Abstract
Given that there are few natural killer T (NKT) cells in the liver of athymic nude mice and in neonatally thymectomized mice, it is still controversial whether all NKT cells existing in the liver are supplied by the thymus or if some such cells develop in the liver. To determine whether or not NKT cells are consistently supplied from the thymus during adult life, thymectomy was conducted in mice at the age of 8 weeks. Interestingly, the proportion and number of CD4+ NKT cells increased or remained unchanged in the liver after adult thymectomy and this phenomenon continued for up to 6 months after thymectomy. The administration of α-galactosylceramide induced severe cytopenia (due to apoptosis) of CD4+ NKT cells in the liver on day 1, but subsequent expansion of these NKT cells occurred in thymectomized mice similar to the case in normal mice. However, in thymectomized mice given lethal irradiation (9·5 Gy) and subsequent bone marrow transfer, the population of CD4+ NKT cells no longer expanded in the liver, although that of CD8+ NKT cells did. These results suggest that thymic CD4+ NKT cells, or their progenitors, may migrate to the liver at a neonatal stage but are not supplied from the thymus in the adult stage under usual conditions. CD8+ NKT cells can be generated in the liver.
Introduction
Attention has been focused on natural killer T (NKT) cells which express an intermediate density of T-cell receptor (TCR)–CD3 complex and one marker of NK cells, NK1.1, on the surface.1–5 This is due to the fact that NKT cells recognize unusual antigens, such as glycolipids, as well as peptides in the context of major histocompatibility complex (MHC) class I-like antigen, CD1d,6–8 and may be associated with immunoregulatory functions in certain autoimmune diseases.9–13 In parallel with these findings, NKT cells are known to express mainly the phenotype of CD4+ or double-negative (DN) CD4−CD8− and to be abundant in the liver and thymus (especially in the medullary region).14–17 These NKT cells preferentially use an invariant chain of Vα14Jα281 gene for TCRα.18
The origin of CD4+ NKT cells in the liver is still controversial: the results of some studies support the concept that all CD4+ NKT cells seen in the liver are of thymic origin, while those of others raise the possibility that at least some NKT cells develop in the liver. The former concept is based on the fact that athymic nude mice19–22 and neonatally thymectomized mice carry extraordinarily few NKT cells.23 Some investigators have also used adult thymectomized mice subjected to fetal liver cell transfer to confirm this theory.24,25
On the other hand, some controversial results have been derived from other experimental designs. For example, parabiosis experiments have shown that NKT cells, but not other T-cell subsets, in the liver did not mix well with partner cells,26 indicating that some NKT cells develop in the liver during adult life. In these parabiosis experiments, we used B6.Ly5.1 and B6.Ly5.2 mice. If some lymphocyte subsets in a specific organ (e.g. NKT cells in the liver) are consistently supplied from other organs, such lymphocyte subsets became a half-and-half mixture of own cells and partner cells by 2 weeks of parabiosis. To characterize further the origin of NKT cells existing in the liver, we conducted adult thymectomy in mice and investigated the level of NKT cells in the liver thereafter with or without the specific stimulation of NKT cells. We propose the possibility that the population of CD4+ NKT cells in the liver is maintained without supply by the thymus, at least under usual conditions in adult life.
Materials and methods
Mice
C57BL/6 (B6) and B6.Ly5.1 mice were used with or without adult thymectomy. Adult thymectomy was performed at the age of 8 weeks. Neonatal thymectomy was performed within 3 days after birth. In some experiments, mice were intraperitoneally administered with α-galactosylceramide (α-GalCer) (KRN 7000) (2 µg/mouse) (Kirin Beer Co., Tokyo, Japan).27 All these mice were maintained under specific pathogen-free conditions in the animal facility of Niigata University.
Lethal irradiation and bone marrow transplantation (BMT)
To eliminate NKT cells and their progenitor cells in the liver and other organs, mice were lethally (9·5 Gy) irradiated. These mice then received bone marrow cells (1 × 107/mouse) which were depleted of Thy-1+ cells. Cell depletion was performed in vitro by using anti-Thy-1 monoclonal antibody (mAb; PharMingen Co., San Diego, CA) and rabbit complement as described previously.28
Cell preparation
Hepatic lymphocytes were isolated by a method described elsewhere.29 Briefly, mice anaesthetized with ether were killed by exsanguination via a cardiac puncture. To obtain lymphocytes, the liver was removed, pressed through a 200-gauge stainless steel mesh, and then suspended in Eagle's minimum essential medium supplemented with 5 mm HEPES (Nissui Pharmaceutical Co., Tokyo, Japan) and 2% heat-inactivated newborn calf serum. After being washed once with the medium, the cell pellet was resuspended in the medium. Lymphocytes were isolated from parenchymal hepatocytes, the nuclei of hepatocytes and Kupffer cells by the Percoll (35% Percoll containing 100 U/ml heparin) gradient method.
