Abstract
Anterior chamber-associated immune deviation (ACAID) is a systemic form of tolerance that is elicited by introducing antigens into the anterior chamber of the eye. ACAID is characterized by deficiencies in delayed-type hypersensitivity and complement-fixing antibodies upon subsequent challenge with antigen. The mechanisms responsible for the generation of this form of tolerance are not yet completely clear. Here we asked whether γδ T cells, which are critical in the induction of oral tolerance and nasal tolerance, play a role in ACAID. The percentage of splenic γδ T cells was higher in mice that received antigen via the anterior chamber compared to untreated mice. In addition, CD44 was up-regulated on some splenic γδ and αβ T cells after the intraocular injection of antigen. Moreover, administration of antigen into the anterior chamber did not induce ACAID in the C57BL/6 mice pretreated with anti-mouse δ-chain monoclonal antibody or in the γδ T-cell-receptor-deficient (δ−/−) mice. γδ T cells from wild-type mice reconstituted ACAID when transferred into the δ−/− mice before injection of antigen, verifying that the deficiency in δ−/− mice results from the lack of γδ T cells rather than from an inadvertent change caused by deletion of the δ-chain. These findings indicate that γδ T cells play a very important role in ocular tolerance.
Introduction
Immunologically privileged sites are anatomical sites in an immunocompetent host that can maintain allogeneic tissues without eliciting rejection for extended periods compared to conventional sites, such as the peritoneal cavity or the skin. The eye has been appreciated as a privileged site for more than 100 years.1 In addition, introduction of antigenic material into the anterior chamber of the eye leads to a deviant form of systemic immunity, called anterior chamber-associated immune deviation (ACAID).3 For example, BALB/c mice bearing progressively growing P815 tumours in the anterior chamber are unable to reject orthotopic DBA/2 skin grafts.2 Recipients of ocular bovine serum albumin (BSA) are refractory to the development of delayed-type hypersensitivity (DTH) when an immunogenic form of BSA is injected subcutaneously (s.c.).3 In contrast to the DTH response, the overall antigen-specific antibody response is preserved. However, the isotypes that fix complement, IgG2a, 2b and 3, are inhibited while IgG1, is unaffected by intraocular antigen.4 The selective deficiency in DTH and immunoglobulin isotypes that fix complement suggests that CD4+ T-cell-mediated immunity is compromised in this form of tolerance. However, the factors involved in the negative regulation of the systemic response in ACAID are yet to be fully clarified.
More than 90% of CD3+ T cells in peripheral blood and conventional lymphoid organs express αβ heterodimeric receptors, while only a minor population of T cells expresses γδ receptors.5 The γδ T cells produce various cytokines and exhibit cytolytic activities and hence they resemble αβ T cells in many functional aspects and are thought to play a role in the first line of defence against invading micro-organisms (summarized in ref. 6). Moreover, this small population has been shown to contain important immunoregulators, controlling both innate and adaptive immune responses by rapidly producing large quantities of regulatory mediators (summarized in ref. 7). γδ T cells have also been implicated in limiting inflammatory reactions and preventing excessive tissue damage.8,9 Generation of tolerance by nasal10,11 and oral12,13 antigens requires γδ T-cell receptor (TCR) -bearing T cells. γδ T cells have also been associated with protection of the testis from autoimmune destruction initiated by bacterial infection14 and with prevention of the rejection of allogeneic fetuses during pregnancy.15,16
To date, no reports investigating the role of γδ T cell in ACAID have been published. Here, we tested the hypothesis that γδ T cells may be required for the development of ACAID.
Materials and methods
Experimental animals
Female C57BL/6 (B6)(H-2b) mice, 7–8 weeks of age, were purchased from the National Cancer Institute (Frederick Cancer Research and Development Center, Frederick, MD). Male or female C57BL/6 J-Tcrdtm1 Mom (δ−/−) (H-2b) mice17 were bred in the animal facilities at Emory University and used at 7–8 weeks of age. All procedures on animals were conducted according to the principles in the guidelines of the Committee on Care and Use of Laboratory Animals, Institute of Laboratory Animal Resources, National Research Council.
Antigens
Chicken egg albumin (OVA, grade VI) and keyhole limpet haemocyanin (KLH) were purchased from Sigma Chemical Co. (St Louis, MO). Complete Freund's adjuvant (CFA) containing Mycobacterium tuberculosis strain H37Ra and incomplete Freund's adjuvant (IFA) were purchased from Difco Laboratories (Detroit, MI). Emulsions of OVA in CFA (2 mg/ml) or OVA in IFA (0·5 mg/ml) were prepared by mixing equal volume of aqueous antigen solution with adjuvant.
Intraocular inoculation
Mice were anaesthetized by injecting 100-µl mixture containing 10 mg/ml ketamine (Sigma Chemical Co.) and 2 mg/ml xylazine (Bayer Corporation, Shawnee Mission, KS) intramuscularly. One drop of Proparacaine HCl (ALCON Inc., Humacao, Puerto Rico) was applied topically on the eye before injection. Under a dissecting microscope, 50 µg OVA or KLH in 2·5 µl phosphate-buffered saline (PBS) solution was injected into the anterior chamber of one eye by using a Hamilton Microliter syringe and a 33-gauge needle (Hamilton Co. Reno, NV).
