Abstract
Control of mycobacterial infection by the cellular immune system relies both on antigen-presenting cells and on T lymphocytes. The quality of an effective cellular immune response is dependent on functional signal transduction residing in the cytoplasmic tails of the T-cell receptor CD3 components. In order to investigate potential effects of mycobacteria on T-cell receptor signalling, we examined the protein expression of T-cell signal transduction molecules (CD3ζ, ZAP-70, p59fyn, p56l ck). In Western blots of peripheral blood mononuclear cells of Mycobacterium tuberculosis infected patients, only the CD3ζ-chain showed a marked reduction in protein expression. To investigate the situation in situ, immunoenzymatic and immunofluorescence stainings for CD3ε and CD3ζ expression were performed on sections of normal lymphoid tissue, M. leprae infected and sarcoid tissue. CD3ε and CD3ζ expression were similar with respect to intensity, localization and the number of cells stained in normal lymphoid tissue and in sarcoid granulomas. In contrast, the granulomas of M. leprae infected tissues showed a significantly reduced expression of CD3ζ compared to CD3ε. Using double immunofluorescence analysis, virtually no CD3ζ expression could be detected in comparison to the CD3ε expression in the lesions. Apparently, mycobacteria are capable of significantly reducing CD3ζ-chain expression, which may be restored by cytokines. IL-2-enhanced ζ-chain expression and T-cell effector functions, defined by interferon-γ release, in M. tuberculosis-specific and human leucocyte antigen-DR restricted CD4+ T cells isolated from granuloma lesions from patients with pulmonary tuberculosis. Because CD3ζ is essential for CD3 signalling and for eliciting T-cell effector functions, reduced CD3ζ protein expression could result in altered signal transduction and inefficient T-cell effector functions. Alternatively, reduced CD3ζ-chain expression may protect T cells from repetitive TCR stimulation associated with anergy or apoptosis.
Introduction
Protective immunity to mycobacterial infection is considered to be a cell-mediated process, in which macrophages and CD8+ or CD4+ αβ T cells, CD4− CD8− αβ T cells, γδ T cells and, presumably, natural killer cells mediate either eradication or containment of viable bacilli.1–9 Clinical manifestation of infection with mycobacteria is thought to result either from immune evasion of viable bacilli, from non-sufficient immune surveillance, or from immune effector mechanisms which are themselves contributing to tissue damage.10 The process of phagocytosis, antigen degradation, presentation, and T-cell stimulation could be blocked at any point along this pathway, leading to ineffective activation of specific T cells and thus impairing the ability of the immune system to resolve infection. For instance, mycobacteria avoid triggering oxidative burst by entry into phagocytes via integrin family proteins,11 phagosome lysosome fusion may be inhibited,12 and the abundantly produced lipoarabinomannan has a number of immunosuppressive effects, such as scavenging of reactive oxygen intermediates and inhibition of transcriptional activation of interferon-γ (IFN-γ) inducible genes.13 Suppression on the level of the T-cell response in mycobacterial infections has been suggested to be mediated by increased production of soluble immunosuppressive factors, including transforming growth factor-β (TGF-β) or interleukin-10 (IL-10).14–16 A key event in the induction of a T-cell response is the repetitive stimulation of the T-cell receptor (TCR)17,18 by classical major histocompatibility complex (MHC) class I, MHC class II or non-polymorphic MHC antigen-presenting molecules (e.g. CD1b, CD1c), leading to the activation of several tyrosine kinases which are responsible for eliciting T-cell responses, e.g. proliferation and cytokine secretion.19–22 Thus, variations in the expression of T-cell signal transduction molecules may be responsible for impaired immune function of T cells. In fact, alterations in expression of T-cell signal transducing molecules in correlation to immune dysfunction have been described in situ as well as in the systemic circulation in tumour-bearing patients,23–25 in patients with systemic lupus erythematosus,26 with rheumatoid arthritis,27 and in human immunodeficiency virus (HIV)-infected individuals.28 To elucidate whether mycobacterial infections may have an impact on T-cell signalling, we investigated protein expression of several key molecules of T-cell signal transduction, ZAP-70, the CD3ζ-chain, p56lck and p59fyn in healthy controls and in patients with tuberculosis, leprosy, or in patients with sarcoidosis, a granulomatous disease of unknown aetiology in which mycobacteria are thought not to be the causative agent.29 Most studies examined alterations in T-cell signal transduction using Western blot analysis;14,30 here, we examined the anatomy of CD3ζ-chain reduction in situ.
Materials and methods
Patients
Biopsies of patients suffering from sarcoidosis or tuberculosis, obtained for diagnostic purposes and no longer needed, were used in this study. Sarcoidosis was diagnosed and assessed by chest radiography, bronchoalveolar lavage, and transbronchial biopsy. Pulmonary tuberculosis was diagnosed by the clinical presentation of the patients and was proven by positive bacterial cultures. Blood samples from tuberculosis patients prior to any drug treatment and from healthy blood donors were obtained by venepuncture after informed consent and was approved by the local ethics committee. Skin biopsies of patients suffering from leprosy were obtained from patients of the leprosy eradication programme performed by the Department of Leprosy of the Ministry of Health and Welfare, Paraguay. Patients were classified according to the clinicopathological criteria of Ridley and Jopling, 1966.31 All leprosy biopsies were taken from untreated patients, snap-frozen in liquid nitrogen and stored until use at −80°.
Antibodies
Antibodies used in this study were rabbit anti-CD3ε antiserum and APAAP-complex from DAKO (Glostrup, Denmark), anti-CD3ζ monoclonal antibody (mAb; clone 8D3, Pharmingen, Hamburg, Germany and clone TIA-2 (now named 2H2D9), Immunotech/Coulter, Krefeld, Germany), anti-ZAP kinase mAb, anti-p59fyn mAb and anti-p56lck mAb (Transduction Laboratories, Affiniti Research Products Ltd, Exeter, UK), Cy-3 conjugated goat anti-mouse immunoglobulin G (IgG) and fluoroscein isothiocyanate (FITC)-conjugated donkey anti-rabbit IgG from Dianova (Hamburg, Germany). Anti-CD3-phycoerythrin (PE) (clone UCHT1), anti-CD16-FITC (clone 3G8), anti-CD56-PE (clone B159), as well as isotype matched controls were obtained from Beckman/Coulter, Krefeld, Germany.
