to progress through the cell cycle and to differentiate into antibody-secreting cells.3 The generation of IL-2-deficient (IL-2−/−) mice by targeted mutagenesis provides an excellent system to study the function of this cytokine in the context of an otherwise intact immune system. Early reports stated that the immune system in young IL-2−/− mice is functionally normal.4,5 IL-2−/− mice develop normally during the first 3–4 weeks of age but ≈50% of the animals die by the 12 weeks of age owing to a generalized autoimmune disease, which is characterized by anaemia, wasting and massive splenomegaly.6 Previous studies have shown that this systemic inflammatory response is the result of an uncontrolled activation and proliferation of CD4+ T lymphocytes.7 All of the surviving animals develop a chronic, rapidly progressing colitis, which is lethal. The intestinal inflammation develops most severely under conventional housing conditions, is attenuated in the specific pathogen-free (SPF) state,6 and is delayed, mild and focal in a germ-free environment.8 These observations suggest that luminal microbial components provide the persistent antigenic stimulus for this T-cell-mediated chronic inflammation. However, a recent study showed that the systemic inflammatory syndrome, apart from the colitis, persists with the same severity in the germ-free state.9
The intestinal inflammation that develops spontaneously in IL-2−/− mice raised in conventional or SPF conditions resembles human ulcerative colitis.6 The multiorgan inflammation in IL-2−/− mice is characterized by infiltrating mononuclear cells, mainly CD4+ T cells,10 which are thymus dependent and invade the colon and bone marrow.11 Cross-breeding of IL-2−/− animals with B-cell-deficient (JH−/−) mice demonstrated that B cells play no role in the pathogenesis of the colonic inflammation in this model, as these animals developed active disease, whereas IL-2−/− × RAG-2−/− double-mutant (B- and T-cell deficient) mice remained disease free.12 Our own findings show that the level of mucosally secreted immunoglobulins and the number of peripheral B lymphocytes in mesenteric lymph nodes dramatically decrease over time in SPF and germ-free IL-2−/− animals, whereas peripheral T cells are preserved.8 This loss of B cells in IL-2−/− mice could be the result of either direct developmental blockade in the bone marrow, owing to the lack of IL-2 interacting with its receptor (IL-2R) on pre-B II cells,13 or an indirect mechanism, such as the invasion of T lymphocytes into the bone marrow that compete for space with developing B cells.
The aims of this study were to further examine the kinetics of B-lymphocyte depletion in the bone marrow and in the peripheral lymphoid tissue of IL-2−/− mice and to determine whether this decrease in B-cell number is the result of defective production of B cells or peripheral loss.
Materials and methods
Mice
An SPF breeding colony of C57BL/6 × 129/Ola IL-2−/− mice was established at the Laboratory Animal Facilities of the University of North Carolina at Chapel Hill, as described previously.8 Germ-free IL-2−/− mice (C57BL/6 × 129/Ola background) were maintained, as described previously,8 in the Gnotobiotic Facility of the Center for Gastrointestinal Biology and Disease at the College of Veterinary Medicine, North Carolina State University. Offspring of heterozygous (IL-2+/− × IL-2+/−) breeders were genotyped by amplification of the IL-2 gene by the polymerase chain reaction (PCR) using IL-2-specific primers (Nucleic Acid Core Facility, Lineberger Comprehensive Cancer Center, University of North Carolina, Chapel Hill, NC).5 For spleen cell transfer experiments, 6-week-old IL-2−/− mice, bred onto the C57BL/6 background, and normal C57BL/6 mice were obtained from the Jackson Laboratory (Bar Harbor, ME). The use of mice described in this report was approved by the North Carolina State University Institutional Animal Care and Use Committee.
Tissue collection
Mice were killed and blood was collected for determination of serum immunoglobulin levels. Spleens, bone marrow, and peritoneal cells were obtained for flow cytometric analysis.
Immunoglobulin isotype-specific enzyme-linked immunosorbent assay (ELISA)
Detection of serum immunoglobulin isotypes has been described in detail previously.14 Affinity-purified goat anti-mouse immunoglobulin G1 (IgG1), and goat anti-mouse IgG2a (Southern Biotechnology, Birmingham, AL) were used for capturing these isotypes. Horseradish peroxidase-labelled goat anti-mouse IgG1 and goat anti-mouse IgG2a (Southern Biotechnology) were used for detection. The concentration of antibody was calculated by comparison with a standard curve using purified mouse IgG1 and IgG2a (PharMingen, San Diego, CA).