DTH responses
Mice were immunized with 100 µg OVA in 50 µl CFA s.c. at the base of the tail. Seven days later, mice were challenged s.c. with 25 µl IFA containing 12·5 µg OVA in one hind footpad. The same volume of PBS in IFA was used as a negative control in the other hind footpad. We chose to inject the footpad with IFA emulsion because we found that the footpad swelling was a larger response and gave reliable measurements. The footpad thickness was measured 24 hr after challenge using a micrometer (Mitutoyo 227-101; MTI Corporation, Paramus, NJ). The footpad swelling in response to OVA was calculated by the following formula: antigen-specific swelling (mm) = the thickness of antigen in IFA footpad (mm) − the thickness of PBS in IFA footpad (mm). All experiments were performed at least three times and the data for all animals were pooled and presented in the figures. Data were subjected to analysis of variance and Student's t-test. A value of P < 0·05 was considered to be statistically significant.
T-cell purification
αβ or γδ T cells were positively selected from single cell suspensions of spleens from B6 mice using antibody-coated beads and MACS Magnetic Separation Column (Miltenyi Biotec, Auburn, CA) according to the manufacture's instructions. Magnetic antibody cell sorting (MACS) super-paramagnetic MicroBeads are extremely small, approximately 50 nm in diameter, comparable to the size of a virus. Their size and composition (iron oxide and polysaccharide) make the MicroBeads biodegradable, so labelled cells retain their physiological function and positive selection does not activate the cells in general. B cells were first depleted from the splenocyte suspension using either anti-mouse CD19-coated MicroBeads and separation through LS+ MACS columns (for purification of γδ T cells), or a mouse T-cell enrichment column (R & D Systems, Minneapolis, MN). Enriched T cells were subsequently treated with Fc-block (Pharmingen, San Diego, CA) before incubating with biotin-conjugated anti-mouse TCR β antibody (H57–597, Pharmingen) or biotin-conjugated anti-mouse TCR δ-chain antibody (GL3, Pharmingen) on ice for 30 min. Cells treated with biotinylated antibody were washed, treated with Streptavidin MicroBeads, and passed through MS+ MACS columns. Both positive and negative subsets were recovered for αβ and γδ T-cell populations. In our hands, approximately 0·5–1·0% of lymphocytes in the spleens of B6 mice are TCR γδ+ (GL3+) cells. Using the magnetic positive purification, 0·4–0·6% of spleen cells were recovered in the GL3+ population.
Flow cytometry
Spleen cells from individual mice were stained with: fluorescein isothiocyanate (FITC) -conjugated anti-CD3 ε-chain (145-2C11), phycoerythrin (PE) -conjugated anti-TCR δ-chain (GL3) and Cy-chrome-conjugated anti-TCR β-chain (H57–597); or FITC-conjugated anti-CD3 ε-chain, Cy-chrome-conjugated anti-CD44 (IM7) and biotin-conjugated anti-TCR δ-chain counterstained with allophycocyanin-conjugated streptavidin (all reagents were obtained from Pharmingen). Fluorochrome conjugates of matched isotypes were used as negative controls in all experiments. All staining was performed in 100 µl staining buffer (PBS pH 7·4 containing 1% BSA and 0·1% sodium azide) and incubated for 30 min on ice. The cells were washed three times with staining buffer after each treatment and analysed immediately thereafter. Three-colour analysis was performed with a FACScan Cytofluorimeter using Cellquest software (Becton Dickinson, San Jose, CA) and the data were analysed using FlowJo software (Tree Star, San Carlos, CA). Forward angle and side light scatter were used to exclude dead and aggregated cells. Two-colour analyses are displayed on log scales.
Results
Splenic accumulation and activation of γδ T cells upon induction of ACAID
CD4+ and CD8+ αβ T cells have been implicated as afferent and efferent regulatory cells in the ACAID animal model.18 However, the role of γδ T cells has not been elucidated in this model. The negative regulatory role of γδ T cells at other immune privilege sites,14–16 as well as their requirement for oral and nasal tolerance10,13 suggests that γδ T cells could play a role in ocular tolerance.
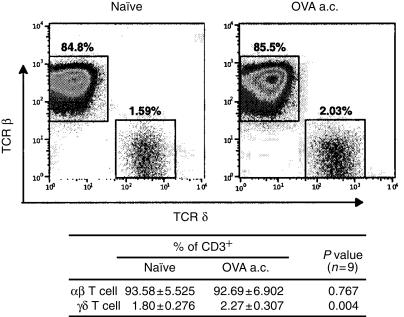
The postulate that γδ T cells are important in ACAID was first assessed by looking for an ACAID-associated increase of γδ T cells in the spleen. Spleens of B6 mice that were inoculated with 50 µg of OVA in the anterior chamber of the eye 7 days earlier or left untreated were extirpated and dissociated into single cell suspensions. Nine spleens per group were analysed individually by flow cytometry after staining for CD3, TCR β-chain and TCR δ-chain. CD3+ cells were gated and we collected 250 000 events in this gate. The flow cytometry data showed that the percentage of γδ T cells in CD3 gate was increased (about 0·47% of the total CD3+ T cells) in mice received anterior chamber treatment, which was statistically significant. By contrast, the percentage of αβ T-cells decreased slightly, however, the difference was not statistically significant (Fig. 1).