Splenocytes were obtained by pressing the spleen through a 200-gauge stainless steel mesh; erythrocytes in the spleen were lysed with 0·83% NH4Cl–Tris buffer (pH 7·6). Bone marrow cells were obtained by flushing femurs with the medium.
Immunofluorescence tests
The surface phenotypes of cells were identified by a two-colour immunofluorescence.30 Fluorescein isothiocyanate (FITC), phycoerythrin (PE), or biotin-conjugated mAbs were used and biotin-conjugated reagents were developed with TRIcolor- conjugated streptavidin (Caltag Lab., San Francisco, CA). The mAb used here included anti-CD3 (145-2C11), anti-IL-2Rβ (anti-interleukin-2 receptor β; TM-β1), anti-NK1.1 (PK136), anti-CD4 (PM4-5), and anti-CD8 (53-6.7) (PharMingen Co.). Cells were analysed by FACScan (Becton Dickinson Co., Mountain View, CA). To prevent non-specific binding of mAbs, CD16/32 (2.4G2) was added before staining with labelled mAb. Dead cells were excluded by forward scatter, side scatter and propidium iodide gating.
Measurement of serum transaminases
Sera were obtained from mice at the indicated time-points in each experiment. The level of serum transaminases, glutamic oxaloacetic transaminase (GOT) and glutamic pyruvic transaminase (GPT), was determined by a previously described method.31
Statistical analysis
Differences were determined by Student's t-test.
Results
Effects of adult thymectomy on the population size of NKT cells
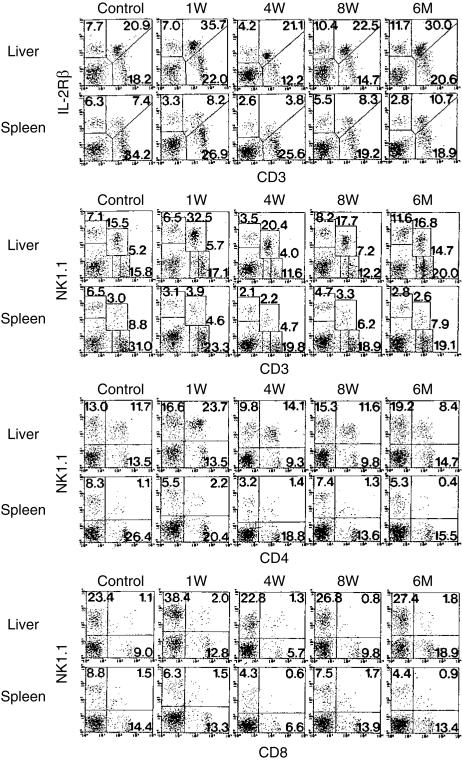
B6 mice were thymectomized at the age of 8 weeks and the proportions of IL-2Rβ+ CD3int cells and NK1.1+ CD3int cells were determined at the indicated time-points (Fig. 1). To identify IL-2Rβ+ CD3int cells, two-colour staining for CD3 and IL-2Rβ was conducted (the top of the figure). As previously found,16 IL-2Rβ+ CD3int cells were abundant in the liver (20·9%) but were scarce in the spleen (7·4%) in control mice. At 1 week after thymectomy, it was found that the proportion of IL-2Rβ+ CD3int cells did not decrease or even increased in the liver and spleen. This was also true at 4 and 8 weeks after thymectomy. Even at 6 months after thymectomy, the proportion of IL-2Rβ+ CD3int cells did not decrease.
Figure 1.
Phenotypic characterization of lymphocytes in the liver and spleen of mice with adult thymectomy. B6 mice at the age of 8 weeks were thymectomized. Lymphocytes were then obtained from the liver and spleen at the indicated weeks after thymectomy. Two-colour staining for CD3 and IL-2Rβ, that for CD3 and NK1.1, that for CD4 and NK1.1, and that for CD8 and NK1.1 were conducted. Numbers in the figure represent the percentages of fluorescence-positive cells. The data shown here are representative of three experiments.
