Abstract
Interleukin-1 (IL-1) consists of two molecules, IL-1α and IL-1β, and IL-1 receptor antagonist (IL-1Ra) is a natural inhibitor of these molecules. Although the adjuvant effects of exogenously administered IL-1 in the humoral immune response are well known, the roles of endogenous IL-1 and the functional discrimination between IL-1α and IL-1β have not been elucidated completely. In this report, we investigated the role of IL-1 in the humoral immune response using gene-targeted mice. Both primary and secondary antibody production against T-dependent antigen, sheep red blood cells (SRBC), was significantly reduced in IL-1α/β−/− mice, and was enhanced in IL-1Ra−/− mice. The intrinsic functions of B cells, such as antibody production against type 1 T-independent antigen, trinitrophenyl–lipopolysaccharide and proliferative responses against mitogenic stimuli, were normal in IL-1α/β−/− mice. The proliferative response of T cells and cytokine production upon stimulation with anti-CD3 monoclonal antibody were also normal, as was the phagocytotic ability of antigen-presenting cells (APCs). However, SRBC-specific proliferative response and cytokine production of T cells through the interaction with APCs were markedly impaired in IL-1α/β−/− mice, and enhanced in IL-1Ra−/− mice. Moreover, we show that SRBC-specific antibody production was reduced in IL-1β−/− mice, but not in IL-1α−/− mice. These results show that endogenous IL-1β, but not IL-1α, is involved in T-cell-dependent antibody production, and IL-1 promotes the antigen-specific T-cell helper function through the T-cell–APC interaction.
Introduction
Interleukin-1 (IL-1) has been found to be involved in various reactions, including inflammation, acute-phase responses, host defence against bacterial and viral infections, fever development and stress responses.1,2 IL-1 has two forms, IL-1α and IL-1β, which are produced from distinct genes and exert similar, although not completely overlapping, biological activities through the IL-1 type I receptor (IL-1RI; CD121a).3 In addition, another member of the IL-1 gene family, the IL-1 receptor antagonist (IL-1Ra), binds to IL-1RI without exerting agonistic activity.3
In the immune system, IL-1 is known as a lymphocyte activating factor.4 IL-1 acts on monocytes and macrophages to induce production of IL-1, tumour necrosis factor-α, IL-6, IL-8, prostaglandin E2 and nitric oxide, enhancing their killing activities against bacteria, protozoa and tumour cells.4 IL-1 also acts on natural killer cells in collaboration with IL-2 and interferon-γ (IFN-γ) to potentiate their cytotoxic activity.4 Furthermore, it was shown that IL-1 promotes T-cell proliferation in response to antigens and lectins, and induces expression of IL-2 and IL-2R.5,6 IL-1 is involved in the proliferation of T helper type 2 (Th2) cells synergistically with IL-2 and IL-4,7 or independently of IL-4,8 and is required for IL-12-induced Th1 cell development.9 Moreover, IL-1 potentiates γδ T-cell proliferation synergistically with IL-7.10 IL-1 enhances proliferation and differentiation of B cells synergistically with IL-4 and IL-6, and potentiates antibody production.11 Although the precise molecular mechanisms of this activation remain largely unknown, IL-1 seems to play an important role in host defence mechanisms against microbes through these effects on immune cells.
When mice were immunized with protein antigens together with IL-1, serum antibody production was enhanced, suggesting that IL-1 has an adjuvant effect.12,13 It was also reported that IL-1 enhanced antibody production to thymus (T-cell)-dependent (TD) and thymus (T-cell)-independent (TI) antigens in vitro and in vivo.14 On the other hand, when mice were immunized with TD antigen, sheep red blood cells (SRBC), IL-1β potentiated antibody production, while IL-1α suppressed the IL-1β effect.15 Therefore, it is suggested that IL-1α and IL-1β have distinct roles in antibody production. However, administration of anti-IL-1RI antibody or recombinant IL-1Ra during immunization with SRBC or another TD antigen, trinitrophenyl-conjugated keyhole limpet haemocyanin (TNP-KLH), did not affect antibody production.16 Furthermore, specific serum antibody levels were normal in IL-1RI−/− mice when mice were immunized with TNP-KLH together with alum or complete Freund's adjuvant (CFA).17,18 On the other hand, we found that IL-1Ra−/− mice developed chronic inflammatory arthropathy spontaneously and production of autoantibodies against immunoglobulins, type II collagen and double-stranded DNA, increased in these mice.19 Thus, these apparently disparate findings indicate that the mechanisms of action of IL-1 and the individual roles of IL-1α and IL-1β in antibody production still remain to be elucidated.
The current investigation studied the functions of IL-1 in the humoral immune response using IL-1α−/−, IL-1β−/−, IL-1α/β−/− and IL-1Ra−/− mice. We show that IL-1β, but not IL-1α, is involved in T-dependent antibody production via antigen-specific T-cell activation.
Materials and methods
Mice
IL-1α−/−, IL-1β−/−, IL-1α/β−/− and IL-1Ra−/− mice were generated by homologous recombination as described previously and backcrossed to BALB/cA mice for seven or eight generations.20 All the mice were housed under specific pathogen-free conditions in an environmentally controlled clean room at the Centre for Experimental Medicine, Institute of Medical Science, University of Tokyo. The experiments were conducted according to the institutional ethical guidelines for animal experiments and the safety guideline for gene manipulation experiments. Sex- and age-matched 8–12-week-old adult mice were used for the experiments.
Immunization of mice
Mice were immunized intraperitoneally with either 1 × 108 SRBC in phosphate-buffered saline (PBS), 25 µg TNP-Ficoll (kindly provided by Dr Hideo Nariuchi; Institute of Medical Science, University of Tokyo), or 50 µg TNP-lipopolysaccharide (LPS) (Paesel + Lorei GmbH & Co., Frankfurt, Germany). Primary and secondary responses were examined after immunization with SRBC and TNP-Ficoll, while only the primary response was examined under TNP-LPS immunization. Blood samples were collected from the tail vein before the immunization, and 1 and 2 weeks after the primary immunization. At 15 days after the primary immunization, mice were given the secondary immunization and blood samples were collected 1 and 2 weeks later.