Figure 1.
Generation of ACAID correlates with a small but significant increase in the percentage of γδ T cells in the spleen. Spleen cells from normal B6 or B6 mice injected with 50 µg of OVA in the anterior chamber 7 days earlier were stained with FITC-conjugated anti-CD3, PE-conjugated anti-TCR δ-chain and Cy-chrome-conjugated anti-TCR β-chain. Lymphocytes were gated based on forward angle and side light scatter, then CD3+ cells were gated and 250 000 events were collected. Dual colour staining of a representative mouse for each group is illustrated on a log scale. Nine spleens per group were analysed individually using three-colour flow cytometry and the percentages of αβ and γδ T subsets in CD3+ cell population are indicated as average (mean±SD) of each group. P values were calculated using Students t-test.
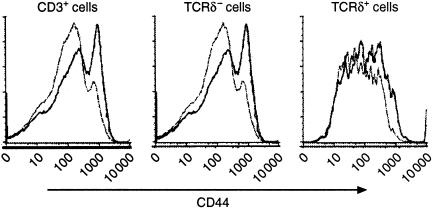
We also examined the expression of the activation marker, CD44, on the surface of the spleen cells after treatment with soluble antigen in the anterior chamber Spleen cells from mice that had received the same treatment as Fig. 1 were stained for CD3, CD44 and TCR δ-chain. Cell gating and collection were also done as described above. Statistically significant up-regulation of CD44 was observed in the total T-cell population as well as in the αβ T-cell population (Fig. 2). A clear shift was also seen in the γδ T-cell subset. However, due to the nature of this small population and the broad curve of the fluorescence histogram, we were unable to apply the statistical analysis to this population. Nevertheless, the data indicated that a portion of both αβ and γδ T cells were activated by injecting antigen into the anterior chamber.
Figure 2.
Expression of CD44 was up-regulated on T cells upon the induction of ACAID. Spleen cells from mice that received the same treatment as in Figure 1 were stained for CD3 (FITC), CD44 (Cy-chrome) and TCR δ-chain (allophycocyanin). Flow cytometry analysis was also carried out as described in Figure 1. Representative data from a control (thin line) and injected mice (thick line) are displayed as the relative number of cells (ordinate) against the increasing florescence channels (abscissa) that identify CD44-labelled cells. Statistics were calculated from six mice pre group and based on the gated regions. *P < 0·005.
ACAID can be induced by soluble antigen in an antigen-specific manner in C57BL/6 mice but not in γδ-deficient mice
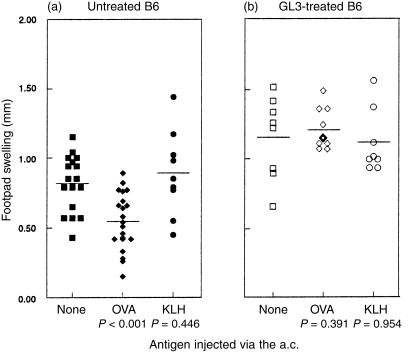
To examine directly the involvement of the γδ T cells in the development of ACAID, we tested the potential of immune deviation induced in mice that were deficient in γδ T cells by soluble antigen in the anterior chamber. We tested the CD4+ T-cell-mediated DTH response to OVA challenge after pretreatment of the mice with OVA in the anterior chamber B6 mice received no treatment, 50 µg of OVA, or 50 µg of KLH in the anterior chamber of one eye a week prior to immunizing s.c. with OVA in CFA. DTH responses to OVA were tested after another week by injecting OVA in IFA in the footpads. Mice that were pretreated with intraocular OVA exhibited impaired DTH responses to OVA challenge, compared to the positive controls that received no anterior chamber treatment (Fig. 3a). Mice pretreated with the irrelevant protein KLH still mounted a strong DTH response to OVA that was comparable to the positive controls.
Figure 3.
ACAID was not induced in B6 mice pretreated with GL3. (a) B6 mice (filled symbols) or (b) B6 mice that were injected intraperitoneally with 200 µg of purified hamster anti-mouse TCR δ-chain antibody (GL3) 3 days before (open symbols), received no injection (▪, □), 50 µg of OVA (♦, ◊) or KLH (•, ○) in the anterior chamber of eye. After 7 days, all mice were immunized with 100 µg OVA in CFA s.c. at base of the tail. DTH responses to OVA were detected by challenging the hind footpad with 12·5 µg OVA in IFA s.c. 7 days after immunization. Footpad swelling was measured 24 hr after challenge. Each symbol represents an individual mouse and the results are the accumulation of several experiments using the same protocol. The bar represents the average swelling and P values were calculated using Student's t-test comparing the control group that received no intraocular treatment with those receiving either OVA or KLH.
