Abstract
It is important to identify characteristics that confer on proteins the potential to induce allergenic sensitization and allergenic disease. Protein allergens carry T-cell epitopes that are capable of inducing a type 2 T helper (Th2) cell response. There is limited information regarding factors that govern the allergenicity of proteins. We previously reported that a decrease in the conformational stability of hen-egg lysozyme (HEL) enhanced its capacity to activate HEL-specific T cells owing to the increased susceptibility to intracellular antigen processing. To determine whether the conformational stability of HEL makes for a critical contribution to allergenic sensitization in vivo, we immunized BALB/c mice with HEL derivatives of different conformational stability, but which retained a similar three-dimensional structure. The magnitude of in vivo T-cell responses, evaluated by ex vivo proliferative responses of lymph node T cells from mice primed with various HEL derivatives, was inversely correlated with conformational stability, as was interferon-γ (IFN-γ) and interleukin-4 (IL-4) production by splenic T cells in response to HEL. Immunization of the least stable derivative led to a potent IL-4 response and to immunoglobulin E (IgE) antibody production. We propose that the intrinsic allergenicity of proteins can be determined by the degree of conformational stability.
Introduction
CD4+ T cells recognize peptides derived from protein antigens bound to class II major histocompatibility complex (MHC) molecules on the surface of antigen-presenting cells (APC). Prior to the recognition of peptide–MHC class II complexes by their T-cell receptors, protein antigens are incorporated into APC and then degraded by endosomal proteases.1–3 A globular protein is in equilibrium between native and denatured (non-native) states, and the proteases preferentially digest proteins in a denatured state rather than those in the native state.4–6 The free energy difference between the two states of a protein is thermodynamically defined as conformational stability, which is of the order of 5–20 kcal/mol and independent of the size of the protein.7,8 An increase in the conformational stability of hen-egg lysozyme (HEL) leads to a decrease in antigenic peptide generation by increasing the resistance for processing enzymes such as cathepsin B and D,9 thus indicating that the conformational stability of protein antigens is a factor regulating antigen-processing efficiency, an event which may affect the dose of antigenic peptides on APC surfaces. Hence, the T-cell triggering response is governed by the conformational stability of protein antigens.
Naturally occurring disulphide bonds make substantial contributions to the conformational stability of proteins. A single disulphide bond contributes over 2–4 kcal/mol to the conformational stability of proteins.10–12 Intramolecular disulphide bonds of a protein have been found to participate in allergenicity. Preventing disulphide bonding through site-directed mutagenesis yields allergens that no longer bind immunoglobulin E (IgE) derived from allergic patients.13–15 However, thermodynamically engineered allergen molecules are less stable than the original and thus would have an increased T-cell stimulatory capacity. Most features of atopy and asthma, especially IgE synthesis, are closely related to induction of type 2 helper T (Th2) cells.16 In a series of studies,13–15 the possibility that the engineered allergens facilitate the induction of Th2 cells in vivo was not investigated.
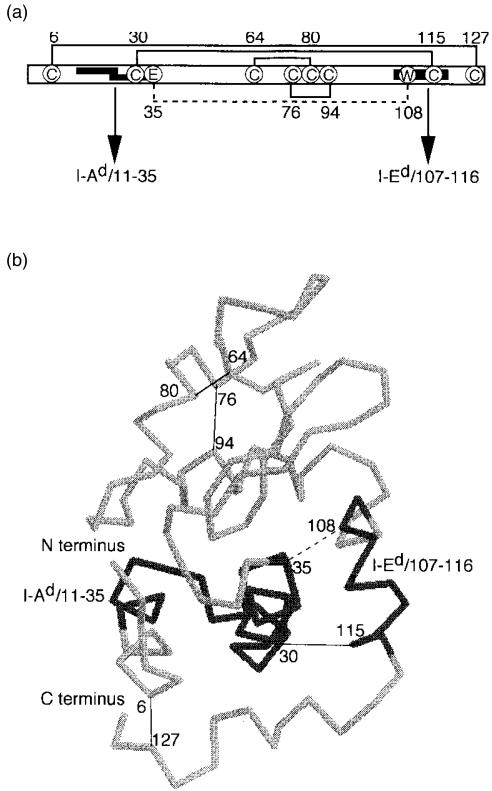
Thus it seemed important to determine whether the conformational stability of a protein antigen provides a critical contribution to Th2 development in vivo. To address this question, we used a well-characterized protein, HEL, as the representative protein antigen. HEL has eight cysteines which form four disulphide bonds between cysteine residue pairs 6–127, 30–115, 64–80 and 76–94 (Fig. 1). The HEL-specific T-cell response in the H-2d haplotype mice is directed toward two regions: the immunodominant determinant HEL107–116 presented by I-Ed molecules and the subdominant determinant HEL11–35 presented by I-Ad molecules (Fig. 1, refs. 17 and 18). We prepared HEL derivatives with a similar conformation and varied conformational stabilities. Less stable 6,127CM-HEL was produced by selective deletion of the intramolecular disulphide bridge19 and more stable 35–108CL-HEL was prepared by selective cross-linking of intramolecular residues through an ester bond.20 We found that the magnitude of in vivo T-cell responses, including the Th2 response, was inversely correlated to the conformational stability of HEL.
Figure 1.
The structure of hen-egg lysozyme (HEL) and its antigenic determinants. (a) Schematic representation of the disulphide bonds and the identified T-cell-epitope regions of HEL. Disulphide bridges between the cysteine residues (C) in HEL are indicated with solid lines. An ester linkage between glutamic acid (E) and tryptophan (W) in 35–108CL-HEL is indicated with a dashed line. The dominant epitope HEL107–116 presented by I-Ed molecules and the subdominant epitope HEL11–35 presented by I-Ad molecules are indicated by bold lines. The subdominant region was reported to contain two determinants, HEL11–25 and HEL20–35.17,49 (b) View of the alpha-carbon backbone of HEL, showing the locations of the disulphide bonds in HEL, the ester bond in 35–108CL-HEL, and the T-cell-determinant regions. This figure was prepared using RasMol version 2·5, with some modifications.
Materials and methods
Antigens
Five times recrystallized HEL was kindly donated by QP Co. (Tokyo, Japan). Preparation of 6,127CM-HEL and 35–108CL-HEL was performed according to Radford et al.19 and Imoto et al.,20 respectively. HEL derivatives used in this study were subjected to assays immediately after purification by cation-exchange chromatography (CM-Toyopearl 650S; Tosoh, Tokyo, Japan). Denatured HEL was prepared with an S-alkylating reagent of (3-bromopropyl)trimethylammonium bromide following reduction of HEL with 2-mercaptoethanol (2-ME), to maintain water solubility of the cysteine-containing denatured proteins by lowering the hydrophobicity.21 To prepare peptide fragments of HEL, HEL was reduced and reversibly S-alkylated with trimethylammoniopropyl methanethiosulphonate (Wako, Osaka, Japan). The denatured HEL was digested with V 8 protease from Staphylococcus aureus (Sigma, St. Louis, MO) and lysylendopeptidase from Achromobacter lyticus (Wako), respectively, followed by separation by reverse-phase high performance liquid chromatography (HPLC), using Mightysil RP-18 GP (4·6 × 250 mm; Kanto, Tokyo, Japan), as described previously.22 Resultant peptides were reduced with dithiothreitol to liberate the sulphydryl group and the final products were repurified by reverse-phase HPLC. HEL8–35 and HEL98–116 were identified using matrix-assisted laser desorption/ionization time-of-flight mass spectrometry and their concentrations were determined by amino acid analysis. A purified protein derivative of Mycobacterium tuberculosis H37Ra (PPD) was purchased from Kainosu Inc. (Tokyo, Japan).