Measurement of antibody titres
Immunoglobulin levels in sera or culture supernatants were measured by enzyme-linked immunosorbent assay (ELISA) as described previously.21 To detect SRBC or TNP-specific antibodies, soluble SRBC antigen (2 µg/ml) or TNP-BSA in PBS (10 µg/ml), which was prepared as described previously,22,23 was coated on Falcon 3912 Micro Test III™ Flexible Assay Plates (Becton Dickinson, Oxnard, CA) at 4° overnight. After incubation, the well was washed three times with Tris-buffered saline (TBS)+0·05% Tween-20, and appropriate diluted serum samples were applied and incubated for 1 hr at room temperature. After washing, alkaline phosphatase (AP)-conjugated goat anti-mouse IgM and IgG (ZYMED, San Francisco, CA) were added and incubated for 1 hr at room temperature. Alkaline phosphatase activity was measured using Substrate Phosphatase Sigma104® (Sigma, St Louis, MO) as the substrate, and the absorbancy at 415 nm is shown.
Preparation of cells from lymphoid tissues
A single-cell suspension was prepared from the spleen or lymph nodes (axillary, inguinal and brachial). Spleen cells were treated with a haemolysis buffer (17 mm Tris–HCl, 140 mm NH4Cl, pH 7·2) to remove red blood cells. Adherent cells and non-adherent cells were separated after incubation for 1 hr on a 10-cm dish. Splenic adherent cells (SACs) were collected and used as antigen-presenting cells (APCs) in the SRBC-specific T-cell proliferative response assay. To prepare splenic and lymph node T cells, non-adherent cells were passed through a nylon wool column. T cells were purified by treating the T-cell preparation with anti-mouse B220 and anti-mouse Mac-1 magnetic beads (Miltenyi Biotec GmbH, Bergisch Gladbach, Germany) and then passing them through a MACS column. The purity of T cells was monitored by FACScan and was usually about 90%.
B-cell proliferative response
Spleen cells (2 × 105 cells/well) were prepared from wild-type or gene-targeted mice and were plated on a 96-well flat-bottom plate with 2 µg/ml of LPS (Difco Laboratories, Detroit, MI) or 10 µg/ml anti-IgM (cappel, ICN Phamaceuticals, Inc., Aurora, OH) in a final volume of 200 µl RPMI-1640/10% FCS and cultured for 48 hr. After 48 hr of culture, [3H]thymidine (0·25 µCi/ml) (Amersham, Buckinghamshire, UK) was added in the culture. After 6 hr, cells were harvested using a Micro 96 cell harvester (Skatron, Lier, Norway) and incorporated [3H]thymidine was measured using Micro Beta System (Pharmacia Biotech, Piscataway, NJ).
T-cell proliferative response
For CD3 treatment, splenic T cells or lymph node T cells (2 × 105 cells/well) were plated on a 96-well plate coated with anti-mouse CD3 antibody (1 µg/ml) (145-2C11; PharMingen, San Diego, CA) in the presence or absence of 10 µg/ml of anti-mouse CD28 antibody (PV-1; kind gift from Dr Ryo Abe; Research Institute for Biological Science, Science University of Tokyo, Japan).
For the SRBC-specific T-cell proliferative response assay, T cells were prepared from the spleen 7–10 days after immunization with SRBC. The T cells (5 × 105 cells/well) and the irradiated SACs from non-immunized mice were then co-cultured for 5 days in the presence or absence of soluble SRBC antigen (50 µg/ml). SACs from IL-1α/β−/− mice were plated at a concentration of 5 × 104 cells/well. In contrast, SACs from IL-1Ra−/− mice were plated at 1 × 103 cells/well in 96-well plates. Cell proliferation was measured by the incorporation of [3H]thymidine as described above.
Phagocytic abilities of APCs
To measure phagocytic activity, 3 × 105 cells of SACs or peritoneal exude cells (PECs) collected by washing the peritoneal cavity with PBS were incubated for 1 hr at 37° with fluorescein isothiocyanate (FITC)-latex beads (provided by Dr Yoshitsugu Matsumoto; School of Agriculture and Life Sciences, University of Tokyo, Japan), FITC-Dextran (10 µg/ml, Sigma) or Lucifer Yellow (2 µg/ml, Sigma). Cells were then washed and the fluorescence was analysed with a FACScan (Becton Dickinson).
Measurement of cytokine levels
The culture supernatant in SRBC-specific T-cell response was collected after 2 days (IL-2) or 5 days (other cytokines). IL-2, IL-4, IL-5, IL-6 and granulocyte–macrophage colony-stimulating factor (GM-CSF) levels were determined by Titer Zyme EIA kit (PerSeptive Diagnostics, Inc., Cambridge, MA). As standard recombinant cytokines, mouse IL-2 (Genzyme, Cambridge, MA), IL-4, GM-CSF (Peprotech, London, UK), IL-5 and IL-6 (PerSeptive Diagnostics) were used. IFN-γ levels were determined by an ELISA system using the following reagents: a monoclonal hamster anti-mouse IFN-γ (Genzyme) as a capture antibody, a rabbit anti-mouse IFN-γ antibody (provided by Dr Masayoshi Kohase; Department of Viral Disease and Vaccine Control, National Institute of Health Japan) as the second antibody, AP-conjugated goat anti-rabbit IgG as the third antibody, and Substrate Phosphate Sigma104® (Sigma) as the substrate for alkaline phosphatase. A recombinant mouse IFN-γ (Shionogi & Co., Ltd, Osaka, Japan) was used as a standard. A TMB One-Step Substrate System was purchased from DAKO (Carpinteria, CA).
Statistics
Student's t-test was used for statistical evaluation of the results.