To generate mice that are deficient of γδ T cells, B6 mice were treated intraperitoneally with 200 µg of GL3, a monoclonal antibody specific for a δ-chain determinant that is expressed by all murine γδ T cells.19 GL3 treatment predominantly down-regulates γδ TCR rather than depleting the T cells.13 Mice were treated with GL3 3 days before receiving an injection of 50 µg OVA or KLH in the anterior chamber followed by OVA immunization and challenge for DTH, as described previously. In contrast to the responses observed in normal B6 mice, potent footpad swelling to OVA challenge was evoked in mice that were pretreated with GL3, regardless of the antigen injected in the anterior chamber (Fig. 3b). These results suggest that γδ T cells contribute to the tolerance induced by delivering antigen into the anterior chamber.
One mechanism that could account for the finding that GL3 treatment inhibited tolerance is that the antibody activated the γδ T cells, causing them to release lymphokines that subsequently interfered with the generation of tolerance. Indeed, some antibody specific for γδ T cells, such as UC7-13D520 and GL421 have been reported to activate γδ T cells in vitro. A prediction of this interpretation is that ACAID should be observed in TCR δ-chain knockout (δ−/−) B6 mice22 as it is in B6 mice. To test this prediction, δ−/− B6 mice were primed with antigen in a.c and assessed for DTH responses as described in Fig. 2. No significant differences in DTH responses to OVA challenge were observed among the three groups that received either no antigen, OVA, or KLH injection in the anterior chamber (Fig. 4). In addition, the magnitude of the DTH response of these δ−/− mice was similar to those seen in the B6 controls (Fig. 3). These results are contrary to the prediction and thus negate the interpretation that GL3 activated γδ T cells that could interfere with ACAID. However, these data do support the alternative hypothesis that γδ T cells are required for induction of ocular tolerance.
Figure 4.
TCR δ-chain knockout mice were deficient for the generation of ACAID. δ−/− mice received either no antigen (○), 50 µg of OVA (◊) or KLH (□) in the anterior chamber and were primed and tested for DTH responses as described in Figure 3.
Transfer of γδ T cells from wild-type (wt) B6 to δ−/− B6 mice reconstitutes ACAID
During the generation of δ−/− mice, it is conceivable that disruption of the δ-chain gene may have inadvertently caused effects on the expression of other genes. To address the possibility that the abrogation of ACAID in δ−/− mice might be the result of something other than the deletion of γδ T lymphocytes, we tested whether ACAID could be restored in δ−/− mice by adoptive transfer of wt B6 spleen cells. One group of δ−/− mice received no splenocytes and the other received 25 × 106 wt splenocytes intravenously 2 hr before OVA was injected via the anterior chamber. B6 mice also received the same number of wt B6 splenocytes with or without anterior chamber injection of OVA. Both groups of δ−/− and wt B6 mice were then immunized and challenged with OVA as described in Fig. 3. ACAID was observed in the B6 control mice (Fig. 5) similar to that previously observed in unreconstituted B6 mice (Fig. 3). As observed previously, anterior chamber injection of OVA did not attenuate footpad swelling in the unreconstituted δ−/− mice. However, δ−/− mice that received splenocytes from normal B6 mice developed reduced DTH responses that were similar to the ACAID control group of normal B6 mice (Fig. 5). Suppression of DTH responses was similar in these two groups and both were significantly different from the control B6 group that had no anterior chamber treatment. Reconstitution of tolerance in δ−/− mice by wt B6 splenocytes further supports the idea that the deficiency in the generation of ACAID was due to the absence of functional γδ T cells. There is a potential concern with this experiment that δ−/− mice theoretically might be able to recognize γδ T cells as foreign and reject them. If γδ T cells had not reconstituted ACAID in δ−/− mice, this would have been a concern. However, transfer of wt spleen cells restored ACAID to the δ−/− mice, which cannot easily be explained by elimination of the γδ T cells.
Figure 5.
Restoration of ACAID by the transfer of WT spleen cells in δ−/− mice. B6 mice received 25 × 106 B6 spleen cells (▪) or 25 × 106 spleen cells and OVA via the anterior chamber (♦). δ−/− mice received anterior chamber treatment only (◊) or 25 × 106 B6 spleen cells before OVA via the anterior chamber (○). All mice were primed and tested for DTH as described in Figure 3.
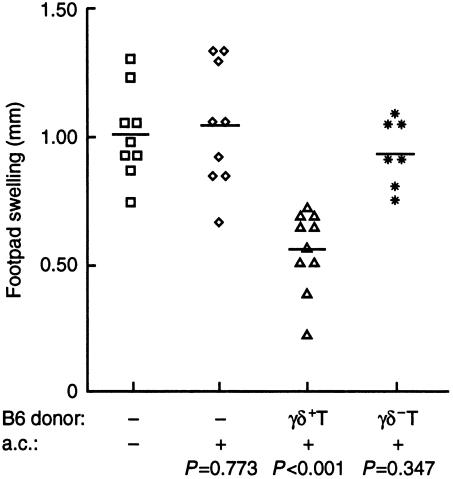
To verify that it was the γδ+ T cells in the B6 splenocytes that reconstituted tolerance in δ−/− mice, B-cell-depleted spleen cells from B6 mice were separated into γδ+ and γδ− subsets. Positively selected γδ+ T cells from B6 mice were found to be ≥80% pure (FACS data not shown). δ−/− mice received no B6 T cells, 25 × 106 γδ− T cells, or 0·25 × 106 γδ+ T cells, which is approximately equal to percentage of γδ T cells in the unfractionated splenocytes that were used to reconstitute ACAID in Fig. 5. The adoptive recipients and one group of non-transplanted δ−/− mice were injected with OVA a.c and all mice received OVA in CFA s.c. Non-transplanted δ−/− mice given OVA anterior chamber developed DTH responses that were equivalent to those of the untreated controls, verifying the lack of tolerance induction in δ−/− mice (Fig. 6). Priming for the DTH response was inhibited in the δ−/− mice that were reconstituted with WT γδ+ T cells, but not in those that received WT γδ− T cells.
