Introduction
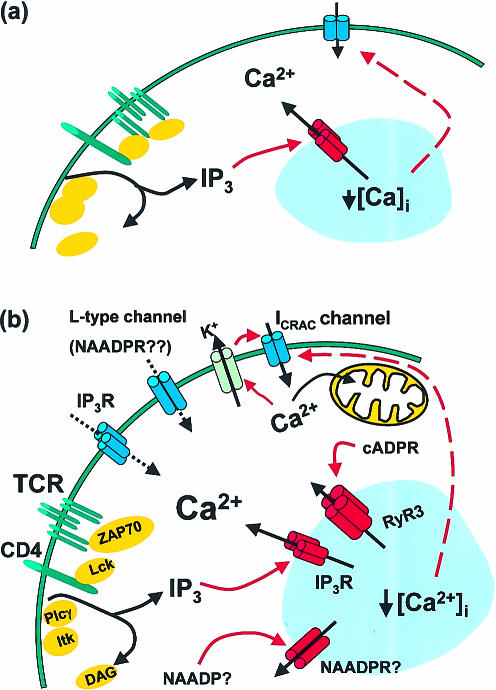
On B and T lymphocytes, ligation of the antigen receptor (AgR) induces a biphasic Ca2+ response. In the initial phase there is a large elevation in the intracellular Ca2+ concentration as a consequence of Ca2+ release from intracellular stores. This is followed by a lower, but prolonged elevation that is dependent on extracellular Ca2+.1,2 This simple description belies the complexity of the response. The initial phase may involve as many as three different intracellular Ca2+ channels, while the second phase depends not only on plasma membrane Ca2+ channels, but also on at least two different intracellular channels. The complexity of the signal, and the many opportunities for regulation of individual components of the signalling mechanism, lead to a tremendous flexibility in outcome, ranging from single, brief elevated Ca2+ transients, through a range of oscillatory responses, each of which can be decoded by the cell into a differing outcome.2 In this review we concentrate on the Ca2+ channels involved in the AgR-mediated Ca2+ signal, but we briefly discuss other Ca2+ channels present in lymphocytes. Figure 1 shows two possible schemes for the involvement of Ca2+ channels in TCR signalling, and Fig. 2 shows possible roles for Ca2+ channels in B cells.
Figure 1.
A possible scheme for the involvement of Ca2+ channels in TCR signalling (a) depicts the simplest possible scheme for the role of Ca2+ channels in TCR-induced Ca2+ signalling. TCR-induced Ins(1,4,5)P3 production causes Ca2+ release from intracellular stores, which in turn relays a signal to the plasma membrane store-operated Ca2+ channel (ICRAC channel), causing it to open. In an alternative scheme (b) intracellular Ca2+ flux results from the TCR-induced production of Ins(1,4,5)P3, cADPR and possibly NAADP, in concert with the activation of the ICRAC channel in the plasma membrane. The Ca2+ signal is sustained by the activity of mitochondria (shown in yellow), KCa channels, and cADPR. Note that the localization of the NAADP receptor is unknown, and that RyR3 and InsP3R may not be present on the same intracellular stores. The roles of plasma membrane InsP3Rs and the l-type Ca2+ channel are unknown – the possibility that they may mediate Ca2+ influx is indicated by dotted lines. The identification of the l-type Ca2+ channel as an NAADP receptor is speculative. Intracellular stores are depicted in blue, and activation steps are shown by red arrows.
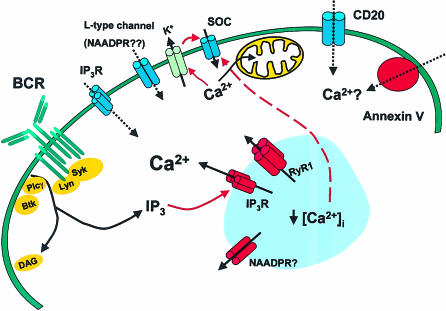
Figure 2.
Possible roles for Ca2+ channels in B cells. The BCR-induced Ca2+ signal involves the production of Ins(1,4,5)P3 and the release of Ca2+ from intracellular stores gated by InsP3Rs and RyR1. This is followed by an influx of Ca2+ through an unidentified store-operated channel (SOC). The mechanism of activation of RyR1 is unknown. Note that RyR1 and InsP3R are unlikely to be present on the same intracellular stores. The possible involvement of NAADP receptors in BCR signalling is highly speculative. The roles of plasma membrane InsP3Rs and the l-type Ca2+ channel are unknown – the possibility that they may mediate Ca2+ influx is indicated by dotted lines. The identification of the l-type Ca2+ channel as an NAADP receptor is speculative. CD20 and annexin V are shown as possible Ca2+ channels. Intracellular stores are depicted in blue, and activation steps are shown by red arrows.
INTRACELLULAR Ca2+ CHANNELS
A plethora of studies of AgR signalling have highlighted the role of inositol trisphosphate [Ins(1,4,5)P3]-mediated release of Ca2+ from internal stores (reviewed in refs 1–3). However, it is becoming apparent that there is more to the regulated release of intracellular Ca2+ in lymphocytes than inositol trisphosphate receptors (InsP3Rs). Recent studies are beginning to unravel roles for ryanodine receptors (RyRs) and the newly described and little understood NAADP receptor.
Inositol trisphosphate receptors
Three types of InsP3R are known, and they vary in their sensitivities to Ins(1,4,5)P3 and in the properties of their activation by Ca2+. InsP3Rs must bind Ins(1,4,5)P3 for Ca2+ release to occur. The response of the InsP3R can be regulated by phosphorylation, by various accessory proteins and by ATP, but by far the most important regulator is Ca2+. The exact mechanism is disputed4–6 but it is apparent that the differing sensitivities of the InsP3R isoforms to regulation by Ca2+ allow cells to fine-tune the temporal and spatial aspects of the Ca2+ signal.5
Much recent work has been directed towards determining the roles of the various isoforms. B and T cells express all three types of InsP3R to varying degrees depending on their stage of differentiation.7–11 It is not clear why lymphocytes simultaneously express all three isoforms, particularly since Sugawara et al.8 clearly demonstrated the redundancy of InsP3R expression. In a series of knockouts in DT40 B lymphocytes the BCR-induced Ca2+ signal could not be ablated until all three InsP3R isoforms were simultaneously knocked out.8
InsP3R3 was the first isoform to have a specific role ascribed to it. In both T and B cells, InsP3R3 is up-regulated in cells undergoing apoptosis.10 Inhibition of InsP3R3 expression using antisense RNA prevents TCR-induced apoptosis.10 However, Sugawara et al.8 provided convincing evidence against a specific role for InsP3R3 in apoptosis when they showed that knockout of any two isoforms in DT40 cells inhibited BCR-induced apoptosis.
