Abstract
Expression of CD154/CD40 ligand (CD154/CD40L), an important molecular component of CD4+ T-cell help, can be triggered by T-cell receptor (TCR) stimulation. Dephosphorylation of the transcriptional element Nuclear Factor of Activated T cells-1 (NFAT1) is a critical activation step in the TCR-initiated signal transduction cascade which promotes CD154/CD40L expression. Cyclosporin A (CsA), which interferes with NFAT1 activation, has been shown to be an effective inhibitor of TCR-triggered CD154/CD40L expression by resting T cells. We now report that recombinant interleukin-2 (rIL-2) is also capable of inducing CD154/CD40L on CD4+ T lymphoblasts via a pathway triggered independently of the CD3/TCR receptor complex. Recombinant IL-2-mediated CD154/CD40L expression, in contrast to that triggered by CD3/TCR stimulation, is only partially inhibited by CsA. The capacity of rIL-2 to induce CD154/CD40L expression by T lymphoblasts also extends to a restricted number of cytokines sharing the cytokine receptor common γ chain, including IL-15, and, to a lesser extent, IL-7, but not IL-4. A similar CsA-resistant CD154/CD40L induction pathway can be triggered in primary T cells by the combination of anti-CD3 stimulation and recombinant lymphokines. In contrast to T lymphoblasts, the CsA-resistant CD154/CD40L induction in primary lymphocytes can be efficiently triggered by multiple cytokines which bind the common γ chain receptor family. The data outline a novel pathway of CD154/CD40L induction which is, at least in part, independent of NFAT1 and resistant to CsA. A more complete understanding of the mechanisms governing CD154/CD40L expression may facilitate the rational design of specifically targeted immunotherapeutic agents.
Introduction
CD154/CD40 ligand (CD154/CD40L) is a critical molecular component of CD4+ T-cell help. There is extensive evidence supporting a role for CD154/CD40L in the maturation of both humoral and cellular immune responses to antigenic challenge (reviewed in refs. 1–3). Although CD154/CD40L is not found on resting CD4+ T cells, its expression can be induced by T-cell receptor (TCR) stimulation. Triggering stimuli include antibodies directed against the CD3 complex,1–4 TCR engagement during presentation of antigens5 or superantigens,6 and mitogen exposure.7–14
Two independent lines of investigation have implicated the transcriptional element Nuclear Factor of Activated T cells (NFAT) as a key determinant of CD154/CD40L induction. First, the human CD154/CD40L gene has two NFAT binding sites within its promoter region.15 Both sites mediate non-redundant promoter function in reporter gene assays.15 Second, TCR-mediated induction of CD154/CD40L is substantially inhibited by cyclosporin A (CsA).16–19 The inhibitory activity of CsA is governed by its capacity to alter the function of the cellular phosphatase calcineurin. Elevated levels of intracellular calcium initiate calcineurin activation, which docks with and removes phosphate groups from N-terminal residues of NFAT (reviewed in refs. 19, 20). The dephosphorylated NFAT is then transported into the nucleus, where, along with the companion transcriptional element AP-1, it functions as a transactivator of selected genes,19,21–23 including CD154/CD40L. Although the inhibitory activity of CsA may in some cases extend to transcriptional events mediated by Rel family members,24 its inhibition of CD154/CD40L induction has been ascribed to interference with the functional activation of NFAT1, the most abundant NFAT isoform found in resting T cells.19,25–27
Despite unequivocal evidence tying CD154/CD40L induction to NFAT1, NFAT1−/− knockout mice have been shown to express CD154/CD40L, albeit at reduced levels,28 suggesting the existence of NFAT1-independent induction pathway(s) for CD154/CD40L. In the present report, we provide evidence for a novel cytokine-triggered CD154/CD40L induction pathway which is, at least in part, resistant to the inhibitory activity of CsA and independent of NFAT1.
Materials and methods
Cells and cell lines
The human T-cell line Jurkat E6-1 and murine hybridomas OKT3 and 5c8 were obtained from the American Type Culture Collection (Manassas, VA).
Peripheral blood mononuclear cells (PBMC) were isolated from whole blood obtained from healthy human donors by density gradient sedimentation, as previously described.29 After plastic adherence, primary T-cell populations were depleted of contaminating monocytes and B cells by incubation with Lympho-Kwik T (One Lambda, Canoga Park, CA) at 37° for 20 min, in accordance with the manufacturer's protocol. Cells were cultured in RPMI-1640 medium (GibcoBRL, Grand Island, NY) supplemented with l-glutamine, penicillin/streptomycin, and 10% fetal bovine serum (GibcoBRL) (complete medium). T lymphoblasts were generated from stimulation of human PBMC. After plastic adherence, PBMC were placed in a 48-well plate (Costar, Corning, NY) at a density of 2 × 105 cells per well in the presence of 50 ng/ml staphylococcal enterotoxin B (SEB) (Sigma, St Louis, MO). After a 6-day incubation, T-cell blasts were harvested, sedimented over Ficoll-Paque Plus (Amersham Pharmacia, Uppsala, Sweden), and washed. Contaminating monocytes and B cells were lysed by exposure to Lympho-Kwik T (One Lambda). In some experiments, T-cell lymphoblasts were partially depleted of CD8+ T cells by exposure to anti-CD8-coated microbeads (Miltenyi Biotec, Auburn, CA), followed by passage over a MS+ column (Miltenyi Biotec) in a magnetic field. Lymphoblasts which failed to bind to the column were used as responder cells after extensive washing. T lymphoblast responder cell populations were found to be > 98% CD3-positive in routine preparations.
Proliferation assay
Responder T lymphoblasts were plated at 2 × 105 cells per well in a 96-well plate (Becton Dickinson Labware, Franklin Lake, NY) in the presence of serial dilutions of Proleukin/recombinant interleukin-2 (rIL-2; Chiron, Emeryville, CA), rIL-4, rIL-7, rIL-12, or rIL15 (PeproTech, Rocky Hill, NJ). The final volume in all wells was 200 µl. Each well was pulsed with 0·5 µCi [3H]thymidine (ICN, Costa Mesa, CA) during the last 16 hr of a 72-hr culture period and harvested onto a glass fibre filter. [3H]thymidine incorporation was assessed by a Betaplate scintillation counter (Wallac, Gaithersburg, MD).