Immunoblotting
Peripheral blood mononuclear cells (PBMC) were obtained from heparinized blood by centrifugation through Ficoll-Hypaque (Pharmacia, Freiburg, Germany) gradients followed by two washing steps. Frequency of CD3 positive cells, and CD16 CD56 double-positive staining cells was determined using an aliquot of this preparation by flow cytometry. Approximately 60–70% of the cells stained positive for CD3. Samples corresponding to 2 × 106 CD3 positive cells were analysed in Western blots. In order to reduce artefacts with respect to T-cell receptor regulation induced by anti-CD3 antibodies freshly isolated cells were implemented in in vitro assays and Western blot analysis without manipulation of positive or negative selection. Individual samples were adjusted to the same volume and were lysed for 20 min at 4° in 50 mm HEPES (pH 7·2), 150 mm NaCl, 5 mm ethylenediaminetetraacetic acid (EDTA), 1 mm orthovanadate, 2·5% Triton-X-100, 200 µg/ml chymostatin (Boehringer Mannheim, Mannheim, Germany), 200 µg/ml trypsin–chymotrypsin inhibitor and 2 mm phenylmethylsulphonyl fluoride (PMSF) (both from Sigma, Deisenhofen, Germany). The lysates were subjected to 15% sodium dodecyl sulphate–polyacrylamide gel electrophoresis (SDS–PAGE) under reducing conditions and blotted onto polyvinylidendifluoride membranes (Immobilon-P, Millipore, Eschborn, Germany). Residual protein binding sites were blocked with 3% skim milk powder/1% bovine serum albumin (BSA) for 1 hr, incubated with primary antibody for 1 hr, washed in TBS/0·05% Tween-20, incubated with the peroxidase-conjugated secondary antibody and developed.
Immunoenzymatic staining
Cryostat frozen sections were fixed in acetone for 30 min, followed by fixation in chloroform for 30 min. After fixation the leprosy sections were preincubated with rabbit normal serum for 30 min to block non-specific binding. Paraffin sections were dewaxed (10 min xylene, 10 min acetone, 10 min 1 : 1 acetone and Tris buffered saline (50 mm Tris, 150 mm NaCl, pH 7·5)) and were cooked in a pressure cooker for 1 min for antigen retrieval before application of rabbit normal serum. Incubation with the primary antibody was performed for 30 min, and immunostaining was undertaken according to the APAAP (alkaline phosphatase anti-alkaline phosphatase) method with New Fuchsin development.32 Finally, slides were counterstained with haematoxylin and mounted. Immunostainings were controlled by implementing the secondary reagents alone in order to confirm specificity or enzyme development alone to rule out endogenous enzyme activities.
Double immunofluorescence
Double immunofluorescence staining was performed after a blocking step with 10% TBS–BSA with the primary antibodies for 30 min. After a washing step, the secondary antibodies were added and incubated for 30 min. Specimens were mounted in DABCO anti-fading solution (2·5% DABCO (1,4-diazabicyclo [2,2,2] octane) in 90% glycerol, pH 8·6) and viewed under a fluorescence microscope. Stainings were controlled by using only the secondary antibodies to detect unspecific and background fluorescence. Double exposures on film were performed using stained tonsillar tissue as a measure for the exposure time.
Functional assays
Granuloma associated lymphocytes (GAL) were obtained from pulmonary granuloma lesions by dissection of tissue samples in small (2 µm) pieces which were placed into 48-well plates and cultured with Dulbecco's modified Eagles minimal essential medium (DMEM) supplemented with 10% fetal calf serum, l-glutamine and penicillin (all reagents from GIBCO-BRL, Heidelberg, Germany) supplemented with 50 ng human recombinant IL-7. T-cells which could be rapidly expanded after a 2-day culture period were analyzed for CD4, CD8 and TCR ζ-chain expression by flow cytometry. CD4+ T cells were obtained by negative sorting, removing CD8+ T cells using immunomagnetic beads (Miltenyi, Bergisch Gladbach, Germany). Pulmonary granuloma tissue was obtained by explorative thoracotomy from patients who underwent surgery to establish a definite diagnosis for pulmonary lesions, representing either a malignancy or tuberculosis. Samples used in this study were from patients with pulmonary tuberculosis (MHC typing: patient #1, human leucocyte antigen (HLA)-A3, 11, B32, 53, Cw4, 12, DRB1*01, 11, DQB1*03, 05; patient #2, HLA-A26, 29, B45, 52, Cw6, 12, DRB1*12, 15, DQB1*03, 06). HLA-DR-matched macrophages were implemented as antigen-presenting cells in a 24-hr IFN-γ-release assay. Briefly, macrophages were obtained from PBMC by adherence to plastic for 2 hr followed by three consecutive washing steps to assure minimal contamination with lymphocytes. Macrophages were infected with mycobacteria (virulent M. tuberculosis Erdman strain) 24 hr prior to the assay and infection evaluated by Ziehl–Neelsen staining. Cells were fixed with 1% formaldehyde, washed twice and incubated with CD4+ GAL for 24 hr. The effector : target ratio was 10 : 1; T cells were adjusted to 5 × 105 cells/well in duplicates. Blocking antibodies (10 µg/well) included the anti-HLA-DR mAb L243 and (as a negative control) the anti-MHC class I mAb w6/32. The anti-CD3 directed mAb OKT3 (2 µg/ml, 50 µl/well) attached to plastic served as the positive control for IFN-γ secretion determined by enzyme-linked immunosorbent assay (ELISA; R & D Systems, Wiesbaden, Germany).
Flow cytometry
All antibodies were obtained from Beckman/Coulter, Krefeld, Germany, except for the anti-TCR ζ-chain mAb (clone 6B10.2) coupled to FITC, which was purchased from Santa Cruz Biotechnology, Heidelberg, Germany. T cells were stained with anti-CD4 (clone SFCI12T4D11) coupled to energy-coupled dye (ECD) and anti-CD8 mAb (clone B9.11) coupled to R-PE-cyanin 5 (PC5). TCR ζ-chain detection was carried out using the IntraPrep® permeabilization reagent (Beckman/Coulter) and the anti-TCR ζ-chain mAb 2H2D9 coupled to PE, or the clone 6B10.2 (see above). Appropriate murine isotype matched non-specific mAbs served as controls. Staining was visualized using a Coulter® Epics® XL flow cytometer with the XL software version 2.1.