InsP3R3 can be expressed on the external surface of the plasma membrane of T and B cells and in T cells it can cocap with the TCR.9,10 The validity of this observation has been questioned, but support was provided by Tanimura et al.12 who demonstrated InsP3R3 expression on the external surface of Jurkat T cells. They showed that this pattern of expression was not limited to InsP3R3, and in fact the predominant isoforms in the plasma membrane were InsP3R1 and InsP3R2.12 The role of plasma membrane InsP3Rs is unknown, but Putney13 has suggested that, in some cell types, InsP3R3 may be expressed as an integral plasma membrane protein and function as all or part of a store-operated Ca2+ channel. However, the properties of InsP3R, in particular its Ca2+ selectivity profile, do not accord with those of known store-operated Ca2+ channels and the prevailing evidence strongly indicates that these channels are formed by distinct molecules from InsP3Rs (discussed below).
In an attempt to explore the role of InsP3R1, Jayaraman et al.11 used antisense RNA to show that TCR-induced Ca2+ signals are exclusively transduced through this subtype. However, this is a controversial finding since the construct they used could cross-react with the type 2 and type 3 receptors. Hirota et al.14 subsequently demonstrated that T lymphocytes could develop normally in InsP3R1 knockout mice and showed normal Ca2+ fluxes in response to anti-CD3 stimulation, contradicting the claim that InsP3R1 was absolutely required for TCR signalling. In the most convincing study to ascribe roles for the various isotypes, Miyakawa et al.15 showed that InsP3R3 was involved in the generation of monophasic single Ca2+ transients following BCR ligation, whereas InsP3R1 and InsP3R2 were involved in the generation of Ca2+ oscillations with differing frequencies.
The knockouts studied by Sugawara et al.8 suggest that inhibition of downstream events may be achieved simply by reducing the overall levels of InsP3Rs, rather than the specific levels of one particular isotype. This raises the possibility that cells do not express homotetramers of InsP3Rs; rather they may express heterotetramers of varying amounts of each isoform, allowing the cell to express a graded array of hybrid receptors with a wide variety of subtly different properties made up of combinations of the properties of the ‘pure’ homotetramers. Clearly much remains to be done to unravel the role of InsP3Rs in AgR signalling.
Ryanodine receptors
RyRs are large (∼560 kDa) homotetrameric receptors that mediate Ca2+ release from the endoplasmic reticulum stores. Three isoforms, encoded by separate genes, have been described. RyRs can be gated by allosteric coupling to plasma membrane voltage-gated Ca2+ channels (in the case of RyR1) and by Ca2+ (all isoforms). All three isoforms can be activated by cyclic ADP ribose (cADPR). cADPR can initiate Ca2+ release and sensitise the RyR to further activation by Ca2+. This feed-forward mechanism, known as Ca2+-induced Ca2+ release, can lead to the prolonged propagation of Ca2+ signals.16,17 In contrast to InsP3Rs, only one isotype of RyR is expressed in lymphocytes, RyR1 in B lymphocytes and RyR3 in T lymphocytes, therefore there is no question of heterotetramer formation.18–22
The role of RyRs in AgR signalling is beginning to be addressed and appears to differ between B and T cells. In T cells the initial peak of Ca2+ release following TCR engagement is due to Ins(1,4,5)P3-mediated Ca2+ release from intracellular stores. This peak is followed by a sustained Ca2+ flux that requires both the influx of extracellular Ca2+ and the production of cADPR. Guse et al.22 showed that cADPR is produced following TCR ligation and that antagonists of cADPR inhibited TCR-induced proliferation and the expression of early and late activation markers. They showed that the long-lasting Ca2+ influx, rather than the initial Ca2+ peak, depended on cADPR production. In contrast, in B cells depletion of RyR1-gated stores significantly inhibits the BCR-induced Ca2+ peak, suggesting that in B cells the RyR is activated at the same time as Ins(1,4,5)P3-induced Ca2+ release.18
The mechanism that activates RyRs in B cells is unknown, but in T cells Guse et al.22 showed that it is TCR-induced cADPR production. The source of TCR-induced cADPR is controversial. cADPR is produced from β-NAD by the action of ADP-ribosyl cyclase. This enzyme was first isolated from Aplysia, and its mammalian homologues include CD38. CD38 is an ecto-enzyme having both ADP-ribosyl cyclase and cADPR-hydrolase activities. The extracellular location of CD38 has proved puzzling since the site of action of its product (cADPR) is intracellular. One study suggests that cADPR produced by CD38 on human haemopoietic precursors can raise intracellular Ca2+ levels via a specific plasma membrane cADPR transporter.23 However, da Silva et al.24 have shown that the TCR-induced production of cADPR is intracellular and independent of extracellular production by CD38. Supporting the intracellular origin of TCR-induced cADPR, Guse et al.22 demonstrated the presence of an ADP ribosyl cyclase activity in the cytosol of T cells.
The presence of RyR in lymphocytes is an interesting recent discovery. The Ca2+-induced Ca2+ release properties of these receptors suggest a role in maintaining a long-term Ca2+ signal. In turn, this may suggest that InsP3Rs are more concerned with triggering of the Ca2+ signal than with its long-term propagation.
NAADP receptors
Nicotinic acid adenine dinucleotide phosphate (NAADP) is a recently described intracellular Ca2+ mobilizing agent. NAADP is synthesized from NADP by the actions of ADP-ribosyl cyclase. Its actions are poorly understood, but it is increasingly evident that it mobilizes Ca2+ via a specific receptor, distinct from InsP3Rs and RyRs, and which is a channel in its own right.25 The actions of NAADP appear to vary depending on cell type (reviewed in refs 25 and 26). In Jurkat T lymphocytes, recent evidence suggests that mobilization of NAADP is an absolute requirement for signalling through the TCR.27 NAADP appears to act before Ins(1,4,5)P3 and cADPR to provide sufficient Ca2+ to sensitize the InsP3R and RyR to Ins(1,4,5)P3 and cADPR, respectively.
The nature of the NAADP receptor is unknown, but recent data suggest that it is an intracellular Ca2+ channel. Its pharmacology is beginning to be explored. It is known to be inhibited by high concentrations of l-type voltage-gated Ca2+ channel inhibitors, and curiously also by activators of these channels. It is also sensitive to inhibitors of some classes of voltage-gated K+ channels.28 There are currently no reports of NAADP involvement in B-cell signalling, but the persistent reports of the effects of inhibitors of l-type Ca2+ channels on BCR signalling (discussed below) suggest the possibility of this system also being involved in the BCR-induced Ca2+ flux.