Figure 6.
Restoration of ACAID in δ−/− mice by the transfer of γδ+ T cells from B6 mice. δ−/− mice without transfer or OVA via the anterior chamber (□) or with OVA via the anterior chamber (◊) were compared to δ−/− mice that received 0·25–0·3 × 106 B6 γδ+ T cells and anterior chamber injection of OVA (▵) or 25 × 106 B6 γδ− T cells before anterior chamber inoculation (⋆). Mice were primed and DTH responses measured as described in Figure 3.
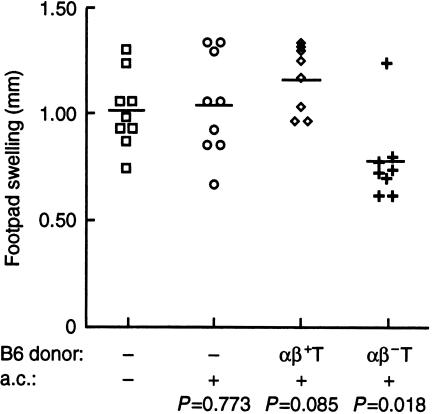
It is conceivable that the reconstitution of ACAID in δ−/− mice by WT γδ+ T cells was due to the transfer of activated T cells, which might have resulted from treatment with anti-TCR δ-chain antibody (GL3) during the positive selection. To address the issue, we transferred B6 spleen cells that have positively selected αβ+ T and αβ− T cells into δ−/− mice before the induction of ACAID. ACAID did not develop in δ−/− recipients of 12 × 106 wt αβ+ T cells upon OVA challenge, showing no difference from the control group without reconstitution and treatment in the anterior chamber (Fig. 7). However, priming of DTH in δ−/− was inhibited by the transfer of 1 × 106 αβ− T cells from normal B6 mice before intraocular treatment. Therefore, these results verify that wt γδ+ T cells restored ACAID in δ−/− mice. Together, our data support the conclusion that γδ T cells are required in the immune suppression of CD4+ T-cell-mediated DTH responses induced by administration of soluble antigen via the anterior chamber of the eye.
Figure 7.
ACAID was induced in δ−/− mice by the transfer of αβ– T cells from B6 mice. δ−/− mice without transfer or OVA via the anterior chamber (□) or with OVA via the anterior chamber (○) were compared to δ−/− mice that received 12 × 106 B6 αβ+ T cells and anterior chamber injection of OVA (◊) or 1 × 106 B6 αβ– T cells before anterior chamber inoculation (+). Mice were primed and DTH responses were measured as described in Figure 3.
Discussion
The studies reported above demonstrate that injection of antigen via the anterior chamber of the eye induces a small, but significant increase in the percentage of γδ T cells in the spleen. In addition, the activation antigen CD44 was up-regulated on a significant portion of CD3+ splenic T cells by ocular injection of OVA as manifest in both the αβ and γδ T-cell subsets. However, it is currently unclear whether the increase in activated γδ T cells is due to recruitment to the spleen or proliferation of resident T cells.
Not only were γδ T cells increased in the spleen by intraocular injection of antigen, but also DTH responses were not inhibited in mice that were genetically deficient in γδ T cells nor in B6 mice treated in vivo with the GL3 antibody. Humoral responses were also tested in the same mice by assessing OVA-specific antibody production in serum collected 14 days after OVA in CFA immunization. Consistent with previous reports,23 we found that the overall humoral response to the intraocular antigen was preserved (data not shown). However, we did not detect significant differences in the production of the complement-fixing IgG2a compared to IgG1 antibodies in the mice treated with OVA anterior chamber (data not shown). The explanation for the failure to detect differences in complement-fixing antibody is unknown, but it might be due to the genetic differences between the B6 and BALB/c mice. Differences could be due to observation that commercial antibodies, which are raised against IgG2a from BALB/c mice, do not react efficiently to antibodies from the Igh1-b in B6 mice.24 Indeed, mice expressing the Igh1-b allotype do not express the IgG2a gene but rather a novel gene encoding a different isotype, IgG2c.25 Further studies will be needed to determine whether regulation of the IgG2a response in ACAID also holds true for the IgG2c isotype.