Results
Antibody production to SRBC in IL-1α/β−/− and IL-1Ra−/− mice
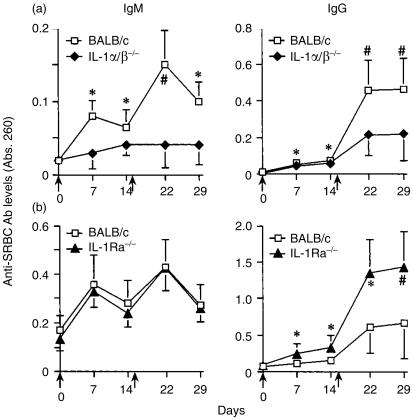
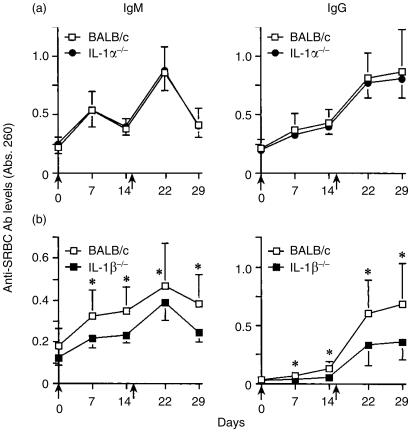
To evaluate whether or not IL-1 is involved in antibody production, wild-type, IL-1α/β−/− and IL-1Ra−/− mice were immunized with SRBC intraperitoneally, and SRBC-specific serum antibody levels were measured by ELISA. Consistent with our previous data,21 SRBC-specific antibody levels of IgM and IgG in IL-1α/β−/− mice were significantly lower than those in wild-type mice after both primary and secondary immunization (Fig. 1a). In contrast, SRBC-specific IgG levels in IL-1Ra−/− mice were increased compared with those in wild-type mice, although IgM levels were comparable in both mice (Fig. 1b). These results clearly show that naturally occurring IL-1 plays important roles in the production of antigen-specific antibodies.
Figure 1.
Efficiency of antibody production against SRBC in IL-1α/β−/− and IL-1Ra−/− mice. Mice were immunized with SRBC (arrow point), and sera were collected 1 and 2 weeks after the primary and secondary immunizations. After appropriate dilution of the serum (IgM and IgG: 1/100 dilution), SRBC-specific antibody levels in the sera were measured by ELISA. (a) Wild-type mice: n = 10, IL-1α/β−/− mice: n = 10. (b) IL-1Ra−/− mice: n = 7. The average and standard deviation (SD) are shown. *P < 0·05; #P < 0·005.
Effects of IL-1-deficiency on intrinsic B-cell and T-cell function and on phagocytic abilities of APCs
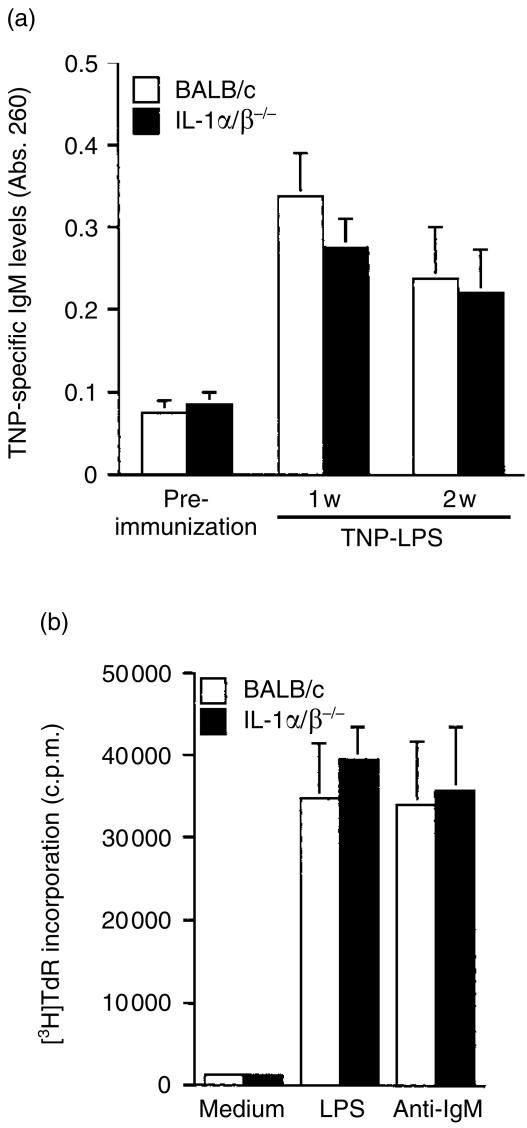
In order to elucidate the mechanism by which IL-1 activates antibody production, we examined antibody production against TI antigens. It is known that antibody production against TNP-LPS which belongs to a type 1 TI antigen does not require T-cell help. When IL-1α/β−/− mice were immunized with TNP-LPS intraperitoneally, antibody production was normal (Fig. 2a), indicating that antibody-producing ability of B cells without T-cell help is normal in those mice.
Figure 2.
Intrinsic functions of B cells in IL-1α/β−/− mice. (a) Mice were immunized with TNP-LPS, and the sera were collected before immunization, and 1 and 2 weeks after immunization. After 1/1000 dilution, anti-TNP-specific IgM levels in sera were measured by ELISA. Wild-type mice: n = 4, IL-1α/β−/− mice: n = 4. Average±SD is shown. (b) Proliferative responses of splenocytes against mitogenic stimulations were measured by the incorporation of [3H]thymidine using IL-1α/β−/− mice. Bars represent an average of two or three mice with SD. One representative result from three independent experiments is shown.
To examine the direct effect of IL-1-deficiency on intrinsic B-cell functions, B-cell responses to LPS and anti-IgM antibody stimulus were investigated. The results showed that the proliferative responses of splenocytes were not different between IL-1α/β−/− and wild-type mice (Fig. 2b). Two to three days after stimulation with anti-IgM antibody, the expression levels of I-Ad, CD80 and CD86 on B220+ cells were not different among these mice (data not shown). These results indicate that the lack of IL-1 does not have any direct effect on B-cell function.