Perhaps the most interesting feature of the NAADP receptor is its inactivation properties. Prior exposure to high concentrations of NAADP, such as might be expected if the TCR had previously been cross-linked, render the receptor inactive and unable to respond to further stimulation. In effect it allows the cell to retain a memory of a previous Ca2+ signal. This property led Berg et al.27 to speculate that the NAADP/Ca2+ system may provide a mechanism underlying anergy in T cells. Together, InsP3Rs, RyRs and the NAADP receptor form a complex web for priming, initiating and maintaining a signal, and then ‘remembering’ that is has been transduced.
Plasma membrane calcium channels
Much more is known about the AgR-activated Ca2+ channel in T cells than in B cells. In T cells there is strong evidence that the TCR-activated Ca2+ channel is identical to the store-operated ICRAC channel (reviewed in detail in ref 2). For example, both store depletion and TCR ligation activate a channel with identical properties29 and in a human primary immunodeficiency caused by an absence of ICRAC, there is no TCR-activated Ca2+ influx.30 Ca2+ influx occurs following BCR ligation or pharmacological depletion of intracellular stores. However, to date, there have been no electrophysiological studies demonstrating the presence of the ICRAC channel in B lymphocytes and it is not known whether B cells express this channel.
While the majority of studies on transmembrane Ca2+ flux have been aimed at identifying the AgR-activated Ca2+ channel, it is unlikely to be the only plasma membrane Ca2+ channel present in lymphocytes. A number of other potential Ca2+ channels have been identified but their unambiguous identification as bona fide Ca2+ channels is still lacking.
Store-operated calcium channels
Properties
AgR ligation leads to a release of Ca2+ from intracellular stores. The decrease in store Ca2+ concentration causes the activation of Ca2+ channels (store-operated Ca2+ channel: SOC) in the plasma membrane by an as yet unresolved mechanism.31–33 Store-operated Ca2+ channels have been the subject of intensive research, but are still best described in terms of the current passing through the channel. The best characterized store-operated Ca2+ current in haemopoietic cells is the Ca2+-release-activated Ca2+ current (ICRAC), which was first identified in mast cells34 and subsequently in Jurkat T lymphocytes.29 The ICRAC channel appears to be widely distributed, but other types of store-operated current have been described, including the Ca2+-release activated non-selective cation current (ICRANC) in Jurkat T cells.35 There are several defining features of the ICRAC Ca2+ influx pathway. These include a high selectivity for Ca2+,36 a very small single channel conductance and blockade by divalent cations with a characteristic selectivity profile.29,37,38 The other notable feature of the ICRAC channel is Ca2+-dependent modulation. Ca2+ influx can be enhanced through the binding of Ca2+ to an extracellular site on the channel39 and the channel can be rapidly inactivated due to accumulation of Ca2+ close to its intracellular mouth.40
The properties of the ICRAC channel have been determined by electrophysiological means and obviously must be met by any candidate protein. In this context, it is important to note that, although these properties describe one current, they may represent the averaged properties of a number of individual different subunits. This in turn may explain why the molecular identity of the ICRAC channel has proved to be so hard to determine (discussed in more detail below).
Regulation
The importance of Ca2+ entry in normal lymphocyte function was highlighted when defects in this pathway were discovered. In 1994, Partesi et al.30 described a patient with an immunodeficiency associated with defective T lymphocyte proliferation, where the defect was shown to be in the Ca2+ entry pathway. This defect was sufficient to alter T lymphocyte expansion and function. More recently, two severe-combined immunodeficiency patients have been shown to suffer from defective Ca2+ influx in both T and B lymphocytes resulting in impaired lymphocyte activation.41
It is not surprising, then, that Ca2+ entry turns out to be a regulated event in lymphocyte activation. The entry of Ca2+ through ICRAC channels is ultimately driven by the membrane potential. Although lymphocytes are non-excitable cells, both B and T lymphocytes express voltage-gated K+ channels and Ca2+-activated K+ channels (KCa) whose function is to maintain the resting membrane potential.42 Following the AgR-induced rise in intracellular Ca2+ concentration, KCa are activated and hyperpolarize the membrane. This makes the membrane potential more negative and enhances Ca2+ influx by increasing the inward driving force for Ca2+ entry. Ca2+ entry can be sustained by the action of mitochondria strategically placed close to the mouth of the ICRAC channel. They sequester Ca2+ following rapid Ca2+ entry preventing the Ca2+-dependent inactivation of the channel.43
Co-receptors on lymphocytes can also regulate Ca2+ entry. Activation of acidic sphingomyelinase following Fas (CD95) ligation on T lymphocytes results in the release of ceramide which is further metabolized to sphingosine. Both ceramide and sphingosine can inhibit Ca2+ currents through ICRAC channels and this may be the mechanism behind Fas-dependent immune suppression and impairment of lymphocyte function.44 It has been suggested that blockade of ICRAC channels could be responsible for anergy in lymphocytes.2 In B lymphocytes, engagement of FcγRIIb is important in the negative regulation of Ca2+ influx. SHIP, a lipid phosphatase, is recruited to FcγRIIb following coligation with the BCR, and plays a key role in down-regulating the BCR-induced Ca2+ influx.45
Ca2+ influx can be regulated by protein kinases activated downstream of AgRs. B lymphocytes lacking Btk activity are deficient in Ca2+ influx following BCR ligation.46 Similarly, in Itk-deficient T cells there is defective TCR-induced Ca2+ influx.47 These effects are due to a defect in the sustained production of Ins(1,4,5)P3, rather than a direct effect of the kinases on the Ca2+ channel. Using Lyn-knockout DT40 B cells, Hasimoto et al.48 showed that Lyn negatively regulates BCR-induced Ca2+ influx, possibly through phosphorylation of the channel. PKCβ1 is reported to have a role in the down-regulation of Ca2+ entry in Jurkat T lymphocytes, although the exact mechanism is unclear.49
There are several estimates for the number of ICRAC channels expressed on T lymphocytes. Kerschbaum and Cahalan50 estimated that there are 100–400 channels per T lymphocyte, whereas Fomina et al.38 estimated that resting T cells have 15 channels, rising to 140 channels in activated T cells. Fomina et al.38 suggested that this up-regulation in the number of channels during T cell activation is necessary for sustaining proliferation and for enhancing Ca2+ signalling during secondary T lymphocyte activation.