Results
Expression of ZAP-70, p56lck, p59fyn and CD3ζ in PBMC from healthy donors and tuberculosis patients
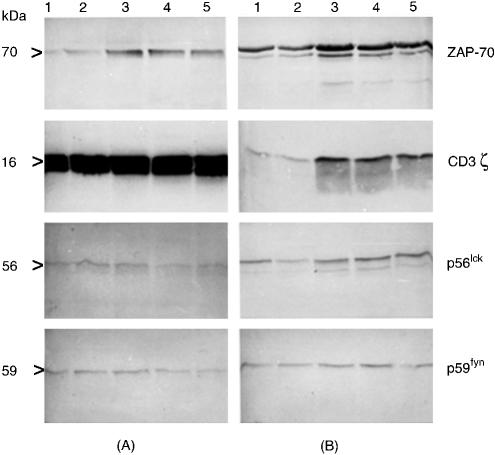
Peripheral blood mononuclear cells of five age-matched healthy controls and five patients suffering from tuberculosis were analysed in Western blots with respect to the expression of ZAP-70, CD3ζ, p56lck and p59fyn. As controlled by flow-cytometric analysis, CD3ε positive cells varied slightly between 60 and 70% of total cells. Samples corresponding to 2 × 106 CD3ε positive cells were applied per lane, to guarantee a comparable signal ratio for the investigated proteins. As shown in Fig. 1, protein expression levels of ZAP-70, p56lck and p59fyn appear to be similar in the patient and the control group whereas CD3ζ protein expression is significantly reduced in the tuberculosis patients compared to the controls.
Figure 1.
Reduced CD3ζ-chain expression in peripheral PBMCs from patients with tuberculosis. Freshly isolated PBMCs of five healthy donors and five patients with tuberculosis were adjusted to 2 × 106 CD3ε positive staining cells as determined by flow cytometry before Western blot analysis. Detection of individual T-cell signalling molecules was performed with specific mAbs as described in the material and methods section. (a) Results obtained from PBMC of five different age-matched healthy donors (lanes 1–5); (b) results of five different tuberculosis patients (lanes 1–5). The expression level of ZAP-70, p56lck and p59fyn appeared to be similar in both groups. In contrast, CD3ζ protein expression was significantly reduced in patients with tuberculosis. The additional band in p56lck or p59fyn was observed in some samples obtained from patients with tuberculosis. The nature of this double band has not yet been defined, it may be associated with the activation status of peripheral T cells from patients with tuberculosis: Upon activation, lck or fyn are enriched in membrane lipid rafts, phosphorylate TCR invariant chains and associate with ZAP-70. These different configurations of lck or fyn (e.g. non-associated or associated with ZAP-70) may provide a reason for a different behaviour in SDS–PAGE analysis and also in Western blot.
Expression of CD3ε and CD3ζ in normal lymphoid tissue
Expression of CD3ε and the CD3ζ-chain was investigated in normal lymphoid tissue (n = 10) by immunoenzymatic staining of serial sections and by double immunofluorescence analyis of hyperplastic tonsillar tissue specimens. As shown in Fig. 2(a,b), for example, CD3ε and CD3ζ expression appeared to be equally intense, expressed on an equal number of cells and also localized to the same area in this tissue. This observation was substantiated by double immunofluorescence analyses (Fig. 3a). All CD3ζ expressing cells in tonsil tissue (red fluorescence, Fig. 3b) coexpress CD3ε (green fluorescence, Fig. 3a) as demonstrated by the double exposure of both fluorochromes (yellow fluorescence, Fig. 3c). Depending on the localization in the tissue, this congruent expression was up to 100% in the T-cell rich mantel zone of germinal centres whereas small numbers of CD3ε positive and CD3ζ negative cells could be discerned adjacent to the T-cell zone or in close proximity to the endothelium. CD3ε negative and CD3ζ positive cells were rarely observed (one in Fig. 3c).
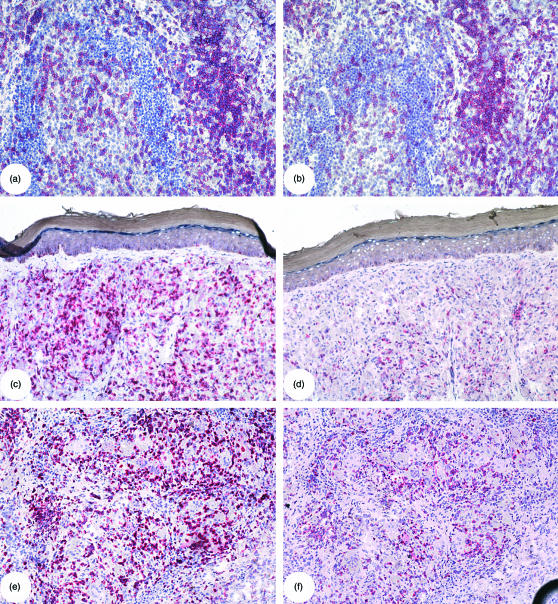
Figure 2.
Reduced in situ CD3ζ expression in tissues with mycobacterial infection. Serial sections of a hyperplastic tonsil (a, b), a skin biopsy of a lepromatous leprosy patient (c, d) and a lymph node biopsy of a sarcoidosis patient (e, f) were stained for the presence of CD3ε (a, c, e) and CD3ζ (b, d, f) with the APAAP technique. In hyperplastic tonsil, the expression of CD3ε (a) and CD3ζ (b) was localized to the same region, expressed by an equal number of cells, and was of similar intensity. In the representative example of M. leprae-infected tissue, CD3ε (c) expression was distributed uniformly throughout the granuloma, whereas CD3ζ (d) expression was greatly reduced and limited to a few isolated cells throughout the granuloma. In granulomatous tissue of a sarcoid lymph node, CD3ε expression (e) was detected as intense staining of cells localized around epithelioid cells in the granuloma. CD3ζ staining (f) was not as intense as CD3ε staining and appeared not to stain as many cells but was localized to the same areas in the tissue.