The interpretation that γδ T cells are required for the development of ACAID is further corroborated by the observation that spleen cells from B6 mice reconstituted ACAID in δ-chain knockout mice. The relevant T cells were found in the αβ− and γδ+ subsets purified with magnetic beads. This expands the previously identified role of γδ T cells in nasal10,11 and oral tolerance,12,13 prolongation of skin graft survival26,27 and protection of an allogeneic fetus during pregnancy.14,16
A common feature of the above-mentioned forms of tolerance, excluding ACAID, is the abundant local distribution of γδ T cells at the site of exposure to antigen, including the respiratory tract, small intestine, epidermis and vagina. However, neither αβ nor γδ T cells are normally found in the eye. Rather, antigen injected into the anterior chamber of the eye is thought to be processed locally by antigen-presenting cells, in the presence of transforming growth factor-β (TGF-β), and subsequently migrate into the blood stream to take up residence in the spleen where they induce ACAID.28 Peripheral antigen-presenting cells that process antigen in the presence of TGF-β also acquire the ability to induce tolerance rather than immunity.29
The antigenic epitopes recognized by γδ T cells are less well-defined than those recognized by αβ T cells and they include non-peptide epitopes that can be presented by non-classical major histocompatibility complex molecules and a number of autologous, cellular antigens (reviewed in ref. 7). The question of whether the γδ T cells that play a role in ACAID recognize the nominal antigen administered via the anterior chamber has not yet been addressed. However, this scenario is feasible since the splenic γδ T cells that transfer IgE-specific tolerance appear to be antigen-specific.30
Another feature of the γδ T cells that are involved in various forms of tolerance is their potency. As few as 0·25 × 106 splenic γδ T cells from naïve B6 mice reconstituted ACAID in δ−/− mice. Although the lower limit of γδ T cells required for reconstitution of ACAID is not known, McMenamin et al. have shown that as few as 10 000 splenic γδ T cells transferred IgE-specific suppression in rats10 and mice.30
We not only showed that splenic γδ T cells reconstituted ACAID in δ−/− mice, but also observed an ACAID-associated increase in the percentage of splenic γδ T cells. These data again support the hypothesis that the spleen is the critical site for the generation of regulatory cells in ACAID.31,32 Splenic B cells have also been reported to be necessary in the generation of ACAID.33 A study on the distribution of avian T cells showed that γδ T cells were present primarily in the sinusoids of the spleen.34 Thus, direct contact between γδ T cells and B cells, might be possible at the sinusoids of the spleen, and such interactions might be critical in the ACAID.
It was recently reported by Sonoda et al.35 that the percentage of NK1.1+ αβ+ T cells was increased in the spleens of mice in which ACAID was induced. In addition, CD1 knockout mice were found to be deficient in ACAID induction35 suggesting that CD1-reactive, natural killer (NK) T cells were required for the induction of ACAID. Since depletion of either NK1.1+αβ T cells35 or γδ T cells (shown here) prevents the induction of ACAID, it suggests that neither population alone is sufficient to mediate this ACAID. Rather, both NK1.1+αβ T cells and γδ T cells are required, which raises the question of whether these cells interact with each other or whether they each contribute a distinct, but requisite, signal.
NK1.1 expression by T cells was first identified on a subset of αβ T cells.36 More recently, NK1.1+ γδ T cells have been described in the peritoneal cavity37 and thymus.38 Such double-positive cells that were detected in the peritoneal cavity of B6 mice infected with Salmonella choleraesuis account for about 20% of γδ+ T cells.37 Moreover, they proliferated and produced interferon-γ (IFN-γ) in vitro when exposed to self major histocompatibility complex class II, suggesting that NK1.1+ γδ T cells may play a regulatory role in the immune response.37 These observations raise the possibility that splenic γδ T cells might also express NK1.1. If so, it will be interesting to determine whether the NK1.1+ γδ+ T cells express a limited repertoire of TCR as has been described for NK1.1+αβ T cells [reviewed in ref. 39], whether they are detected in CD1 knockout mice, and what role they might play in ACAID.
Acknowledgments
This work was supported by a research grant and a centre grant from the Foundation Fighting Blindness and NEI core grant (P30 EYO06360) and a gift from Malcolm E. and Musette Powell. J. A. Kapp is the recipient of the Jules and Doris Stein Professorship in Ophthalmology awarded by Research to Prevent Blindness. Authors thank Dr Wayne Streilein for generously providing time to train us in the techniques used for ACAID induction and his encouragement of our work in this area.
Abbreviations
- ACAID
anterior chamber-associated immune deviation
- APC
allophycocyanin
- B6
C57BL/6
- CFA
complete Freund's adjuvant
- δ−/−
TCR δ-chain knockout
- DTH
delayed-type hypersensitivity
- KLH
keyhole limpet haemocyanin
- IFA
incomplete Freund's adjuvant
- OVA
chicken egg albumin
- s.c.
subcutaneous
- wt
wild-type.