It is clear that ICRAC can be regulated by a number of mechanisms. Regulation by intracellular Ca2+ and by lymphocyte co-receptors allows lymphocytes the opportunity to fine-tune the Ca2+ signal in the short term. In the longer term, activation can be sustained by up-regulation of the number of channels expressed on the cell surface.
Identity
The molecular identity of the ICRAC channel is unclear but the current view is that it is formed by one or more members of the Trp family. Trp proteins were originally discovered in Drosophila photoreceptor cells as ion channels required for sustained Ca2+ entry. Mammalian homologues of Trp fall into three subfamilies: TrpC, TrpV and TrpM.51 TrpC1, TrpC3, TrpC5 and TrpC6 have all been reported to be expressed in Jurkat T lymphocytes.52 Su et al.35 described a store-operated non-selective cation channel (ICRANC) in Jurkat. They suggest that TrpC3 and TrpC6 are likely to be subunits of this channel since, when TrpC3 and TrpC6 are expressed together, they form a channel with very similar properties to ICRANC.
It is possible that Trp proteins are regulators of Ca2+ currents, are accessory subunits in a channel complex, or form the channel themselves. It may be necessary for a number of different Trp proteins to form heteromeric complexes in order to produce functional ion channels. Many of the studies that have sought to assign roles to ion channels formed by Trp proteins have relied on the ectopic overexpression of these proteins. This is likely to produce a channel that does not represent the native stoichiometry. In turn, this may explain some of the conflicting data on the functions of Trp family members. For just about every Trp family member, it is possible to find studies claiming and refuting its role as a store-operated Ca2+ channel.53,54
Currently the best candidate for the ICRAC channel is the TrpV family member CaT1, which was originally cloned from small intestine55 and subsequently recloned from Jurkat T cells.56 Yue et al.56 showed that transient expression of CaT1 in CHO-K1 cells results in a Ca2+ current with properties similar (but not identical) to those of ICRAC. These similarities include activation by store depletion, cation selectivity, and estimates of single channel conductance. By determining some of the properties in CHO-K1 cells expressing submaximal levels of CaT1, Yue et al. address the issue of stoichiometry and association with other regulatory proteins. Their data provide compelling evidence that CaT1 may form all or a component of the ICRAC channel. As yet the expression of CaT1 in B lymphocytes has not been addressed.
Other Ca2+ channels
Store-operated Ca2+ channels are unlikely to be the sole Ca2+ channels present in lymphocytes. There are numerous reports suggesting the presence of other types of Ca2+ channel such as the ICRANC channel (discussed above), annexin V, CD20 and channels related to voltage-gated Ca2+ channels.
Annexins potentially form Ca2+ channels in lymphocytes. The annexins are a large diverse family of Ca2+-dependent phospholipid binding proteins that can form voltage-gated Ca2+ channels in cell-free systems.57,58 Annexin V (also known as endonexin II) is expressed intracellularly in B and T lymphocytes where its physiological function is unknown.58
Two recent studies have addressed the involvement of annexin V in Ca2+ influx in lymphocytes. In CEM T cells, the pharmacological extracellular application of annexin V was associated with an increased intracellular Ca2+ concentration, which in turn was associated with an inhibition of etoposide-induced apoptosis.59 However, the extracellular application of large amounts of a protein that normally has an intracellular localization does not represent a physiologically realistic situation. Nor was the mechanism of formation and activation of the supposed annexin V Ca2+ channel explored. In a more convincing study, Kubista et al.57 demonstrated annexin V-mediated H2O2-induced Ca2+ influx in B lymphocytes. Using DT40 B lymphocytes with targeted gene disruptions in annexin V, they showed that thapsigargin- and anti-IgM-induced store-operated Ca2+ entry was normal in these cells but that Ca2+ elevations induced by 2 mm H2O2 were reduced. However, this study could not rule out a role of annexin V as an activator of a Ca2+ channel rather than an authentic Ca2+ channel in its own right.
Possibly the best known candidate for a non-store-operated Ca2+ channel is CD20. CD20 is a marker of B lymphocytes that is expressed on resting and activated B cells60 and on some T cell malignancies.61–63 Transfection of CD20 into non-lymphoid cells induces the expression of a Ca2+ conductance identical to that seen when CD20 is overexpressed in T and B cells. This Ca2+ conductance is enhanced following the binding of anti-CD20 monoclonal antibodies to CD20+ lymphoblastoid cells.64 However, the anti-CD20 induction of Ca2+ influx is not a universal observation.65 In some cases where anti-CD20 Ca2+ influx has been demonstrated there is evidence suggesting that it is a downstream consequence of phospholipase C activation.66,67 Even in those studies that suggest that CD20 is a Ca2+-permeable cation channel68–70 the possibility that CD20 could be a regulatory subunit of a Ca2+ channel complex, rather than a Ca2+ channel in its own right, cannot be excluded. The role of CD20 in Ca2+ influx therefore remains unresolved.
An intriguing and persistent observation is that lymphocytes express non-voltage-gated Ca2+ channels that are related to classical voltage-gated Ca2+ channels. Early work relied heavily on pharmacological agents, typically the classical l-type Ca2+ channel antagonists such as diltiazem and verapamil. These were used at higher concentrations than required to block voltage-gated Ca2+ channels and at concentrations far higher than estimated therapeutic levels. Using l-type channel antagonists a number of studies showed profound effects on T- and B-cell activation and AgR-induced Ca2+ influx.71–77 Whilst these pharmacological studies provide some evidence for the expression of Ca2+ channels related to voltage-gated Ca2+ channels, they should at the same time be treated with some caution due to the high concentrations of drugs used.
Akha et al.78 demonstrated that anti-Ig induced Ca2+ influx in rat B lymphocytes occurred through a dihydropyridine-sensitive channel with similarities to the α1D (CaV1.3) subtype of l-type Ca2+ channels. l-type channel antagonists and an anti-α1D antibody blocked the anti-Ig induced Ca2+ response. The presence of α1C (CaV1.2) and α1S (CaV1.1) l-type Ca2+ channel transcripts in Jurkat T lymphocytes has been demonstrated by RT-PCR.79 Recent studies show that a dihydropyridine-sensitive Ca2+ channel is involved in HgCl2 and TCR-induced IL-4 synthesis in mouse T lymphocytes.80,81 Savignac et al.81 showed surface staining of mouse T lymphocytes with an anti-α1D specific antibody, and the expression of an l-type Ca2+ channel transcript in these cells. These studies demonstrate the presence of an l-type channel transcript and protein in lymphocytes, but there appears to be some confusion over which pore-forming subunit is expressed.