Cell preparation and flow cytometric analysis of bone marrow cells
Bone marrow cells were collected by injecting 1·5 ml of ice-cold Hanks' balanced salt solution (HBSS; calcium-, magnesium- and phenol red-free; Gibco, Grand Island, NY) into the femur. Cells were washed several times in HBSS and the red blood cells lysed. The following antibodies were used for detection of mature cells and B-cell precursors: fluorescein isothiocyanate (FITC)-labelled anti-B220; biotinylated anti-CD43, anti-c-kit and anti-CD25 (anti-IL-2Rα); and phycoerythrin (PE)-labelled anti-IgMa and anti-IgMb (all PharMingen). Cells were first incubated with combinations of FITC-labelled and biotinylated antibodies for 30 min in HBSS on ice, then washed twice and incubated for a further 30 min with PE-labelled antibodies and Streptavidin Red 670 (Gibco) to reveal binding of the biotin-labelled antibody. T cells were identified using FITC-labelled anti-CD4 and PE-labelled anti-CD8 (Caltag, Burlingame, CA). Stained cells were analysed by flow cytometry using a fluorescence-activated cell sorter (FACScan; Becton-Dickinson Immunocytometry Systems, San Jose, CA), with acquisition and analysis software from Cytomation, Inc. (Ft. Collins, CO) or Becton-Dickinson (Cell Quest).
Cell preparation and flow cytometric analysis of splenic lymphocytes and peritoneal extracts
Spleens were removed and single-cell suspensions were prepared by gentle teasing. Peritoneal cells were obtained by injecting cold HBSS into the peritoneal cavity. Spleen cells were incubated with antibodies to detect B lymphocytes (FITC-labelled anti-B220, PE-labelled anti-IgM, or biotinylated anti-CD23; PharMingen), or T lymphocytes (FITC-labelled anti-CD4, PE-labelled anti-CD8; Caltag); peritoneal cells were incubated with antibodies to detect B1 cells (FITC-labelled anti-CD5 and PE-labelled anti-IgM; PharMingen), according to the above protocol.
Splenocyte transfer and flow cytometric analysis of transferred cells
Normal C57BL/6 mice were used as donors for the transfer of splenocytes into IL-2−/− or normal C57BL/6 recipients. It should be noted that donor and recipient mice for the cell transfer experiment were purchased from the Jackson Laboratory to ensure tissue compatibility. Spleens were removed from donor mice, and single-cell suspensions were prepared by teasing. Cells were then washed and concentrated to 5 × 107 cells/ml and incubated for 15 min at 37° with 1 µm CFDA (5-and-6-carboxyfluorescein diacetate, succinimidyl ester; Molecular Probes Inc., Eugene, OR). Cells were washed and concentrated to 1·5 × 108 cells/0·5 ml of phosphate-buffered saline (PBS) and injected intraperioneally into recipient IL-2−/− or normal C57BL/6 mice. CFDA-labelled and endogenous B220+ cells were enumerated in the spleens of recipient mice killed 6 weeks after cell transfer, using Tri-color anti-B220 (Caltag) for flow cytometry. All analyses were performed in duplicate. The percentage of B220+ cells of total transferred cells that were detected was calculated as follows:
Results
Immunoglobulin production
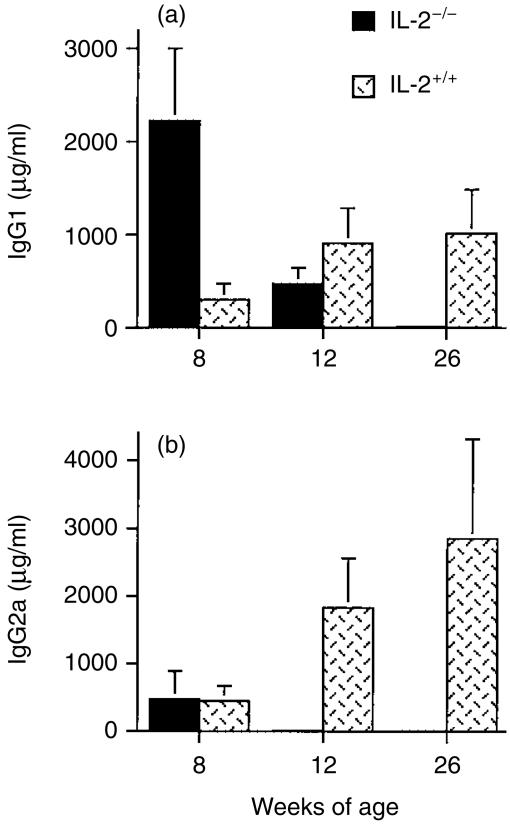
Serum immunoglobulin levels were very low in old IL-2−/− mice (Fig. 1). However, at 8 weeks of age (the time where the first histological signs of intestinal inflammation became apparent), the sera of IL-2−/− mice contained very high levels of IgG1. As mice age, a progressive decline in both IgG1 and IgG2a occurs. The level of both IgG isotypes in sera of IL-2−/− mice was negligible in 26-week-old mice (Fig. 1).
Figure 1.
Serum immunoglobulin G1 (IgG1) (a) and IgG2a (b) levels measured by isotype-specific ensyme-linked immunosorbent assay (ELISA) in 8–26-week-old IL-2−/− and IL-2+/+ mice. The numbers of mice in each group are as follows. IL-2−/−: 8 weeks, n = 4; 12 weeks, n = 3; 26 weeks, n = 4. IL-2+/+: 8 weeks, n = 7; 12 weeks, n = 4; 26 weeks, n = 5. Values represent mean ± SD (in µg/ml) of each isotype, measured in sera collected from mice at the different ages shown.