Animals
Female BALB/c mice (H-2d) were obtained from Japan SLC (Shizuoka, Japan). At 8–12 weeks of age, the mice were immunized. All experiments involving the use of mice were performed in accordance with protocols approved by the Animal Care and Use Committee of the Kyushu University, Faculty of Dental Science.
Sandwich enzyme-linked immunosorbent assay (ELISA)
ELISA was performed to test the conformational integrity of different HEL derivatives, using two monoclonal antibodies (mAbs) specific for conformational epitopes of HEL. HEL-specific immunoglobulin M (IgM) mAb was generated from transgenic mice, obtained from the Jackson Laboratory (Bar Harbor, ME), expressing high-affinity HEL-specific immunoglobulin receptors.23 The IgM mAb has the same antigen specificity as HyHEL-10 mAb, which recognizes the discontinuous epitope of HEL comprising the residues 16, 21 73, 93, 96, 97, 100, 101 and 102.24 An anti-HEL immunoglobulin G1 (IgG1) mAb was produced from BALB/c mice immunized with native HEL. The specificity of IgG1 mAb has not been determined. Wells of microtitre ELISA plates (Nunc, Roskilde, Denmark) were coated with the IgM mAb (at a concentration of 1 µg/ml in 0·1 m sodium carbonate buffer, pH 9·6) overnight at 4°. Blocking was carried out with 2% non-fat dry milk in phosphate-buffered saline (PBS) containing 0·05% Tween-20 (PBST). The blocking buffer was used as a diluent, and PBST was used for washing. HEL derivatives were added to precoated wells and allowed to react with the coated IgM for 1 hr at room temperature. The anti-HEL IgG1 mAb was then added to each well followed by incubation for an additional 1 hr at room temperature. Binding of the IgG1 was subsequently probed with an alkaline phosphatase-conjugated goat anti-mouse IgG (Zymed, South San Francisco, CA). p-nitrophenyl phosphate was used as a substrate for alkaline phosphatase and, following development, the absorbance at 405 nm (A405) of each well was recorded, using ImmunoMini NJ-2300 (InterMed, Tokyo, Japan).
8-Anilino-1-naphthalenesulphonic acid (ANS) fluorescence study
The environmentally sensitive fluorescent probe, ANS, is useful for determining the degree of exposure of hydrophobic sites as the structure of a protein is perturbed. ANS is weakly fluorescent in an aqueous environment, but becomes appreciably fluorescent with blue-shifted emission spectra in non-polar environments.25,26 To measure the binding of ANS to HEL derivatives, 5 µm of each HEL derivative and 200 µm of ANS in 20 mm N-2-hydroxyethylpiperazine-N′-2-ethanesulphonic acid (HEPES)-buffered saline, pH 7·5, were incubated for 10 min at 37°. The fluorescence spectra from 400 to 640 nm were recorded with excitation at 372 nm on a spectrofluorimeter (Model F-2000; Hitachi, Tokyo, Japan).
Protease degradation
HEL derivatives at 1 mg/ml in 0·5 ml of 20 mm HEPES-buffered saline, pH 7·5, were incubated at 37° with 100 µg of alpha-chymotrypsin from bovine pancreas (Wako) and carboxypeptidase Y from yeast (Oriental Yeast, Tokyo, Japan). These digests were subjected to sodium dodecyl sulphate–polyacrylamide gel electrophoresis (SDS–PAGE), through a 15% acrylamide gel and under reducing conditions, then stained with Coomassie Brilliant Blue (Rapid CBB KANTO; Kanto).
Antigen-presentation assay
T-cell hybridomas specific for HEL8–35/I-Ad and HEL98–116/I-Ed were established by immunizing BALB/c mice with HEL8–35 and native HEL, respectively. The determination of T-cell MHC restriction was made by the inhibition of antigen presentation using mAbs 34.5.3S (anti-I-Ad) and 14.4.4S (anti-I-Ed). Assays were performed essentially as described previously.9 Briefly, T-hybridoma cells were cultured with various concentrations of HEL derivatives or saline in the presence of a B-lymphoma cell line, A20 cells (American Type Culture Collection [ATCC], Rockville, MD), as APC. Interleukin (IL)-2 produced by T-hybridoma cells was measured based on the proliferative response of an IL-2-dependent cell line, CTLL-2.27 The culture medium used for maintenance and stimulation of the cell lines was Dulbecco's modified Eagle's medium (DMEM; Sigma) containing 4·5 g/l of glucose supplemented with l-glutamine (216 µg/ml), l-asparagine (36 µg/ml), l-arginine–HCl (116 µg/ml), folic acid (6 µg/ml), sodium pyruvate (110 µg/ml), HEPES (10 mm), 2-ME (5 × 10−5 m), penicillin (100 U/ml), streptomycin (100 µg/ml) and 10% heat-inactivated fetal bovine serum (GibcoBRL™, Life Technologies Oriental Inc, Osaka, Japan).
T-cell proliferation assay
Mice were immunized subcutaneously with HEL derivatives in 0·1 ml of complete Freund's adjuvant (CFA; Difco Laboratories, Detroit, MI). These mice were killed 9 days after immunization; draining lymph nodes were removed and cell suspensions were prepared using Hanks' balanced salt solution (Sigma). Cells resuspended in serum-free HL-1 medium (BioWhittaker, Walkersville, MD) supplemented with l-glutamine (216 µg/ml), 2-ME (5 × 10−5 m), penicillin (100 U/ml) and streptomycin (100 µg/ml), were cultured in a flat-bottom 96-well plate (4 × 105 cells/200 µl). Proliferation was measured after 96 hr, using a colorimetric assay based on tetrazolium salt – 3-(4,5-dimethylthiazol-2-yl)-2,5-dimethyltetrazolium bromide (MTT) – as described previously.28
Measurement of cytokine production
The levels of interferon-γ (IFN-γ) and IL-4 were measured, as described previously.29 Briefly, antibody-coated plates provided in murine cytokine ELISA kits (BioSource International, Camarillo, CA) were first treated with sterile PBS containing penicillin (100 U/ml), streptomycin (100 µg/ml) and 2% bovine serum albumin (BSA) at 37° for 24 hr. After three washes with sterile PBS, spleen cells at 106 cells/well in 100 µl of DMEM supplemented with 1% heat-inactivated syngenic mouse serum, with or without antigens, were cultured in these plates for 48 hr. The cultures were terminated by extensive washing with PBS containing 1% Triton-X-100 and 0·5% Tween-20. IFN-γ and IL-4 associated with the solid phase were measured following the ELISA protocol described in the manufacturer's instructions.