It is known that IL-2Rβ+ CD3int cells mainly consist of the NK1.1+ subset (i.e. NKT cells) and that these NKT cells are CD4+, including some DN CD4− CD8− cells.16 To determine the proportion of these NKT cells and their subsets, two-colour staining for NK1.1 and CD3 (or CD4 or CD8) was then conducted (lower rows of the figure). In parallel with the increase in the proportion of IL-2Rβ+ CD3int cells in the liver after thymectomy, the proportion of NK1.1+ CD3int cells increased in this organ. It is known that these NKT cells are mainly CD4+, but not CD8+. This was also the case before adult thymectomy (control) and at any indicated time-points after adult thymectomy.
In these experiments, we also enumerated the number of lymphocytes yielded by the liver and spleen (data not shown). The variation in the absolute numbers of CD4+ NK1.1+ cells in the liver of mice with or without adult thymectomy (ATx) was calculated (Table 1). The absolute number of CD4+ NKT cells did not decrease and even increased (on 1 week, P < 0·05) after adult thymectomy. Even 6 months after thymectomy, a significant number of CD4+ NKT cells remained in the liver. In a subsequent study, these ATx mice were kept for 12 months but the level of CD4+ NKT cells did not decrease in the liver (data not shown).
Table 1.
A comparison of the absolute number of CD4+ NK1.1+ cells in the liver between untreated mice (None) and ATx mice (ATx)
| Number of cells (× 106/liver) | ||||
|---|---|---|---|---|
| 0 week | 1 week* | 4 weeks | 6 months | |
| None | 0·8 ± 0·1 | 1·0 ± 0·2 | 1·1 ± 0·2 | 0·6 ± 0·1 |
| ATx | 0·8 ± 0·1 | 1·7 ± 0·4† | 1·3 ± 0·3 | 0·7 ± 0·2 |
Interval after adult thymectomy.
P < 0·05.Thymectomy was performed at the age of 8 weeks. Six mice were used to produce the mean and one SD at each point of time.
Expansion of NKT cells by the administration of α-GalCer in the liver of mice with adult thymectomy
We previously reported that a single injection of α-GalCer induced the apoptosis of NKT cells in the liver, with a subsequent prominent expansion of NKT cells.31 It is still unknown whether these NKT cells expand or are supplied from the thymus. In this experiment, we used thymectomized adult mice for this purpose (Fig. 2). As expected, a prominent decrease in the proportions of IL-2Rβ+ CD3int cells and NK1.1+ CD3int cells was seen in the liver of these mice on day 1 when α-GalCer (2 µg/mouse) was administered (top two rows of the figure). The proportion of CD4+ NKT cells eventually decreased, as estimated by two-colour staining for CD4 and NK1.1. However, similar to the case in normal mice (data not shown), the expansion of NKT cells was observed in the liver even of thymectomized mice on days 3 and 7. In other words, NKT cells remaining in the liver may begin to expand when no such cells are supplied by the thymus.
Figure 2.
Administration of α-GalCer to thymectomized mice. Mice were kept for 2 weeks after adult thymectomy and were then intraperitoneally injected with α-GalCer (2 µg/mouse). Two-colour staining for the indicated combinations was conducted on days 1, 3 and 7 after the injection. Numbers in the figure represent the percentages of fluorescence-positive cells. The data shown here are representative of three experiments.
Similar to the case of normal mice,31 mice with adult thymectomy showed severe signs of hepatic injury, by the elevation of the transaminases GOT and GPT, when these mice were administered α-GalCer (Fig. 3). Many areas of massive necrosis were seen in the livers of these mice (data not shown).
Figure 3.
Induction of hepatic injury by administration of α-GalCer in mice with adult thymectomy. Mice were kept for 2 weeks after adult thymectomy and were then intraperitoneally injected with α-GalCer (2 µg/mouse). The data for serum transaminases, GOT and GPT, at each point were produced using four mice.