Flow cytometric analysis of lymphocytes in bone marrow, spleen and peritoneal exudate
IL-2−/− mice of all ages had very brittle bones, and the bone marrow of these mice was white, in contrast to the dark-red appearance of the bone marrow of wild-type animals. Bone marrow from 8 to 10-week-old IL-2−/− mice contained ≈ 2·5-fold fewer B220+ IgM− pre-B cells than bone marrow of age-matched wild-type littermates (Table 1). The number of these cells decreased further by 11 weeks of age and, by 22–26 weeks of age, IL-2−/− mice had sevenfold fewer pre-B cells than wild-type mice of the same age (Table 1). Splenic B-cell numbers in 8–10-week-old IL-2−/− mice were variable but tended to be lower than the number of B cells in the spleens of IL-2+/+ mice (Table 1). Splenic B-cell numbers declined dramatically in IL-2−/− mice 11 weeks of age and older (Table 1). As a result of the severe splenomegaly that occurs in older IL-2−/− mice (we have observed spleen weights as high as 1·3 g, or up to 5% of body weight), total numbers of spleen cells were not determined and therefore this comparison was not made for splenic B cells of mice that were older than 13 weeks of age.
Table 1.
B-cell and total cell numbers in the bone marrow and spleen of IL-2−/− mice *
| Source of cells | |||
|---|---|---|---|
| Cell subpopulation | Age (weeks) | IL-2−/− | IL-2+/+ |
| Bone marrow | |||
| B220+/IgM− | 8–10† | 1·7 ± 1·1‡ | 4·1 ± 1·6 |
| 11–13 | 1·3 ± 0·6 | 4·4 ± 0·1 | |
| 22–26 | 0·5 ± 0·1 | 3·7 ± 0·9 | |
| Total cells | 8–10† | 26·5 ± 8·9 | 19·4 ± 6·2 |
| 11–13 | 35·9 ± 13·2 | 21·8 ± 4·5 | |
| 22–26 | 10·7 ± 3·9 | 20·2 ± 5·4 | |
| Spleen | |||
| B220+ | 8–10 | 26·7 ± 11·3 | 57·7 ± 26·5 |
| 11–13 | 4·3 ± 2·3 | 46·7 ± 18·4 | |
| Total cells | 8–10† | 142·6 ± 54·0 | 105·9 ± 49·1 |
| 11–13 | 110·6 ± 2·3 | 100·0 ± 31·6 | |
Bone marrow and spleen cells were collected from IL-2−/− and IL-2+/+ mice between the ages of 8 and 26 weeks. Cell subpopulations were identified by flow cytometry.
Number of mice per group. IL-2−/−: 8–10 weeks, n = 8; 11–13 weeks, n = 3; 22–26 weeks, n = 2. IL-2+/+: 8–10 weeks, n = 7; 11–13 weeks, n = 2; 22–26 weeks, n = 5.
Values represent the mean ± SD of the number of cells(×106) in each subpopulation determined by multiplying the % of bone marrow B220+/IgM− cells (bone marrow gate includes lymphocytes and large granular cells) or splenic B220+ cells (spleen gate includes lymphocytes only) by the total number of cells obtained from bone marrow (femurs) or spleen of each animal.
Considerably higher numbers of T cells were consistently found in the bone marrow of IL-2−/− mice compared to wild-type mice (Table 2). In contrast to the progressive loss of bone marrow B cells observed as IL-2−/− mice age (see Table 1), CD4+ cell numbers, whilst higher and more variable in the group of 11–13-week-old IL-2−/− mice, were similar in the oldest and the youngest mice examined. The CD8+ T cells in the bone marrow decreased somewhat in number as the IL-2−/− mice aged, consistent with low total cell numbers in the bone marrow of old IL-2−/− mice. As shown in Table 1, the total number of bone marrow cells of 22–26-week-old IL-2−/− mice was 10·7 ± 3·9 × 106 and of 22–26-week-old IL-2+/+ mice was 20·2 ± 5·4 × 106. In the spleen, high numbers of CD4+ and CD8+ T cells were also observed in IL-2−/− mice (Table 2). Elevated numbers of T cells were evident prior to the dramatic enlargement of the spleens of IL-2−/− mice that we observed in animals over 13 weeks of age.
Table 2.
T-cell numbers in the bone marrow and spleen of IL-2−/− mice*
| Source of cells | |||
|---|---|---|---|
| Cell subpopulation | Age (weeks) | IL-2−/− | IL-2+/+ |
| Bone marrow | |||
| CD4+ | 8–10† | 3·9 ± 1·7‡ | 0·7 ± 0·5 |
| 11–13 | 7·7 ± 5·0 | 0·9 ± 0·1 | |
| 22–26 | 3·2 ± 0·9 | 0·8 ± 0·1 | |
| CD8+ | 8–10 | 9·9 ± 3·7‡ | 1·0 ± 0·0 |
| 11–13 | 7·0 ± 1·8 | 1·2 ± 0·5 | |
| 22–26 | 3·5 ± 1·2 | 0·9 ± 0·3 | |
| Spleen | |||
| CD4+ | 8–10 | 32·0 ± 18·5 | 9·6 ± 4·0 |
| 11–13 | 21·6 ± 11·1 | 10·5 ± 8·2 | |
| CD8+ | 8–10 | 57·2 ± 34·5 | 11·9 ± 3·8 |
| 11–13 | 27·1 ± 16·9 | 13·4 ± 8·7 | |
Bone marrow and spleen cells were collected from IL-2−/− and IL-2+/+ mice between the ages of 8 and 26 weeks. Cell subpopulations were identified by flow cytometry.
