Abstract
Normal human B lymphocytes activate the alternative pathway of complement via complement receptor type 2 (CR2, CD21), that binds hydrolysed C3 (iC3) and thereby promotes the formation of a membrane-bound C3 convertase. We have investigated whether this might lead to the generation of a C5 convertase and consequent formation of membrane attack complexes (MAC). Deposition of C3 fragments and MAC was assessed on human peripheral B lymphocytes in the presence of 30% autologous serum containing 4·4 mm MgCl2/20 mm EGTA, which abrogates the classical pathway of complement without affecting the alternative pathway. Blockade of the CR2 ligand-binding site with the monoclonal antibody FE8 resulted in 56 ± 13% and 71 ± 9% inhibition of the C3-fragment and MAC deposition, respectively, whereas the monoclonal antibody HB135, directed against an irrelevant CR2 epitope, had no effect. Blockade of the CR1 binding site with the monoclonal antibody 3D9 also resulted in a minor reduction in MAC deposition, while FE8 and 3D9, in combination, markedly reduced deposition of both C3 fragments (91 ± 5%) and C9 (95 ± 3%). The kinetics of C3-fragment and MAC deposition, as well as the dependence of both processes on CR2, indicate that MAC formation is a consequence of alternative pathway activation.
Introduction
Normal human B lymphocytes have been shown to activate the alternative pathway of the complement cascade, resulting in the deposition of C3 fragments on the B-cell surface.1,2 The activation is mediated by complement receptor type 2 (CR2, CD21), which binds the hydrolysed form of C3 (iC3) and thereby promotes the formation of a membrane-associated C3 convertase, consisting of iC3, Bb and properdin.3 CR2 also serves as the preferred acceptor site for C3b on the B-cell surface.3,4 C3b deposited at acceptor sites on the B-cell membrane is subsequently degraded to iC3b and thereafter to C3dg, with B-cell complement receptor type 1 (CR1, CD35) acting as co-factor in both cleavage processes.5 These membrane-bound C3dg fragments can then act as a ligand for interaction with CR2 expressed either on the same cell or on other CR2-positive cells, i.e. B cells, follicular dendritic cells,6 or activated T cells.7
The biological implications of this activation remain poorly defined. Early studies with Epstein–Barr virus-transformed lymphoblastoid cell lines indicated that C3-fragment deposition rendered these cells more susceptible to cell-mediated cytolysis, presumably involving interaction of deposited iC3b on the target cells with complement receptor type 3 (CR3, CD11b/CD18) on the effector cells.8 However, since the majority of these cell lines lack CR18 and thereby are unable to protect themselves by converting iC3b to C3dg,9 the relevance of this finding for normal CR1-expressing B cells remains questionable. More recent studies, in the murine system, suggest that C3dg deposited on B cells, and other antigen-presenting cells, may promote the response to soluble antigen by enhancing interactions between the cells involved in the response.10 No such role has yet been demonstrated in humans.
A further possibility requiring investigation is that formation of a C3 convertase in the ligand-binding site of CR2 may be succeeded by the generation of a C5 convertase, either at the same site or on C3b deposited at secondary sites on the cell surface. This would potentiate the formation of membrane attack complexes (MAC) on the B cells. In the present study, we demonstrate deposition of MAC on B cells, following alternative pathway activation, and report on the influence of the complement receptors, CR1 and CR2, on this process.
Materials and methods
Cells and serum
Peripheral blood mononuclear cells (PBMC) were isolated by the centrifugation, over Lymphoprep (Nycomed, Oslo, Norway), of blood drawn from healthy consenting donors into sodium citrate-containing tubes (Meda, Copenhagen, Denmark). Serum was harvested from the same donors by collecting blood in anticoagulant-free tubes, which were held for 1 hr at room temperature, before centrifugation at 400 g for 5 min.