Inability of the generation of CD4+ NKT cells in mice with adult thymectomy when they are lethally irradiated and subjected to BMT
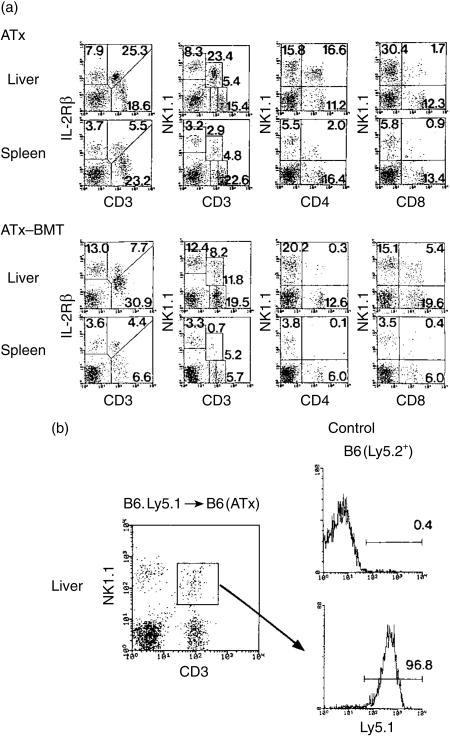
To completely eliminate NKT cells from mice with adult thymectomy (ATx), these mice were lethally irradiated (9·5 Gy). BMT was then conducted to determine whether NKT cells were able to be generated in the absence of the thymus (Fig. 4a). Control data were produced by mice with only adult thymectomy (the top row of the figure). Many T cells were found to recover, especially in the liver, of ATx-BMT mice; these cells were estimated to be IL-2Rβ+ (a little low) CD3int and a mixture of NK1.1+ and NK1.1−. However, CD4+ NK1.1+ cells were almost completely absent in both the liver and spleen. Instead, CD8+ NK1.1+ cells (as well as DN CD4− CD8− cells as shown by calculation) were found to be generated especially in the liver. The recovery of NK1.1− CD3int cells from the liver was also an interesting phenomenon.
Figure 4.
A comparison of the phenotypic characterization of lymphocytes in the liver between thymectomized mice and thymectomized mice irradiated lethally and subjected to BMT. (a) ATx and ATx-BMT, (b) ATx-BMT (Ly5.1 origin). Lymphocytes were obtained from the liver of thymectomized mice lethally irradiated and subjected to BMT at 2 months after such treatments. Age-matched control (thymectomized) mice were also used. Two-colour staining for the indicated combinations was conducted. In (b), BMT of B6.Ly5.1 origin was conducted to determine the origin of newly generated CD8+ NKT cells in ATxBMT mice (B6.Ly5.2+). Three-colour staining for CD8, NK1.1, and Ly5.1 was conducted. The data shown here are representative of three experiments.
To determine the actual origin (donor or recipient origin) of CD8+ NKT cells generated in the liver, we performed BMT using B6.Ly5.1 origin cells in this experiment (Fig. 4b). Two month after BMT, the origin was determined by three-colour staining for CD8, NK1.1 and Ly5.1. Gated analysis of CD8+ NKT cells, the majority of them (up to 96%) expressed Ly5.1 antigen (donor origin).
NKT cells in the liver and bone marrow in mice with neonatal or adult thymectomy
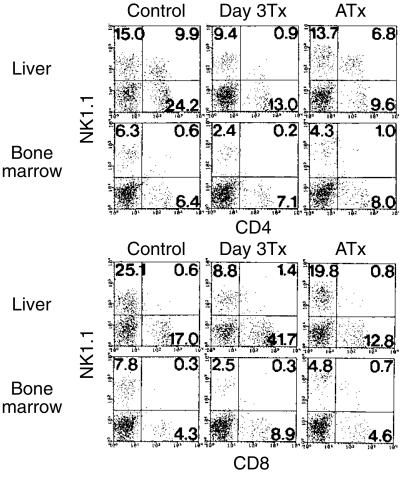
In an earlier study, it was reported that the bone marrow is an important site for the regeneration of NKT cells when they are impaired (not a lethal impairment).32 In this experiment, we compared the effect of thymectomy on size of the population of CD4+ and CD8+ NKT cells (Fig. 5). Both neonatal and adult thymectomies were conducted. As already shown,23 neonatal thymectomy reduced the size of the CD4+ NKT cell population in the liver. However, it was confirmed that adult thymectomy did not change the number of CD4+ NKT cells. CD8+ NKT cell numbers in the liver were always low. Under the same conditions, the proportions of both CD4+ and CD8+ NKT cells, both of which were primarily a few in the bone marrow, did not change in this organ of adult mice, irrespective of neonatal or adult thymectomy. In other words, a specific expansion of CD8+ NKT cells was seen in neither the liver nor the bone marrow.
Figure 5.
Size of the population of CD4+ and CD8+ NKT cells in the liver and bone marrow after neonatal or adult thymectomy. Neonatal thymectomy was performed by day 3 after birth (Day 3 Tx) while adult thymectomy was done at the age of 8 weeks (ATx). All mice, including untreated B6 mice, were used at the age of 6 months. Two-colour staining for the indicated combinations was conducted. The data shown here are representative of three experiments.