The numbers of mice per group and the total cell numbers are given in Table 1.
Values represent the mean ± SD of the number of cells (×106) in each subpopulation determined by multiplying the % of bone marrow or spleen CD4+ or CD8+ cells by the total number of cells obtained from bone marrow (femurs) or spleen of each animal. Bone marrow and spleen gates include lymphocytes only.
The different stages in B-cell development can be identified by their expression of certain cell-surface proteins, as shown in Table 3. All B-lineage cells expressed the pan B-cell markers B220 or CD19. Pro-B and pre-BI cells, the earliest identifiable stages and corresponding to Hardy fractions A, B, and C,15 have either no H-chain gene rearrangements or D-J rearrangements. They are CD43hi, CD25−, and c-kit+. Pre-BI cells undergo VH rearrangement and those cells that produce H chains become pre-BII cells (fraction D). They are identifiable as CD43lo, CD25+, and c-kit−. Initially these cells are large and undergo multiple rounds of cell division before becoming smaller, non-dividing cells. As a result of the cell division, pre-BII cells are the largest pre-B-cell fraction in normal mouse bone marrow. These cells undergo L-chain gene rearrangement and become immature B cells (fraction E; IgM+, B220hi, and CD43−) that exit the bone marrow and migrate to the spleen. In the spleen they differentiate into mature B cells. Some mature B cells are present in the bone marrow because they recirculate back from the periphery.
Table 3.
Expression of cell-surface molecules on B-cell subpopulations during development in bone marrow
| B-lineage cell fractions | |||
|---|---|---|---|
| Cell-surface molecule | A,B,C* | D | E |
| B220 | Positive | Positive | High |
| CD19 | Positive | Positive | Positive |
| CD43 | High | Low | Negative |
| CD25 | Negative | Positive | Positive |
| c-kit | Positive | Negative | Negative |
| IgM | Negative | Negative | Positive |
Fractions designated according to Hardy et al15.
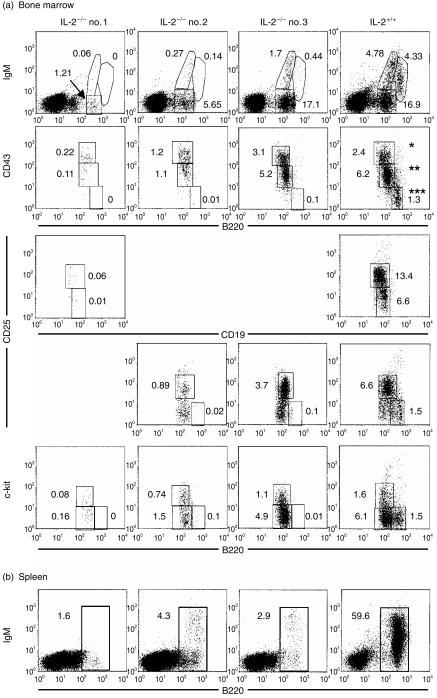
In order to understand the basis of the progressive B-cell loss in IL-2−/− mice, we compared the phenotype of bone marrow cells from IL-2−/− mice and their wild-type littermates. Shown in Fig. 2a are flow cytometric analyses of bone marrow from three, 23–24-week-old IL-2−/− mice. Two of these mice (IL-2−/−, no. 1; and IL-2−/−, no. 2) had significantly fewer than normal numbers of B-lineage cells at all stages, including those of the least mature pro/pre-BI stage (CD43hi, CD25−, and c-kit+) that precedes expression of the IL-2Rα (CD25). Interestingly, the pre-BII fraction (CD43lo, CD25+, and c-kit−) was affected more in both IL-2−/− mice than in the less mature pro/pre-BI cells. This could indicate a direct effect of the absence of IL-2 as these cells express CD25. The immature B cells (CD43− B220hi) are severely affected in these two IL-2−/− mice. None are seen in the bone marrow of IL-2−/− no. 1, and 0·01% of the bone marrow of IL-2−/− no. 2 are CD43− B220hi versus 1·3% in the bone marrow of a representative IL-2+/+ mouse. The bone marrow of the third IL-2−/− mouse (Fig. 2a, IL-2−/−, no. 3), a germ-free littermate of IL-2−/− no. 2, also contained very few immature B cells, but was not as severely depleted of the pre-BII cells (B220+ CD25+ cells are 3·7% in IL-2−/− no. 3 versus 0·89% in IL-2−/− no. 2 and 0·06% CD19+ CD25+ cells in IL-2−/− no. 1). Nevertheless, very few splenic B cells were detected in this mouse (Fig. 2b; 2·9% B220+ versus 59·6% in IL-2+/+). Thus, the more mature cells of the B-lineage that develop in bone marrow appear to be affected first, indicating progressive loss of cells from the most mature to the least mature fractions. However, all fractions are affected and eventually all are lost, including fractions that precede the expression of IL-2R (CD25).