Complement activation
The PBMC were washed twice in 10 ml veronal buffer or RPMI-1640, suspended in low-absorbing polypropylene tubes (Life Technologies, Paisley, UK), at a density of 106 cells per ml, in veronal buffer or RPMI-1640 containing 30% v/v autologous serum and 20 mm ethyleneglycoltetraacetic acid (EGTA)/4·4 mm MgCl2 or 20 mm ethylenediaminetetraacetic acid (EDTA), and incubated at 37°C for the indicated times. The cells were then washed once in phosphate-buffered saline containing 0·5% bovine serum albumin.
Experiments with a C8-deficient serum (kindly donated by Dr Claus Koch, Statens Seruminstitut, Copenhagen, Denmark) were performed with PBMC from a blood group O donor, using autologous serum and sera from three other donors as positive controls.
Antibodies against complement receptors
The binding-site-blocking anti-CR2 monoclonal antibody (mAb) FE8 [immunoglobulin G1 (IgG1)] was prepared as described11 and used for receptor-blockade at a concentration of 1 µg/ml. The mAb 3D9 (IgG1), which blocks the C3b binding site of CR1,12 was a kind gift from Dr J. O'Shea (Frederick Cancer Research and Development Center, Frederick, MD), and was used either singly or in combination with FE8 at a final concentration of 1 µg/ml. HB135 (IgG2a, anti-CR2, non-function-blocking, 1 µg/ml) and HB8592 (IgG1, anti-CR1, non-function-blocking, 1 µg/ml) were purchased from the American Type Culture Collection (Manassas, VA) and served as controls. The antibodies were added to the cell samples prior to mixture with serum and were present during the entire incubation period.
Measurement of C3 and C9 complement fragment deposition on PBMC
After incubation with serum, the cells were incubated for 1 hr at room temperature with either (i) fluorescein isothiocyanate (FITC)-conjugated polyclonal rabbit anti-human C3d (DAKO, Copenhagen, Denmark); (ii) a monoclonal murine anti-C3d IgG1 antibody (Quidel Corporation, San Diego, CA), conjugated to a FITC:antibody ratio of 4·0:1 in this laboratory; or (iii) the anti-human C9 mAb E1113 directed against a neoepitope only exposed on C9 associated with MAC (kindly donated by Dr T. E. Mollnes, Nordland Central Hospital, Bodø, Norway), which was conjugated to a FITC:antibody ratio of 4·3:1. B cells, in the PBMC preparations, were labelled concurrently by inclusion of phycoerythrin-conjugated anti-CD19 in the incubation.
After one further wash, the cells were suspended in Sheath-buffer (Becton-Dickinson, Copenhagen, Denmark) and analysed by flow cytometry, using a FACScalibur cytometer (Becton Dickinson) with Cellquest software. B cells were identified by a combination of morphological (forward and side scatter) and fluorescence gating. Specific C3-fragment and C9 deposition on B cells was measured as the mean fluorescence intensity (MFI) of CD19-positive cells minus the MFI for CD19-negative lymphocytes and is given as ‘Net MFI’.
Statistics
The Student's t-test was used to calculate P-values and confidence intervals for comparison of the means in C3-fragment and C9 deposition on B cells.
Results
Demonstration of C9 deposition
In view of our previous demonstration that CR2 on normal peripheral B cells activates the alternative pathway of complement,1–3 resulting in the deposition of C3 fragments at secondary acceptor sites, we wished to establish whether this activation also led to the formation of MAC in the B-cell membrane. To this end, PBMC were incubated for 90 min with 30% autologous serum in veronal buffer containing 4·4 mm MgCl2 and 20 mm EGTA, to block classical pathway contribution, and were then probed with FITC-labelled anti-C9 mAb E11. In parallel, FITC-conjugated polyclonal rabbit anti-human C3d was used to assess C3-fragment deposition. As a negative control, PBMC incubated with autologous serum in the presence of 20 mm EDTA were treated alike.