Discussion
In the present study, we demonstrated that adult thymectomy did not decrease the number and proportion of NKT cells in the liver up to 6 months after thymectomy. We previously reported that a single administration of α-GalCer induced severe apoptosis of hepatic NKT cells on day 1 with subsequent intensive expansion of NKT cells in the liver.31 At that time, we did not know whether these expanding NKT cells in the liver were supplied by the thymus or not. In the present study, it was found that these NKT cells expanded in the liver. The present results suggest that NKT cells seen in the liver are able to maintain their population size without a supply of NKT cells from the thymus, at least at adult stage.
In parallel with these experiments, adult thymectomized mice were subjected to lethal irradiation (9·5 Gy) and subsequent BMT. These mice were not able to produce CD4+ NKT cells in the liver. In contrast, CD8+ NKT cells, which are an extremely minor population in the liver of normal mice,33,34 began to expand in the liver of these mice. It is conceivable that CD4+ NKT cells might not newly arise in the liver when such cells are completely impaired. However, this was not the case with CD8+ NKT cells. In conjunction with the former data, the present findings indicated that CD4+ NKT cells may be able to renew in the liver if some CD4+ NKT cells remain in this organ. We, as well as other investigators, have reported that NKT cells in the liver are characterized by an intensive uptake of bromodeoxyuridine when it is injected in vivo.25,35
It has already been reported by two groups that CD4+ NKT cells which use an invariant chain of Vα14Jα281 for TCRα, did not appear in the liver of adult thymectomized mice which received fetal liver cells.24,25 In contrast, such CD4+ NKT cells appeared in the liver of mice with an intact thymus. These results suggest that CD4+ NKT cells are eventually of thymic origin and have the ability to migrate into the liver if such cells there are completely impaired. In addition to this fact, we obtained findings indicating that CD4+ NKT cells in the liver might not always be supplied by the thymus in the case of incomplete cell impairment. If this is the case, there is a possibility that CD4+ NKT cells or their progenitors migrate from the thymus to the liver at a neonatal stage and undergo renewal in the liver at an adult stage. This idea is based on the fact that neonatally thymectomized mice show a low level of CD4+ NKT cells in the liver at an adult stage.23
We, and other investigators,33,34 have focused attention on CD8+ NKT cells in the liver and other organs. Although these CD8+ NKT cells are a minor population in the liver, it was found that they begin to expand in a compensatory manner when conventional CD4+ NKT cells were impaired. It was also found that these CD8+ NKT cells are actually of extrathymic origin. In unusual mice such as β2-microglobulin (−/−), CD1d (−/−), or Jα281 (−/−) mice which lack CD4+ NKT cells in the liver and other organs, NKT cells are known to expand in the liver with aging.36 These NKT cells are CD8+ NKT cells and do not show a skewed usage of Vα14Jα281 for TCRα. We recently found that these CD8+ NKT cells may be intimately associated with allogeneic MHC antigens and simultaneously mediate self-reactive cytotoxicity (H. Kawamura, unpublished observation). A similar phenomenon has also been observed by other investigators37 before the concept of NKT cells had been introduced.
In addition to α-GalCer, it was reported that the administration of anti-CD3 mAb or IL-12 into mice induced the apoptosis of NKT cells and subsequent restoration of NKT cells in the liver.32 These authors showed that the bone marrow is important for this NKT cell homeostasis in the liver. We do not deny the possibility that some NKT cells in the liver are derived from the bone marrow in mice administered with α-GalCer. However, they did not compare the phenotype of NKT cells between the liver and bone marrow, especially in terms of CD4 and CD8 in that study. There is the tissue-dependent distribution of NKT cell subsets: CD8+ NKT cells are abundant in the bone marrow while CD4+ NKT cells are abundant in the liver.23 This was true even after the regeneration of NKT cells in the liver and bone marrow after the administrations of α-GalCer and anti-CD3 mAb (our unpublished observation). Therefore, we have to consider another possibility, namely, the renewal of CD4+ NKT cells in the liver (at least, an incomplete impairment of NKT cells or their progenitors in the liver).