Figure 2.
Flow cytometric analysis of
(a) B220 and CD43, IgM, CD25, c-kit, or CD19 and CD25 on bone marrow cells from three IL-2−/− mice (IL-2−/− no. 1, specific pathogen-free 26-weeks old; IL-2−/− no. 2, germ-free 23-weeks old; IL-2−/− no. 3, germ-free 23-weeks old) and one IL-2+/+ mouse (germ-free; 24-weeks old) or (b) B220 and IgM on spleen cells from the same mice. *Represents B-lineage cell fractions A, B, and C that contain pro-B and pre-BI cells; **represents B-lineage cell fraction D that contains pre-BII cells; ***represents B-lineage cell fraction E that contains immature B cells prepared to exit the bone marrow. These fractions have been described previously by Hardy et al.15
Young IL-2−/− mice (6-weeks old) have normal proportions of mature and transitional B cells in their spleens, based on expression of CD23 and the level of the heat-stable antigen (HSA) (CD24) (data not shown). Thus, there is not a disproportionate loss of immature and mature B cells in the early stages of B-cell loss in the periphery of IL-2−/− mice.
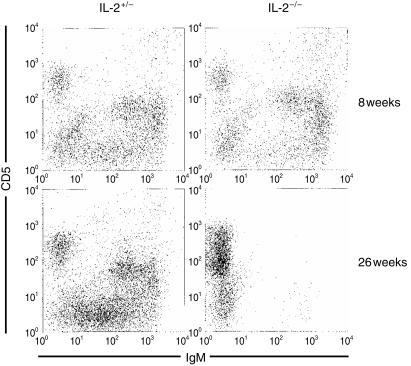
Because B1 cells constitute a B-cell subset that is developmentally and physiologically distinct from conventional or B2 cells, we examined the peritoneums of IL-2−/− mice to determine the fate of these cells in the absence of IL-2. Shown in Fig. 3 are non-elicited peritoneal extracts of 8- and 26-week-old IL-2−/− mice and their age-matched heterozygous littermates. At 8 weeks of age, CD5+ IgM+ B1 cells are clearly evident in both IL-2−/− and IL-2+/− mice. However, at 26 weeks, few peritoneal CD5+ B1 cells are present in IL-2−/− mice, although they are present in heterozygous control mice. Thus, B1 cells, like conventional B2 cells, are lost as IL-2−/− mice age.
Figure 3.
Flow cytometric comparison of non-elicited peritoneal cells isolated from 8-week-old and 26-week-old IL-2−/− and IL-2+/− mice.
Flow cytometric analysis of transferred splenocytes
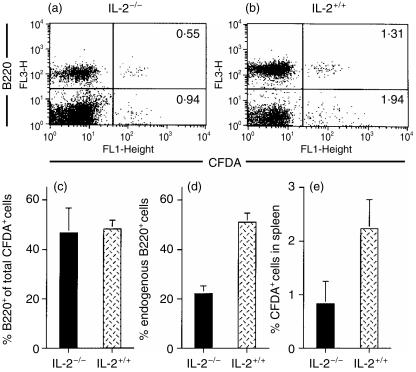
To further investigate the loss of mature B220+ lymphocytes in the spleens of IL-2−/− mice, we designed an experiment to monitor normal B cells transferred into IL-2−/− recipients. For these studies, we labelled normal, unseparated spleen cells from C57BL/6 mice with the fluorescent dye CFDA and injected the labelled cells into either recipient IL-2−/− mice (bred onto the C57BL/6 background; obtained from Jackson Laboratory) or normal C57BL/6 mice. Six weeks after transfer, CFDA-labelled cells were enumerated in the spleens of recipient mice. As shown in Fig. 4, transferred CFDA-labelled B220+ cells were readily identified in the spleen of a representative IL-2−/− mouse (Fig. 4a) and in the spleen of a normal IL-2+/+ (Fig. 4b) recipient mouse. There was no significant difference in the proportions of CFDA-labelled B220+ cells (calculated as the % of total CFDA-labelled cells) between IL-2−/− mice and normal controls (Fig. 4c). These results suggest that the transferred B cells are not eliminated in the spleens of the IL-2−/− recipient mice. As shown in Fig. 4(d), the proportion of B220+ endogenous (CFDA-negative) cells in the spleens of IL-2−/− mice (12 weeks of age) was twofold lower than the proportion of B220+ endogenous cells in the spleens of age-matched IL-2+/+ mice. Thus, the spleens of these IL-2−/− mice were losing endogenous B cells. It is interesting to note, as shown in Fig. 4(e), that transferred spleen cells (CFDA-labelled cells) comprise a lower proportion of cells in the spleens of the IL-2−/− recipient mice compared to the normal recipient mice, even though total numbers of spleen cells were similar in the two groups (103·7 ± 30·5 × 106 total spleen cells in the IL-2−/− recipients versus 106·1 ± 24·1 × 106 total spleen cells in the IL-2+/+ recipients). This observation suggests that both B cells and non-B cells from the spleen of a normal donor do not survive as well in IL-2−/− recipients as they do in normal mice. Taken together, the results of the transfer experiment suggest that peripheral B cells are not selectively eliminated. Thus, the data argue against a mechanism that specifically targets B cells for depletion.