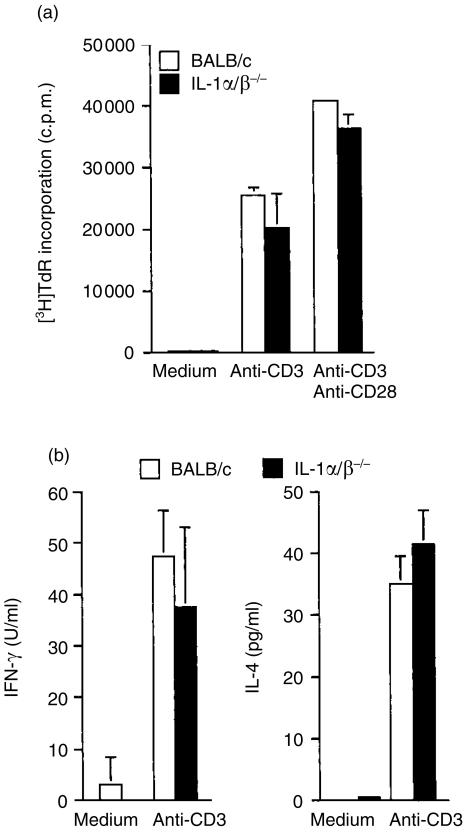
We next analysed the intrinsic functions of T cells in IL-1α/β−/− mice. When splenic T cells from IL-1α/β−/− mice were stimulated with immobilized anti-CD3 antibody or immobilized anti-CD3 antibody plus soluble anti-CD28 antibody, proliferative responses of these cells were normal (Fig. 3a). IL-4 and IFN-γ productions after stimulation with immobilized anti-CD3 antibody were normal in IL-1α/β−/− mice under these conditions (Fig. 3b). Thus, it was shown that intrinsic T-cell function was not affected by the deficiency of IL-1, so far as it was estimated by these tests.
Figure 3.
Intrinsic functions of T cells in IL-1α/β−/− mice. Proliferative responses of splenic T cells against plate-coated anti-CD3 monoclonal antibody only or with soluble anti-CD28 monoclonal antibody were measured by the incorporation of [3H]thymidine using IL-1α/β−/− mice
Cytokine levels in culture supernatants were measured by ELISA
Bars represent an average of two or three mice with SD. One representative result from three independent experiments is shown.
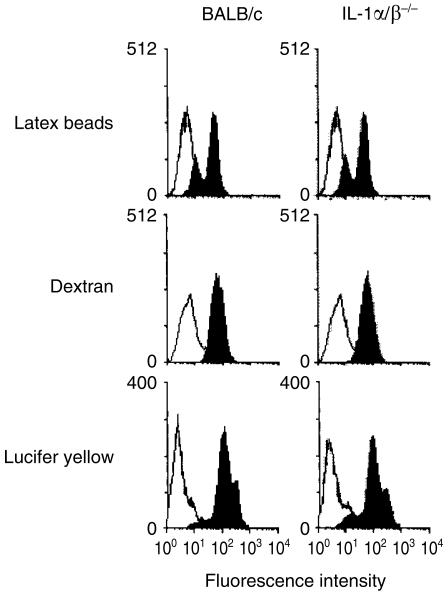
Next, we examined phagocytic activity of macrophages from IL-1α/β−/− mice. As shown in Fig. 4(e), phagocytic activities of the peritoneal macrophages to both FITC-latex beads and FITC-dextran were normal (Fig. 4). Pinocytotic activity of splenic adherent cells (SACs) from IL-1α/β−/− mice to lucifer yellow was also normal. These results suggest that IL-1 is neither involved in the phagocytic activity of APCs, nor in T- and B-cell intrinsic functions.
Figure 4.
Phagocytic abilities of APCs of IL-1α/β−/− mice. Phagocytic abilities of FITC-latex beads and FITC-dextran of PECs and pinocytic abilities for lucifer yellow of SACs from wild-type and IL-1α/β−/− mice were assessed by flow cytometry. One representative result from at least three independent experiments is shown.
The role of IL-1 in T-cell–APC interaction
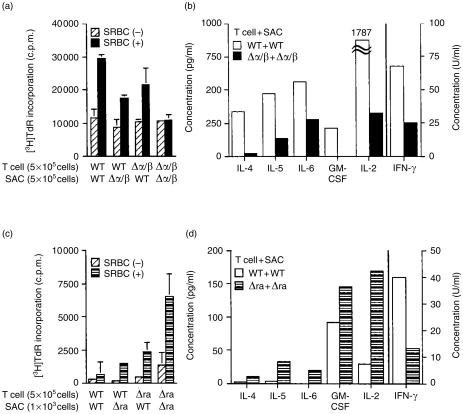
We then examined the role of IL-1 in activating antigen-specific T-cell helper function upon interaction with APCs. As shown in Fig. 5(a), when splenic T cells from IL-1α/β−/− mice immunized with SRBC (T[Δα/β]) and APCs from non-immunized wild-type mice (APC[WT]) as well as in the (T[WT] + APC[Δα/β]) culture were co-cultured in the presence of soluble SRBC antigens, the T-cell proliferative response decreased by one-third of that observed in (T[WT] + APC[WT]) culture. The response in the (T[Δα/β] + APC[Δα/β]) co-culture was most severely impaired among these combinations (T[WT] + APC[WT]: 100% versus T[WT] + APC[Δα/β]: 57% ± 21%, P < 0·005; T[Δα/β + APC[WT]: 63% ± 19%, P < 0·005; T[Δα/β] + APC[Δα/β]: 19% ± 16%, P < 0·0005, respectively. The average±SD after subtraction of the background response in the absence of antigen from six independent experiments is shown). The (T[Δα/β] + APC[Δα/β]) culture also showed a markedly decreased production of cytokines, such as IL-2, IL-4, IL-5, IL-6, GM-CSF and IFN-γ (Fig. 5b).