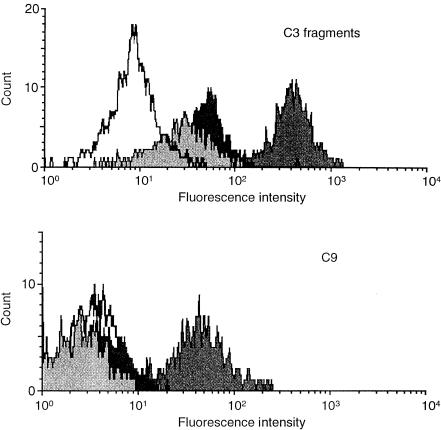
A significant level of C9 deposition was observed on B cells but not other lymphocytes in the preparation, while little or no deposition was seen when EDTA was present (Fig. 1, lower panel). A similar pattern was observed for C3-fragment deposition (Fig. 1, upper panel), although the background values in EDTA-serum or RPMI-1640 were somewhat higher, reflecting the presence of in vivo-deposited C3d fragments on the B cells.1
Figure 1.
Deposition of C3 fragments and C9 on peripheral B cells. Histograms displaying the fluorescence intensity of B cells stained with FITC-conjugated polyclonal anti-human C3d (upper panel) and the anti-C9 mAb, E11, following incubation for 90 min in normal human serum (NHS)/Mg/EGTA (dark grey peak), NHS/EDTA (black peak) or RPMI-1640 (light grey peak). The white peak depicts non-B lymphocytes in the PBMC preparation incubated in NHS/Mg/EGTA, which was used in all subsequent experiments as the background value.
Kinetics of alternative-pathway-mediated C3-fragment and C9 deposition on B cells
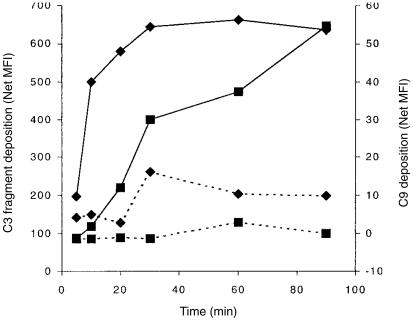
In order to compare the kinetics of the two processes, PBMC were incubated for various times with autologous serum in RPMI-1640 containing Mg/EGTA or EDTA and then probed with antibodies against C3d or C9. The deposition of C3 fragments occurred rapidly, reaching a plateau value after about 30 min (Fig. 2) while incorporation of C9 into the B-cell membrane was delayed in relation to that for C3 fragments, consistent with dependence of MAC formation upon the activation of C3. Furthermore, the C9 deposition failed to reach a plateau value during the observed time-span (Fig. 2). On this basis, a 90-min incubation period was chosen for all further studies.
Figure 2.
The kinetics of AP-mediated C3-fragment deposition and C9 incorporation. The mean fluorescence intensity (MFI) of B cells stained with polyclonal anti-C3d (♦) or mAb E11 (▪) following incubation for various times with normal human serum (NHS)/Mg/EGTA (solid lines) or NHS/EDTA (broken lines). The data shown are for a representative experiment from the four performed.
Dependency of C9 incorporation on C8
In order to confirm that the deposited C9 was in the form of a MAC, PBMC from a blood group O donor were incubated with either autologous serum, sera from three healthy donors, or serum from a C8-deficient individual. Although the extent of C3-fragment deposition with C8-deficient serum was only 19·7% of that seen with autologous serum and considerably lower than that seen with the other normal donor sera (36·1 ± 2·9%), it was nevertheless appreciable (see Table 1). On the other hand, no C9 deposition was observed with the C8-deficient serum while, with the normal donor sera, C9 incorporation was roughly proportional to the C3-fragment deposition capacity (Table 1).
Table 1.
C3-fragment deposition and MAC formation on B cells following incubation with various sera
| Serum | C3-fragment deposition* | % of autologous | C9 deposition* | % of autologous | Ratio‡ |
|---|---|---|---|---|---|
| Autologous† | 142 | 100 | 43·3 | 100 | 1:1 |
| Donor 1 | 71·6 | 50·4 | 20·1 | 46·4 | 1:0·92 |
| Donor 2 | 83·6 | 58·9 | 17·2 | 39·7 | 1:0·67 |
| Donor 3 | 78·1 | 55·0 | 18·1 | 41·8 | 1:0·76 |
| C8 deficient† | 28·0 | 19·7 | 0·2 | 0·5 | 1:0·025 |
Difference in values (mean fluoresence intensity units, MFI) for cells incubated in normal human serum (NHS) or NHS/EDTA, upon probing with FITC-conjugated anti-C3d and anti-C9 mAb, respectively.