Figure 4.
Flow cytometric analysis of B220+ 5-and-6-carboxyfluorescein diacetate, succinimidyl ester (CFDA)-labelled and B220+ endogenous spleen cells 6 weeks after transfer of CFDA-labelled normal spleen cells into IL-2−/− and IL-2+/+ (C57BL/6) mice. B220 (FL3) and CFDA (FL1) were detected on spleen cells from a representative IL-2−/− recipient (a) and from a representative IL-2+/+ (C57BL/6) recipient (b). Values represent the % of B220+/CFDA+ cells (upper right) and B220−/CFDA+ cells (lower right). In (c), values represent the mean ± SE of the % of B220+ cells of the total CFDA-labelled cells detected in spleens of IL-2−/− (n = 3) and IL-2+/+ (C57BL/6) (n = 3) mice. Values in (d) represent the mean ± SE of endogenous B220+ cells in spleens of the same mice. Values in (e) represent the mean ± SE of the % of CFDA+ cells of the total spleen cells from the same mice.
Discussion
IL-2−/− mice provide a model of human ulcerative colitis, but in addition to the progressive and lethal colitis, these mice express multiple immune system abnormalities. Early reports demonstrated normal immune function in young IL-2−/− mice with a normal development of lymphoid organs and the presence of all major lymphocyte subsets, in particular no obvious B-cell abnormality.6 However, in-depth descriptions of mice lacking the IL-2 gene pointed out various defects in the immune system in IL-2−/− mice older than 4 weeks of age, including a decreased number of peripheral B lymphocytes.7 Our kinetic analysis of serum immunoglobulin levels (Fig. 1) showed that the levels of IgG1 in serum are higher in 8-week-old IL-2−/− mice than in IL-2+/+ littermates, but IgG2a levels are similar. However, by 12 weeks of age, IgG1 levels are low and IgG2a is undetectable in the sera of IL-2−/− mice. These observations indicate that in early stages, preceding clinical colitis, functional B cells are present in IL-2−/− mice. Sadlack et al. demonstrated serum anti-colon autoantibodies in young IL-2−/− mice, but the proportion of these antibodies decreased as the B-lymphocyte numbers decreased, with further progression of intestinal disease.7 The high level of serum IgG1 in IL-2−/− mice is interesting because interleukin-4 (IL-4) induces the production of IgG1 in mice.16 Although not addressed in this study, perturbations in the cytokine network caused by the absence of IL-2 could explain the different kinetics for IgG1 and IgG2a production. While changes in cytokine levels could account for the decline in serum IgG1 and IgG2a in older mice, the major factor is likely to be B-cell loss.
Peripheral B-cell loss could occur by attrition because of a failure of the bone marrow to generate new B cells, or it could occur by elimination of peripheral B cells. Our data suggest that both occur. The loss of peritoneal B1 cells makes a compelling argument for peripheral B cell-elimination in IL-2−/− mice. B1 cells are long-living, self-renewing B cells; upon adoptive transfer peritoneal B1 cells are capable of reconstituting themselves. Thus, they are not dependent on replenishment from progenitors in the bone marrow, arguing that their loss is unlikely to be caused by attrition related to a bone marrow deficiency. Why they are lost is unknown, but the absence of IL-2 could affect the function of non-B cells that provide important survival signals to B1 cells. Alternatively, the uncontrolled activation of T cells that occurs in IL-2−/− mice could cause the destruction of peritoneal B1 cells directly, as has been suggested for bone marrow B-cell precursors in these mice,11 or indirectly by simple crowding. Interestingly, mice deficient in the apoptotic receptor Fas also develop a peritoneal B1-cell deficiency as adults.17 This has been suggested to be caused by a cytokine imbalance. Perhaps a similar cytokine imbalance initiated by the absence of IL-2 could result in B1-cell loss.
The number of B cells in the spleens of IL-2−/− mice compared to wild-type littermates decreased dramatically between 11 and 13 weeks of age (Table 1). A destructive mechanism acting on mature splenic B cells could contribute to the loss of conventional splenic B cells in IL-2−/− mice. However, the lack of evidence for specific elimination of B cells after transfer of labelled IL-2+/+ spleen cells into IL-2−/− recipients (Fig. 4) suggests that this mechanism is probably not a significant cause of splenic B-cell loss. Conventional B cells are not self-renewing and therefore they are entirely dependent on replenishment from the bone marrow. Thus, whereas B1 cells would be affected minimally by the loss of bone marrow precursors, the effect on conventional B2 cells would be severe and could explain conventional B-cell loss in IL-2−/− mice. An alternative explanation for the loss of B cells as IL-2−/− mice age is the uncontrolled activation and proliferation of T cells with a defect in apoptosis that has been previously described.7,18 Crowding by T cells or cytokine-related alteration of B-lymphocyte development, differentiation, or lifespan, may explain the loss of B cells in the spleen, peritoneal cavity and bone marrow in older IL-2−/− mice. If B cells required IL-2 for their survival, it is unlikely that B cells or antibody would be found even in young IL-2−/− mice.