References
- 1.Medawar P. Immunity tohomologous grafted skin: III. The fate of skin homografts transplanted to the brain, to subcutaneous tissue, and to the anterior chamber of the eye. Br J Exp Pathol. 1948;29:58–69. [PMC free article] [PubMed] [Google Scholar]
- 2.Streilein JW, Niederkorn JY, Shadduck JA. Systemic immune unresponsiveness induced in adult mice by anterior chamber presentation of minor histocompatibility antigens. J Exp Med. 1980;152:1121–5. doi: 10.1084/jem.152.4.1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mizuno K, Clark AF, Streilein JW. Anterior chamber-associated immune deviation induced by soluble antigens. Invest Ophthalmol Vis Sci. 1989;30:1112–19. [PubMed] [Google Scholar]
- 4.Wilbanks GA, Streilein JW. Distinctive humoral immune responses following anterior chamber and intravenous administration of soluble antigen. Evidence for active suppression of IgG2-secreting B lymphocytes. Immunology. 1990;71:566–72. [PMC free article] [PubMed] [Google Scholar]
- 5.Haas W, Pereira P, Tonegawa S. γ/δ T cells. Annu Rev Immunol. 1993;11:637–85. doi: 10.1146/annurev.iy.11.040193.003225. [DOI] [PubMed] [Google Scholar]
- 6.Kaufmann SH. γ/δ and other unconventional T lymphocytes: what do they see and what do they do? Proc Natl Acad Sci USA. 1996;93:2272–9. doi: 10.1073/pnas.93.6.2272. 10.1073/pnas.93.6.2272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Born W, Cady C, Jones-Carson J, Mukasa A, Lahn M, O'Brien R. Immunoregulatory functions of γδ T cells. Adv Immunol. 1999;71:77–144. [PubMed] [Google Scholar]
- 8.Fu YX, Roark CE, Kelly K, Drevets D, Campbell P, O'Brien R, Born W. Immune protection and control of inflammatory tissue necrosis by γδ T cells. J Immunol. 1994;153:3101–15. [PubMed] [Google Scholar]
- 9.Rosat JP, MacDonald HR, Louis JA. A role for γδ+ T cells during experimental infection of mice with Leishmania major. J Immunol. 1993;150:550–5. [PubMed] [Google Scholar]
- 10.McMenamin C, McKersey M, Kuehnlein P, Henig T, Holt PG. γδ T cells down-regulate primary IgE responses in rats to inhaled soluble protein antigens. J Immunol. 1995;154:4390–4. [PubMed] [Google Scholar]
- 11.Harrison LC, Dempsey-Collier M, Kramer DR, Takahashi K. Aerosol insulin induces regulatory CD8 γδ T cells that prevent murine insulin-dependent diabetes. J Exp Med. 1996;184:2167–74. doi: 10.1084/jem.184.6.2167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wildner G, Hunig T, Thurau SR. Orally induced, peptide-specific γ/δ TCR+ cells suppress experimental autoimmune uveitis. Eur J Immunol. 1996;26:2140–8. doi: 10.1002/eji.1830260927. [DOI] [PubMed] [Google Scholar]
- 13.Ke Y, Pearce K, Lake JP, Ziegler HK, Kapp JA. γδ T lymphocytes regulate the induction and maintenance of oral tolerance. J Immunol. 1997;158:3610–18. [PubMed] [Google Scholar]
- 14.Mukasa A, Hiromatsu K, Matsuzaki G, O'Brien R, Born W, Nomoto K. Bacterial infection of the testis leading to autoaggressive immunity triggers apparently opposed responses of αβ and γδ T cells. J Immunol. 1995;155:2047–56. [PubMed] [Google Scholar]
- 15.Heyborne KD, Cranfill RL, Carding SR, Born WK, O'Brien RL. Characterization of γδ T lymphocytes at the maternal–fetal interface. J Immunol. 1992;149:2872–8. [PubMed] [Google Scholar]
- 16.Suzuki T, Hiromatsu K, Ando Y, Okamoto T, Tomoda Y, Yoshikai Y. Regulatory role of γδ T cells in uterine intraepithelial lymphocytes in maternal antifetal immune response. J Immunol. 1995;154:4476–84. [PubMed] [Google Scholar]
- 17.Itohara S, Mombaerts P, Lafaille J, et al. T cell receptor δ gene mutant mice: independent generation of αβ T cells and programmed rearrangements of γδ TCR genes. Cell. 1993;72:337–48. doi: 10.1016/0092-8674(93)90112-4. [DOI] [PubMed] [Google Scholar]
- 18.Wilbanks GA, Streilein JW. Characterization of suppressor cells in anterior chamber-associated immune deviation (ACAID) induced by soluble antigen. Evidence of two functionally and phenotypically distinct T-suppressor cell populations. Immunology. 1990;71:383–9. [PMC free article] [PubMed] [Google Scholar]
- 19.Goodman T, Lefrancois L. Intraepithelial lymphocytes: anatomical site, not T cell receptor form, dictates phenotype and function. J Exp Med. 1989;170:1569–81. doi: 10.1084/jem.170.5.1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sandor M, Houlden B, Bluestone J, Hedrick SM, Weinstock J. In vitro and in vivo activation of murine γ/δ T cells induces the expression of IgA, IgM, and IgG Fc receptors. J Immunol. 1992;148:2363–9. [PubMed] [Google Scholar]