Mean values from two experiments.
Ratio of relative efficiency for C3-fragment:C9 deposition.
The role of CR1 and CR2 in promoting C3-fragment deposition and MAC formation following activation via the alternative pathway
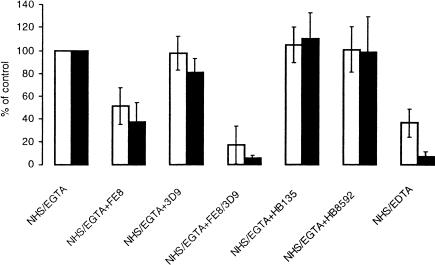
Since CR2 has previously been shown to be instrumental in the alternative-pathway-mediated C activation process that leads to C3-fragment deposition on B cells,1,2 we chose to investigate whether the formation of MAC was equally dependent on the function of this receptor. The PBMC suspensions were incubated with the CR2-blocking mAb FE8, while the non-blocking anti-CR2 mAb HB135 was employed as a negative control. Whereas HB135 did not affect either C3-fragment deposition or the formation of MAC on B cells, a marked inhibition of both processes (48·6 ± 20·6 and 62·9 ± 21·9% inhibition from six experiments, respectively) was seen with FE8 (Fig. 3).
Figure 3.
Influence of CR2 and CR1 blockade on C3-fragment deposition and MAC formation on B cells. The percentage of C3d-specific (open columns) and C9-specific (solid columns) fluorescence, respectively, relative to incubation in normal human serum (NHS)/Mg/EGTA, in the absence of blocking antibodies, is given. B cells were incubated under the given conditions in the presence of the CR2-blocking mAb FE8, the CR1-blocking mAb 3D9 or the control mAbs HB135, for CR2 and HB8592, for CR1. The error bars display the 95% confidence intervals for the values obtained with sera from six donors.
Furthermore, the CR1-blocking mAb 3D9, alone, had no significant influence on C3-fragment deposition, but mediated a slight, though significant, decrease in the deposition of MAC (18·8 ± 15·3%, n = 6, P < 0·05). No effect on either process was observed with the control anti-CR1 mAb HB8592. Used in combination with FE8, however, 3D9 markedly enhanced the inhibitory effect on both processes (83·0 ± 20·7% and 94·9 ± 3·3% inhibition, for C3 fragment deposition and MAC formation, respectively), as compared to FE8 alone (Fig. 3).
Discussion
We report here the novel finding that the activation of the alternative pathway of complement, mediated by CR2 on normal B cells, results not only in the deposition of C3 fragments at the cell surface, but also in the formation of MAC. This observation is in line with previous reports concerning Epstein–Barr virus-transformed B-lymphoblastoid cell-lines such as Raji.14 The kinetics of C9 deposition, which are delayed with respect to C3-fragment deposition, are consistent with the requirement of prior C3 activation, while the failure to detect cell-bound C9, following incubation with C8-deficient serum, confirms that C9 is being deposited as a MAC component. Formation of the C5 convertase, required to initiate MAC assembly, could occur via two mechanisms: first, C3b generated by the alternative pathway convertase on CR2 might deposit on the convertase itself thereby generating an effective C5 convertase; or second, nascent C3b deposited at an acceptor site elsewhere on the cell surface might combine with factor B to form first a C3 convertase and thereafter, upon incorporation of a further C3b molecule, the C5 convertase. The data derived in this study do not permit distinction between these possibilities.