The number of B220+ IgM− precursors in the bone marrow of IL-2−/− mice is consistently lower than in wild-type mice. However, we observed significant fluctuation among individual mice in the developmental stage of pre-B-cell loss. Analysis of the pre-B-cell populations in older IL-2−/− mice reveals differences between mice that provides clues to the mechanism of the B-cell developmental blockade. IL-2−/− mouse no. 3 (Fig. 2a) appeared to have a disruption of B-cell development at a late stage. This mouse had relatively normal proportions of pro/pre-BI and pre-BII cells, but was deficient in IgM+ cells. While the absence of mature recirculating B cells in the bone marrow was expected, as this mouse is deficient in splenic B cells, the absence of normal numbers of IgMlo immature B cells, and particularly IgMhi transitional B cells, suggested a deficiency in the differentiation and survival of IgM+ B cells in the bone marrow. In contrast, IL-2−/− mice nos 1 and 2 showed loss at the pro- and pre-B cell stages. The pre-BII cell population, which constitutes the majority of pre-B cells in normal mice, was most severely decreased, but the proportion of pro/pre-BI cells were also decreased, particularly in IL-2−/− mouse no. 1. These data suggest that there is a progressive loss of B-cell development from the most mature to the least mature stages in the bone marrow of IL-2−/− mice.
It is interesting to note that while the microbial status (SPF or germ-free) of the animals has a dramatic effect on the development of intestinal inflammation in IL-2−/− mice,6,8,9 bacteria and other environmental antigens do not appear to play a role in the progressive loss of B cells, confirming the results of Contractor et al. who also reported that germ-free IL-2−/− mice develop the anaemia and uncontrolled lymphoid cell proliferation that are characteristic pathological consequences of IL-2 deficiency.9
The blockade in B-cell development could be a result of the infiltration of activated, dysregulated T cells into the bone marrow.7 This was suggested based on the fact that athymic IL-2−/− mice have normal B-cell development.11 Normal proportions of B cells in athymic IL-2−/− mice provide the most compelling evidence to support the conclusion that IL-2 is not required for B-cell survival. Moreover, transfer of peripheral lymphocytes from IL-2−/− mice to athymic IL-2+/+ recipients results in an influx of T cells to the bone marrow and loss of B-cell development.11 Thus, activated, IL-2−/− T cells may have a central role in the destruction of developing B cells in the bone marrow. Their action may be to crowd out the B-cell progenitors, preventing contact with appropriate stromal cells and access to survival signals, or they may have a more direct role in B-cell destruction. Our experiments appear to exclude a direct role for the absence of IL-2 on the loss of pre-B cells, as differentiative stages before and after expression of the IL 2Rα chain (CD25) are eventually lost.
In conclusion, we demonstrate that the conventional B-lymphocyte depletion in IL-2−/− mice is caused principally by disrupted B-cell development in the bone marrow. It is probable that the loss of peritoneal B1 cells occurs by a separate mechanism that operates on B1 cells themselves, as this population is not dependent on new B-cell development in the bone marrow. The loss of progenitor B cells in the bone marrow seems to be progressive. It is evident first at the pre-BII to immature B-cell transition and eventually affects the pro- and pre-BI population. The loss of progenitor B cells in the bone marrow is independent of the expression of the IL-2R, indicating that the blockade is secondary to some other effect caused by the loss of IL-2. Whether this is a result of selective cytotoxic effects of IL-2−/− T cells on these early B-cell progenitors, invasion and replacement of the bone marrow by T cells, or decreased survival of B-cell precursors, will be addressed in future studies.
Acknowledgments
We thank Julie Kwon and Lisa Wiltron for expert technical assistance. This work was supported by NIH grants DK 40249, DK 53347, and a grant from the Crohn's and Colitis Foundation of America to R.B.S., by NIH Center Grant PO DK34987, by NIH grants AI-29596, AI-43587, American Cancer Society grant IM-772 and a grant from the Arthritis Foundation to S.H.C. and by the Deutsche Forschungsgemeinschaft Schu 1131 to M.S.
Abbreviations
- CFDA
5-and-6-carboxyfluorescein diacetate, succinimidyl ester
- IL-2
interleukin-2
- IL-2−/−
interleukin-2-deficient
- SPF
specific pathogen-free.