Figure 5.
Impaired T-cell responses through T-cell–APC interaction in IL-1α/β−/− mice. Proliferative responses and cytokine production of antigen-specific T cells upon interaction with SACs were determined. T cells (2 × 105 cells) from the spleen of SRBC-immunized wild-type (WT), IL-1α/β−/− (Δα/β)(a), and IL-1Ra−/− (Δra) mice (c) were cultured with SACs from non-immunized mice in the absence (hatched column) or presence (closed column) of soluble SRBC antigen, and the proliferative responses were measured by the incorporation of [3H]thymidine after 5 days. Cytokine levels in the supernatants of the IL-1α/β−/− mouse (b) or IL-1Ra−/− mouse (d) T-cell cultures were measured. Average±SD is shown in (a) and (c), while bars in (b) and (d) show the total amount of the cytokine in triplicate culture supernatants. One representative result from three independent experiments is shown.
On the other hand, co-culture of splenic T cells from IL-1Ra−/− mice immunized with SRBC (T[Δra]) and APCs from non-immunized IL-1Ra−/− mice (APC[Δra]) in the presence of soluble SRBC antigens showed the highest response (Fig. 5c) (T[WT] + APC[WT]: 100% versus T[WT] + APC[Δra]: 147% ± 18%, P < 0·05; T[Δra] + APC[WT]: 417% ± 255%, P < 0·05; T[Δra] + APC[Δra]: 901% ± 509%, P < 0·05, respectively. The average ±SD after subtraction of the background response in the absence of antigen from four independent experiments is shown). In addition, IL-2, IL-4, IL-5, IL-6 and GM-CSF levels in the (T[Δra] + APC[Δra]) culture fluid were increased compared with the levels in the (T[WT] + APC[WT]) culture fluid (Fig. 5d). In contrast, the IFN-γ level in the (T[Δra] + APC[Δra]) culture fluid was lower than that in the (T[WT] + APC[WT]) culture fluid (Fig. 5d). IL-1α, IL-1β and TNF-α could not be detected in those culture fluids (data not shown). It is known that wild-type APCs produce IL-1 and IL-1Ra. However, the change of T-cell response by IL-1 deficiency or IL-1Ra deficiency was not ameliorated by co-culture with wild-type APCs. These results suggest that IL-1 is involved in the induction of antigen-specific memory T cells in T-cell-priming in vivo. These results indicate that IL-1 is involved in the activation of T cells through interaction with APCs, suggesting that the defect of antibody production in IL-1α/β−/− mice may be caused by the reduced responses of antigen-specific T cells.
Antibody production to SRBC in IL-1α−/− and IL-1β−/− mice
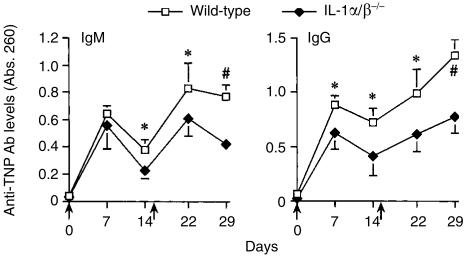
It was suggested that IL-1α and IL-1β play distinct roles in antibody production.15 When antibody production was examined in either IL-1α−/− or IL-1β−/− mice, SRBC-specific IgM and IgG levels in IL-1β−/− mice were clearly lower than those in wild-type mice, whereas those in IL-1α−/− mice were normal (Fig. 6a,b). These results indicate that IL-1β, but not IL-1α, plays a major role in T-cell-dependent antibody production.
Figure 6.
Efficiency of antibody production against SRBC in IL-1α−/− and IL-1β−/− mice. Mice were immunized with SRBC (arrow point), and sera were collected 1 and 2 weeks after the primary and secondary immunization. After appropriate dilution of the serum (IgM and IgG: 1/100 dilution), SRBC-specific antibody levels in the sera were measured by ELISA. (a) Wild-type mice: n = 8, IL-1α−/− mice: n = 7. (b) IL-1β−/− mice: n = 7. The average and standard deviation (SD) are shown. *P < 0·05, and #P < 0·005.
Antibody production to type 2 TI antigens in IL-1α/β−/− mice
It is known that antibody production against TNP-LPS, which belongs to a type 1 TI antigen, does not require T-cell help, while that to type 2 TI antigens such as TNP-Ficoll still requires T-cell help, although the mechanism of the humoral immune response to type 2 TI antigens is considered to be different from that to TD antigens.24 When IL-1α/β−/− mice were immunized with TNP-Ficoll intraperitoneally, TNP-specific IgM and IgG levels were significantly reduced in IL-1α/β−/− mice both after primary and secondary immunization (Fig. 7). This reduction was seen in all the IgG subclasses (data not shown). These results indicate that IL-1 is also involved in the antibody production to type 2 TI antigens.
Figure 7.
Antibody production against type 2 TI antigens in IL-1α/β−/− mice. Mice were immunized with TNP-Ficoll (arrow point), and the sera were collected 1 and 2 weeks after the primary and secondary immunization. After 1/1000 dilution, anti-TNP-specific immunoglobulin levels in sera were measured by ELISA. Wild-type mice: n = 5, IL-1α/β−/− mice: n = 5. Average±SD is shown. *P < 0·05, and #P < 0·005.