In the case of the complete eradication of NKT cells (or progenitors) by lethal irradiation, there exists an essential role of the thymus for the recovery of CD4+ NKT cells in the liver. At this time, CD8+ NKT cells might be of bone marrow origin, because BMT was done in this case. Alternatively, the progenitors are of bone marrow origin but CD8+ NKT cells develop thereafter in the liver. This idea comes from the fact that we could never see the expansion of CD8+ NKT cells in the bone marrow under all tested conditions (see Fig. 5) and under conditions of the lethal irradiation and subsequent BMT (data not shown).
We have obtained additional information on the self-renewing ability of CD4+ NKT cells in the liver during adult life. It is conceivable that they are supplied from the thymus at the neonatal stage but not during adult life. However, if they are completely impaired in the liver during adult life, the thymus is again required for maintaining CD4+ NKT cells in the liver. We, as well as other investigators, have always produced these conditions by using lethal irradiation. However, we do not yet know whether such severe circumstances occur naturally under conditions other than lethal irradiation.
Although we paid attention mainly to NKT cells in this study, there was other new evidence (see Fig. 4), namely, the generation of NK1.1− IL-2Rβ+ CD3int cells in the liver of mice with ATx which were then lethally irradiated and subjected to BMT. This population appeared in the liver and resembled T cells seen in the liver of athymic nude mice. In the absence of the thymus, these nude mice lacked NKT cells under usual conditions19–22 but carried NK1.1− IL-2Rβ+ CD3int cells (manuscript in preparation). Therefore, we conclude that these T cells are truly of extrathymic, hepatic origin.
Acknowledgments
This work was supported by a grant-in-aid for Scientific Research from the Ministry of Education, Science and Culture, Japan. The authors thank Mrs Masako Watanabe for manuscript preparation and Mr Tetsuo Hashimoto for animal maintenance.
Abbreviations
- BMT
bone marrow transplantation
- DN
double-negative
- α-GalCer
α-galactosylceramide
- IL-2Rβ
interleukin-2 receptor β-chain
- MNC
mononuclear cells
- NKT cells
natural killer T cells
- PE
phycoerythrin
- TCRint cells
intermediate TCR cells.
References
- 1.Bendelac A. Mouse NK1+ T cells. Curr Opin Immunol. 1995;7:367–74. doi: 10.1016/0952-7915(95)80112-x. [DOI] [PubMed] [Google Scholar]
- 2.MacDonald HR. NK1.1+ T cells receptor-α/β+ cells: new clues to their origin, specificity, and function. J Exp Med. 1995;182:633–8. doi: 10.1084/jem.182.3.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bix M, Locksley RM. Natural T cells: Cells that co-express NKRP-1 and TCR. J Immunol. 1995;155:1020–2. [PubMed] [Google Scholar]
- 4.Vicari AP, Zlotnik A. Mouse NK1.1+ T cells: a new family of T cells. Immunol Today. 1996;17:71–6. doi: 10.1016/0167-5699(96)80582-2. [DOI] [PubMed] [Google Scholar]
- 5.Abo T, Kawamura T, Watanabe H. Physiological responses of extrathymic T cells in the liver. Immunol Rev. 2000;174:135–49. doi: 10.1034/j.1600-0528.2002.017415.x. [DOI] [PubMed] [Google Scholar]
- 6.Bendelac A, Lantz O, Quimby ME, Yewdell JW, Bennink JR, Brutkiewicz RR. CD1 recognition by mouse NK1+ T lymphocytes. Science. 1995;268:863–5. doi: 10.1126/science.7538697. [DOI] [PubMed] [Google Scholar]
- 7.Kawano T, Cui J, Koezuka Y, et al. CD1d-restricted and TCR-mediated activation of Vα 14 NKT cells by glycosylceramides. Science. 1997;278:1626–9. doi: 10.1126/science.278.5343.1626. [DOI] [PubMed] [Google Scholar]