Measurement of serum antibody levels
To induce serum IgE responses, three groups of five BALB/c mice were immunized with 6,127CM-HEL, native HEL, or 35–108CL-HEL. In the first experiment, mice were immunized subcutaneously with 1 µg of each antigen in CFA, in a total volume of 0·1 ml, on day 0 and given booster immunizations intraperitoneally with the same antigen in incomplete Freund's adjuvant (IFA), in a total volume of 0·1 ml, on day 35. In the second experiment, mice were immunized intraperitoneally with 0·5 µg (on days 0 and 21) and 5 µg (on days 53 and 87) of each antigen adsorbed onto 1 mg of Al(OH)3 adjuvant30 in a total volume of 0·5 ml. Serum antibody levels to HEL, after immunization, were measured using ELISA. Wells of microtitre ELISA plates were coated with 50 µl of antigen at 3 µg/ml (50 pmol/50 µl) in 0·1 m sodium carbonate buffer, pH 9·6 (the concentration which absorbed the same amount of these HELs onto the solid phase at 7 pmol/well; data not shown). Blocking was carried out using 2% non-fat dry milk in PBST. The blocking buffer was used as a diluent for all antibody reagents, including serum specimens, and PBST was used for washes. We used alkaline phosphatase-conjugated rat monoclonal anti-mouse IgG1, IgG2a and immunoglobulin A (IgA) (Zymed) to evaluate antibody levels. Binding of the antibody was detected following development with p-nitrophenyl phosphate, and the A405 of each well was recorded, as described above. To detect IgE responses, ELISA plates were coated with antigen, blocked, and immune sera were added to the antigen-coated wells. After a wash with PBST, bound mouse IgE was probed with a rat mAb (LO-ME-2; Zymed) that was specific for mouse IgE.31 Subsequently, the IgG were probed with biotinylated goat anti-rat IgG (Chemicon International Inc., Temecula, CA; preadsorbed with mouse, human, bovine and horse sera), followed by the alkaline phosphatase–biotin/avidin complex (Vector Laboratories, Burlingame, CA). The A405 of the wells was recorded, as described above.
Results
Structural characterization of HEL derivatives
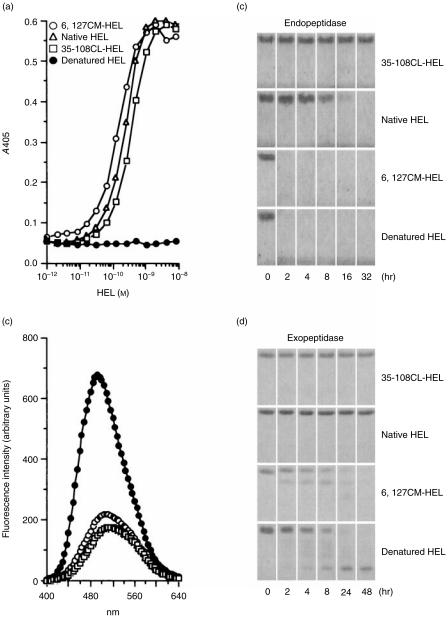
To examine the influence of conformational stability of antigens on the generation of antigen-specific Th2 cells in vivo, unstable and stable HELs were prepared. Selective reduction and S-carboxymethylation of the disulphide bond Cys6–Cys127 of HEL (Fig. 1) dramatically decreased the conformational stability. The difference in conformational stability between this unstable 6,127CM-HEL and native HEL was 10·4 kcal/mol, as determined in this study based on guanidinium hydrochloride denaturation curves at pH 7·0 and 37° (data not shown; ref. 32). On the other hand, cross-linking between Glu35 and Trp108 through an ester bond (Fig. 1) increased the conformational stability. The difference in conformational stability between this stable 35–108CL-HEL and native HEL was 5·1 kcal/mol, which we determined previously by differential scanning calorimetry.33 Regardless of the different conformational stabilities, both unstable and stable HELs took on a native-like conformation that was readily distinguishable from the denatured conformation (Fig. 2a).
Figure 2.
The structural profiles of hen-egg lysozyme (HEL) derivatives. (a) Detection of the tertiary conformation of HEL by sandwich enzyme-linked immunosorbent assay (ELISA). Two different monoclonal antibodies (mAbs), recognizing different conformational epitopes of HEL, were used according to the procedure described in the Materials and Methods. (b) Binding of 8-anilino-1-naphthalenesulphonic acid (ANS) to HEL derivatives. The different HEL derivatives shown on the figure were incubated with ANS at 37° and pH 7·5. After a 10-min incubation, the fluorescence spectra (400–640 nm) were measured by excitation at 372 nm. The control spectrum was measured under the same experimental conditions but in the absence of HEL. The spectra of native HEL and 35–108CL-HEL were almost the same as that of the control spectrum. (c) and (d) Susceptibility of HEL derivatives to proteolysis. HEL derivatives were digested with alpha-chymotrypsin (c) and carboxypeptidase Y (d) at 37° and pH 7·5. Each incubation was terminated at various time-points and the digested samples were stored at −20° until analysed by sodium dodecyl sulphate–polyacrylamide gel electrophoresis (SDS–PAGE).
To characterize in detail the structure of HEL derivatives, surface hydrophobicity and the conformational flexibility were examined by measuring the ANS-binding activity and the protease susceptibility, respectively. Unbound ANS was weakly fluorescent, with an emission maximum at ≈ 520 nm, and ANS in the presence of native HEL or 35–108CL-HEL had essentially the same emission spectra (Fig. 2b). In the presence of denatured HEL, however, the fluorescence was increased, and the spectra was blue shifted. In the presence of 6,127CM-HEL, a relatively small fluorescence enhancement and blue shift was observed, thus indicating that 6,127CM-HEL binds some ANS. Therefore, unstable 6,127CM-HEL took on a partially unfolded structure characterized by an increase in surface hydrophobicity. Alpha-chymotrypsin is an endopeptidase which cleaves at the carboxyl terminus of aromatic amino acids; carboxypeptidase Y is an exopeptidase which removes amino acids sequentially from the carboxyl terminus. Using these peptidases, we next examined the conformational difference among HEL derivatives from a different aspect. Alpha-chymotrypsin digestion is specific for unstable conformation (Fig. 2c), hence, conformational stability of proteins determines their resistance/susceptibility to proteolytic digestion, as previously demonstrated.6,9 On the other hand, carboxypeptidase Y could not degrade stable 35–108CL-HEL and native HEL, but did gradually cleave unstable 6,127CM-HEL and denatured HEL (Fig. 2d), which means that the disulphide bridge Cys6–Cys127 determines the sensitivity of HEL to carboxypeptidase degradation. These results mean that the global conformations of HEL derivatives are similar but the regional conformation of 6,127CM-HEL differs slightly from the other two HELs.