- 21.Gramzinski RA, Adams E, Gross JA, Goodman TG, Allison JP, Lefrancois L. T cell receptor-triggered activation of intraepithelial lymphocytes in vitro. Int Immunol. 1993;5:145–53. doi: 10.1093/intimm/5.2.145. [DOI] [PubMed] [Google Scholar]
- 22.Itohara S, Mombaerts P, Lafaille J, et al. T cell receptor δ gene mutant mice: independent generation of αβ T cells and programmed rearrangments of γδ TCR genes. Cell. 1993;72:337–48. doi: 10.1016/0092-8674(93)90112-4. [DOI] [PubMed] [Google Scholar]
- 23.Jager MJ, Gregerson DS, Streilein JW. Regulators of immunological responses in the cornea and the anterior chamber of the eye. Eye. 1995;9:241–6. doi: 10.1038/eye.1995.47. [DOI] [PubMed] [Google Scholar]
- 24.Martin RM, Brady JL, Lew AM. The need for IgG2c specific antiserum when isotyping antibodies from C57BL/6 and NOD mice. J Immunol Methods. 1998;212:187–92. doi: 10.1016/s0022-1759(98)00015-5. 10.1016/s0022-1759(98)00015-5. [DOI] [PubMed] [Google Scholar]
- 25.Jouvin-Marche E, Morgado MG, LeGuern C, Voegtle D, Bonhomme F, Cazenave P-A. The mouse Igh-1a and Igh1b H chain constant regions are derived from two distinct isotypic genes. Immunogenetics. 1989;29:92. doi: 10.1007/BF00395856. [DOI] [PubMed] [Google Scholar]
- 26.Gorczynski RM, Cohen Z, Leung Y, Chen Z. γδ TCR+ hybridomas derived from mice preimmunized via the portal vein adoptively transfer increased skin allograft survival in vivo. J Immunol. 1996;157:574–81. [PubMed] [Google Scholar]
- 27.Shiohara T, Moriya N, Hayakawa J, Itohara S, Ishikawa H. Resistance to cutaneous graft-vs.-host disease is not induced in T cell receptor δ gene-mutant mice. J Exp Med. 1996;183:1483–9. doi: 10.1084/jem.183.4.1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Streilein JW, Ksander BR, Taylor AW. Immune deviation in relation to ocular immune privilege. J Immunol. 1997;158:3557–60. [PubMed] [Google Scholar]
- 29.Wilbanks GA, Mammolenti M, Streilein JW. Studies on the induction of anterior chamber-associated immune deviation (ACAID). III. Induction of ACAID depends upon intraocular transforming growth factor-β. Eur J Immunol. 1992;22:165–73. doi: 10.1002/eji.1830220125. [DOI] [PubMed] [Google Scholar]
- 30.McMenamin C, Pimm C, McKersey M, Holt PG. Regulation of IgE responses to inhaled antigen in mice by antigen-specific γ/δ T cells. Science. 1994;265:1869–71. doi: 10.1126/science.7916481. [DOI] [PubMed] [Google Scholar]
- 31.Streilein JW, Niederkorn JY. Induction of anterior chamber-associated immune deviation requires an intact, functional spleen. J Exp Med. 1981;153:1058–67. doi: 10.1084/jem.153.5.1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wilbanks GA, Streilein JW. Macrophages capable of inducing anterior chamber associated immune deviation demonstrate spleen-seeking migratory properties. Reg Immunol. 1992;4:130–7. [PubMed] [Google Scholar]
- 33.D'Orazio TJ, Niederkorn JY. Splenic B cells are required for tolerogenic antigen presentation in the induction of anterior chamber-associated immune deviation (ACAID) Immunology. 1998;95:47–55. doi: 10.1046/j.1365-2567.1998.00581.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bucy RP, Chen CL, Cihak J, Losch U, Cooper MD. Avian T cells expressing gamma delta receptors localize in the splenic sinusoids and the intestinal epithelium. J Immunol. 1988;141:2200–5. [PubMed] [Google Scholar]
- 35.Sonoda K-H, Exley M, Snapper S, Balk SP, Stein-Streilein J. CD1-reactive natural killer T cells are required for development of systemic tolerance through an immune-privileged site. J Exp Med. 1999;190:1215–25. doi: 10.1084/jem.190.9.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Makino Y, Kanno R, Ito T, Higashino K, Taniguchi M. Predominant expression of invariant Vα14+ TCR α chain in NK1.1+ T cell populations. Int Immunol. 1995;7:1157–61. doi: 10.1093/intimm/7.7.1157. [DOI] [PubMed] [Google Scholar]
- 37.Nishimura H, Washizu J, Naiki Y, Hara T, Fukui Y, Sasazuki T, Yoshikai Y. MHC class II-dependent NK1.1+ γδ T cells are induced in mice by Salmonella infection. J Immunol. 1999;162:1573–81. [PubMed] [Google Scholar]
- 38.Vicari AP, Mocci S, Openshaw P, O'Garra A, Zlotnik A. Mouse γδ TCR+NK1.1+ thymocytes specifically produce interleukin-4, are major histocompatibility complex class I independent, and are developmentally related to αβ TCR+NK1.1+ thymocytes. Eur J Immunol. 1996;26:1424–9. doi: 10.1002/eji.1830260704. [DOI] [PubMed] [Google Scholar]
- 39.Vicari AP, Zlotnik A. Mouse NK1.1+ T cells: a new family of T cells. Immunol Today. 1996;17:71–6. doi: 10.1016/0167-5699(96)80582-2. [DOI] [PubMed] [Google Scholar]