- 8.Tangri S, Brossay L, Burdin N, Lee DJ, Corr M, Kronenberg M. Presentation of peptide antigens by mouse CD1 requires endosomal localization and protein antigen processing. Proc Natl Acad Sci USA. 1998;95:14314–9. doi: 10.1073/pnas.95.24.14314. 10.1073/pnas.95.24.14314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lehuen A, Lantz O, Beaudoin L, Laloux V, Carnaud C, Bendelac A, Bach J-F, Monteiro RC. Overexpression of natural killer T cells protects Vα14-Jα281 transgenic nonobese diabetic mice against diabetes. J Exp Med. 1998;188:1831–9. doi: 10.1084/jem.188.10.1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hammond KJL, Poulton LD, Palmisano LJ, Silveira PA, Godfrey DI, Baxter AG. α/β-T cell receptor (TCR)+ CD4−CD8− (NKT) thymocytes prevent insulin-dependent diabetes mellitus in nonobese diabetic (NOD) /Lt mice by the influence of interleukin (IL)-4 and/or IL-10. J Exp Med. 1998;187:1047–56. doi: 10.1084/jem.187.7.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Takeda K, Dennert G. The development of autoimmunity in C57BL/6 lpr mice correlates with the disappearance of natural killer type 1-positive cells: evidence for their suppressive action on bone marrow stem cell proliferation, B cell immunoglobulin secretion, and autoimmune symptoms. J Exp Med. 1993;177:155–64. doi: 10.1084/jem.177.1.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Falcone M, Yeung B, Tucker L, Rodriguez E, Sarvetnick N. A defect in interleukin 12-induced activation and interferon γ secretion of peripheral natural killer T cells in nonobese diabetic mice suggests new pathogenic mechanisms for insulin-dependent diabetes mellitus. J Exp Med. 1999;190:963–72. doi: 10.1084/jem.190.7.963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mieza MA, Itoh T, Cui JQ, et al. Selective reduction of Vα14+ NK T cells associated with disease development in autoimmune-prone mice. J Immunol. 1996;156:4035–40. [PubMed] [Google Scholar]
- 14.Crispe IN, Moore MW, Husmann LA, Smith L, Bevan MJ, Shimonkevitz RP. Differentiation potential of subsets of CD4−8− thymocytes. Nature. 1987;329:336–9. doi: 10.1038/329336a0. [DOI] [PubMed] [Google Scholar]
- 15.Kikly K, Dennert G. Evidence for extrathymic development of TNK cells. NK1+CD3+ cells responsible for acute marrow graft rejection are present in thymus-deficient mice. J Immunol. 1992;149:403–12. [PubMed] [Google Scholar]
- 16.Watanabe H, Miyaji C, Kawachi Y, Iiai T, Ohtsuka K, Iwanaga T, Takahashi-Iwanaga H, Abo T. Relationships between intermediate TCR cells and NK1.1+T cells in various immune organs. NK1.1+T cells are present within a population of intermediate TCR cells. J Immunol. 1995;155:2972–83. [PubMed] [Google Scholar]
- 17.Kimura M, Watanabe H, Ohtsuka K, Iiai T, Tsuchida M, Sato S, Abo T. Radioresistance of intermediate TCR cells and their localization in the body of mice revealed by irradiation. Microbiol Immunol. 1993;37:641–52. doi: 10.1111/j.1348-0421.1993.tb01687.x. [DOI] [PubMed] [Google Scholar]
- 18.Makino Y, Yamagata N, Sasho T, Adachi Y, Kanno R, Koseki H, Kanno M, Taniguchi M. Extrathymic development of Vα14-positive T cells. J Exp Med. 1993;177:1399–408. doi: 10.1084/jem.177.5.1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hsu D-H, Moore KW, Spits H. Differential effects of IL-4 and IL-10 on IL-2-induced IFN-γ synthesis and lymphokine-activated killer activity. Int Immunol. 1992;4:563–9. doi: 10.1093/intimm/4.5.563. [DOI] [PubMed] [Google Scholar]
- 20.Emoto M, Emoto Y, Kaufmann SHE. IL-4 producing CD4+ TCRαβint liver lymphocytes: influence of thymus, β2-microglobulin and NK1.1 expression. Int Immunol. 1995;7:1729–39. doi: 10.1093/intimm/7.11.1729. [DOI] [PubMed] [Google Scholar]
- 21.Emoto M, Emoto Y, Kaufmann SHE. CD8αβ+ TCRαβintermediate lymphocytes expressing skewed TCRVβ repertoire in the liver of aged athymic nu/nu mice. J Immunol. 1997;158:1041–50. [PubMed] [Google Scholar]
- 22.Emoto M, Mittrücker H-W, Schmits R, Mak TW, Kaufmann SHE. Critical role of leukocyte function-associated antigen-1 in liver accumulation of CD4+NKT cells. J Immunol. 1995;162:5094–8. [PubMed] [Google Scholar]