Discussion
In previous studies, the adjuvant effects of exogenous IL-1 were shown in humoral immune responses;12,13 however, the roles of endogenous IL-1 have not been elucidated clearly. The effects of IL-1RI deficiency were not observed in the humoral immune responses against TNP-KLH when mice were immunized with alum or CFA.17,18 Furthermore, administration of anti-IL-1RI antibody or recombinant IL-1Ra during immunization with SRBC or TNP-KLH did not affect the antibody production.16 In this report, we have first shown that antibody production against SRBC was severely reduced in IL-1α/β−/− mice, showing that endogenous IL-1 plays a crucial role in the humoral immune response under physiological conditions. This apparent discrepancy seems to be caused by differences in the immunization protocols used; i.e. other studies used CFA in their experiments, while we immunized without adjuvant. It is probable that immunization with adjuvant may induce various inflammatory cytokines that substitute for the function of IL-1. Consistent with this theory, we found that antibody production after immunization with SRBC without adjuvant was reduced in IL-1RI−/− mice (Nakae et al. unpublished data). Mycobacteria in CFA also affect the development of collagen-induced arthritis by stimulating expansion of the Mac-1+ cell population,25 and IFN-γ causes apparently opposite effects on the development of the arthritis depending on the presence of CFA. Enhancing effects of CFA on antibody production were also observed in CD80/CD86−/− and ICOS−/− mice,26,27 in which the strong inflammatory response elicited by CFA immunization induced CD80 and CD86 expression which compensated the defects of co-signalling molecules.
The immune cell compartment of the thymus, spleen and lymph nodes, as well as the splenic microarchitecture, was normal in IL-1α/β−/− mice (data not shown). Germinal centre formation was also normally observed in the mutant mice (data not shown). Moreover, intrinsic functions of B cells and T cells from IL-1α/β−/− mice were normal. However, SRBC-specific proliferative response and Th1/Th2 cytokine production of T cells through interaction with APCs were markedly impaired in IL-1α/β−/− mice, and enhanced in IL-1Ra−/− mice. These observations suggest that T-cell–APC interaction is impaired in these IL-1 mutant mice. In this context, recently, we demonstrated that IL-1 is involved in T-cell activation during the primary T-cell response through interaction with APCs.21 Therefore, the defect of secondary SRBC-specific T-cell response in IL-1α/β−/− mice is considered to be caused by the failure of T-cell priming and antigen-specific memory T-cell induction.
It is remarkable that the antibody production was reduced only in IL-1β−/− mice, but was normal in IL-1α−/− mice, indicating that two IL-1 molecules play distinct roles in the immune system. With regard to this, it was reported that rIL-1β, as well as a nonapeptide derived from the IL-1β sequence, potentiated antigen-specific antibody production when mice were immunized with SRBC, while rIL-1α suppressed the rIL-1β action.14,15 However, we showed that antibody levels in wild-type and IL-1α−/− mice were similar, indicating that endogenous IL-1α is not inhibitory to antibody production. It is probable that the concentration of rIL-1α was too high in their experiments compared to the levels of physiological IL-1α, causing non-specific suppression of the immune reaction.
The mechanism for this functional discrimination between IL-1α and IL-1β is not completely known, however, some possibilities are conceivable. It is known that T-cell priming occurs in periarteriolar lymphoid sheath (PALS) in lymph nodes or in the T-cell zone in the spleen through interaction with dendritic cells (DC). It was reported in studies of human DC that monocyte-derived DC, termed DC1, synthesized IL-1α but DC2, derived from plasmacytoid cells, did not.28 Furthermore, it was also reported that CD11a− CC81− MyD-1+ DC from bovine afferent lymph synthesized IL-1α and simulated both CD4+ and CD8+ T cells, while CD11a+ CC81+ MyD-1− DC that did not synthesize IL-1α could not stimulate CD8+ T cells.29 Thus, it is possible that CD4+ T cells that are involved in the humoral immune response are stimulated by a particular subpopulation of DC that synthesizes only IL-1β. As another possibility, this discrimination may occur at the B-cell maturation steps, because it was reported that IL-1β, but not IL-1α, is strongly expressed in follicular DCs in the germinal centre that plays an important role in affinity maturation and isotype switch of immunoglobulins through interaction with B cells.25 On the other hand, we previously reported that IL-1α and IL-1β are mutually inductive, and that IL-1α expression is particularly dependent on IL-1β expression.20 Thus, it is also possible that IL-1α deficiency is compensated by IL-1β, while IL-1β deficiency is not, causing detrimental effects on the immune system.
We showed that IFN-γ levels in the culture supernatants of T cells stimulated with SRBC antigens were reduced both in IL-1α/β−/− and IL-1Ra−/− mice. Since IL-1 is required for the development of antigen-specific T-cell priming and memory T-cell induction,21,30 memory T cells may develop only poorly after the first immunization in the absence of IL-1. We think this is the reason why cytokine production of both Th1/Th2 classes was reduced in IL-1-deficient mice upon secondary immunization. On the other hand, a defect of IL-1Ra would cause an excess IL-1 signal to the cells, because IL-1Ra suppresses both IL-1α and IL-1β activity. Thus, the enhancement of IL-4, IL-5 and IL-6 production and reduction of IFN-γ production suggest that IL-1 promotes development of the Th2 population. Consistent with this observation, we found that IL-4 production was enhanced in IL-1Ra−/− mice on a BALB/c background upon Nippostrongylus brasiliensis infection (Nakae et al. unpublished data). However, in contrast to these observations, it was reported that IL-1 promoted Th1 responses in mice on the BALB/c background, but not on the C57BL/6 background.9 It was also reported that Th2 cytokine production was enhanced in IL-1RI−/− mice infected with Leishmania major and immunized with KLH/CFA.18 Therefore, the effects of IL-1 deficiency and of excessive IL-1 signalling on Th1/Th2 balance seem complex. We are now further analysing the role of IL-1 in Th1/Th2 differentiation using the Nippostrongylus brasiliensis infection and Schistosoma egg injection systems (Th2 response) as well as the bacillus Calmette–Guérin infection system (Th1).
We found that IgG production specific to SRBC was enhanced in IL-1Ra−/− mice, indicating that excess IL-1 signal accelerates the immune response. One exception was the effects on IgM production; IL-1 deficiency reduced IgM production, whereas IL-1Ra deficiency did not affect this at all, showing that excess IL-1 signal alone cannot enhance IgM production.