Although a major role for CR2 in the generation of MAC is clearly demonstrated, the finding that blockade with FE8 leads to only partial inhibition of C3-fragment deposition and MAC formation, even though the mAb was employed at a concentration that achieved saturation binding (ref. 11 and data not shown), suggests that factors other than CR2 may also be involved. Indeed, the observation of a marginal, but significant, effect of CR1 blockade on MAC formation, as well as synergy in the inhibition of both C3-fragment deposition and MAC formation upon combined blockade, suggests that CR1 plays a subsidiary role. This finding is, at first sight, somewhat surprising, given the reported function of CR1 as a negative regulator of complement function at several levels including convertase decay acceleration and co-factor activity in the cleavage of C3b and, subsequently, iC3b to C3d.15,16 Thus blockade of CR1's active site with mAb 3D9 might have been expected to enhance, rather than inhibit, the deposition of C3 fragments and MAC formation. The mechanism underlying the subsidiary role of CR1 remains to be defined. One possibility is that, given its reported association with CR2,17 it might serve to stabilize or even promote formation of the convertase(s) by capturing hydrolysed C3 (C3i) from the fluid phase or nascent C3b, generated by the CR2-bound alternative pathway convertase, thereby furnishing CR2 with the components required for generating the C3 and C5 convertases, respectively. This mechanism would require that association of CR1 with CR2 leads to blockade of CR1's function as co-factor in the degradation of C3b.
The finding that alternative pathway activation via CR2 leads to MAC formation at the B-cell surface has clear biological implications. While the B cells bearing such complexes may not, by virtue of their expression of CD59,18 succumb to lytic attack, MAC deposition may induce changes in both membrane polarity19,20 and intracellular free Ca2+ concentration,21–23 leading to the activation of a range of intracellular signalling pathways.24–27 Indeed, it has previously been reported that the C5b–9 complex, incorporated into the membrane of the human lymphoblastoid B-cell line, JY25, couples with heterotrimeric G proteins,28 resulting in the activation of Ras and the mitogen-activated protein kinase pathway.29 Thus MAC incorporated into the membranes of normal B cells may influence a range of functions, including their proliferative response to antigen. Another possibility is that the presence of MAC may influence B-cell survival, either as an inducer of apoptosis30 or by conferring protection against other apoptotic stimuli.31 The consequences of MAC incorporation into the B-cell membrane are currently under investigation.
Acknowledgments
We thank Mrs Nanna Bøgesvang for excellent technical assistance and Dr Michael Schwendinger for many helpful discussions. This work was supported by grants from the Medical Research Council, the Danish Arthritis and Rheumatism Council, Novo Nordisk Fund, The Fund for the Advancement of Medical Sciences, King Christian the Tenth's Fund, Director Jacob Madsen's Fund, Grocer Sven Hansen's Fund, Katherine and Vigo Skovgaard's Fund, Ingemann O. Bucks Fund, Former Director Leo Nielsen and spouse Karen Margrethe Nielsen's Legate, Mimi and Viktor Larsen's Fund, A. J. Andersen and spouse Fund, Helen and Einar Bjørnow's Fund, Henny and Holger Holgersen's Memorial Legate as well as from the Austrian Science Funds (F-202 to WMP).