Processing of dominant and subdominant T-cell epitopes from HEL derivatives
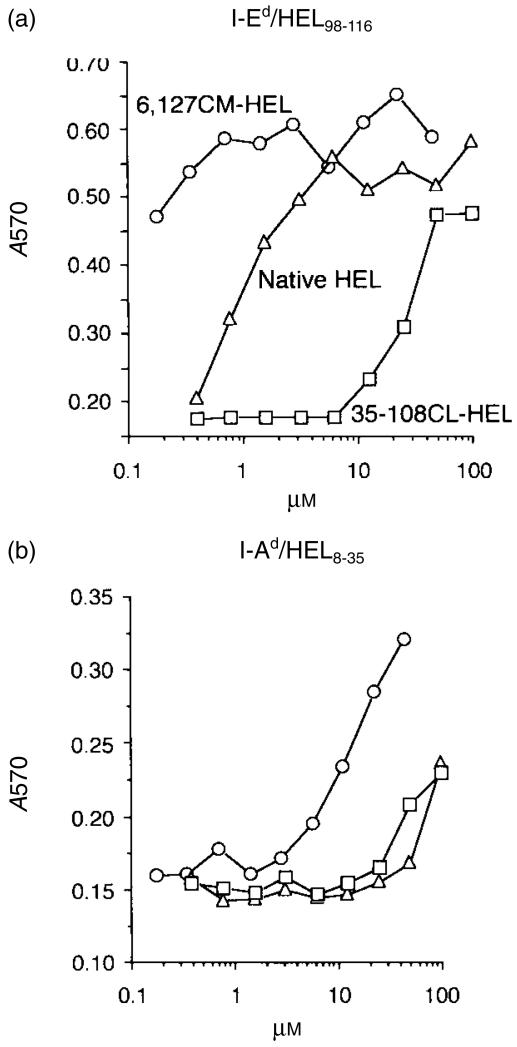
In the course of antigen processing, structural alteration, including disulphide reduction and denaturation, is essential to expose critical peptide bonds of the antigen that are normally buried in the interior of the native structure. To evaluate the processing efficiency of the dominant and the subdominant epitope of HEL, two cloned T-cell hybridomas specific for HEL8–35/I-Ad and HEL107–116/I-Ed were stimulated with A20 cells (I-Ad, I-Ed) in the presence of the HEL derivatives. Chemical modifications introduced into 6,127CM-HEL and 35–108CL-HEL do not alter their internalization capacity into APC and the antigenicity of the epitope regions.9 The presentation efficiency of the dominant region, HEL107–116, was regulated by conformational stability, i.e. increasing the stability led to depression of epitope generation, and recognition of HEL107−116 in stable 35–108CL-HEL required a concentration ≈ 2 logs higher than that of unstable 6,127CM-HEL (Fig. 3a). Similarly, the subdominant region, HEL8–35, in 6,127CM-HEL was efficiently processed from HEL and differences between native HEL and 35–108CL-HEL were not apparent (Fig. 3b). Thus, elimination of the outer disulphide bridge of HEL facilitates presentation of antigenic determinants in HEL.
Figure 3.
Destabilization of hen-egg lysozyme (HEL) facilitates presentation of the antigenic determinants of HEL. Two T-cell hybridoma clones specific for HEL98–116/I-Ed (a) and HEL8–35/I-Ad (b) were cultured with HEL derivatives in the presence of A20 cells. Culture supernatants were harvested at 24 hr, and the levels of interleukin-2 (IL-2) were measured according to growth of CTLL-2, determined by metabolizing MTT and measuring the absorbance at 570 nm (A570).
Contribution of conformational stability of HEL toin vivo T-cell responses
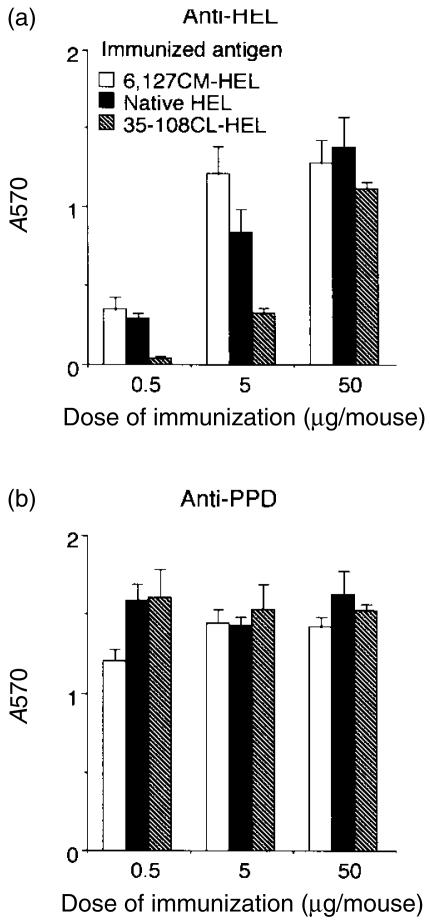
To determine the influence of conformational stability of HEL on in vivo T-cell responses, BALB/c mice were immunized with the HEL derivatives and levels of the proliferative response of lymph node T cells to native HEL were evaluated. In mice given lower doses of immunization (0·5 and 5 µg of HEL derivatives), the ex vivo proliferative response to HEL decreased with an increase in the stability of HEL derivatives immunized, thereby indicating that induction of anti-HEL T cells in vivo is suppressed by enhancing the stability of HEL (Fig. 4a). In mice given 50 µg of derivatives, the difference was not evident, presumably because the high dose may be sufficient to induce T-cell proliferative response without any restriction of the stability. Anti-PPD T-cell proliferative responses were similar in all three dose groups of HEL (Fig. 4b). Thus, the magnitude of the T-cell response in vivo depends on the stability of HEL.
Figure 4.
Destabilization of hen-egg lysozyme (HEL) enhances the priming of HEL-specific T cells in vivo. Nine groups of two BALB/c mice were immunized with unstable 6,127CM-HEL, native HEL, or stable 35–108CL-HEL (0·5, 5, or 50 µg/mouse) in complete Freund's adjuvant (CFA). Nine days later, draining lymph node cells were cultured in the presence of 10 µm of native HEL (a), 10 µg/ml of purified protein derivative of Mycobacterium tuberculosis (PPD) (b), or no antigen, at a concentration of 4 × 105 cells/well. After 4 days of incubation, the number of viable cells in each well was determined by metabolizing MTT and measuring the absorbance at 570 nm (A570). Cultures were set up in triplicate from pooled lymph node cells. Data are expressed as mean absorbance at 570 nm and standard deviation (SD) of triplicate cultures, with background values (cultured with no antigen) subtracted (ΔA570).