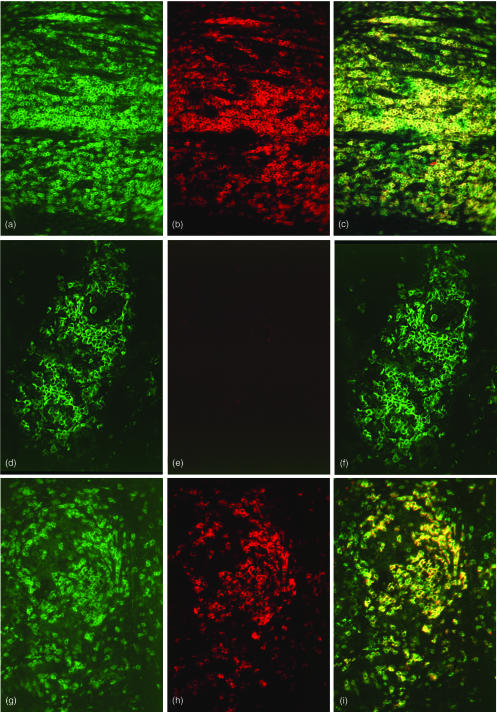
Figure 3.
Double immunofluorescence analysis of CD3ε and CD3ζ expression in situ. Hyperplastic tonsil (a, b, c), a tuberculoid leprosy skin biopsy (d, e, f) and a sarcoid lymphnode (g, h, i) were stained for CD3ε (green fluorescence, a, d, g), CD3ζ (red fluorescence, b, e, h) and double exposed for colocalization of both CD3ε and CD3ζ (yellow fluorescence, c, f, i). In hyperplastic tonsil, CD3ζ expression colocalized 100% with CD3ε expression. In the tissue area chosen close to endothelium in this example, several cells exhibited CD3ε positivity and CD3ζ negativity. In an example of tuberculoid leprosy, CD3ε positive cells were detected throughout the granuloma (d) whereas CD3ζ expression was barely visible (e), resulting in no detection of CD3ε CD3ζ double positive cells (no yellow fluorescence (f). The sarcoid lymph node lesion exhibited CD3ε (g) and CD3ζ (h) expression to approximately the same extent. All CD3ζ cells were CD3ε positive (yellow fluorescence) and only a minor number of cells were CD3ε positive CD3ζ negative (i, green fluorescence).
CD3ε and CD3ζ expression in mycobacterially infected tissue
Serial sections of different tissue specimens infected with M. leprae or M. tuberculosis were stained immunoenzymatically for CD3ε and CD3ζ expression. In 10 different cases of tuberculoid and 11 different cases of lepromatous leprosy, a striking reduction of CD3ζ positive cells was found as compared to CD3 positive cells (Fig. 2c,d). In some cases, no CD3ζ positive cells could be detected in granulomatous lesions. Reduced CD3ζ expression in comparison to CD3ε appeared to be even more evident in double immunofluorescence studies using the same conditions as for the tonsillar tissue (Fig. 3d). Substantial numbers of CD3ε positive cells were observed within the lesions (green fluorescence, Fig. 3d), whereas CD3ζ positive cells were scarcely discernible because of reduced intensity in staining and reduced numbers of positive cells (red fluorescence, Fig. 3e). Thus, no double positive cells (absent yellow fluorescence, only green fluorescence, Fig. 3f) in the double exposures could be detected. With respect to the different clinical forms of leprosy investigated, no evident differences could be observed between the tuberculoid and lepromatous forms of leprosy concerning reduced CD3ζ expression.
The analysis of M. tuberculosis infected tissues was performed on eight different lung tissues. Since these specimens were not enriched with cellular constituents, the difference between CD3ε and CD3ζ positive cells was not as evident as in specimens obtained from patients with leprosy. However, there was a clear trend to decreased expression of CD3ζ compared to CD3ε (data not shown).
CD3ε and CD3ζ expression in sarcoid granulomas
As a comparison to mycobacterially infected tissue, sarcoid granulomatous lesions, a disease in which mycobacteria are presumably not involved, were stained for CD3ε and CD3ζ expression. Immunoenzymatic staining of serial sections of five sarcoid lymphnodes showed that CD3ζ expression was reduced as compared to CD3ε expression (Figs 2e,f) with respect to the intensity of the staining, however, the localization of positive signals and the number of cells stained for either CD3ε or CD3ζ appeared to be approximately the same. Double immunofluorescence analysis indeed resulted in a similar image with respect to the presence of double positive cells, comparable to normal hyperplastic tonsil tissue. However, some CD3ε positive CD3ζ negative cells were present, but the majority of CD3ε positive cells stained also positive for CD3ζ (Fig. 3g–i). This observation appears to be associated with the localization of T cells within the granuloma. T-cell rich zones encircling an epithelioid cell granuloma contained predominantly CD3ε positive CD3ζ positive cells. In contrast, some CD3ε positive CD3ζ negative cells could be detected in the center of the epithelioid granuloma and on the edge of the T-cell zone. Additional analysis of three sarcoid granulomatous lesions in skin biopsies showed no reduction of CD3ζ expression as compared to CD3ε expression.
To summarize, there was a significant difference in the level of CD3ζ-chain expression between healthy PBMC, non-infected tissues or sarcoid tissue and mycobacterially infected tissues. No significant difference in CD3ζ-chain expression relative to CD3ε expression could be observed in healthy PBMC, tonsils and in sarcoid granulomas.