These reports represent an intriguing finding in the light of the reported pharmacology of the NAADP receptor. Could this elusive channel be in some way related to the non-voltage-gated l-type channels so persistently reported in lymphocytes? This question turns us full circle: it is clear that Ca2+ signals in lymphocytes exhibit a complex temporal and spatial pattern. The remarkable sophistication of something seemingly as simple as a Ca2+ flux enables the lymphocyte to translate an extracellular signal into an outcome finely tuned to the environment and needs of the cell. This is achieved via a web of intracellular and transmembrane Ca2+ channels interacting in a complexity of ways that we are only beginning to understand.
Acknowledgments
GG is supported by the Medical Research Council; LT is supported by the University of Birmingham, School of Medicine. We are grateful to Dave Sansom for critical reading of this review.
Abbreviations
- AgR
antigen receptor
- BCR
B-cell antigen receptor
- cADPR
cyclic ADP ribose
- ICRAC
Ca2+-release-activated Ca2+ current
- ICRANC
Ca2+-release-activated non-selective cation current
- InsP3R
inositol trisphosphate receptor
- KCa
Ca2+-activated K+ channel
- NAADP
nicotinic acid adenine dinucleotide phosphate
- RyR
ryanodine receptor
- TCR
T-cell antigen receptor.
References
- 1.Kurosaki T. Genetic analysis of B cell antigen receptor signaling. Annu Rev Immunol. 1999;17:555–92. doi: 10.1146/annurev.immunol.17.1.555. [DOI] [PubMed] [Google Scholar]
- 2.Lewis RS. Calcium signaling mechanisms in T lymphocytes. Annu Rev Immunol. 2001;19:497–396. doi: 10.1146/annurev.immunol.19.1.497. [DOI] [PubMed] [Google Scholar]
- 3.Kurosaki T, Tsukada S. BLNK. Connecting Syk and Btk to calcium signals. Immunity. 2000;12:1–5. doi: 10.1016/s1074-7613(00)80153-3. [DOI] [PubMed] [Google Scholar]
- 4.Bootman MD, Lipp P. Calcium signalling: ringing changes to the ‘bell-shaped curve’. Curr Biol. 1999;9:R876–R78. doi: 10.1016/s0960-9822(00)80072-x. 10.1016/s0960-9822(00)80072-x. [DOI] [PubMed] [Google Scholar]
- 5.Yule DI. Subtype-specific regulation of inositol 1,4,5-trisphosphate receptors. Controlling calcium signals in time and space. J Gen Physiol. 2001;117:431–4. doi: 10.1085/jgp.117.5.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Taylor CW, Thorn P. Calcium signalling. IP3 rises again… and again. Curr Biol. 2001;11:R352–R55. doi: 10.1016/s0960-9822(01)00192-0. [DOI] [PubMed] [Google Scholar]
- 7.Sugiyama T, Yamamoto-Hino M, Miyawaki A, Furuichi T, Mikoshiba K, Hasegawa M. Subtypes of inositol 1,4,5-trisphosphate receptor in human hematopoietic cell lines: dynamic aspects of their cell-type specific expression. FEBS Lett. 1994;349:191–6. doi: 10.1016/0014-5793(94)00662-8. [DOI] [PubMed] [Google Scholar]
- 8.Sugawara H, Kurosaki M, Takata M, Kurosaki T. Genetic evidence for involvement of type 1, type 2 and type 3 inositol 1,4,5-trisphosphate receptors in signal transduction through the B-cell antigen receptor. EMBO J. 1997;16:3078–88. doi: 10.1093/emboj/16.11.3078. 10.1093/emboj/16.11.3078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Khan AA, Steiner JP, Klein MG, Schneider MF, Snyder SHI. P3 receptor localization to plasma membrane of T cells and cocapping with the T cell receptor. Science. 1992;257:815–8. doi: 10.1126/science.1323146. [DOI] [PubMed] [Google Scholar]
- 10.Khan AA, Soloski MJ, Sharp AH, Schilling G, Sabatini DM, Li SH, Ross CA, Snyder SH. Lymphocyte apoptosis: mediation by increased type 3 inositol 1,4,5–trisphosphate receptor. Science. 1996;273:503–7. doi: 10.1126/science.273.5274.503. [DOI] [PubMed] [Google Scholar]
- 11.Jayaraman T, Ondriasova E, Ondrias K, Harnick DJ, Marks AR. The inositol 1,4,5-trisphosphate receptor is essential for T-cell receptor signaling. Proc Natl Acad Sci USA. 1995;92:6007–11. doi: 10.1073/pnas.92.13.6007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tanimura A, Tojyo Y, Turner RJ. Evidence that Type I, II, and III inositol 1,4,5-trisphosphate receptors can occur as integral plasma membrane proteins. J Biol Chem. 2000;275:27488–93. doi: 10.1074/jbc.M004495200. [DOI] [PubMed] [Google Scholar]
- 13.Putney Jw., Jr Type 3 inositol 1,4,5-trisphosphate receptor and capacitative calcium entry. Cell Calcium. 1997;21:257–61. doi: 10.1016/s0143-4160(97)90050-6. [DOI] [PubMed] [Google Scholar]
- 14.Hirota JBM, Matsumoto M, Furuichi T, Takatsu K, Mikoshiba K. T-cell-receptor signalling in inositol 1,4,5-trisphosphate receptor (IP3R) type-1-deficient mice: is IP3R type 1 essential for T-cell-receptor signalling? Biochem J. 1998;333:615–9. doi: 10.1042/bj3330615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miyakawa T, Maeda A, Yamazawa T, Hirose K, Kurosaki T, Iino M. Encoding of Ca2+ signals by differential expression of IP3 receptor subtypes. EMBO J. 1999;18:1303–8. doi: 10.1093/emboj/18.5.1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mackrill JJ. Protein–protein interactions in intracellular Ca2+-release channel function. Biochem J. 1999;337:345–61. 10.1042/0264-6021:3370345. [PMC free article] [PubMed] [Google Scholar]
- 17.Lee HC. Physiological functions of cyclic ADP-Ribose and NAADP as calcium messengers. Annu Rev Pharmacol Toxicol. 2001;41:317–45. doi: 10.1146/annurev.pharmtox.41.1.317. [DOI] [PubMed] [Google Scholar]