Contribution of conformational stability of HEL to induction of Th1 and Th2 responses
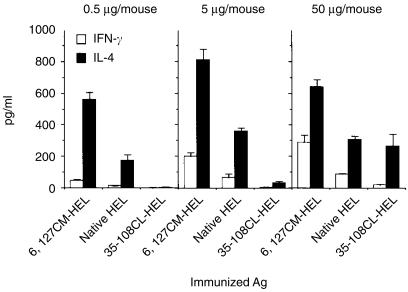
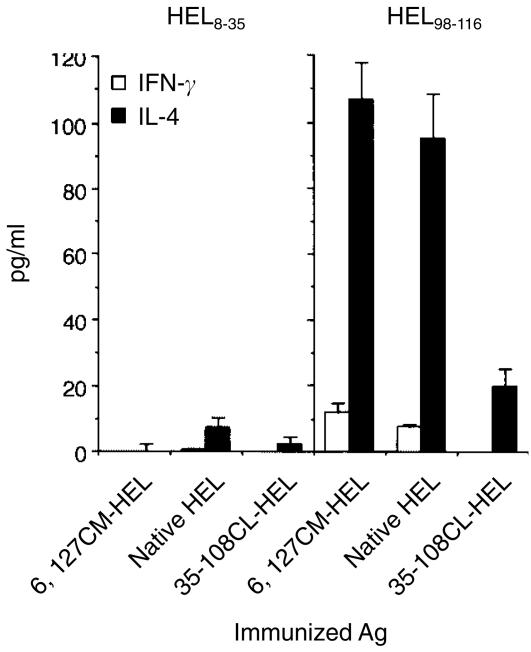
Next, the capacity of HEL derivatives to produce HEL-specific Th1 and Th2 cells in vivo was evaluated by measuring antigen-specific secretions of IFN-γ and IL-4 from splenocytes of primed mice. Production of both the representative Th1 and Th2 cytokines was increased as the conformational stability of HEL decreased (Fig. 5). This result is compatible with the proliferative response shown in Fig. 4(a). The predominant response with all HEL derivatives was a Th2 type. The quantity of IL-4 produced was at least twofold higher than the quantity of IFN-γ produced, at every dose of HEL derivatives. Immunization of the least stable HEL at the smallest dose (0·5 µg/mouse) led to a potent IL-4 response which was never induced by other, more stable, HELs, even at a dose 100 times higher (Fig. 5). In addition, the Th2-dominated response was predominantly directed to the dominant epitope of HEL (Fig. 6). Therefore, the conformational stability of HEL quantitatively governs both Th1 and Th2 responses and is a critical contribution to the Th2 response in BALB/c mice.
Figure 5.
Destabilization of hen-egg lysozyme (HEL) leads to efficient priming of HEL-specific interleukin-4 (IL-4)-secreting cells in the spleen. Nine groups of two BALB/c mice were immunized with 6,127CM-HEL, native HEL, or 35–108CL-HEL (0·5, 5, or 50 µg/mouse) in complete Freund's adjuvant (CFA). Nine days later, spleen cells were cultured in the presence of 10 µm of native HEL, or with no antigen, at a concentration of 106 cells/well in anti-IL-4 antibody- or anti-interferon-γ (IFN-γ) antibody-precoated plates. After 48 hr of incubation, cytokine production was measured, using a sandwich enzyme-linked immunosorbent assay (ELISA). Data are expressed as mean± SD of triplicate cultures with the background values (cultured with no antigen: IFN-γ, 11·0 ± 1·2 pg/ml; IL-4, 15·4 ± 4·9 pg/ml) subtracted. IFN-γ and IL-4 levels in control mice (injected with CFA alone) were 1·6 ± 0·6 pg/ml and 4·5 ± 6·4 pg/ml, respectively.
Figure 6.
Epitope specificities of interferon-γ (IFN-γ)- and interleukin-4 (IL-4)-secreting cells induced in vivo by injection with hen-egg lysozyme (HEL) derivatives. Three groups of two BALB/c mice were immunized with 6,127CM-HEL, native HEL, or 35–108CL-HEL (50 µg/mouse) in complete Freund's adjuvant (CFA). Nine days later, spleen cells were cultured in the presence of 3 µm of HEL8–35, HEL98–116, or no antigen, at 106 cells/well in anti-IL-4 antibody- or anti-IFN-γ antibody-precoated plates. After 48 hr of incubation, cytokine production was measured, using a sandwich enzyme-linked immunosorbent assay (ELISA), as described in the legend to Figure 5.
High IgE-inducing capacity of unstable HEL
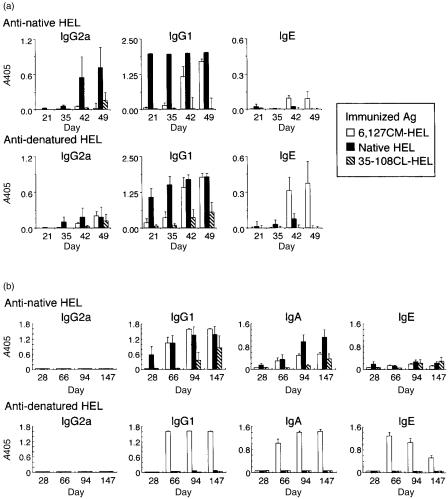
The induction of in vivo T-cell responses was restricted by the stability of HEL and a higher Th2 response was generated by an unstable HEL. We next investigated whether a less-stable HEL would induce a higher IgE response. First, we immunized subcutaneously with 1 µg of each HEL derivative emulsified in CFA, but no detectable response was observed on days 21 and 35 (Fig. 7a). However, booster injection of 1 µg of the same antigen emulsified in IFA on day 35 did induce an anti-HEL IgE response in mice primed with unstable 6,127CM-HEL. Next, to induce a higher HEL-specific IgE response, we used alum adjuvant, which initiates strong antigen-specific Th2 responses.34 When BALB/c mice were intraperitoneally administered each HEL derivative in alum adjuvant, IgG1 and IgE responses, but not an IgG2a response, were generated (Fig. 7b), indicating that the Th2-dominated humoral immune response was induced in BALB/c mice by immunization of HEL plus alum adjuvant. Although gradually elevated anti-native HEL IgG1 and IgA responses were observed, low anti-native HEL IgE responses were induced by all three HEL derivatives (Fig. 7b, upper row). On the other hand, high serum IgE responses against denatured HEL were induced in mice primed with unstable 6,127CM-HEL; these responses peaked on day 66, then showed a gradual decrease (Fig. 7b, lower row). On day 66, the titre of anti-denatured HEL IgE induced by 6,127CM-HEL was at least 1000-fold higher than that induced by the other two HELs (data not shown). Destabilized proteins thus evoke a high serum IgE response, presumably owing to the effective stimulating capacity for Th2 cells.
Figure 7.
Time courses of serum antibody levels against native hen-egg lysozyme (HEL) and denatured HEL. Three groups of five BALB/c mice were immunized subcutaneously with 1 µg/mouse of 6,127CM-HEL, native HEL, or 35–108CL-HEL in complete Freund's adjuvant (CFA) on day 0 and intraperitoneally with 1 µg/mouse of the same antigen in incomplete Freund's adjuvant (IFA) on day 35 (a). Independently, three groups of five BALB/c mice were immunized intraperitoneally with 0·5 µg/mouse (on days 0 and 21) and 5 µg/mouse (on days 53 and 87) of HEL derivatives in alum adjuvant (b). Native HEL (upper row) and denatured HEL (lower row) were coated onto enzyme-linked immunosorbent assay (ELISA) plates and IgG2a, IgG1, IgA and IgE antibody levels in diluted sera were evaluated. Dilution of serum in ELISA: IgG2a, 1/100 for (a), 1/50 for (b); IgG1, 1/500 for (a), 1/50 for (b); IgA, 1/50 for (b); IgE, 1/20 for (a), 1/50 for (b). The data are expressed as mean absorbance at 405 nm (A405) plus the standard error, of five mice.