Enhanced immune effector functions in the presence of IL-2
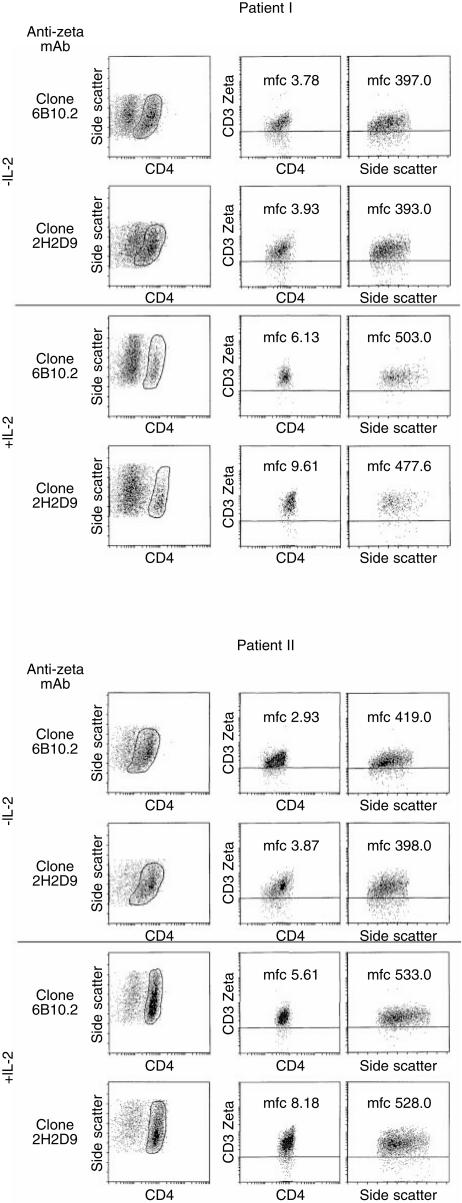
Because no viable tissue was available from patients suffering from leprosy, we took advantage of surgically resected pulmonary granuloma lesions obtained from two patients with tuberculosis. Freshly isolated CD4+ GAL were either cultured with or without IL-2 (3 days) followed by intracellular TCR ζ detection (Fig. 4). CD4+ GAL cultured in the presence of IL-2 exhibited a stronger ζ-chain expression as determined by mean fluorescence intensity. These T-cell populations were also evaluated for differences in immune effector functions as determined by IFN-γ secretion upon antigen exposure. Both CD4+ T-cell lines have been identified to recognize M. tuberculosis-associated antigens in the context of HLA-DR (manuscript in preparation). CD4+ GAL cultured in the presence of IL-2 showed considerably higher IFN-γ secretion as compared to freshly isolated CD4+ GAL without IL-2 (Table 1).
Figure 4.
Enhanced TCR ζ−chain expression in M. tuberculosis granuloma-associated CD4+ T lymphocytes induced by IL-2. GAL were freshly isolated from pulmonary lesions, as described in detail in the Materials and Methods section, cultured for 3 days in the presence or absence of IL-2 (1000 IU/ml), gated on CD4+ T cells and stained for TCR ζ chain expression by flow cytometry using two different mAb as indicated. Differences in mean fluorescence channel (m.f.c.) intensity. CD4 + T-cells correspond to effector cells described in Table 1. ss=side scatter.
Table 1.
Enhanced immune effector functions in CD4+ T cells from pulmonary tuberculosis granuloma lesions in the presence of IL-2
| Patient #1 | Patient #2 | |||
|---|---|---|---|---|
| Culture in the presence of IL-2 | − | + | − | + |
| APC alone IFN-γ (pg/ml/24 hr) | 0 | 0 | 0 | 0 |
| APC+GAL IFN-γ (pg/ml/24 hr) | 0 | 0 | 192 | 326 |
| APC+M. tub.IFN-γ (pg/ml/24 hr) | 0 | 0 | 0 | 0 |
| APC+M. tub. + GALIFN-γ (pg/ml/24 hr) | 769 | 2389 | 1032 | 3467 |
| APC+M. tub. + GAL +anti-HLA-DR IFN-γ (pg/ml/24 hr) | 0 | 36 | 276 | 233 |
| APC+M. tub. + GAL +anti-MHC class I IFN-γ (pg/ml/24 hr) | 548 | 1906 | n.d. | n.d. |
| GAL+anti-CD3 IFN-γ (pg/ml/24 hr) | 3695 | 2091 | > 20 000 | > 20 000 |
Freshly isolated (2 days) granuloma associated CD4+ lymphocytes (GAL) were exposed for 24 hr to HLA-DR matched macrophages (antigen-presenting cells, APC) which had been infected with M. tuberculosis (M. tub.). The antigen-specific and HLA-DR-restricted CD4+ T-cell response was confirmed by blocking with an anti-MHC class II (DR)-specific mAb. Blocking was not observed with the anti-MHC class I-directed mAb. Crosslinking the TCR with anti-CD3 served as the positive control. CD4+ GAL correspond to the T-cell population shown in Fig. 4, which were either short-term (2 days) expanded in medium supplemented only with IL-7, or alternatively in the presence of high dose (1000 IU/ml) IL-2. n.d. = not determined.
Discussion
The salient finding of this report is the ex vivo analysis of reduced CD3ζ TCR protein expression in a comprehensive panel of human tissues infected with mycobacteria. In contrast, reduced CD3ζ TCR protein expression appears not to be present in hyperplastic tonsillar tissue containing T cells that have encountered antigenic stimulation, or in sarcoid lymph nodes, representing a granulomatous disease of unknown aetiology. Reduced TCR ζ-chain expression was observed both systemically in the peripheral blood of tuberculosis patients, as well as locally in the granulomatous lesions of tissue infected with M. leprae, but not in the hyperplastic tonsils nor in the sarcoid lymphnodes.