- 23.Hammond K, Cain W, van Driel I, Godfrey D. Three day neonatal thymectomy selectively depletes NK1.1+ T cells. Int Immunol. 1998;10:1491–9. doi: 10.1093/intimm/10.10.1491. 10.1093/intimm/10.10.1491. [DOI] [PubMed] [Google Scholar]
- 24.Tilloy F, Di Santo JP, Bendelac A, Lantz O. Thymic dependence of invariant Vα14+ natural killer-T cell development. Eur J Immunol. 1999;29:3313–18. doi: 10.1002/(SICI)1521-4141(199910)29:10<3313::AID-IMMU3313>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 25.Coles MC, Raulet DH. NK1.1+ T cells in the liver arise in the thymus and are selected by interactions with class I molecules on CD4+CD8+ cells. J Immunol. 2000;164:2412–18. doi: 10.4049/jimmunol.164.5.2412. [DOI] [PubMed] [Google Scholar]
- 26.Suzuki S, Sugahara S, Shimizu T, et al. Low level of mixing of partner cells seen in extrathymic T cells in the liver and intestine of parabiotic mice: Its biological implication. Eur J Immunol. 1998;28:3719–29. doi: 10.1002/(SICI)1521-4141(199811)28:11<3719::AID-IMMU3719>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 27.Brossay L, Naidenko O, Burdin N, Matsuda J, Sakai T, Kronenberg M. Structural requirements for galactosylceramide recognition by CD1-restricted NK T cells. J Immunol. 1998;161:5124–8. [PubMed] [Google Scholar]
- 28.Sato K, Ohtsuka K, Hasegawa K, et al. Evidence for extrathymic generation of intermediate TCR cells in the liver revealed in thymectomized, irradiated mice subjected to bone marrow transplantation. J Exp Med. 1995;182:759–67. doi: 10.1084/jem.182.3.759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tsukahara A, Seki S, Iiai T, et al. Mouse liver T cells: Their change with aging and in comparison with peripheral T cells. Hepatology. 1997;26:301–9. doi: 10.1002/hep.510260208. [DOI] [PubMed] [Google Scholar]
- 30.Kawamura H, Kawamura T, Kokai Y, et al. Expansion of extrathymic T cells as well as granulocytes in the liver and other organs of G-CSF transgenic mice: Why they lost the ability of hybrid resistance. J Immunol. 1999;162:5957–64. [PubMed] [Google Scholar]
- 31.Osman Y, Kawamura T, Naito T, Takeda K, van Kaer L, Okumura K, Abo T. Activation of hepatic NKT cells and subsequent liver injury following administration of α-galactosylceramide. Eur J Immunol. 2000;30:1919–28. doi: 10.1002/1521-4141(200007)30:7<1919::AID-IMMU1919>3.0.CO;2-3. 10.1002/1521-4141(200007)30:7<1919::aid-immu1919>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 32.Eberl G, MacDonald HR. Rapid death and regeneration of NKT cells in anti-CD3ε- or IL-12-treated mice: a major role for bone marrow in NKT cell homeostasis. Immunity. 1998;9:345–53. doi: 10.1016/s1074-7613(00)80617-2. [DOI] [PubMed] [Google Scholar]
- 33.Hammond KJL, Pelikan SB, Crowe NY, et al. NKT cells are phenotypically and functionally diverse. Eur J Immunol. 1999;29:3768–81. doi: 10.1002/(SICI)1521-4141(199911)29:11<3768::AID-IMMU3768>3.0.CO;2-G. 10.1002/(sici)1521-4141(199911)29:11<3768::aid-immu3768>3.3.co;2-7. [DOI] [PubMed] [Google Scholar]
- 34.Eberl G, Lees R, Smiley ST, Taniguchi M, Grusby MJ, MacDonald HR. Tissue-specific segregation of CD1d-dependent and CD1d-independent NK T cells. J Immunol. 1999;162:6410–19. [PubMed] [Google Scholar]
- 35.Narita J, Miyaji C, Watanabe H, et al. Differentiation of forbidden T cell clones and granulocytes in the parenchymal space of the liver in mice treated with estrogen. Cell Immunol. 1998;185:1–13. doi: 10.1006/cimm.1998.1245. [DOI] [PubMed] [Google Scholar]
- 36.Murakami M, Paul WE. Age-dependent appearance of NK1.1+ T cells in the livers of β2-microglobulin knockout and SJL mice. J Immunol. 1998;160:2649–54. [PubMed] [Google Scholar]
- 37.Glas R, Öhlen C, Höglund P, Kärre K. The CD8+ T cell repertoire in β2-microglobulin-deficient mice is biased towards reactivity against self-major histocompatibility class I. J Exp Med. 1994;179:661–72. doi: 10.1084/jem.179.2.661. [DOI] [PMC free article] [PubMed] [Google Scholar]