Discussion
Our data clearly indicate that conformational stability of HEL quantitatively regulates in vivo priming and differentiation of HEL-specific T cells. Remarkably, BALB/c mice primed with the least stable 6,127CM-HEL induced strong Th2-type immune responses.
The structural uniqueness of 6,127CM-HEL was characterized by X-ray crystallography. Hill et al.35 reported that 6,127CM-HEL retains a substantially native-like conformation. However, specific reduction of the disulphide bond does not free the N-terminal, but does free the C-terminal portion from its interaction with the body of HEL. Thus, the conformational difference in the C-terminal region may be responsible for the ANS-binding activity (Fig. 2b) and the enhanced susceptibility to carboxypeptidase (Fig. 2d). The efficient antigen processing of the C-terminal HEL107–116 region in 6,127CM-HEL (Fig. 3) may be, in part, a result of the regional unstable character. On the other hand, the formation of the internal ester linkage between Glu35 and Trp108 was found to cause no significant alteration in the native structure.36
The efficiency of antigen processing was enhanced when the structure of HEL was destabilized. We previously found that cathepsin B and D degraded 6,127CM-HEL more rapidly than native HEL and 35–108CL-HEL in vitro.9 The increased antigen presentation in 6,127CM-HEL (Fig. 3) was not the result of accelerated cellular internalization to A20 cells9 but rather to structural instability, which resulted in a facilitated intracellular proteolysis. The augmented expression of antigenic peptide–MHC class II complexes on the surface of APC also occurred in vivo, because potent HEL-specific T-cell responses were induced by immunization of 6,127CM-HEL, rather than by two other, more stable, antigens (Figs 4 and 5). Induction of the T-cell response against the subdominant region HEL8–35 in vivo was negligible for all derivatives (Fig. 6), probably because this region is intrinsically unsusceptible to antigen processing. Therefore, the conformational stability of HEL limits the antigen-processing efficiency and influences the magnitude of priming and differentiation of HEL-specific T cells in vivo.
Why did destabilization of HEL favour the development of Th2 cells? Decreasing the conformational stability of HEL could enhance the effective concentration of peptide–MHC complexes on the surface of APC, which may lead to the development of Th2 responses with high IgE production. Several studies have demonstrated that the antigen dose is a critical parameter for priming and differentiation of CD4+ T cells.37–41 In an in vitro system, and using naive CD4+ T cells from DO11.10 T-cell receptor (TCR)-transgenic BALB/c mice, high doses of ovalbumin peptide generated Th2 effector cells.40 Also, a greater level of signalling appears to be required for inducing Th2-type responses.42–44 Considering these data, unstable antigens seem to be a potent Th2 inducer because of their higher T-cell epitope-producing capacity. Indeed, unstable 6,127CM-HEL had 100-fold higher stimulatory capacity for T-hybridoma cells than stable 35–108CL-HEL (Fig. 3), and the kinetics of activation was ≈ 10-fold higher than native HEL.9 Kinetically elevated peptide–MHC complexes on APC in 6,127CM-HEL-primed mice may effectively trigger TCRs of naive CD4+ T cells, followed by their differentiation into Th2-effector cells. This hypothesis needs to be studied experimentally using a TCR transgenic mouse system.
6,127CM-HEL primed Th2-type responses more efficiently than the other two HELs (Figs 5 and 7). These results are inconsistent with the idea that allergen mutants with deleted disulphide bridges are effective agents for allergen-specific immunotherapy.13–15 Certainly, the mutants attenuate the pathogenicity of pre-existing IgE, because the majority of IgE molecules are directed to conformational epitopes that are absent from the denatured allergen. However, the capacity of disulphide-deleted allergens to induce Th2 cells and a specific IgE response in vivo has not been examined. Our data suggest that artificially destabilized proteins, in which the intramolecular disulphide bridges have been removed, function as strong allergens and induce a greater IgE response towards continuous epitopes, as specificity differs from the pre-existing antibodies mainly towards conformational epitopes.
Our data explain the anaphylactic reactions to gelatin contained in vaccine. Gelatin has long been believed to be of low immunogenicity and is thought to be weakly allergenic in humans. For this reason, bovine- or porcine-derived gelatins have been widely used as a stabilizer in vaccines.45 However, gelatin-containing vaccines occasionally induce gelatin-specific IgE and T-cell responses as a cause of anaphylaxis.45–48 As gelatin is a denatured form of collagen and highly susceptible to protease degradation, it should have the potential to effectively prime Th2 responses. Care has to be exercised for the design and manufacture of protein-related drugs, in order not to damage the native protein structure.
In conclusion, our data show that the conformational stability of HEL can quantitatively regulate the Th2 response against the dominant epitope. The possibility that elimination of disulphide bonds from a protein allergen enhances the intrinsic allergenicity needs to be considered. Conformational stability can serve as an index for evaluating not only antigenicity but also allergenicity of protein antigens.
Acknowledgments
We thank Dr Toshitaka Koga for valuable discussions and constant encouragement; Yoshiyuki Tsujihata and Chiaki Kajiyama for preparing T-cell hybridoma cells; Yosuke Mizukami for preparing mAbs; and Chihaya Shiragasawa for measuring serum antibody response. This work was supported by Grant-in-Aid for Encouragement of Young Scientists from the Japan Society for the Promotion of Science (grant nos: 10771293 and 12771404).
Abbreviations
- ANS
8-anilino-1-naphthalenesulphonic acid
- CFA
complete Freund's adjuvant
- ELISA
enzyme-linked immunosorbent assay
- HEL
hen-egg lysozyme
- 35–108CL-HEL
HEL cross-linked between Glu35 and Trp108 with an ester bond
- 6,127CM-HEL
HEL S-carboxymethylated at Cys6 and Cys127
- IFA
incomplete Freund's adjuvant
- IFN-γ
interferon-γ
- IL
interleukin
- PBST
phosphate-buffered saline containing 0·05% Tween-20
- PPD
purified protein derivative of Mycobacterium tuberculosis
- Th1
T helper type 1
- Th2
T helper type 2.