CD154/CD40L expression by FACS analysis
Responder populations were plated in a 48-well plate (1·0 × 106 cells per well for primary T cells, 0·5 × 106 cells per well for T lymphoblasts) in 0·5 ml complete medium. In some cases, the wells had been precoated overnight with 1 µg/ml OKT3. Recombinant cytokines were added at 200 ng/ml. The incubation period for each stimulus was 20 hr, except for ionomycin (500 ng/ml) (Sigma), for which the incubation was 6 hr. CsA (Sigma) or FK506 were added, where indicated, at a final concentration of 5 µg/ml. In some experiments, responder T cells were preincubated with cycloheximide (20 µg/ml) for 30 min and then washed once prior to stimulation with rIL-2. Stimulated lymphoblasts were harvested and stained with fluorescein isothiocyanate (FITC)-conjugated anti-CD4 (DAKO, Carpenteria, CA) plus either phycoerythrin (PE)-conjugated anti-CD154/CD40 (TRAP1) (Pharmingen, San Diego, CA) or a PE-conjugated isotype-matched control monoclonal antibody (MOPC-21) (Pharmingen) in phosphate-buffered saline (PBS) supplemented with 5% bovine serum albumin and 1% sodium azide (Sigma). After a 1-hr incubation, stained cells were washed and analysed on a FACStar flow cytometry machine (Becton Dickinson, San Jose, CA). Statistics were performed using CellQuest 3.0 software (Becton Dickinson).
NFAT1 Western blot
Responder T-lymphoblast populations were harvested and lysed in extraction buffer (1% Triton X-100 in 200 mm Tris pH 7·5, 400 mm NaCl, 1 mm ethylenediaminetetraacetic acid, 1 mm dithiothreitol, 5% glycerol) plus protease inhibitors, using 10 µl of extraction buffer per 106 cells. Cellular lysates were resolved by 7% sodium dodecyl sulphate–polyacrylamide gel electrophoresis (SDS–PAGE) and electrotransferred onto a polyvinyldifluoride filter (NEN, Boston, MA) using a semi-dry transfer cell (BioRad, Hercules, CA). After blocking in 5% milk in PBS, the filter was blotted with a 1 : 2000 dilution of anti-NFAT1 (Transduction Laboratories, Lexington, KY), followed by a 1 : 3000 dilution of horseradish peroxidase-conjugated goat anti-mouse immunoglobulin G (IgG; BioRad). Bands were visualized by incubation with West Pico chemiluminescent substrate (Pierce, Rockford, IL) for 1 min, followed by X-ray film (Fuji Medical, Stamford, CT) exposure and development. In some experiments, responder T-lymphoblast populations were stained for CD154/CD40L expression and sorted using a cell sorter prior to lysis. T lymphoblasts were stained with a 1 : 5 dilution of 5c8 hybridoma supernatant, followed by a 1 : 100 dilution of FITC-conjugated goat anti-mouse IgG (Boehringer Mannheim, Indianapolis, IN). Stained cells were analysed on a flow cytometer and sorted into two populations: CD154/CD40L-negative cells, and lymphoblasts bearing the highest CD154/CD40L levels. These subpopulations, each representing 20% of the total lymphoblasts, were collected and lysed for Western blot analysis.
Results
SEB-stimulated T lymphoblasts proliferate in response to rIL-2, rIL-4, rIL-7, and rIL-15
T lymphoblasts have been shown to express an array of cytokine receptors, including members of the cytokine receptor common γ chain family (e.g. IL-2, IL-4, IL-7, IL-9 and IL-15). Each of these cytokines possesses a unique range of bioactivities, though all are recognized as T-cell growth factors. The defining feature of this receptor family is its heterodi- or -trimeric structure, with high-specificity binding chain(s) unique for each cytokine coupled to a non-binding common γ chain critical for receptor function (reviewed in ref. 30).
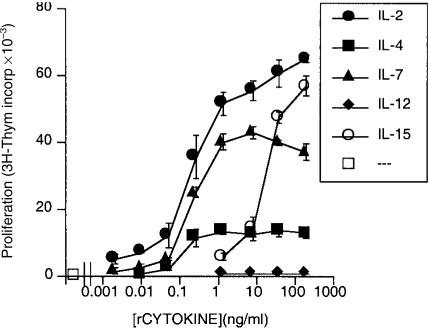
In order to screen for cytokines with the capacity to induce phenotypic changes in helper CD4+ T cells, recombinant cytokines which share the cytokine receptor common γ chain were added to T lymphoblasts which had been initially stimulated with the superantigen SEB. In agreement with previous reports,31–33 rIL-2, rIL-4, rIL-7 and rIL-15 all demonstrated significant activity as growth factors for T-cell lymphoblasts (Fig. 1). Recombinant IL-12, which is not a member of this family of cytokines, failed to stimulate proliferation, and served as a reproducible negative control in these assays.
Figure 1.
Cytokines employing the common receptor γ chain support T-lymphoblast proliferation; 2 × 105 responder T lymphoblasts were cultured in the presence of the indicated concentrations of selected recombinant cytokines. Induction of [3H]thymidine incorporation was determined during the last 18 hr of a 72-hr culture period. Results are presented as the mean of [3H]thymidine incorporation in triplicate wells±standard error. Representative results from one of four experiments are shown.