Reduced CD3ζ-chain expression has been described in situ as well as in the systemic circulation in tumour-bearing patients,23–25 in patients with systemic lupus erythematosus,26 patients suffering from rheumatoid arthritis,27 in HIV-infected individuals,28 and has recently been shown in the periphery of lepromatous leprosy patients by Western blot analysis.30 However, up to now, the in situ situation concerning TCRζ-chain expression has not been characterized in inflammatory diseases. The reduced protein expression of this crucial TCR signalling molecule appears to correlate with immune dysfunction. In the case of T lymphocytes recovered from human tumours or from the peripheral circulation of patients with advanced cancer, reduced CD3ζ expression was correlated with a decreased Ca2+ flux as well as impaired kinase activity.33 In the context of mycobacterial infection, the reduced expression of the CD3ζ-chain is also likely to play an important role in influencing T-cell effector functions. Because CD3ζ expression is essential for TCR signalling and eliciting T-cell effector functions by linking the TCR complex phosphorylation to cytosolic events through association with and activation of ZAP-70,34 this reduction could result in altered signal transduction and reduced T-cell effector functions, possibly culminating in the T-cell anergy often associated with mycobacterial infections. Indeed, freshly isolated M. tuberculosis-specific and HLA-DR-restricted CD4+ T cells from pulmonary granuloma lesions obtained from patients with tuberculosis showed enhanced IFN-γ secretion (Table 1) and a stronger TCR ζ-chain signal (Fig. 4) in the presence of IL-2. This notion has been confirmed in a recent study: peripheral T cells from patients with a M. tuberculosis infection which exhibited a negative delayed-type hypersensitivity (DTH)-response (‘anergy’) produced IL-10, no IFN-γ and showed a defective phosphorylation pattern of the TCR ζ-chain.14
The mechanism for reduced CD3ζ-chain expression following T-cell triggering is poorly understood. Previous reports have demonstrated that insufficient TCR triggering upon recognition of antagonists or partial agonist peptides may lead to a unique pattern of CD3ζ-chain phosphorylation and to insufficient activation of the ZAP-70 kinase.35 Additionally, engagement of the TCR with its ligands leads to reduced CD3 expression, presumably resulting from decreased CD3ζ-chain expression.36 However, these models are based on the fact that specific TCR–MHC/peptide engagement is required to ensure CD3ζ downregulation. Because the number of antigen-specific T cells in patients with tuberculosis may be low, it is unlikely that a general decreased CD3ζ-chain expression in the systemic circulation in patients with mycobacterial infection or even in patients with cancer,23–25 stems from T-cell populations which have encountered their specific ligands. Thus, impaired T-cell function due to impaired CD3ζ-chain expression may be induced by (a) systemic factor(s) affecting the majority of circulating T cells. The search for these compounds has not been very successful up to now. We have tested human or viral IL-10 as well as TFG-β for the capacity to affect CD3ζ-chain expression in peripheral blood cells either from healthy blood donors or from patients with tuberculosis. Neither cytokine resulted in reduced TCR ζ-chain expression. In addition, ‘chronic inflammation’ which may also be present in lesions from patients with sarcoidosis did not lead to ablated CD3ζ-chain expression. The reduction of CD3ζ-chain expression has also been correlated with hydrogen peroxide production of stimulated macrophages.37 In the experiments described, lipopolysaccharide activation of macrophages resulted in reduction of CD3ζ protein expression, which could be inhibited by the addition of catalase. However, in a different experimental setup with live M. tuberculosis we could not inhibit the mycobacterially induced effect on CD3ζ expression by the addition of catalase, arguing for the influence of other factors (data not shown).
An alternative model for TCR dynamics suggests that the TCR ζ-chain may physically dissociate from the rest of the TCR–CD3 complex, including CD3ε upon TCR triggering.38 Although the ζ-chain represents a prerequisite to maintain stable CD3 cell surface expression, the TCR may be freely exchanged with newly synthesized CD3ζ molecules.39 CD3ζ-chains are ubiquinated in a tyrosine-kinase dependent manner after TCR-ligation and subjected to proteolysis. Alternatively, different rates of TCR ζ-chain kinetics as compared with other TCR components also exist, presumably because of retention of intracellular TCR components rather than TCR internalization.38 These data are particularly interesting in the context of the immunohistology data in this report: The TCR–CD3ε component was still detectable in the absence (or reduced level) of ζ-chain expression. Of note, ζ-chain downmodulation may not only indicate ‘anergy’, but also protection from overstimulation, a form of ‘reversible tolerance’ if a vast amount of antigen would potentially be deleterious to T-cell survival.38–40
The loss of signalling proteins is reversible but requires appropriate stimulation, e.g. IL-241–42 or CD3 and CD28 cross-linking.43 In these cases, however, restoration of CD3ζ expression alone was not sufficient to restore specific cytolytic T-cell activity against the nominal target cells. It remains to be investigated whether restoration of CD3ζ expression in T cells affects bacterial survival in cells infected with mycobacteria. Interestingly however, clinical studies have shown that adjunctive IL-2 therapy in multidrug resistant tuberculosis may enhance the antimicrobial response.44,45
In conclusion, we have been able to show that mycobacterial infection significantly reduces the protein expression of the CD3ζ-chain. Because of the essential role of this signalling molecule in eliciting T-cell effector functions, this reduction of CD3ζ protein expression may represent a novel mechanism by which mycobacteria evade cellular immune surveillance.
Acknowledgments
The expert technical assistance of K. Freitag and M. Hahn is gratefully acknowledged. This project was supported in part by grants from the Deutsche Forschungsgemeinschaft (SFB 367/C1 and SFB 490/C4).
Abbreviations
- APAAP
alkaline phosphatase anti-alkaline phosphatase
- BSA
bovine serum albumin
- mAb
monoclonal antibody
- PBMC
peripheral blood mononuclear cells
- TBS
Tris-buffered saline
- TCR
T-cell receptor.