References
- 1.Harding CV, Unanue ER. Cellular mechanisms of antigen processing and the function of class I and II major histocompatibility complex molecules. Cell Regul. 1990;1:499–509. doi: 10.1091/mbc.1.7.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jensen PE. Acidification and disulfide reduction can be sufficient to allow intact proteins to bind class II MHC. J Immunol. 1993;150:3347–56. [PubMed] [Google Scholar]
- 3.Fineschi B, Miller J. Endosomal proteases and antigen processing. Trends Biochem Sci. 1997;22:377–82. doi: 10.1016/s0968-0004(97)01116-x. [DOI] [PubMed] [Google Scholar]
- 4.Matthyssens GE, Simons G, Kanarek L. Study of the thermal-denaturation mechanism of hen egg-white lysozyme through proteolytic degradation. Eur J Biochem. 1972;26:449–54. doi: 10.1111/j.1432-1033.1972.tb01786.x. [DOI] [PubMed] [Google Scholar]
- 5.Pace CN, Barrett AJ. Kinetics of tryptic hydrolysis of the arginine–valine bond in folded and unfolded ribonuclease T1. Biochem J. 1984;219:411–7. doi: 10.1042/bj2190411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Imoto T, Yamada H, Ueda T. Unfolding rates of globular proteins determined by kinetics of proteolysis. J Mol Biol. 1986;190:647–9. doi: 10.1016/0022-2836(86)90250-0. [DOI] [PubMed] [Google Scholar]
- 7.Pace CN. The stability of globular proteins. CRC Crit Rev Biochem. 1975;3:1–43. doi: 10.3109/10409237509102551. [DOI] [PubMed] [Google Scholar]
- 8.Privalov PL. Stability of proteins: small globular proteins. Adv Protein Chem. 1979;33:167–241. doi: 10.1016/s0065-3233(08)60460-x. [DOI] [PubMed] [Google Scholar]
- 9.So T, Ito H-O, Koga T, Watanabe S, Ueda T, Imoto T. Depression of T-cell epitope generation by stabilizing hen lysozyme. J Biol Chem. 1997;272:32136–40. doi: 10.1074/jbc.272.51.32136. [DOI] [PubMed] [Google Scholar]
- 10.Pace CN, Grimsley GR, Thomson JA, Barnett BJ. Conformational stability and activity of ribonuclease T1 with zero, one, and two intact disulfide bonds. J Biol Chem. 1988;263:11820–5. [PubMed] [Google Scholar]
- 11.Kanaya S, Katsuda C, Kimura S, et al. Stabilization of Escherichia coli ribonuclease H by introduction of an artificial disulfide bond. J Biol Chem. 1991;266:6038–44. [PubMed] [Google Scholar]
- 12.Futami J, Tada H, Seno M, Ishikami S, Yamada H. Stabilization of human RNAse 1 by introduction of a disulfide bond between residues 4 and 118. J Biochem (Tokyo) 2000;128:245–50. doi: 10.1093/oxfordjournals.jbchem.a022747. [DOI] [PubMed] [Google Scholar]
- 13.Smith AM, Chapman MD. Reduction in IgE binding to allergen variants generated by site-directed mutagenesis: contribution of disulfide bonds to the antigenic structure of the major house dust mite allergen Der p 2. Mol Immunol. 1996;33:399–405. doi: 10.1016/0161-5890(95)00150-6. [DOI] [PubMed] [Google Scholar]
- 14.Olsson S, van Hage-Hamsten M, Whitley P. Contribution of disulphide bonds to antigenicity of Lep d 2, the major allergen of the dust mite Lepidoglyphus destructor. Mol Immunol. 1998;35:1017–23. doi: 10.1016/s0161-5890(98)00101-1. 10.1016/s0161-5890(98)00101-1. [DOI] [PubMed] [Google Scholar]
- 15.Takai T, Yokota T, Yasue M, Nishiyama C, Yuuki T, Mori A, Okudaira H, Okumura Y. Engineering of the major house dust mite allergen Der f 2 for allergen-specific immunotherapy. Nat Biotechnol. 1997;15:754–8. doi: 10.1038/nbt0897-754. [DOI] [PubMed] [Google Scholar]
- 16.Holgate ST. The epidemic of allergy and asthma. Nature. 1999;402:B2–4. doi: 10.1038/35037000. [DOI] [PubMed] [Google Scholar]
- 17.Gammon G, Geysen HM, Apple RJ, Pickett E, Palmer M, Ametani A, Sercarz EE. T cell determinant structure: cores and determinant envelopes in three mouse major histocompatibility complex haplotypes. J Exp Med. 1991;173:609–17. doi: 10.1084/jem.173.3.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gapin L, Bravo de Alba Y, Casrouge A, Cabaniols JP, Kourilsky P, Kanellopoulos J. Antigen presentation by dendritic cells focuses T cell responses against immunodominant peptides: studies in the hen egg-white lysozyme (HEL) model. J Immunol. 1998;160:1555–64. [PubMed] [Google Scholar]
- 19.Radford SE, Woolfson DN, Martin SR, Lowe G, Dobson CM. A three-disulphide derivative of hen lysozyme. Structure, dynamics and stability. Biochem J. 1991;273:211–7. doi: 10.1042/bj2730211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Imoto T, Hartdegen FJ, Rupley JA. Oxidation of lysozyme by iodine: isolation of an inactive product and its conversion to an oxindolealanine-lysozyme. J Mol Biol. 1973;80:637–48. doi: 10.1016/0022-2836(73)90201-5. [DOI] [PubMed] [Google Scholar]
- 21.Yamada H, Seno M, Kobayashi A, Moriyama T, Kosaka M, Ito Y, Imoto T. An S-alkylating reagent with positive charges as an efficient solubilizer of denatured disulfide-containing proteins. J Biochem (Tokyo) 1994;116:852–7. doi: 10.1093/oxfordjournals.jbchem.a124606. [DOI] [PubMed] [Google Scholar]
- 22.Koga H, Yamada H, Nishimura Y, Kato K, Imoto T. Multiple proteolytic action of rat liver cathepsin B. specificities and pH-dependences of the endo- and exopeptidase activities. J Biochem (Tokyo) 1991;110:179–88. doi: 10.1093/oxfordjournals.jbchem.a123554. [DOI] [PubMed] [Google Scholar]
- 23.Goodnow CC, Crosbie J, Adelstein S, et al. Altered immunoglobulin expression and functional silencing of self-reactive B lymphocytes in transgenic mice. Nature. 1988;334:676–82. doi: 10.1038/334676a0. [DOI] [PubMed] [Google Scholar]
- 24.Smith-Gill SJ. Molecular recognition of lysozyme by monoclonal antibodies. Exs. 1996;75:277–300. doi: 10.1007/978-3-0348-9225-4_15. [DOI] [PubMed] [Google Scholar]
- 25.Stryer L. The interaction of a naphthalene dye with apomyoglobin and apohemoglobin. A fluorescent probe of non-polar binding sites. J Mol Biol. 1965;13:482–95. doi: 10.1016/s0022-2836(65)80111-5. [DOI] [PubMed] [Google Scholar]