Recombinant IL-2, rIL-7 and rIL-15 induce CD154/CD40L expression on CD4+ T-cell lymphoblasts
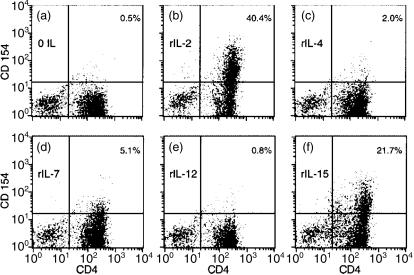
The capacity of this set of recombinant cytokines to induce CD154/CD40L expression by T lymphoblasts was examined next. Recombinant cytokines were used at a concentration which exceeded that necessary to induce lymphoblast growth. There was no significant expression of CD154/CD40L by unstimulated T lymphoblasts (Fig. 2a). Lymphoblasts stimulated for 24 hr with either rIL-2 (Fig. 2b) or rIL-15 (Fig. 2f) demonstrated expression of CD154/CD40L on a significant number of CD4+ cells. Of note, rIL-2-stimulated lymphoblasts expressing CD154/CD40L were predominantly confined to the CD4+ subset, thus mimicking the restricted pattern of CD154/CD40L expression in anti-CD3-stimulated lymphocytes.7–11,14 The percentage of cells expressing CD154/CD40L after rIL-2 stimulation was approximately twice as high as that induced by rIL-15 in multiple experiments (data not shown). T-cell lymphoblasts stimulated by rIL-7 (Fig. 2d) also expressed CD154/CD40L on a small subpopulation of CD4+ cells. The IL-7-stimulated induction, though quantitatively less than that induced by rIL-2 and rIL-15, was reproducibly seen in lymphoblasts from multiple donors (data not shown). In contrast, rIL-4, which showed activity as a T-cell growth factor (Fig. 1), failed to induce CD154/CD40L on a significant number of T lymphoblasts (Fig. 2c). Recombinant IL-12, which has been previously demonstrated to augment anti-CD3-stimulated CD154/CD40L expression,34 failed to induce significant CD154/CD40L expression on T-cell lymphoblasts (Fig. 2e). Assessment of the expression of cytokine receptors by FACS analysis showed high levels of the IL-2 receptor chains; IL-4 receptor expression was low (data not shown). Thus, the differential activity of cytokines to induce CD154/CD40L expression may reflect levels of cytokine receptor expression on CD4+ T lymphoblasts. In summary, induction of CD154/CD40L was confined to a subset of the cytokine family sharing the common receptor γ chain, although all demonstrated some activity as T-cell growth factors (Fig. 1).
Figure 2.
Recombinant IL-2 and IL-15 induce CD154/CD40L expression by CD4+ T lymphoblasts; 5 × 105 responder T lymphoblasts were cultured with the indicated cytokine (50 ng/ml) for 24 hr. Assessment of surface expression of CD4 (x-axis) and CD154/CD40L (y-axis) was performed using directly conjugated monoclonal antibodies and FACS analysis. The percentage of CD154+ CD4+ lymphoblasts is indicated in the upper right hand corner of each panel. Representative results from one of five experiments are shown.
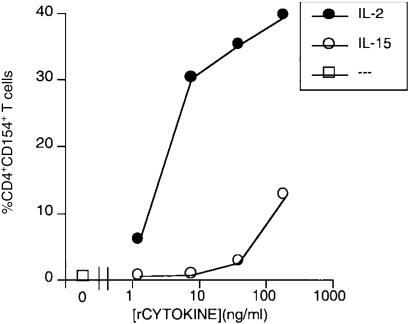
The induction of CD154/CD40L on T lymphoblasts was further characterized by quantifying the induction of CD154/CD40L on T lymphoblasts stimulated by titred amounts of rIL-2 and rIL-15. Recombinant IL-2 demonstrated a lower threshold for inducing both T-lymphoblast proliferation (Fig. 1) and CD154/CD40L expression (Fig. 3) than rIL-15. The concentration of both rIL-2 and rIL-15 for triggering CD154/CD40L expression paralleled that for triggering proliferation.
Figure 3.
Recombinant IL-2 is a potent inducer of CD154/CD40L expression. Responder T lymphoblasts were cultured in the indicated concentrations of either rIL-2 or rIL-15. Assessment of CD154/CD40L expression by CD4+ T cells was performed using directly conjugated monoclonal antibodies and two-colour FACS analysis, and the percentage of CD154+ CD4+ lymphoblasts was graphed as a function of recombinant cytokine concentration. Representative results from one of three experiments are shown.
Cyclosporin A only partially inhibits the rIL-2-triggered induction of CD154/CD40L on T lymphoblasts
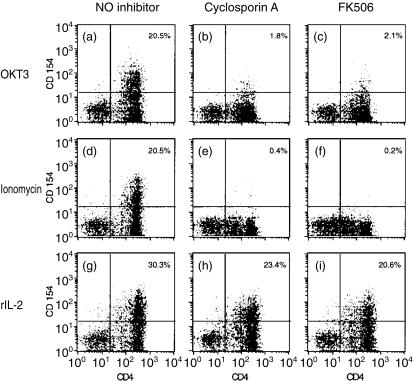
Given the well-established inhibitory activity of CsA for the induction of CD154/CD40L on anti-CD3-stimulated T cells,16–19 we next examined the inhibitory capacity of CsA (and an inhibitor with similar activity, FK506) for cytokine-triggered CD154/CD40L induction. Both CsA and FK506 abrogated CD154/CD40L expression by T lymphoblasts stimulated by either plate-bound anti-CD3 (Fig. 4b,c) or ionomycin (Fig. 4e,f). In contrast, CD154/CD40L expression induced by rIL-2 was only partially inhibited by CsA (Fig. 4h) or FK506 (Fig. 4i). Inhibition of CD154/CD40L expression by CsA or FK506 was routinely 20–30% for rIL-2-stimulated T lymphoblasts, versus inhibition of greater than 90% for anti-CD3-stimulated cells.
Figure 4.
CD154/CD40L expression by CD4+ T lymphoblasts induced by rIL-2 is partially resistant to the inhibitory activity of either CsA or FK506; 5 × 105 responder T lymphoblasts were stimulated by plate-bound OKT3 (a,b,c), ionomycin (0·5 µg/ml) (d,e,f), or rIL-2 (50 ng/ml) (g,h,i). Inhibitors of NFAT activation [CsA (b,e,h) or FK506 (c,f,i)] were added to a final concentration of 5 µg/ml. Assessment of surface expression of CD4 (x-axis) and CD154/CD40L (y-axis) was performed using directly conjugated monoclonal antibodies and FACS analysis. The percentage of CD154+ CD4+ lymphoblasts is indicated in the upper right hand corner of each panel. CD154/CD40L expression by unstimulated lymphoblasts was < 1% in this experiment. Representative results from one of three experiments are shown.