- 18.Sei Y, Gallagher KL, Basile AS. Skeletal muscle type ryanodine receptor is involved in calcium signaling in human B lymphocytes. J Biol Chem. 1999;274:5995–6002. doi: 10.1074/jbc.274.9.5995. 10.1074/jbc.274.9.5995. [DOI] [PubMed] [Google Scholar]
- 19.Sei Y, Gallagher KL, Daly JW. Multiple effects of caffeine on Ca2+ release and influx in human B lymphocytes. Cell Calcium. 2001;29:149–60. doi: 10.1054/ceca.2000.0175. 10.1054/ceca.2000.0175. [DOI] [PubMed] [Google Scholar]
- 20.Hakamata Y, Nishimura S, Nakai J, Nakashima Y, Kita T, Imoto K. Involvement of the brain type of ryanodine receptor in T-cell proliferation. FEBS Lett. 1994;352:206–10. doi: 10.1016/0014-5793(94)00955-4. [DOI] [PubMed] [Google Scholar]
- 21.Bourguignon LYW, Chu A, Jin H, Brandt NR. Ryanodine receptor–ankyrin interaction regulates internal Ca2+ release in mouse T-lymphoma cells. J Biol Chem. 1995;270:17917–22. doi: 10.1074/jbc.270.30.17917. [DOI] [PubMed] [Google Scholar]
- 22.Guse AH, da Silva CP, Berg I, et al. Regulation of calcium signalling in T lymphocytes by the second messenger cyclic ADP-ribose. Nature. 1999;398:70–3. doi: 10.1038/18024. 10.1038/18024. [DOI] [PubMed] [Google Scholar]
- 23.Podesta M, Zocchi E, Pitto A, et al. Extracellular cyclic ADP-ribose increases intracellular free calcium concentration and stimulates proliferation of human hemopoietic progenitors. FASEB J. 2000;14:680–90. doi: 10.1096/fasebj.14.5.680. [DOI] [PubMed] [Google Scholar]
- 24.da Silva CP, Schweitzer K, Heyer P, Malavasi F, Mayr GW, Guse AH. Ectocellular CD38-catalyzed synthesis and intracellular Ca2+-signalling activity of cyclic ADP-ribose in T-lymphocytes are not functionally related. FEBS Lett. 1998;439:291–6. doi: 10.1016/s0014-5793(98)01396-9. 10.1016/s0014-5793(98)01396-9. [DOI] [PubMed] [Google Scholar]
- 25.Patel S, Churchill GC, Galione A. Coordination of Ca2+ signalling by NAADP. Trends Biochem Sci. 2001;26:482–9. doi: 10.1016/s0968-0004(01)01896-5. [DOI] [PubMed] [Google Scholar]
- 26.da Silva CP, Guse AH. Intracellular Ca2+ release mechanisms: multiple pathways having multiple functions within the same cell type? Biochim Biophys Acta. 2000;1498:122–33. doi: 10.1016/s0167-4889(00)00089-6. [DOI] [PubMed] [Google Scholar]
- 27.Berg I, Potter BVL, Mayr GW, Guse AH. Nicotinic acid adenine dinucleotide phosphate (NAADP+) is an essential regulator of T-lymphocyte Ca2+-signaling. J Cell Biol. 2000;150:581–8. doi: 10.1083/jcb.150.3.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Genazzani A, Mezna M, Dickey D, Michelangeli F, Walseth T, Galione A. Pharmacological properties of the Ca2+-release mechanism sensitive to NAADP in the sea urchin egg. Br J Pharmacol. 1997;121:1489–95. doi: 10.1038/sj.bjp.0701295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zweifach A, Lewis RS. Mitogen-regulated Ca2+ current of T lymphocytes is activated by depletion of intracellular Ca2+ stores. Proc Natl Acad Sci USA. 1993;90:6295–9. doi: 10.1073/pnas.90.13.6295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Partiseti M, Le Deist F, Hivroz C, Fischer A, Korn H, Choquet D. The calcium current activated by T cell receptor and store depletion in human lymphocytes is absent in a primary immunodeficiency. J Biol Chem. 1994;269:32327–35. [PubMed] [Google Scholar]
- 31.Barritt GJ. Receptor-activated Ca2+ inflow in animal cells: a variety of pathways tailored to meet different intracellular Ca2+ signalling requirements. Biochem J. 1999;337:153–69. [PMC free article] [PubMed] [Google Scholar]
- 32.Elliott AC. Recent developments in non-excitable cell calcium entry. Cell Calcium. 2001;30:73–93. doi: 10.1054/ceca.2001.0215. [DOI] [PubMed] [Google Scholar]
- 33.Putney Jw, Jr, Broad LM, Braun F-J, Lievremont J-P, Bird GSJ. Mechanisms of capacitative calcium entry. J Cell Sci. 2001;114:2223–2. doi: 10.1242/jcs.114.12.2223. [DOI] [PubMed] [Google Scholar]
- 34.Hoth M, Penner R. Depletion of intracellular calcium stores activates a calcium current in mast cells. Nature. 1992;355:353–6. doi: 10.1038/355353a0. [DOI] [PubMed] [Google Scholar]
- 35.Su Z, Csutora P, Hunton D, Shoemaker RL, Marchase RB, Blalock JE. A store-operated nonselective cation channel in lymphocytes is activated directly by Ca2+ influx factor and diacylglycerol. Am J Physiol. 2001;280:C1284–113. doi: 10.1152/ajpcell.2001.280.5.C1284. [DOI] [PubMed] [Google Scholar]
- 36.Hoth M. Calcium and barium permeation through calcium release-activated calcium (CRAC) channels. Pflugers Arch. 1995;430:315–22. doi: 10.1007/BF00373905. [DOI] [PubMed] [Google Scholar]
- 37.Parekh AB, Penner R. Store depletion and calcium influx. Physiol Rev. 1997;77:901–30. doi: 10.1152/physrev.1997.77.4.901. [DOI] [PubMed] [Google Scholar]
- 38.Fomina AF, Fanger CM, Kozak JA, Cahalan MD. Single channel properties and regulated expression of Ca2+ release-activated Ca2+ (CRAC) channels in human T cells. J Cell Biol. 2000;150:1435–120. doi: 10.1083/jcb.150.6.1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zweifach A, Lewis RS. Calcium-dependent potentiation of store-operated calcium channels in T lymphocytes. J General Physiol. 1996;107:597–610. doi: 10.1085/jgp.107.5.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zweifach A, Lewis RS. Rapid inactivation of depletion-activated calcium current (ICRAC) due to local calcium feedback. J General Physiol. 1995;105:209–26. doi: 10.1085/jgp.105.2.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Feske S, Giltnane J, Dolmetsch R, Staudt LM, Rao A. Gene regulation mediated by calcium signals in T lymphocytes. Nat Immunol. 2001;2:316–24. doi: 10.1038/86318. [DOI] [PubMed] [Google Scholar]