References
- 1.Marquart HV, Svehag S-E, Leslie RGQ. CR2 is the primary acceptor site for C3 during alternative pathway activation of complement on human peripheral B lymphocytes. J Immunol. 1994;153:307–15. [PubMed] [Google Scholar]
- 2.Olesen EH, Johnson AA, Damgaard G, Leslie RGQ. The requirement of localised, CR2-mediated, alternative pathway (AP) activation of complement for covalent deposition of C3 fragments on normal B Cells. Immunology. 1998;93:177–83. doi: 10.1046/j.1365-2567.1998.00429.x. 10.1046/j.1365-2567.1998.00429.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schwendinger MG, Spruth M, Schoch J, Dierich MP, Prodinger WM. A novel mechanism of alternative pathway complement activation accounts for the deposition of C3-fragments on CR2-expressing homologous cells. J Immunol. 1997;158:5455–63. [PubMed] [Google Scholar]
- 4.Mold C, Nemerow GR, Bradt BM, Cooper NR. CR2 is a complement activator and the covalent binding site for C3 during alternative pathway activation by Raji cells. J Immunol. 1988;140:1923–9. [PubMed] [Google Scholar]
- 5.Leslie RGQ. The influence of complement receptor type 1 (CD35) and decay accelerating factor (CD55) on complement receptor type 2-(CD21-) mediated alternative activation by B cells. Immunology. 1999;97:371–3. doi: 10.1046/j.1365-2567.1999.00846.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reynes M, Aubert JP, Cohen JH, Audouin J, Tricottet V, Diebold J, Kazatchkine MD. Human follicular dendritic cells express CR1, CR2 and CR3 complement receptor antigens. J Immunol. 1985;135:2687–94. [PubMed] [Google Scholar]
- 7.Fischer EM, Delibrias C, Kazatchkine MD. Expression of CR2 (the C3dg/EBV receptor, CD21) on normal human peripheral blood T lymphocytes. J Immunol. 1991;146:865–9. [PubMed] [Google Scholar]
- 8.Ramos OF, Sármay G, Klein E, Yefenof E, Gergely J. Complement-dependent cellular cytotoxicity: Lymphoblatoid lines that activate complement component 3 (C3) and express C3 receptors have increased sensitivity to lymphocyte-mediated lysis in the presence of fresh human serum. Proc Nat Acad Sci USA. 1985;82:5470–4. doi: 10.1073/pnas.82.16.5470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marquart HV, Olesen EH, Johnson AA, Damgaard G, Leslie RGQ. A comparative study of normal B cells and the EBV-positive Burkitt's lymphoma cell line, Raji, as activators of the complement system. Scand J Immunol. 1997;46:246–53. doi: 10.1046/j.1365-3083.1997.d01-122.x. [DOI] [PubMed] [Google Scholar]
- 10.Kerekes K, Prechl J, Bajtay Z, Mihály J, Erdei A. A further link between innate and adaptive immunity: C3 deposition on antigen-presenting cells enhances the proliferation of antigen-specific T cells. Int Immunol. 1998;10:1923–30. doi: 10.1093/intimm/10.12.1923. [DOI] [PubMed] [Google Scholar]
- 11.Prodinger WM, Schwendinger MG, Schoch J, Kochle M, Larcher C, Dierich MP. Characterization of C3dg binding to a recess formed between the short consensus repeats 1 and 2 of complement receptor type 2 (CR2; CD21) J Immunol. 1998;161:4604–10. [PubMed] [Google Scholar]
- 12.O'Shea JJ, Brown EJ, Seligman BE, Metcalf JA, Frank MM, Gallin JI. Evidence for distinct intracellular pools of receptors for C3b and C3bi in human neutrophils. J Immunol. 1985;134:2580–7. [PubMed] [Google Scholar]
- 13.Mollnes TE, Lea T, Harboe M, Tschopp J. Monoclonal antibodies recognising a neoantigen of poly (C9) detect the human terminal complement complex in tissue and plasma. Scand J Immunol. 1985;22:183–95. doi: 10.1111/j.1365-3083.1985.tb01870.x. [DOI] [PubMed] [Google Scholar]
- 14.Li YP, Mold C, Du Clos TW. Sublytic complement attack exposes C-reactive protein binding sites on cell membranes. J Immunol. 1994;152:2995–3005. [PubMed] [Google Scholar]