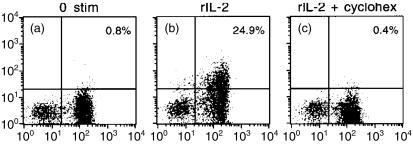
One plausible explanation for this resistance to CsA is that rIL-2 drives the mobilization of previously synthesized pools of CD154/CD40L from cellular stores. Cycloheximide, an inhibitor of protein synthesis, completely abrogated CD154/CD40L induction in T lymphoblasts (Fig. 5), suggesting that rIL-2-stimulated surface expression of CD154/CD40L reflects new protein synthesis.
Figure 5.
Cycloheximide inhibits rIL-2-driven CD154/CD40L induction. Responder cells were stimulated with rIL-2 (50 ng/ml) after a 30 minute preincubation in the absence (panel B) or presence (panel C) of cycloheximide. Cell staining and FACS analysis was performed as described in Figure 4. Shown are representative results from one of three experiments.
rIL-2 induces NFAT1 dephosphorylation in CD154/CD40L-expressing cells
CD154/CD40L expression induced by rIL-2 stimulation, unlike that induced by anti-CD3 stimulation, was only partially inhibited by CsA and FK506 (Fig. 4). Three hypotheses were put forward to explain this differential susceptibility to CsA inhibition: (1) rIL-2-induced CD154/CD40L expression results from a pathway entirely independent of NFAT1, with partial inhibition reflecting an interaction between CsA and a target other than NFAT; (2) rIL-2-induced CD154/CD40L expression results from NFAT1 activation and dephosphorylation by a phosphatase other than calcineurin; (3) rIL-2-induced CD154/CD40L expression results from calcineurin-mediated NFAT1 dephosphorylation, although this pathway's standard CsA susceptibility is altered. In order to discriminate between these hypotheses, the phosphorylation status of NFAT1 in T lymphoblasts was investigated.
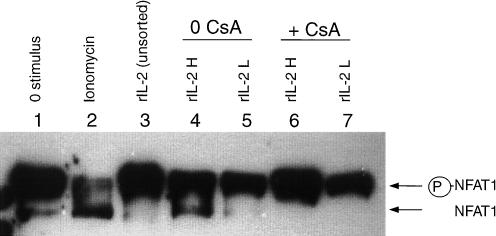
The phosphorylation status of NFAT1 was evaluated by Western blot. Dephosphorylation of NFAT1 by calcineurin results in an activated NFAT1 band which migrates faster in SDS–PAGE than its resting counterpart.22,24,35 The calcium ionophore ionomycin induced substantial dephosphorylation of NFAT1 (Fig. 6, lane 2) and served as a reproducible positive control in these experiments. Anti-CD3 stimulation induced partial NFAT1 dephosphorylation at early time-points after stimulation (1–4 hr) (data not shown), consistent with earlier reports.35 NFAT1 dephosphorylation was not appreciated in the cellular lysates of unseparated T lymphoblasts stimulated with rIL-2 (Fig. 6, lane 3); after cell sorting, however, NFAT1 dephosphorylation was noted on lymphoblasts expressing higher levels of CD154/CD40L (Fig. 6, lane 4). In contrast, NFAT1 dephosphorylation was not seen in rIL-2-stimulated T lymphoblasts treated with CsA (Fig. 6, lanes 6, 7). Thus, analysis of the NFAT1 activation state by Western blotting disclosed this key finding: rIL-2-stimulated T lymphoblasts expressed substantial amounts of surface CD154/CD40L (Fig. 4, panel H) even when NFAT1 dephosphorylation was completely inhibited by CsA (Fig. 6, lanes 6, 7), suggesting that NFAT1 dephosphorylation is not an essential determinant of rIL-2-triggered CD154/CD40L induction.
Figure 6.
Recombinant IL-2 induces CsA-susceptible NFAT1 dephosphorylation in CD154/CD40L-expressing T lymphoblasts. T lymphoblasts were cultured in the presence of rIL-2 (lanes 3–7) or left unstimulated (lane 1). CsA (5 µg/ml) was added to a subset of rIL-2-stimulated cells (lanes 6,7). After a 24-hr culture, surface expression of CD154/CD40L by stimulated lymphoblasts was assessed using indirect immunofluorescence and FACS analysis. Lymphoblasts were sorted into rIL-2-stimulated populations expressing high levels of CD154/CD40L (rIL-2H, designating 20% of the cells with the highest expression) (lanes 4,6), and populations expressing no detectable CD154/CD40L (rIL-2L) (lanes 5,7). Cells were then lysed, and the NFAT1 activation state was assessed by Western blotting. The positions of the inactivated (phosphorylated) and activated (non-phosphorylated) forms of NFAT1 are indicated to the right of the blot. Lysates from lymphoblasts which had been stimulated with ionomycin (and showed substantial NFAT1 dephosphorylation) were used as a positive control (lane 2). Shown are representative results from one of three experiments.
Multiple cytokines sharing the common receptor γ chain induce CsA-resistant CD154/CD40L expression in primary T cells
Having documented CD154/CD40L induction by rIL-2 and rIL-15 for T lymphoblasts, CsA susceptibility patterns for CD154/CD40L induction in primary T lymphocytes were investigated. We anticipated that rIL-2 and rIL-15 might induce significant CsA-resistant CD154/CD40L expression in primary T cells. Responder T cells were stimulated with the combination of plate-bound anti-CD3 in the presence of recombinant cytokines (Fig. 7a–f). Each of the selected cytokines induced a modest increase in the percentage of lymphocytes expressing CD154/CD40L. In all cases, CD154/CD40L-expressing lymphocytes were predominantly confined to the CD4+ subpopulation (Fig. 7a–f).