- 26.Runnels HA, Moore JC, Jensen PE. A structural transition in class II major histocompatibility complex proteins at mildly acidic pH. J Exp Med. 1996;183:127–36. doi: 10.1084/jem.183.1.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gillis S, Ferm MM, Ou W, Smith KA. T cell growth factor: parameters of production and a quantitative microassay for activity. J Immunol. 1978;120:2027–32. [PubMed] [Google Scholar]
- 28.So T, Ito H-O, Koga T, Ueda T, Imoto T. Reduced immunogenicity of monomethoxypolyethylene glycol-modified lysozyme for activation of T cells. Immunol Lett. 1996;49:91–7. doi: 10.1016/0165-2478(95)02488-3. [DOI] [PubMed] [Google Scholar]
- 29.Ito H-O, So T, Hirata M, Koga T, Ueda T, Imoto T. Tolerogenic activity of polyethylene glycol-conjugated lysozyme distinct from that of the native counterpart. Immunology. 1998;93:200–7. doi: 10.1046/j.1365-2567.1998.00414.x. 10.1046/j.1365-2567.1998.00414.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Levine BB, Vaz NM. Effect of combinations of inbred strain, antigen, and antigen dose on immune responsiveness and reagin production in the mouse. A potential mouse model for immune aspects of human atopic allergy. Int Arch Allergy Appl Immunol. 1970;39:156–71. doi: 10.1159/000230343. [DOI] [PubMed] [Google Scholar]
- 31.Moreau MC, Gaboriau RV. The absence of gut flora, the doses of antigen ingested and aging affect of the long-term peripheral tolerance induced by ovalbumin feeding in mice. Res Immunol. 1996;147:49–59. doi: 10.1016/0923-2494(96)81548-3. [DOI] [PubMed] [Google Scholar]
- 32.Inoue M, Yamada H, Yasukochi T, Kuroki R, Miki T, Horiuchi T, Imoto T. Multiple role of hydrophobicity of tryptophan-108 in chicken lysozyme: structural stability, saccharide binding ability, and abnormal pKa of glutamic acid-35. Biochemistry. 1992;31:5545–53. doi: 10.1021/bi00139a017. [DOI] [PubMed] [Google Scholar]
- 33.Ueda T, Masumoto K, Ishibashi R, So T, Imoto T. Remarkable thermal stability of doubly intramolecularly cross-linked hen lysozyme. Protein Eng. 2000;13:193–6. doi: 10.1093/protein/13.3.193. [DOI] [PubMed] [Google Scholar]
- 34.Brewer JM, Conacher M, Hunter CA, Mohrs M, Brombacher F, Alexander J. Aluminium hydroxide adjuvant initiates strong antigen-specific Th2 responses in the absence of IL-4- or IL-13-mediated signaling. J Immunol. 1999;163:6448–54. [PubMed] [Google Scholar]
- 35.Hill CP, Johnston NL, Cohen RE. Crystal structure of a ubiquitin-dependent degradation substrate: a three-disulfide form of lysozyme. Proc Natl Acad Sci USA. 1993;90:4136–40. doi: 10.1073/pnas.90.9.4136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Beddell CR, Blake CC, Oatley SJ. An X-ray study of the structure and binding properties of iodine-inactivated lysozyme. J Mol Biol. 1975;97:643–54. doi: 10.1016/s0022-2836(75)80064-7. [DOI] [PubMed] [Google Scholar]
- 37.Parish CR. The relationship between humoral and cell-mediated immunity. Transplant Rev. 1972;13:35–66. doi: 10.1111/j.1600-065x.1972.tb00059.x. [DOI] [PubMed] [Google Scholar]
- 38.Bretscher PA, Wei G, Menon JN, Bielefeldt OH. Establishment of stable, cell-mediated immunity that makes ‘susceptible’ mice resistant to Leishmania major. Science. 1992;257:539–42. doi: 10.1126/science.1636090. [DOI] [PubMed] [Google Scholar]
- 39.Murray JS. How the MHC selects Th1/Th2 immunity. Immunol Today. 1998;19:157–63. doi: 10.1016/s0167-5699(97)01237-1. [DOI] [PubMed] [Google Scholar]
- 40.Hosken NA, Shibuya K, Heath AW, Murphy KM, O'Garra A. The effect of antigen dose on CD4+ T helper cell phenotype development in a T cell receptor-alpha beta-transgenic model. J Exp Med. 1995;182:1579–84. doi: 10.1084/jem.182.5.1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Constant S, Pfeiffer C, Woodard A, Pasqualini T, Bottomly K. Extent of T cell receptor ligation can determine the functional differentiation of naive CD4+ T cells. J Exp Med. 1995;182:1591–6. doi: 10.1084/jem.182.5.1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Croft M, Swain SL. Recently activated naive CD4 T cells can help resting B cells, and can produce sufficient autocrine IL-4 to drive differentiation to secretion of T helper 2-type cytokines. J Immunol. 1995;154:4269–82. [PubMed] [Google Scholar]
- 43.Ranger AM, Das MP, Kuchroo VK, Glimcher LH. B7-2 (CD86) is essential for the development of IL-4-producing T cells. Int Immunol. 1996;8:1549–60. doi: 10.1093/intimm/8.10.1549. [DOI] [PubMed] [Google Scholar]
- 44.Rulifson IC, Sperling AI, Fields PE, Fitch FW, Bluestone JA. CD28 costimulation promotes the production of Th2 cytokines. J Immunol. 1997;158:658–65. [PubMed] [Google Scholar]
- 45.Sakaguchi M, Hori H, Ebihara T, Irie S, Yanagida M, Inouye S. Reactivity of the immunoglobulin E in bovine gelatin-sensitive children to gelatins from various animals. Immunology. 1999;96:286–90. doi: 10.1046/j.1365-2567.1999.00696.x. 10.1046/j.1365-2567.1999.00696.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kumagai T, Yamanaka T, Wataya Y, et al. Gelatin-specific humoral and cellular immune responses in children with immediate- and nonimmediate-type reactions to live measles, mumps, rubella, and varicella vaccines. J Allergy Clin Immunol. 1997;100:130–4. doi: 10.1016/s0091-6749(97)70204-5. [DOI] [PubMed] [Google Scholar]
- 47.Taniguchi K, Fujisawa T, Ihara T, Kamiya H. Gelatin-induced T-cell activation in children with nonanaphylactic-type reactions to vaccines containing gelatin. J Allergy Clin Immunol. 1998;102:1028–32. doi: 10.1016/s0091-6749(98)70342-2. [DOI] [PubMed] [Google Scholar]
- 48.Ohsaki M, Tsutsumi H, Kumagai T, et al. The relevance of TH1 and TH2 cells in immediate and nonimmediate reactions to gelatin-containing vaccine. J Allergy Clin Immunol. 1999;103:276–81. doi: 10.1016/s0091-6749(99)70502-6. [DOI] [PubMed] [Google Scholar]
- 49.Drakesmith H, O'Neil D, Schneider SC, Binks M, Medd P, Sercarz E, Beverley P, Chain B. In vivo priming of T cells against cryptic determinants by dendritic cells exposed to interleukin 6 and native antigen. Proc Natl Acad Sci USA. 1998;95:14903–8. doi: 10.1073/pnas.95.25.14903. 10.1073/pnas.95.25.14903. [DOI] [PMC free article] [PubMed] [Google Scholar]