- 42.Lewis RS, Cahalan MD. Potassium and calcium channels in lymphocytes. Annu Rev Immunol. 1995;13:623–53. doi: 10.1146/annurev.iy.13.040195.003203. [DOI] [PubMed] [Google Scholar]
- 43.Hoth M, Button DC, Lewis RS. Mitochondrial control of calcium-channel gating: a mechanism for sustained signaling and transcriptional activation in T lymphocytes. Proc Natl Acad Sci USA. 2000;97:10607–12. doi: 10.1073/pnas.180143997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lepple-Wienhues A, Belka C, Laun T, et al. Stimulation of CD95 (Fas) blocks T lymphocyte calcium channels through sphingomyelinase and sphingolipids. Proc Natl Acad Sci USA. 1999;96:13795–800. doi: 10.1073/pnas.96.24.13795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Okada H, Bolland S, Hashimoto A, Kurosaki M, Kabuyama Y, Iino M, Ravetch JV, Kurosaki T. Role of the inositol phosphatase SHIP in B cell receptor-induced Ca2+ oscillatory response. J Immunol. 1998;161:5129–32. [PubMed] [Google Scholar]
- 46.Fluckiger AC, Li ZM, Kato RM, et al. Btk/Tec kinases regulate sustained increases in intracellular Ca2+ following B-cell receptor activation. EMBO J. 1998;17:1973–85. doi: 10.1093/emboj/17.7.1973. 10.1093/emboj/17.7.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liu KQ, Bunnell SC, Gurniak CB, Berg LJ. T cell receptor-initiated calcium release is uncoupled from capacitative calcium entry in Itk-deficient T cells. J Exp Med. 1998;187:1721–7. doi: 10.1084/jem.187.10.1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hashimoto A, Hirose K, Kurosaki T, Iino M. Negative control of store-operated Ca2+ influx by B cell receptor cross-linking. J Immunol. 2001;166:1003–740. doi: 10.4049/jimmunol.166.2.1003. [DOI] [PubMed] [Google Scholar]
- 49.Haverstick DM, Dicus M, Resnick MS, Sando JJ, Gray LS. A role for protein kinase CβI in the regulation of Ca2+ entry in Jurkat T cells. J Biol Chem. 1997;272:15426–33. doi: 10.1074/jbc.272.24.15426. 10.1074/jbc.272.24.15426. [DOI] [PubMed] [Google Scholar]
- 50.Kerschbaum HH, Cahalan MD. Single-channel recording of a store-operated Ca2+ channel in Jurkat T lymphocytes. Science. 1999;283:836–9. doi: 10.1126/science.283.5403.836. 10.1126/science.283.5403.836. [DOI] [PubMed] [Google Scholar]
- 51.Clapham DE, Runnels LW, Strubing C. The trp ion channel family. Nat Rev Neurosci. 2001;2:387–96. doi: 10.1038/35077544. 10.1038/35077544. [DOI] [PubMed] [Google Scholar]
- 52.Garcia RL, Schilling WP. Differential expression of mammalian TRP homologues across tissues and cell lines. Biochem Biophys Res Commun. 1997;239:279–83. doi: 10.1006/bbrc.1997.7458. [DOI] [PubMed] [Google Scholar]
- 53.Harteneck C, Plant TD, Schultz G. From worm to man. three subfamilies of TRP channels. Trends Neurosci. 2000;23:159–66. doi: 10.1016/s0166-2236(99)01532-5. 10.1016/s0166-2236(99)01532-5. [DOI] [PubMed] [Google Scholar]
- 54.Montell C. An end in sight to a long TRP. Neuron. 2001;30:3–5. doi: 10.1016/s0896-6273(01)00254-9. [DOI] [PubMed] [Google Scholar]
- 55.Peng J-B, Chen X-Z, Berger UV, Vassilev PM, Tsukaguchi H, Brown EM, Hediger MA. Molecular cloning and characterization of a channel-like transporter mediating intestinal calcium absorption. J Biol Chem. 1999;274:22739–46. doi: 10.1074/jbc.274.32.22739. [DOI] [PubMed] [Google Scholar]
- 56.Yue L, Peng JB, Hediger MA, Clapham DE. CaT1 manifests the pore properties of the calcium-release-activated calcium channel. Nature. 2001;410:705–9. doi: 10.1038/35070596. [DOI] [PubMed] [Google Scholar]
- 57.Kubista H, Hawkins TE, Patel DR, Haigler HT, Moss SE. Annexin 5 mediates a peroxide-induced Ca2+ influx in B cells. Curr Biol. 1999;9:1403–6. doi: 10.1016/s0960-9822(00)80085-8. 10.1016/s0960-9822(00)80085-8. [DOI] [PubMed] [Google Scholar]
- 58.Kourie JI, Wood HB. Biophysical and molecular properties of annexin-formed channels. Prog Biophys Mol Biol. 2000;73:91–134. doi: 10.1016/s0079-6107(00)00003-1. [DOI] [PubMed] [Google Scholar]
- 59.Gidon-Jeangirard C, Solito E, Hofmann A, Russo-Marie F, Freyssinet JM, Martinez MC. Annexin V counteracts apoptosis while inducing Ca2+ influx in human lymphocytic T cells. Biochem Biophys Res Commun. 1999;265:709–15. doi: 10.1006/bbrc.1999.1752. [DOI] [PubMed] [Google Scholar]
- 60.Dorken B, Moller P, Pezzutto A, Schwartz-Albiez R, Moldenhauer G. B-cell antigens: CD20. In: W Knapp, B Dorken, WR Gilks, EP Rieber, RE Schmidt, H von Stein, AEGK dem Borne., editors. B-Cell Antigens: CD20. Oxford: Oxford University Press; 1989. pp. 46–8. [Google Scholar]
- 61.Hultin LE, Hausner MA, Hultin PM, Giorgi JV. CD20 (pan-B cell) antigen is expressed at a low level on a subpopulation of human T lymphocytes. Cytometry. 1993;14:196–204. doi: 10.1002/cyto.990140212. [DOI] [PubMed] [Google Scholar]