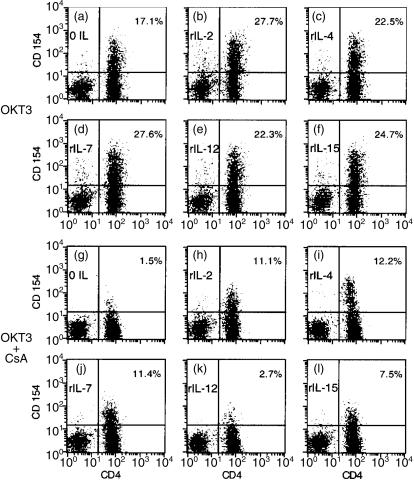
Figure 7.
Multiple cytokines which share the common γ chain induce CsA-resistant CD154/CD40L expression in anti-CD3-stimulated primary CD4+ T cells; 1 × 106 monocyte- and B-cell-depleted PBMC were stimulated by plate-bound OKT3 (1 µg/ml) and the indicated recombinant cytokine in the absence (a–f) or presence (g–l) of CsA (5 µg/ml). Assessment of surface expression of CD4 (x-axis) and CD154/CD40L (y-axis) after 18-hr culture was performed using directly conjugated monoclonal antibodies and FACS analysis. The percentage of CD154+ CD4+ lymphocytes is indicated in the upper right hand corner of each panel. CD154/CD40L expression by unstimulated lymphocytes was < 1% in this experiment. No cytokine induced a significant amount of CD154/CD40L surface expression in the absence of OKT3 stimulation (data not shown). Representative results from one of three experiments are shown.
CsA inhibited anti-CD3-stimulated CD154/CD40L expression in CD4+ T lymphocytes by over 90% (Fig. 7g). As was the case with T lymphoblasts (Fig. 2), both rIL-2, and, to a lesser extent, rIL-15 induced significant CsA-resistant CD154/CD40L expression in anti-CD3-stimulated T cells (Fig. 7h,l). Surprisingly, both rIL-4 and rIL-7 also induced substantial CsA-resistant CD154/CD40L expression. Although rIL-4 was a poor inducer of CD154/CD40L T lymphoblasts (Fig. 2c), this cytokine was the most potent inducer of CsA-resistant CD154/CD40L expression in primary lymphocytes obtained from multiple donors (Fig. 7i). Thus, the capacity to induce CsA-resistant gene expression is a property shared by multiple members of the common receptor γ chain family of cytokines.
Discussion
The induction of CD154/CD40L triggered by TCR stimulation has been previously shown to be dependent upon NFAT binding sites within the CD154/CD40L promoter region.15,18 As a consequence, TCR-triggered CD154/CD40L induction can be inhibited by CsA.16–19 Surprisingly, NFAT1−/− knockout mice maintain some capacity to produce CD154/CD40L.28 This might be ascribed to redundant functional activities by other NFAT isoforms (e.g. NFAT-2 and -4) found in the lymphocytes of NFAT1−/− knockout animals. It must be noted, however, that these NFAT family members remain fully susceptible to CsA inhibition.24,36,37 Recombinant IL-2-triggered CD154/CD40L induction, in contrast, is substantially resistant to the action of CsA (Figs 4, 7), suggesting a degree of independence from the NFAT family of transcriptional factors.
Recombinant IL-2 initiates CD154/CD40L induction with two distinct patterns of CsA susceptibility. First, rIL-2-triggered CD154/CD40L expression remains susceptible to CsA in a subset of lymphoblasts, with a 20–30% loss of signal in the presence of inhibitor (Fig. 4). This was accompanied by CsA-susceptible NFAT1 activation in a subpopulation of CD154/CD40L-expressing T lymphocytes (Fig. 6). TCR stimulation induces a transient, high-amplitude calcium flux which activates calcineurin, and subsequently results in NFAT dephosphorylation. The pattern of TCR-triggered calcium fluxes that initiates NFAT activation has not been previously described in rIL-2-stimulated lymphoblasts. It should be noted, however, that NFAT dephosphorylation and nuclear translocation can be induced by a sustained calcium flux of low amplitude.38 This contrasts with the high-amplitude calcium flux required for the activation of other transcription factors, including the Rel family member NFκB.38 Thus, rIL-2 stimulation triggers CsA-susceptible CD154/CD40L expression apparently mediated by NFAT in a subpopulation of T lymphoblasts.
Significantly, a second pattern of CsA susceptibility is demonstrated for the larger population of stimulated lymphoblasts. CD154/CD40L induction on the majority of rIL-2-stimulated T lymphoblasts is resistant to the inhibitory activity of both CsA and FK506 (Fig. 4). Substantial CD154/CD40L expression in the face of complete inhibition of NFAT activation (Fig. 6, lanes 6, 7) suggests the existence of an NFAT-independent CD154/CD40L induction pathway initiated through the IL-2 receptor. A recent report by Skov et al. has demonstrated a similar pattern of CsA susceptibility for rIL-2-stimulated CD154/CD40L expression in T lymphoblasts stimulated by the combination of anti-CD3 and anti-CD28.39 The cytoplasmic tail of the IL-2 receptor β chain is a known interaction site for a variety of signalling kinases, including JAKs, STATs, src family kinases and PI-3 kinase (reviewed in refs 30, 40–42). Each of these represents a possible site of initiation for the CsA-resistant signalling cascade leading to CD154/CD40L expression. We speculate that these two different CsA susceptibility patterns reflect two discrete (and possibly independent) pathways for the induction of CD154/CD40L. The capacity of rIL-4 (as well as the other common γ receptor cytokines) to induce CsA-resistant CD154/CD40L expression in primary CD4+ lymphocytes (Fig. 7h–l) suggests that triggering the CsA-resistant pathway is a function shared by the cytoplasmic domains of each of the specific cytokine-binding receptors within this family. Alternatively, the common receptor γ chain itself may be key signal-transducing component.