We also found that TNP-specific antibody production of both IgM and IgG subclasses was markedly reduced in IL-1α/β−/− mice when those mice were immunized with TNP-Ficoll. Although antibody production to type 2 TI antigens requires T-cell help, the mechanism of this function is thought to be different from that of TD antigens.24 Recently, we have shown that IL-1 enhances T-dependent antibody production through induction of CD40L and OX40 on T cells.21 It is known, however, that the antibody production against type 2 TI antigens is neither dependent on interactions between TCR and major histocompatibility complex class II, CD28 and CD80/CD86, CD40L and CD40, nor OX40 and OX40L.31–35 Thus, the mechanisms in which IL-1 is involved may be different between responses against type 2 TI antigens and TD antigens.
In conclusion, these observations indicate that IL-1β, but not IL-1α, is a potent activator of the humoral immune response, and that IL-1Ra has important regulatory functions in the immune system. The immune enhancing activity of IL-1 should be of benefit for the host defence mechanism because IL-1 produced upon infection with bacteria or viruses would enhance the immune response against these pathogens.
Acknowledgments
We thank Dr Hideo Nariuchi (Institute of Medical Science, University of Tokyo) for critically reading the manuscript and for valuable discussions. We would also like to thank Dr Hideo Nariuchi for TNP-Ficoll, Dr Ryo Abe (Research Institute for Biological Science, Science University of Tokyo) for anti-mouse CD28 monoclonal antibody and Dr Masayoshi Kohase (Department of Viral Disease and Vaccine Control, National Institute of Health, Japan) for anti-mouse IFN-γ monoclonal antibody. We also thank all the members of the laboratory for their kind discussion and help in animal care. This work was supported by grants from the Ministry of Education, Culture, Sport and Science of Japan, The Ministry of Health and Welfare of Japan, Core Research for Evolutional Science and Technology (CREST), the Japan Society for the Promotion of Science, and Pioneering Research Project in Biotechnology.
References
- 1.Dinarello CA. Biologic basis for interleukin-1 in disease. Blood. 1996;87:2095–147. [PubMed] [Google Scholar]
- 2.Tocci MJ, Schmidt JA. Interleukin-1: Structure and Function. New York: Marcel Dekker; 1997. Cytokines in Health and Disease; pp. 1–27. [Google Scholar]
- 3.Dinarello CA. Interleukin-1. Cytokine Growth Factor Rev. 1997;8:253–65. doi: 10.1016/s1359-6101(97)00023-3. [DOI] [PubMed] [Google Scholar]
- 4.Dinarello CA. Interleukin-1 and interleukin-1 antagonism. Blood. 1991;77:1627–52. [PubMed] [Google Scholar]
- 5.Falk W, Mannel DN, Darjes H, Krammer PH. IL-1 induces high affinity IL-2 receptor expression of CD4−8− thymocytes. J Immunol. 1989;143:513–7. [PubMed] [Google Scholar]
- 6.Taira S, Kato T, Yamamoto K, Inoue T, Nariuchi H. Differential requirement for humoral factors for IL-2R expression of murine T cell subsets, Th1, Th2, and CD8Th clones. Cell Immunol. 1993;147:41–50. doi: 10.1006/cimm.1993.1046. [DOI] [PubMed] [Google Scholar]
- 7.Kurt-Jones EA, Hamberg S, Ohara J, Paul WE, Abbas AK. Heterogeneity of helper/inducer T lymphocytes. I. Lymphokine production and lymphokine responsiveness. J Exp Med. 1987;166:1774–87. doi: 10.1084/jem.166.6.1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hüber M, Beuscher HU, Rohwer P, Kurrle R, Rollinghoff M, Lohoff M. Costimulation via TCR and IL-1 receptor reveals a novel IL-1α-mediated autocrine pathway of Th2 cell proliferation. J Immunol. 1998;160:4242–7. [PubMed] [Google Scholar]
- 9.Shibuya K, Robinson D, Zonin F, et al. IL-1α and TNFα are required for IL-12-induced development of Th1 cells producing high levels of IFN-γ in BALB/c but not C57BL/6 mice. J Immunol. 1998;160:1708–16. [PubMed] [Google Scholar]
- 10.Skeen MJ, Ziegler HK. Intercellular interactions and cytokine responsiveness of peritoneal α/β and γ/δ T cells from Listeria-infected mice: synergistic effects of interleukin 1 and 7 on γ/δ T cells. J Exp Med. 1993;178:985–96. doi: 10.1084/jem.178.3.985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maliszewski CR, Sato TA, Vanden Bos T, Waugh S, Dower SK, Slack J, Beckmann MP, Grabstein KH. Cytokine receptors and B cell functions. I. Recombinant soluble receptors specifically inhibit IL-1- and IL-4-induced B cell activities in vitro. J Immunol. 1990;144:3028–33. [PubMed] [Google Scholar]
- 12.Staruch MJ, Wood DD. The adjuvanticity of interleukin 1 in vivo. J Immunol. 1983;130:2191–4. [PubMed] [Google Scholar]
- 13.Reed SG, Pihl DL, Conlon PJ, Grabstein KH. IL-1 as adjuvant. Role of T cells in the augmentation of specific antibody production by recombinant human IL-1α. J Immunol. 1989;142:3129–33. [PubMed] [Google Scholar]
- 14.Nencioni L, Villa L, Tagliabue A, Antoni G, Presentini R, Perin F, Silvestri S, Boraschi D. In vivo immunostimulating activity of the 163–171 peptide of human IL-1β. J Immunol. 1987;139:800–4. [PubMed] [Google Scholar]
- 15.Boraschi D, Villa L, Volpini G, et al. Differential activity of interleukin 1α and interleukin 1β in the stimulation of the immune response in vivo. Eur J Immunol. 1990;20:317–21. doi: 10.1002/eji.1830200213. [DOI] [PubMed] [Google Scholar]