References
- 1.Beckman EM, Melian A, Behar SM, et al. CD1c restricts responses of mycobacteria-specific T-cells. Evidence for antigen presentation by a second member of the human CD1 family. J Immunol. 1996;157:2795–803. [PubMed] [Google Scholar]
- 2.D'Sousa CD, Cooper AM, Frank AA, Orme IM. The role of CD8 cells in acquired immunity and pulmonary tuberculosis in the mouse model. Molecular Methods and Immunological Aspects, Keystone Symposia. 1998;47 Abstract. [Google Scholar]
- 3.Flynn JL, Goldstein MM, Triebold KJ, Koller B, Bloom BR. Major histocompatibility complex class I-restricted T-cells are required for resistance to Mycobacterium tuberculosis infection. Proc Natl Acad Sci USA. 1992;89:12013–7. doi: 10.1073/pnas.89.24.12013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jullien D, Sieling PA, Uyemura K, Mar ND, Rea TH, Modlin RL. IL-15, an immunomodulator of T-cell responses in intracellular infection. J Immunol. 1997;158:800–6. [PubMed] [Google Scholar]
- 5.Kaufmann SHE, Ladel CH. Role of T-cell subsets in immunity against intracellular bacteria: experimental infections of knock-out mice with Listeria monocytogenes and Mycobacterium bovis BCG. Immunobiology. 1994;191:509–19. doi: 10.1016/S0171-2985(11)80457-2. [DOI] [PubMed] [Google Scholar]
- 6.Porcelli S, Morita CT, Brenner MB. CD1b restricts the response of human CD4− 8− T lymphocytes to a microbial antigen. Nature. 1992;260:593–7. doi: 10.1038/360593a0. [DOI] [PubMed] [Google Scholar]
- 7.Tanaka Y, Morita T, Tanaka Y, Nieves E, Brenner MB, Bloom BR. Natural and synthetic nonpeptide antigens recognized by human γδ T-cells. Nature. 1995;375:155–8. doi: 10.1038/375155a0. [DOI] [PubMed] [Google Scholar]
- 8.Tsugaguchi K, Balaji KN, Boom WH. CD4+ αβ T-cell and γδ T-cell responses to Mycobacterium tuberculosis, similarities and differences in antigen recognition, cytotoxic effector function, and cytokine production. J Immunol. 1995;154:1786–96. [PubMed] [Google Scholar]
- 9.Zhu X, Venkataprasad N, Thangeraj HS, Hill M, Singh M, Invayi J, Vordermeier HM. Functions and specificity of T-cells following nucleic acid vaccination of mice against Mycobacterium tuberculosis. J Immunol. 1997;158:5921–6. [PubMed] [Google Scholar]
- 10.Rook GAW, Stanford JL. The Koch phenomenon and the immunopathology of tuberculosis. In: Shinnick TM, editor. Tuberculosis Current Topics in Microbiology and Immunology. New York: Springer; 1996. pp. 239–62. [DOI] [PubMed] [Google Scholar]
- 11.Wright SD, Silverstein SC. Receptors for C3b and C3bi promote phagocytosis but not the release of toxic oxygen from human phagocytes. J Exp Med. 1983;158:2016–23. doi: 10.1084/jem.158.6.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goren MB, D'Arcy Hart P, Young MR. Ammonia inhibits phagosome–lysosome fusion in macrophages. Nature. 1980;286:79–80. doi: 10.1038/286079a0. [DOI] [PubMed] [Google Scholar]
- 13.Chan J, Fan X-D, Hunter SW, Brennan PJ, Bloom BR. Lipoarabinomannan, a possible virulence factor involved in persistence of Mycobacterium tuberculosis within macrophages. Infect Immun. 1991;59:1755–61. doi: 10.1128/iai.59.5.1755-1761.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Boussiotis VA, Tsai EY, Yunis EJ, et al. IL-10-producing T cells suppress immune responses in anergic tuberculosis patients. J Clin Invest. 2000;105:1317–25. doi: 10.1172/JCI9918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Toossi Z, Gogate P, Shiratsuchi H, Young T, Ellner JJ. Enhanced production of TGF-β by blood monocytes from patients with active tuberculosis and presence of TGF-β in tuberculous granulomatous lung lesions. J Immunol. 1995;154:465–73. [PubMed] [Google Scholar]
- 16.VanHeyningen TK, Collins HL, Russell DG. IL-6 produced by macrophages infected with Mycobacterium species suppresses T-cell responses. J Immunol. 1997;158:330–7. [PubMed] [Google Scholar]
- 17.Hudrisier D, Kessler B, Valitutti S, Horvath C, Cerottini JC, Luescher IF. The efficiency of antigen recognition by CD8+ CTL clones is determined by the frequency of serial TCR engagement. J Immunol. 1998;161:553–62. [PubMed] [Google Scholar]
- 18.Valitutti S, Müller S, Cella M, Padovan E, Lanzavecchia A. Serial triggering of many T-cell receptors by a few peptide–MHC complexes. Nature. 1995;375:148–51. doi: 10.1038/375148a0. [DOI] [PubMed] [Google Scholar]
- 19.Appleby MW, Gross JA, Cooke MP, Levin SD, Qian X, Perlmutter RM. Defective T-cell receptor signaling in mice lacking the thymic isoform of p59fyn. Cell. 1992;70:751–63. doi: 10.1016/0092-8674(92)90309-z. [DOI] [PubMed] [Google Scholar]
- 20.Cooke MP, Abraham KM, Forbush KA, Perlmutter RM. Regulation of T-cell receptor signaling by a src family protein-tyrosine kinase (p59fyn) Cell. 1991;65:281–91. doi: 10.1016/0092-8674(91)90162-r. [DOI] [PubMed] [Google Scholar]
- 21.Klausner RD, Samelson LE. T-cell antigen receptor activation pathways: the tyrosine kinase connection. Cell. 1991;64:875–8. doi: 10.1016/0092-8674(91)90310-u. [DOI] [PubMed] [Google Scholar]
- 22.Koretzky GA, Picus J, Schultz T, Weiss A. Tyrosine phosphatase CD45 is required for T-cell antigen receptor and CD2-mediated activation of a protein tyrosine kinase and interleukin 2 production. Proc Natl Acad Sci USA. 1991;88:2037–41. doi: 10.1073/pnas.88.6.2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Buggins AGS, Hirst WJR, Pagliuca A, Mufti GJ. Variable expression of CD3-zeta and associated protein tyrosine kinases in lymphocytes from patients with myeloid malignancies. Br J Haematol. 1998;100:784–92. doi: 10.1046/j.1365-2141.1998.00654.x. 10.1046/j.1365-2141.1998.00654.x. [DOI] [PubMed] [Google Scholar]
- 24.Finke JH, Zea AH, Stanley J, et al. Loss of T-cell receptor zeta-chain and p56lck in T-cells infiltrating human renal cell carcinoma. Cancer Res. 1993;53:5613–6. [PubMed] [Google Scholar]