Along with one other very recent report,39 this is the first demonstration of cyclosporin-resistant CD154/CD40L induction. The phenomenon of cyclosporin resistance, however, has been described in other genes for which NFAT is a major transcriptional regulator. For example, TCR-triggered IL-2 production by T lymphocytes can be inhibited by CsA,16–19 reflecting the presence of critical NFAT sites within the promoter region of the IL-2 gene.15,18 The addition of CD28 co-stimulation triggers IL-2 expression which is no longer susceptible to CsA inhibition.43–46 It has been suggested that the well-documented capacity of anti-CD28 co-stimulation to induce IL-2 production may contribute to the CsA resistance seen for some gene products.45 Similar patterns of CsA resistance mediated by CD28 co-stimulation have been documented for several additional cytokines, including tumour necrosis factor, interferon-γ, and granulocyte–macrophage colony-stimulating factor.44
Triggering stimuli other than anti-CD28 co-stimulation have also been implicated in conferring CsA resistance. For example, production of IL-5 by activated T lymphocytes can be induced by rIL-2 via a CsA-resistant pathway.47 In a similar fashion, we have demonstrated CD154/CD40L induction in SEB-stimulated T lymphoblasts by either rIL-2 or rIL-15 via a CsA-resistant pathway. The relationship between the CsA-resistant pathway induced by CD28 co-stimulation and that induced by exogenous recombinant cytokines has not been determined.
Given its central position in the maturation of immune responses to antigenic stimulation, the regulatory control of CD154/CD40L is a matter of some importance. Consequently, CD154/CD40L represents a particularly attractive target for immunotherapeutic intervention. Monoclonal antibodies with a specificity for CD154/CD40L have shown substantial efficacy in clinical solid organ transplantation.48,49 Previous studies have emphasized the susceptibility of TCR-triggered CD154/CD40L induction to CsA inhibition.16–19 Data presented here demonstrate that, in some cases, CD154/CD40L induction can be resistant to the inhibitory activity of CsA. Induction of CD154/CD40L via a TCR-independent pathway could generate a subset of helper T cells which contribute to the pathogenesis of autoimmune diseases. In this regard, the description of aberrant CD154/CD40L-expressing T cells, which are frequently found in patients with systemic lupus erythematosus, is of particular interest.50,51 A more complete understanding of the mechanisms governing CD154/CD40L expression may allow the design of additional immunotherapeutics specifically targeted to alternative induction pathway(s) for this key mediator of T-cell help.
Acknowledgments
I would like to acknowledge Dr Mark Tykocinski (University of Pennsylvania) for his support in the initiation of this work. I would like to thank Dr Dennis Templeton (CWRU) for helpful discussions, as well as for his continued support. I thank Drs Man-Sun Sy, Paul Lehman and Peter Heger (CWRU) for reviewing this manuscript and offering useful suggestions. I wish to thank Barbara Staron and Susan Brill for expert secretarial assistance.
References
- 1.Foy TM, Aruffo A, Bajorath J, Buhlmann JE, Noelle RJ. Immune regulation by CD40 and its ligand. Annu Rev Immunol. 1996;14:591–617. doi: 10.1146/annurev.immunol.14.1.591. [DOI] [PubMed] [Google Scholar]
- 2.Grewal IS, Flavell RA. A central role of CD40 ligand in the regulation of CD4+ T-cell responses. Immunol Today. 1996;17:410–14. doi: 10.1016/0167-5699(96)10030-x. [DOI] [PubMed] [Google Scholar]
- 3.van Kooten C, Banchereau J. CD40–CD40 ligand. J Leuk Biol. 2000;67:2–17. doi: 10.1002/jlb.67.1.2. [DOI] [PubMed] [Google Scholar]
- 4.Armitage RJ, Fanslow WC, Strockbine L, et al. Molecular and biological characterization of a murine ligand for CD40. Nature. 1992;357:80–2. doi: 10.1038/357080a0. [DOI] [PubMed] [Google Scholar]
- 5.Jaiswal AI, Dube C, Swain SL, Croft M. Regulation of CD40 ligand expression on naive CD4 T cells: a role for TCR but not co-stimulatory signals. Int Immunol. 1996;8:275–85. doi: 10.1093/intimm/8.2.275. [DOI] [PubMed] [Google Scholar]
- 6.Ludewig B, Henn V, Schroder JM, Graf D, Kroczek RA. Induction, regulation, and function of soluble TRAP (CD40L) during interaction of primary CD4+ CD45RA+ T cells with dendritic cells. Eur J Immunol. 1996;26:3137–43. doi: 10.1002/eji.1830261246. [DOI] [PubMed] [Google Scholar]
- 7.Lane P, Traunecker A, Hubele S, Inui S, Lanzavecchia A, Gray D. Activated human T cells express a ligand for the human B cell-associated antigen CD40 which participates in T cell-dependent activation of B lymphocytes. Eur J Immunol. 1992;22:2573–8. doi: 10.1002/eji.1830221016. [DOI] [PubMed] [Google Scholar]
- 8.Spriggs MK, Armitage RJ, Strockbine L, Clifford KN, Macduff BM, Sato TA, Maliszewski CR, Fanslow WC. Recombinant human CD40 ligand stimulates B cell proliferation and immunoglobulin E secretion. J Exp Med. 1992;176:1543–50. doi: 10.1084/jem.176.6.1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lederman S, Yellin MJ, Inghirami G, Lee JJ, Knowles DM, Chess L. Molecular interactions mediating T-B lymphocyte collaboration in human lymphoid follicles. Roles of T cell-B-cell-activating molecule (5c8 antigen) and CD40 in contact-dependent help. J Immunol. 1992;149:3817–26. [PubMed] [Google Scholar]