- 15.Iida K, Nussenzweig V. Complement receptor 1 is an inhibitor of the complement cascade. J Exp Med. 1981;153:1138–50. doi: 10.1084/jem.153.5.1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Medicus RG, Melamed J, Arnaout MA. Role of human factor I and C3b receptor in the cleavage of surface-bound C3bi molecules. Eur J Immunol. 1983;13:465–70. doi: 10.1002/eji.1830130607. [DOI] [PubMed] [Google Scholar]
- 17.Tuveson DA, Ahearn JM, Matsumoto AK, Fearon DT. Molecular interactions of complement receptors on B lymphocytes: a CR1/CR2 complex distinct from the CR2/CD19 complex. J Exp Med. 1991;173:1083–9. doi: 10.1084/jem.173.5.1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nagakura S, Nakakuma H, Harikawa K, Hidika M, Kagimoto T, Kawakita M, Tomita M, Takatsuki K. Expression of decay-accelerating factor and CD59 in lymphocyte subsets of healthy individuals and paroxysmal nocturnal hemoglobulinuria patients. Am J Hematol. 1993;43:14–18. doi: 10.1002/ajh.2830430105. [DOI] [PubMed] [Google Scholar]
- 19.Wiedmer T, Sims PJ. Cyanine dye fluorescence used to measure membrane potential changes due to the assembly of complement proteins C5b-9. J Membr Biol. 1985;84:249–58. doi: 10.1007/BF01871388. [DOI] [PubMed] [Google Scholar]
- 20.Wiedmer T, Sims PJ. Effect of complement proteins C5b-9 on blood platelets. Evidence for reversible depolarization of membrane potential. J Biol Chem. 1985;260:8014–19. [PubMed] [Google Scholar]
- 21.Wiedmer T, Andbo B, Sims PJ. Complement C5b-9-stimulated platelet secretion is associated with a Ca2+-initiated activation of cellular protein kinases. J Biol Chem. 1987;262:13674–81. [PubMed] [Google Scholar]
- 22.Papadimitriou JC, Phelps PC, Shin ML, Smith MW, Trump BF. Effects of Ca2+ deregulation on mitochondrial membrane potential and cell viability in nucleated cells following lytic complement attack. Cell Calcium. 1994;15:217–27. doi: 10.1016/0143-4160(94)90061-2. [DOI] [PubMed] [Google Scholar]
- 23.Laffafian I, Davies EV, Campbell AK, Hallett MB. Complement component C9-dependent cytosolic free Ca2+ rise and recovery in neutrophils. Cell Calcium. 1995;17:279–86. doi: 10.1016/0143-4160(95)90074-8. [DOI] [PubMed] [Google Scholar]
- 24.Nicholson-Weller A, Halpern JA. Membrane signalling by complement C5b-9, the membrane attack complex. Immunol Res. 1993;12:244–57. doi: 10.1007/BF02918256. [DOI] [PubMed] [Google Scholar]
- 25.Cybulsky AV, Monge JC, Papillon J, McTavish AJ. Complement C5b-9 activates cytosolic phospholipase A2 in glomerular epithelial cells. Am J Physiol. 1995;269:F739–49. doi: 10.1152/ajprenal.1995.269.5.F739. [DOI] [PubMed] [Google Scholar]
- 26.Cybulsky AV, Takano T, Papillon J, McTavish AJ. Complement C5b-9 induces tyrosine kinase transactivation in glomerular epithelial cells. Am J Pathol. 1999;155:1701–11. doi: 10.1016/S0002-9440(10)65485-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kraus S, Fishelson Z. Cell desensitisation by sublytic C5b-9 complexes and calcium ionophores depends on the activation of protein kinase C. Eur J Immunol. 2000;30:1272–80. doi: 10.1002/(SICI)1521-4141(200005)30:5<1272::AID-IMMU1272>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 28.Niculescu F, Rus H, Shin ML. Receptor-independent activation of guanine-binding regulatory proteins by terminal complement complexes. J Biol Chem. 1994;269:4417–23. [PubMed] [Google Scholar]
- 29.Niculescu F, Rus H, van Biessen T, Shin ML. Activation of Ras and mitogen-activated protein kinase pathway by terminal complement complexes is G-protein dependent. J Immunol. 1997;158:4405–12. [PubMed] [Google Scholar]
- 30.Sato T, van Dixhoorn MG, Prins FA, et al. The terminal sequence of complement plays an essential role in antibody-mediated renal cell apoptosis. J Am Soc Nephrol. 1999;10:1242–52. doi: 10.1681/ASN.V1061242. [DOI] [PubMed] [Google Scholar]
- 31.Soane L, Rus H, Niculescu F, Shin M. Inhibition of oligodendrocyte apoptosis by sublytic C5b-9 is associated with enhanced synthesis of Bcl-2 and mediated by inhibition of caspase-3 activation. J Immunol. 1999;163:6132–8. [PubMed] [Google Scholar]