- 16.Faherty DA, Claudy V, Plocinski JM, Kaffka K, Kilian P, Thompson RC, Benjamin WR. Failure of IL-1 receptor antagonist and monoclonal anti-IL-1 receptor antibody to inhibit antigen-specific immune responses in vivo. J Immunol. 1992;148:766–71. [PubMed] [Google Scholar]
- 17.Glaccum MB, Stocking KL, Charrier K, et al. Phenotypic and functional characterization of mice that lack the type I receptor for IL-1. J Immunol. 1997;159:3364–71. [PubMed] [Google Scholar]
- 18.Satoskar AR, Okano M, Connaughton S, Raisanen-Sokolwski A, David JR, Labow M. Enhanced Th2-like responses in IL-1 type 1 receptor-deficient mice. Eur J Immunol. 1998;28:2066–74. doi: 10.1002/(SICI)1521-4141(199807)28:07<2066::AID-IMMU2066>3.0.CO;2-X. 10.1002/(sici)1521-4141(199807)28:07<2066::aid-immu2066>3.3.co;2-o. [DOI] [PubMed] [Google Scholar]
- 19.Horai R, Saijo S, Tanioka H, et al. Development of chronic inflammatory arthropathy resembling rheumatoid arthritis in interleukin 1 receptor antagonist-deficient mice. J Exp Med. 2000;191:313–20. doi: 10.1084/jem.191.2.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Horai R, Asano M, Sudo K, Kanuka H, Suzuki M, Nishihara M, Takahashi M, Iwakura Y. Production of mice deficient in genes for interleukin (IL)-1α, IL-1β, IL-1α/β, and IL-1 receptor antagonist shows that IL-1β is crucial in turpentine-induced fever development and glucocorticoid secretion. J Exp Med. 1998;187:1463–75. doi: 10.1084/jem.187.9.1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nakae S, Asano M, Horai R, Sakaguchi N, Iwakura Y. IL-1 enhances T cell-dependent antibody production through induction of CD40 ligand and OX40 on T cells. J Immunol. 2001;167:90–7. doi: 10.4049/jimmunol.167.1.90. [DOI] [PubMed] [Google Scholar]
- 22.Kelly BS, Levy JG, Sikora L. The use of the enzyme-linked immunosorbent assay (ELISA) for the detection and quantification of specific antibody from cell cultures. Immunology. 1979;37:45–52. [PMC free article] [PubMed] [Google Scholar]
- 23.Gustavsson S, Hjulstrom-Chomez S, Lidstrom BM, Ahlborg N, Andersson R, Heyman B. Impaired antibody responses in H-2Ab mice. J Immunol. 1998;161:1765–71. [PubMed] [Google Scholar]
- 24.Mond JJ, Lees A, Snapper CM. T cell-independent antigens type 2. Annu Rev Immunol. 1995;13:655–92. doi: 10.1146/annurev.iy.13.040195.003255. [DOI] [PubMed] [Google Scholar]
- 25.Toellner KM, Scheel-Toellner D, Sprenger R, Duchrow M, Trumper LH, Ernst M, Flad HD, Gerdes J. The human germinal centre cells, follicular dendritic cells and germinal centre T cells produce B cell-stimulating cytokines. Cytokine. 1995;7:344–54. doi: 10.1006/cyto.1995.0044. 10.1006/cyto.1995.0044. [DOI] [PubMed] [Google Scholar]
- 26.Borriello F, Sethna MP, Boyd SD, et al. B7–1 and B7–2 have overlapping, critical roles in immunoglobulin class switching and germinal center formation. Immunity. 1997;6:303–13. doi: 10.1016/s1074-7613(00)80333-7. [DOI] [PubMed] [Google Scholar]
- 27.McAdam AJ, Greenwald RJ, Levin MA, Chernova T, Malenkovich N, Ling V, Freeman GJ, Sharpe AH. ICOS is critical for CD40-mediated antibody class switching. Nature. 2001;409:102–5. doi: 10.1038/35051107. 10.1038/35051107. [DOI] [PubMed] [Google Scholar]
- 28.Rissoan MC, Soumelis V, Kadowaki N, Grouard G, Briere F, de Waal Malefyt R, Liu YJ. Reciprocal control of T helper cell and dendritic cell differentiation. Science. 1999;283:1183–6. doi: 10.1126/science.283.5405.1183. [DOI] [PubMed] [Google Scholar]
- 29.Hope JC, Sopp P, Collins RA, Howard CJ. Differences in the induction of CD8+ T cell responses by subpopulations of dendritic cells from afferent lymph are related to IL-1a secretion. J Leukoc Biol. 2001;69:271–9. [PubMed] [Google Scholar]
- 30.Nakae S, Naruse-Nalajima C, Sudo K, Horai R, Asano M, Iwakura I. IL-1alpha, but not IL-1beta, is required for contact-allergen-specific T cell activation during the sensitization phase in contact hypersensitivity. Int Immunol. 2001. in press. [DOI] [PubMed]
- 31.Gosgrove D, Gray D, Dierich A, Kaufman J, Lemeur M, Benoist C, Mathis D. Mice lacking MHC class II molecules. Cell. 1991;66:1051–66. doi: 10.1016/0092-8674(91)90448-8. [DOI] [PubMed] [Google Scholar]
- 32.Sethna MP, van Parijs L, Sharpe AH, Abbas AK, Freeman GJ. A negative regulatory function of B7 revealed in B7–1 transgenic mice. Immunity. 1994;1:415–21. doi: 10.1016/1074-7613(94)90072-8. [DOI] [PubMed] [Google Scholar]
- 33.Xu J, Foy TM, Laman JD, et al. Mice deficient for the CD40 ligand. Immunity. 1994;1:423–31. doi: 10.1016/1074-7613(94)90073-6. [published erratum appears in Immunity 1994; 1:613] [DOI] [PubMed] [Google Scholar]
- 34.Stüber E, Strober W. The T cell–B cell interaction via OX40-OX40L is necessary for the T cell-dependent humoral immune response. J Exp Med. 1996;183:979–89. doi: 10.1084/jem.183.3.979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pippig SD, Pena-Rossi C, Long J, et al. Robust B cell immunity but impaired T cell proliferation in the absence of CD134 (OX40) J Immunol. 1999;163:6520–9. [PubMed] [Google Scholar]