- 10.Gauchat JF, Aubry J-P, Mazzei G, Life P, Jomotte T, Elson G, Bonnefoy J-Y. Human CD40-ligand: molecular cloning, cellular distribution and regulation of expression by factors controlling IgE production. FEBS. 1993;315:259–66. doi: 10.1016/0014-5793(93)81175-y. [DOI] [PubMed] [Google Scholar]
- 11.Roy M, Waldschmidt T, Aruffo A, Ledbette JA, Noelle RJ. The regulation of the expression of gp39, the CD40 ligand, on normal and cloned CD4+ T cells. J Immunol. 1993;151:2497–510. [PubMed] [Google Scholar]
- 12.Graf D, Korthauer U, Mages HW, Senger G, Kroczek RA. Cloning of TRAP, a ligand for CD40 on human T cells. Eur J Immunol. 1992;22:3191–4. doi: 10.1002/eji.1830221226. [DOI] [PubMed] [Google Scholar]
- 13.Grabstein KH, Maliszewski CR, Shanebeck K, Sato TA, Spriggs MK, Fanslow WC, Armitage RJ. The regulation of T cell-dependent antibody formation in vitro by CD40 ligand and IL-2. J Immunol. 1993;150:3141–7. [PubMed] [Google Scholar]
- 14.Nishioka Y, Lipsky PE. The role of CD40–CD40 ligand interaction in human T cell-B cell collaboration. J Immunol. 1994;153:1027–36. [PubMed] [Google Scholar]
- 15.Schubert LA, King G, Cron RQ, Lewis DB, Aruff A, Hollenbaugh D. The Human gp39 promoter. Two distinct nuclear factors of activated T cell protein-binding elements contribute independently to transcriptional activation. J Biol Chem. 1995;270:29624–7. doi: 10.1074/jbc.270.50.29624. [DOI] [PubMed] [Google Scholar]
- 16.Fuleihan R, Ramesh N, Horner A, et al. Cyclosporin A inhibits CD40 ligand expression in T lymphocytes. J Clin Invest. 1994;93:1315–20. doi: 10.1172/JCI117089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Splawski JB, Nishioka J, Nishioka Y, Lipsky PE. CD40 ligand is expressed and functional on activated neonatal T cells. J Immunol. 1996;156:119–27. [PubMed] [Google Scholar]
- 18.Tsytsykova AV, Tsitsikov EN, Geha RS. The CD40L promoter contains nuclear factor of activated T cells-binding motifs which require AP-1 binding for activation of transcription. J Biol Chem. 1996;271:3763–70. doi: 10.1074/jbc.271.7.3763. 10.1074/jbc.271.7.3763. [DOI] [PubMed] [Google Scholar]
- 19.Rao A, Luo C, Hogan PG. Transcription factors of the NFAT family: regulation and function. Annu Rev Immunol. 1997;15:707–47. doi: 10.1146/annurev.immunol.15.1.707. [DOI] [PubMed] [Google Scholar]
- 20.Crabtree GR. Generic signals and specific outcomes: Signaling through Ca+2, calcineurin, and NF-AT. Cell. 1999;96:611–14. doi: 10.1016/s0092-8674(00)80571-1. [DOI] [PubMed] [Google Scholar]
- 21.Luo C, Burgeon E, Rao A. Mechanisms of transactivation by nuclear factor of activated T cells-1. J Exp Med. 1996;184:141–7. doi: 10.1084/jem.184.1.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cho C-W, Rincon M, Davis RJ. Requirement for transcription factor NFAT in interleukin-2 expression. Mol Cell Biol. 1999;19:2300–7. doi: 10.1128/mcb.19.3.2300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Boise LH, Petryniak B, Mao X, et al. The NFAT-1 DNA binding complex in activated T cells contains Fra-1 and JunB. Mol Cell Biol. 1993;13:1911–19. doi: 10.1128/mcb.13.3.1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Aramburu J, Yaffe MB, Lopez-Rodriguez C, Cantley LC, Hogan PG, Rao A. Affinity-driven peptide selection of an NFAT inhibitor more selective than cyclosporin A. Science. 1999;285:2129–33. doi: 10.1126/science.285.5436.2129. 10.1126/science.285.5436.2129. [DOI] [PubMed] [Google Scholar]
- 25.McCaffrey PG, Luo C, Kerppol TK, et al. Isolation of the cyclosporin-sensitive T cell transcription factor NFATp. Science. 1993;262:750–4. doi: 10.1126/science.8235597. [DOI] [PubMed] [Google Scholar]
- 26.Hoey T, Sun Y-L, Williamson K, Xu X. Isolation of two new members of the NF-AT gene family and functional characterization of the NF-AT proteins. Immunity. 1995;2:461–72. doi: 10.1016/1074-7613(95)90027-6. [DOI] [PubMed] [Google Scholar]
- 27.Lyakh L, Ghosh P, Rice NR. Expression of NFAT-family proteins in normal human T cells. Mol Cell Biol. 1997;17:2475–84. doi: 10.1128/mcb.17.5.2475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hodge MR, Ranger AM, de la Brousse FC, Hoey T, Grusby MJ, Glimcher LH. Hyperproliferation and dysregulation of IL-4 expression in NF-ATp-deficient mice. Immunity. 1996;4:397–405. doi: 10.1016/s1074-7613(00)80253-8. [DOI] [PubMed] [Google Scholar]
- 29.Fayen J, Huang J-H, Ferrone S, Tykocinski ML. Negative signaling by anti-HLA class I monoclonal antibodies is dependent upon two triggering events. Int Immunol. 1998;10:1347–58. doi: 10.1093/intimm/10.9.1347. [DOI] [PubMed] [Google Scholar]
- 30.He Y-W, Malek TR. The structure and function of γc-dependent cytokines and receptors: Regulation of T lymphocyte development and homeostasis. Crit Rev Immunol. 1998;18:503–24. doi: 10.1615/critrevimmunol.v18.i6.20. [DOI] [PubMed] [Google Scholar]
- 31.Bulfone-Paus S, Durkop H, Paus R, Krause H, Pohl T, Onu A. Differential regulation of human T lymphoblast functions by IL-2 and IL-15. Cytokine. 1997;9:507–13. doi: 10.1006/cyto.1996.0194. 10.1006/cyto.1996.0194. [DOI] [PubMed] [Google Scholar]