- 62.Quintanilla-Martinez L, Preffer F, Rubin D, Ferry JA, Harris NL. CD20+ T-cell lymphoma. Neoplastic transformation of a normal T-cell subset. Am J Clin Pathol. 1994;102:483–9. doi: 10.1093/ajcp/102.4.483. [DOI] [PubMed] [Google Scholar]
- 63.Yao X, Teruya-Feldstein J, Raffeld M, Sorbara L, Jaffe ES. Peripheral T-cell lymphoma with aberrant expression of CD79a and CD20: a diagnostic pitfall. Mod Pathol. 2001;14:105–10. doi: 10.1038/modpathol.3880265. [DOI] [PubMed] [Google Scholar]
- 64.Bubien JK, Zhou LJ, Bell PD, Frizzell RA, Tedder TF. Transfection of the CD20 cell surface molecule into ectopic cell types generates a Ca2+ conductance found constitutively in B lymphocytes. J Cell Biol. 1993;121:1121–32. doi: 10.1083/jcb.121.5.1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Holder M, Grafton G, MacDonald I, Finney M, Gordon J. Engagement of CD20 suppresses apoptosis in germinal center B cells. Eur J Immunol. 1995;25:3160–4. doi: 10.1002/eji.1830251126. [DOI] [PubMed] [Google Scholar]
- 66.Deans JP, Schieven GL, Shu GL, Valentine MA, Gilliland LA, Aruffo A, Clark EA, Ledbetter JA. Association of tyrosine and serine kinases with the B cell surface antigen CD20. Induction via CD20 of tyrosine phosphorylation and activation of phospholipase C-gamma 1 and PLC phospholipase C-gamma 2. J Immunol. 1993;151:4494–504. [PubMed] [Google Scholar]
- 67.Hofmeister JK, Cooney D, Coggeshall KM. Clustered CD20 induced apoptosis. Src-family kinase, the proximal regulator of tyrosine phosphorylation, calcium influx, and caspase 3-dependent apoptosis. Blood Cells Mol Dis. 2000;26:133–43. doi: 10.1006/bcmd.2000.0287. [DOI] [PubMed] [Google Scholar]
- 68.Tedder TF, Engel P. CD20, a regulator of cell-cycle progression of B lymphocytes. Immunol Today. 1994;15:450–4. doi: 10.1016/0167-5699(94)90276-3. [DOI] [PubMed] [Google Scholar]
- 69.Kanzaki M, Shibata H, Mogami H, Kojima I. Expression of calcium-permeable cation channel CD20 accelerates progression through the G1 phase in Balb/c 3T3 cells. J Biol Chem. 1995;270:13099–104. doi: 10.1074/jbc.270.22.13099. [DOI] [PubMed] [Google Scholar]
- 70.Kanzaki M, Lindorfer MA, Garrison JC, Kojima I. Activation of the calcium-permeable cation channel CD20 by alpha subunits of the Gi protein. J Biol Chem. 1997;272:14733–9. doi: 10.1074/jbc.272.23.14733. 10.1074/jbc.272.23.14733. [DOI] [PubMed] [Google Scholar]
- 71.Birx DL, Berger M, Fleisher TA. The interference of T cell activation by calcium channel blocking agents. J Immunol. 1984;133:2904–9. [PubMed] [Google Scholar]
- 72.Dugas B, Vazquez A, Delfraissy JF, Gérard JP, Rannou MT, Galanaud P. Human B cell activation: selective sensitivity of the early stages to calcium channel-blocking drugs. Eur J Immunol. 1986;16:162–7. doi: 10.1002/eji.1830160210. [DOI] [PubMed] [Google Scholar]
- 73.Kubista H, Hawkins T, Moss SE. Characterisation of calcium signalling in DT40 chicken B-cells. Biochim Biophys Acta. 1998;1448:299–310. doi: 10.1016/s0167-4889(98)00132-3. [DOI] [PubMed] [Google Scholar]
- 74.Guse AH, de Wit C, Klokow T, Schweitzer K, Mayr GW. Unique properties of the capacitative Ca2+-entry antagonist LU 52396: its inhibitory activity depends on the activation state of the cells. Cell Calcium. 1997;22:91–7. doi: 10.1016/s0143-4160(97)90109-3. [DOI] [PubMed] [Google Scholar]
- 75.Young W, Chen J, Jung F, Gardner P. Dihydropyridine bay K 8644 activates T lymphocyte calcium-permeable channels. Mol Pharmacol. 1988;34:239–44. [PubMed] [Google Scholar]
- 76.Ricci A, Bisetti A, Bronzetti E, Felici L, Ferrante F, Veglio F, Amenta F. Pharmacological characterisation of Ca2+ channels of the 1-type in human peripheral blood lymphocytes. Eur J Pharmacol. 1996;301:189–94. doi: 10.1016/0014-2999(96)00016-7. 10.1016/0014-2999(96)00016-7. [DOI] [PubMed] [Google Scholar]
- 77.Morgano A, Pierri I, Stagnaro R, Setti M, Puppo F, Indiveri F. Decreased lymphocyte blastogenesis, IL2 production and NK activity following nifedipine administration to healthy humans. Eur J Clin Pharmacol. 1990;39:545–50. doi: 10.1007/BF00316092. [DOI] [PubMed] [Google Scholar]
- 78.Akha AAS, Willmott NJ, Brickley K, Dolphin AC, Galione A, Hunt SV. Anti-Ig-induced calcium influx in rat B lymphocytes mediated by cGMP through a dihydropyridine-sensitive channel. J Biol Chem. 1996;271:7297–300. doi: 10.1074/jbc.271.13.7297. [DOI] [PubMed] [Google Scholar]
- 79.Brereton HM, Harland ML, Froscio M, Petronijevic T, Barritt GJ. Novel variants of voltage-operated calcium channel alpha 1-subunit transcripts in a rat liver-derived cell line: deletion in the IVS4 voltage sensing region. Cell Calcium. 1997;22:39–52. doi: 10.1016/s0143-4160(97)90088-9. [DOI] [PubMed] [Google Scholar]
- 80.Badou A, Savignac M, Moreau M, Leclerc C, Pasquier R, Druet P, Pelletier L. HgCl2-induced Interleukin-4 gene expression in T cells involves a protein kinase C-dependent calcium influx through 1-type calcium channels. J Biol Chem. 1997;272:32411–8. doi: 10.1074/jbc.272.51.32411. 10.1074/jbc.272.51.32411. [DOI] [PubMed] [Google Scholar]
- 81.Savignac M, Badou A, Moreau M, et al. Protein kinase C-mediated calcium entry dependent upon dihydropyridine sensitive channels: a T cell receptor-coupled signaling pathway involved in IL-4 synthesis. FASEB J. 2001;15:1577–9. doi: 10.1096/fj.00-0733fje. [DOI] [PubMed] [Google Scholar]