References
- 1.Smith KA. Interleukin-2: inception, impact and implications. Science. 1988;240:1169–76. doi: 10.1126/science.3131876. [DOI] [PubMed] [Google Scholar]
- 2.Swain SL. Lymphokines and the immune response: the central role of interleukin-2. Curr Opin Immunol. 1991;3:304–10. doi: 10.1016/0952-7915(91)90028-y. [DOI] [PubMed] [Google Scholar]
- 3.Tigges MA, Casey LS, Koshland ME. Mechanism of interleukin-2 signaling: mediation of different outcomes by a single receptor and transduction pathway. Science. 1989;243:781–6. doi: 10.1126/science.2492678. [DOI] [PubMed] [Google Scholar]
- 4.Kündig TM, Schorle H, Bachmann MF, Hengartner H, Zinkernagel RM, Horak I. Immune responses in IL-2-deficient mice. Science. 1993;262:1059–61. doi: 10.1126/science.8235625. [DOI] [PubMed] [Google Scholar]
- 5.Schorle H, Holtschke T, Hünig T, Schimpl A, Horak I. Development and function of T cells in mice rendered interleukin-2-deficient by gene targeting. Nature. 1991;352:621–4. doi: 10.1038/352621a0. [DOI] [PubMed] [Google Scholar]
- 6.Sadlack B, Merz H, Schorle A, Schimpl A, Feller AC, Horak I. Ulcerative colitis-like disease in mice with a disrupted interleukin-2 gene. Cell. 1993;75:253–61. doi: 10.1016/0092-8674(93)80067-o. [DOI] [PubMed] [Google Scholar]
- 7.Sadlack B, Lohler J, Schorle H, Klebb H, Haber H, Sickel E, Noelle RJ, Horak I. Generalized autoimmune disease in interleukin-2-deficient mice is triggered by an uncontrolled activation of CD4+ T cells. Eur J Immunol. 1995;25:3053–9. doi: 10.1002/eji.1830251111. [DOI] [PubMed] [Google Scholar]
- 8.Schultz M, Tonkonogy SL, Sellon RK, et al. Interleukin-2 deficient mice raised under germfree conditions develop delayed mild focal intestinal inflammation. Am J Physiol. 1999;276:G1461–72. doi: 10.1152/ajpgi.1999.276.6.G1461. [DOI] [PubMed] [Google Scholar]
- 9.Contractor NV, Bassiri H, Reya T, Park AY, Baumgart DC, Wasik MA, Emerson SG, Carding SR. Lymphoid hyperplasia, autoimmunity, and compromised intestinal intraepithelial lymphocyte development in colitis-free gnotobiotic IL-2 deficient mice. J Immunol. 1998;160:385–94. [PubMed] [Google Scholar]
- 10.Simpson SJ, Mizoguchi E, Allen D, Bahn AK, Terhorst C. Evidence that CD4+, but not CD8+ T cells are responsible for murine interleukin-2-deficient colitis. Eur J Immunol. 1995;25:2618–25. doi: 10.1002/eji.1830250932. [DOI] [PubMed] [Google Scholar]
- 11.Krämer S, Schimpl A, Hünig T. Immunopathology of interleukin-2-deficient mice: thymus dependence and suppression by thymus-dependent cells with an intact interleukin-2 gene. J Exp Med. 1995;182:1769–76. doi: 10.1084/jem.182.6.1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ma A, Datta M, Margosian E, Chen J, Horak I. T cells, but not B cells, are required for inflammatory bowel disease in interleukin-2-deficient mice. J Exp Med. 1995;183:1567–72. doi: 10.1084/jem.182.5.1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rolink A, Grawunder U, Winkler TH, Karasuyama H, Melchers F. IL-2 receptor α chain (CD25, TAC) expression defines a crucial stage in pre-B cell development. Int Immunol. 1994;6:1257–64. doi: 10.1093/intimm/6.8.1257. [DOI] [PubMed] [Google Scholar]
- 14.Tonkonogy SL, McKenzie DT, Swain SL. Regulation of isotype production by IL-4 and IL-5. Effects of lymphokines on Ig production depend on the state of activation of the responding B cells. J Immunol. 1989;142:4351–60. [PubMed] [Google Scholar]
- 15.Hardy RR, Carmack CE, Shinton SA, Kemp JD, Hayakawa K. Resolution and characterization of Pro-B and Pre-Pro-B cell stages in normal mouse bone marrow. J Exp Med. 1991;173:1213–25. doi: 10.1084/jem.173.5.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Snapper CM, Paul WE. B cell stimulatory factor-1 (interleukin 4) prepares resting murine B cells to secrete IgG1 upon subsequent stimulation with bacterial lipopolysaccharide. J Immunol. 1987;139:10–17. [PubMed] [Google Scholar]
- 17.Reap EA, Sobel ES, Cohen PL, Eisenberg RA. Conventional B cells, not B-1 cells, are responsible for producing autoantibodies in lpr mice. J Exp Med. 1993;177:69–78. doi: 10.1084/jem.177.1.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kneitz B, Herrmann T, Yonehara S, Schimpl A. Normal clonal expansion but impaired Fas-mediated cell death and anergy induction in interleukin-2-deficient mice. Eur J Immunol. 1995;25:2572–67. doi: 10.1002/eji.1830250925. [DOI] [PubMed] [Google Scholar]