- 32.Vella AT, Dow S, Potter TA, Kappler J, Marrack P. Cytokine-induced survival of activated T cells in vitro and in vivo. Proc Natl Acad Sci USA. 1998;95:3810–15. doi: 10.1073/pnas.95.7.3810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Akbar AN, Borthwich NJ, Wickremasinghe RG, et al. Interleukin-2 receptor common γ-chain signaling cytokines regulate activated T cell apoptosis in response to growth factor withdrawal: selective induction of anti-apoptotic (bcl-2, bcl-xL) but not pro-apoptotic (bax, bcl-xs) gene expression. Eur J Immunol. 1996;26:294–9. doi: 10.1002/eji.1830260204. [DOI] [PubMed] [Google Scholar]
- 34.Peng X, Remacle JE, Kasran A, Huylebroeck D, Ceuppens JL. IL-2 up-regulates CD40 ligand (CD154) expression on human T cells. J Immunol. 1998;160:1166–72. [PubMed] [Google Scholar]
- 35.Loh C, Carew JA, Kim J, Hogan PG, Rao A. T-cell receptor stimulation elicits an early phase of activation and a later phase of deactivation of the transcription factor NFAT1. Mol Cell Biol. 1996;16:3945–54. doi: 10.1128/mcb.16.7.3945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chow C-W, Rincon M, Cavanagh J, Dickens M, Davis RJ. Nuclear accumulation of NFAT4 opposed by the JNK signal transduction pathway. Science. 1997;278:1638–41. doi: 10.1126/science.278.5343.1638. [DOI] [PubMed] [Google Scholar]
- 37.Timmerman LA, Healy JI, Ho SN, Chen L, Goodnow CC, Crabtree GR. Redundant expression but selective utilization of nuclear factor of activated T cells family members. J Immunol. 1997;159:2735. [PubMed] [Google Scholar]
- 38.Dolmetsch RE, Lewis RS, Goodnow CC, Healy JI. Differential activation of transcription factors induced by Ca+2 response amplitude and duration. Nature. 1997;368:855–8. doi: 10.1038/386855a0. [DOI] [PubMed] [Google Scholar]
- 39.Skov S, Bonyhadi M, Odum N, Ledbetter JA. IL-2 and IL-15 regulate CD154 expression on activated CD4 T cells. J Immunol. 2000;164:3500–5. doi: 10.4049/jimmunol.164.7.3500. [DOI] [PubMed] [Google Scholar]
- 40.Johnston JA, Bacon CM, Riedy MC, O'Shea JJ. Signaling by IL-2 and related cytokines: JAKS, STATs, and relationship to immunodeficiency. J Leuk Biol. 1996;60:441–52. doi: 10.1002/jlb.60.4.441. [DOI] [PubMed] [Google Scholar]
- 41.Waldmann T, Tagaya Y, Bamford R. Interleukin-2, interleukin-15, and their receptors. Int Rev Immunol. 1998;16:205–26. doi: 10.3109/08830189809042995. [DOI] [PubMed] [Google Scholar]
- 42.Gespert F, Delespine-Carmagnat M, Bertoglio J. Recent advances in the understanding of interleukin-2 signal transduction. J Clin Immunol. 1998;18:307–20. doi: 10.1023/a:1023223614407. [DOI] [PubMed] [Google Scholar]
- 43.June CH, Ledbetter JA, Gillespie MM, Lindsten T, Thompson CB. T-cell proliferation involving the CD28 pathway is associated with cyclosporin-resistant interleukin 2 gene expression. Mol Cell Biol. 1987;7:4472–81. doi: 10.1128/mcb.7.12.4472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Thompson CB, Lindsten T, Ledbetter JA, Kunkel SL, Young HA, Emerson SG, Leiden JM, June CH. CD28 activation pathway regulates the production of multiple T-cell-derived lymphokines/cytokines. Proc Natl Acad Sci USA. 1989;86:1333–7. doi: 10.1073/pnas.86.4.1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.van Gool SW, Kasran A, Wallays G, de Boer M, Ceuppens JL. Accessory signalling by B7–1 for T cell activation induced by anti-CD2: Evidence for IL-2-independent CTL generation and CsA-resistant cytokine production. Scand J Immunol. 1995;41:23–30. doi: 10.1111/j.1365-3083.1995.tb03529.x. [DOI] [PubMed] [Google Scholar]
- 46.Ghosh P, Sica A, Cippitelli M, Subleski J, Lahesmaa R, Young HA, Rice NA. Activation of nuclear factor of activated T cells in a cyclosporin A-resistant pathway. J Biol Chem. 1996;271:7700–4. doi: 10.1074/jbc.271.13.7700. [DOI] [PubMed] [Google Scholar]
- 47.Valentine JE, Sewell WA. Induction of IL-5 expression by IL-2 is resistant to the immunosuppressive agents cyclosporin A and rapamycin. Int Immunol. 1997;9:975–82. doi: 10.1093/intimm/9.7.975. 10.1093/intimm/9.7.975. [DOI] [PubMed] [Google Scholar]
- 48.Kirk AD, Harlan DM, Armstrong NN, et al. CTLA4-Ig and anti-CD40 ligand prevent renal allograft rejection in primates. Proc Natl Acad Sci USA. 1997;94:8789–94. doi: 10.1073/pnas.94.16.8789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kenyon NS, Chatzipetrou M, Masetti M, et al. Long-term survival and function of intrahepatic islet allografts in rhesus monkeys treated with humanized anti-CD154. Proc Natl Acad Sci USA. 1999;96:8132–7. doi: 10.1073/pnas.96.14.8132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Desai-Mehta A, Lu L, Ramsey-Goldman R, Datta SK. Hyperexpression of CD40 ligand by B and T cells in human lupus and its role in pathogenic autoantibody production. J Clin Invest. 1996;97:2063–73. doi: 10.1172/JCI118643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yellin MJ, Thienel U. T cells in the pathogenesis of system lupus erythematosus: potential roles of CD154–CD40 interactions and costimulatory molecules. Curr Rheumatol Rep. 2000;2:24–31. doi: 10.1007/s11926-996-0065-8. [DOI] [PubMed] [Google Scholar]