- 25.Matsuda M, Petersson M, Lenkei R, Taupin JL, Magnussen L, Mellstedt H, Andersson P, Kiessling R. Alterations in the signal-transducing molecules of T-cells and NK cells in colorectal tumor-infiltrating, gut mucosal and peripheral lymphocytes: correlation with the stage of the disease. Int J Cancer. 1995;61:765–72. doi: 10.1002/ijc.2910610605. [DOI] [PubMed] [Google Scholar]
- 26.Liossis S-NC, Ding XZ, Dennis GJ, Tsokos GC. Altered pattern of TCR/CD3-mediated protein-tyrosyl phosphorylation in T-cells from patients with systemic lupus erythematosus. Deficient expression of the T-cell receptor zeta chain. J Clin Invest. 1998;101:1448–57. doi: 10.1172/JCI1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Matsuda M, Ulfgren A-K, Lenkei R, et al. Decreased expression of signal-transducing CD3ζ chains in T-cells from the joints and peripheral blood of rheumatoid arthritis patients. Scand J Immunol. 1998;47:254–62. doi: 10.1046/j.1365-3083.1998.00296.x. 10.1046/j.1365-3083.1998.00296.x. [DOI] [PubMed] [Google Scholar]
- 28.Trimble LA, Liebermann J. Circulating CD8 T lymphocytes in human immunodeficiency virus-infected individuals have impaired function and downmodulate CD3ζ, the signaling chain of the T-cell receptor complex. Blood. 1998;91:585–94. [PubMed] [Google Scholar]
- 29.Richter E, Greinert U, Kirsten D, et al. Assessment of mycobacterial DNA in cells and tissues of mycobacterial and sarcoid lesions. Am J Respir Crit Care Med. 1995;153:375–80. doi: 10.1164/ajrccm.153.1.8542146. [DOI] [PubMed] [Google Scholar]
- 30.Zea AH, Ochoa MT, Ghosh P, et al. Changes in expression of signal transducing proteins in T lymphocytes of patients with leprosy. Infect Immun. 1998;66:499–504. doi: 10.1128/iai.66.2.499-504.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ridley DS, Jopling WH. Classification of leprosy according to immunity. A five group system. Int J Leprosy. 1996;34:255–73. [PubMed] [Google Scholar]
- 32.Cordell JL, Fallini B, Erber WN, et al. Immunoenzymatic labeling of monoclonal antibodies using immune complexes of alkaline phosphatase and monoclonal anti-alkaline phosphatase (APAAP complexes) J Histochem Cytochem. 1984;32:219–29. doi: 10.1177/32.2.6198355. [DOI] [PubMed] [Google Scholar]
- 33.Reichert TE, Rabinowich H, Johnson JT, Whiteside TL. Mechanisms responsible for signaling and functional defects. J Immunother. 1998;21:295–306. doi: 10.1097/00002371-199807000-00007. [DOI] [PubMed] [Google Scholar]
- 34.Weiss A, Littmann DR. Signal transduction by lymphocyte antigen receptors. Cell. 1994;76:263–74. doi: 10.1016/0092-8674(94)90334-4. [DOI] [PubMed] [Google Scholar]
- 35.Kersh GJ, Allen PM. Essential flexibility in the T-cell recognition of antigen. Nature. 1996;380:495–8. doi: 10.1038/380495a0. [DOI] [PubMed] [Google Scholar]
- 36.Valitutti S, Müller S, Salio M, Lanzavecchia A. Degradation of T-cell receptor (TCR)–CD3-ζ complexes after antigenic stimulation. J Exp Med. 1997;185:1859–64. doi: 10.1084/jem.185.10.1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kono K, Salazar OF, Petersson M, et al. Hydrogen peroxide secreted by tumor-derived macrophages down-modulates signal transducing zeta molecules and inhibits tumor-specific T-cell and natural killer cell mediated cytotoxicity. Eur J Immunol. 1996;26:1308–13. doi: 10.1002/eji.1830260620. [DOI] [PubMed] [Google Scholar]
- 38.Liu H, Rhodes M, Wiest DL, Vignali DA. On the dynamics of TCR. CD3 complex cell surface expression and downmodulation. Immunity. 2000;13:665–75. doi: 10.1016/s1074-7613(00)00066-2. [DOI] [PubMed] [Google Scholar]
- 39.Dietrich J, Kastrup J, Lauritsen JP, Menne C, von Bulow F, Geisler C. TCRzeta is transported to and retained in the Golgi apparatus independently of other TCR chains: implications for TCR assembly. Eur J Immunol. 1999;29:1719–28. doi: 10.1002/(SICI)1521-4141(199905)29:05<1719::AID-IMMU1719>3.0.CO;2-M. 10.1002/(sici)1521-4141(199905)29:05<1719::aid-immu1719>3.3.co;2-d. [DOI] [PubMed] [Google Scholar]
- 40.Cai Z, Kishimoto H, Brunmark A, Jackson MR, Peterson PA, Sprent J. Requirements for peptide-induced T cell receptor downregulation on naive CD8+ T cells. J Exp Med. 1997;185:641–51. doi: 10.1084/jem.185.4.641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Guarini A, Riera L, Cignetti A, Montacchini L, Massaia M, Foa R. Transfer of the interleukin-2 gene into human cancer cells induces specific antitumor recognition and restores the expression of CD3/T-cell receptor associated signal transduction molecules. Blood. 1997;89:212–8. [PubMed] [Google Scholar]
- 42.Yoong KF, Adams DH. Interleukin 2 restores CD3-zeta chain expression but fails to generate tumour-specific lytic activity in tumour-infiltrating lymphocytes derived from human colorectal hepatic metastases. Br J Cancer. 1998;77:1072–81. doi: 10.1038/bjc.1998.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Renner C, Ohnesorge S, Held G, Bauer S, Jung W, Pfitzenmaier JP, Pfreundschuh M. T-cells from patients with Hodgkin's disease have a defective T-cell receptor zeta chain expression that is reversible by T-cell stimulation with CD3 and CD28. Blood. 1996;88:236–41. [PubMed] [Google Scholar]
- 44.Johnson BJ, Bekker LG, Rickman R, et al. rhuIL-2 adjunctive therapy in multidrug resistant tuberculosis: a comparison of two treatment regimens and placebo. Tuber Lung Dis. 1997;78:195–203. doi: 10.1016/s0962-8479(97)90026-5. [DOI] [PubMed] [Google Scholar]
- 45.Johnson BJ, Estrada I, Shen Z, Ress S, Willcox P, Colston MJ, Kaplan G. Differential gene expression in response to adjunctive recombinant human interleukin-2 immunotherapy in multidrug-resistant tuberculosis patients. Infect Immun. 1998;66:2426–33. doi: 10.1128/iai.66.6.2426-2433.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]