Abstract
Adrenaline is a catecholamine hormone secreted by the adrenal medulla in response to acute stress. Previous studies have shown that adrenaline suppresses the nitric oxide (NO) response of murine macrophages (Mφs) stimulated in vitro with lipopolysaccharide (LPS). We have now extended these studies to examine the effects of adrenaline on the production of tumour necrosis factor alpha (TNF-α) and interleukin-10 (IL-10). Our results showed that NO, TNF-α and IL-10 were concurrently produced following in vitro LPS (10 µg/ml) stimulation of murine peritoneal Mφs. Adrenaline suppressed both NO and TNF-α with concomitant up-regulation of the IL-10 response above that seen with LPS alone. In this in vitro model of LPS stimulation we demonstrated that TNF-α was required for NO production, as the TNF-α neutralizing monoclonal antibody, TN3.19.12, abolished the response; in contrast, IL-10 suppressed NO. In order to determine any functional consequence of adrenaline-mediated IL-10 augmentation on NO production, Mφs were stimulated with LPS and specific neutralizing anti-IL-10 antibodies were added to the cultures. The LPS NO response was suppressed to 43% of the control value by adrenaline (10−8 m) and an irrelevant control antibody had no effect on the adrenaline-mediated inhibition of NO, but anti-IL-10 treatment restored the NO response to levels similar to those observed with LPS alone. Furthermore, we demonstrated that exogenous TNF-α, at a dose range of 1·9–50 ng per ml, also restored the nitrite response to LPS in the presence of adrenaline. Together, the observations that neutralization of IL-10 and addition of TNF-α abrogate adrenaline's inhibition of NO, suggest that this hormone suppresses NO partly through up-regulation of IL-10 which, in turn, may suppress TNF-α that is required for NO production. Finally, we also observed that the Mφ-activating cytokine, interferon-γ (IFN-γ), attenuated the inhibitory effect of adrenaline on the LPS NO response.
Introduction
Lipopolysaccharide (LPS) stimulates macrophages (Mφs) to produce an array of inflammatory mediators. Many of these contribute to the pathogenesis of endotoxic shock, for example the potent vasodilator nitric oxide (NO) and the cytokines tumour necrosis factor-α (TNF-α), interleukin (IL)-1, IL-6 and IL-12.1–5 In addition to these proinflammatory mediators, Mφs concurrently secrete IL-10 and transforming growth factor-beta (TGF-β). These are inhibitory cytokines that suppress the inflammatory response and thus prevent excessive and/or chronic inflammation. IL-10 and TGF-β are examples of Mφ deactivating factors, which act in an autocrine manner to reduce production of proinflammatory mediators.6,7
Alterations in the balance between proinflammatory versus inhibitory cytokines have profound effects on host immunity. This has been demonstrated through the use of exogenously administered cytokines, neutralizing monoclonal antibodies (mAbs) and genetically engineered cytokine and/or cytokine-receptor knockout mice. These studies have elegantly displayed the dual roles of proinflammatory cytokines such as TNF-α in contributing to septic shock and also in combating infection.8 In contrast, inhibitory cytokines such as IL-10 are generally important in controlling excessive inflammation during endotoxaemia,9–11 but in excess they may exacerbate infections.12,13 There is increasing evidence that agents present in the Mφ microenvironment at the time of stimulation can skew the balance of proinflammatory versus inhibitory cytokines. Immunomodulators such as prostaglandin E2,14 cAMP inducers15 and immune complexes,16 suppress TNF-α but enhance IL-10 secretion above stimulant-alone values. Conversely, the priming of Mφs with interferon-γ (IFN-γ) prior to activation augments the TNF-α response but inhibits that of IL-10.13
It is now acknowledged that products of immune cells activate the neuroendocrine system, and in turn neurotransmitters and hormones regulate immunity (reviewed in ref. 17). The effect of stress on host resistance is a good example of this interaction. Adrenaline is a catecholamine hormone produced by the adrenal medulla during acute stress. Adrenaline levels increase during endotoxaemia in response to IL-1 and IL-6 (reviewed in ref. 18). We have previously reported that adrenaline suppresses LPS-stimulated NO from murine Mφs in vitro through β1- and β2-adrenergic receptors.19 In this study, we now show that inhibition of NO by adrenaline is associated with concurrent differential regulation of TNF-α and IL-10, and furthermore that potentiation of IL-10 contributes to suppression of NO in this model.
Materials and methods
Mice
Male 8–12-week-old BALB/c mice, bred at the Animal House of the University of Zimbabwe, were used in these experiments.
Reagents
LPS from Salmonella typhosa (Sigma, St Louis, MO) was used to activate Mφs for NO production. Propranolol was obtained from Sigma. Adrenaline was purchased from Datlabs (Harare, Zimbabwe). Recombinant murine TNF-α and IL-10 were from Cambridge Bioscience (Cambridge, UK). Recombinant murine IFN-γ (specific activity 5 × 106 U/mg) was obtained from Genzyme (Genzyme Corporation, Cambridge, MA). TN3.19.12, the neutralizing mAb against TNF, was kindly provided by Professor R. D. Schreiber (Department of Pathology, Washington University School of Medicine, St Louis, MO). Neutralizing anti-IL-10 mAb, SXC-1, and its isotype-matched control, J51D, were kindly provided by Schering Plough (Kenilworth, NJ).
Mφ cultures
Resident peritoneal exudate cells were harvested by lavage of the peritoneal cavities of mice with ice-cold RPMI-1640 (Highveld Biological, Lyndhurst, South Africa), supplemented with 1% heat-inactivated fetal calf serum (FCS) (Highveld Biological), 100 U/ml penicillin (Sigma), 100 µg/ml streptomycin (Sigma), 25 mm N-2hydroxyethylpiperazine-N′-2-ethanesulphonic acid (HEPES) (Sigma) and 10 mm l-glutamine (Highveld Biological) (R1). Cells were centrifuged at 4° for 7 min at 150 g and resuspended in RPMI-1640 supplemented as for R1 but containing 10% FCS (R10). Cells were counted and viability assessed using Trypan Blue dye exclusion (Sigma). For in vitro stimulation with LPS, 100 µl of the cell suspension was plated onto 96-well sterile flat-bottom tissue culture plates (Nunc, Roskilde, Denmark) to produce a final concentration of 3 × 105 cells in each well. Cells were adhered at 37° for 2 hr in a 5% CO2 incubator, then non-adherent cells were removed by washing three times in warm R10. Medium alone, LPS, or LPS together with various drugs, were added to the wells to produce a final volume of 200 µl per well. All cultures were performed in duplicate. Plates were incubated at 37° for 48 hr, after which supernatants were harvested for immediate determination of nitrite, or stored at −70° until required for cytokine analysis.
Determination of nitrite production
The NO produced by activated Mφs reacts rapidly with oxygen to produce nitrite. Therefore, nitrite levels in the supernatants of Mφ cultures were measured using the Griess reaction.20 The reactions were performed in duplicate by addition of 100 µl of fresh supernatant to 100 µl of Griess reagent (0·1% naphthlyenediamine dihydrochloride/1% sulphanilamide/2·5% H3PO4) (Sigma). After incubation at room temperature for 15 min, the absorbance was read at 562 nm using a Multiscan Plus microplate reader (Labsystems, Helsinki, Finland). The nitrite concentration was determined by comparison to a sodium nitrite (Hopkins & Williams, Chadwell Heath, UK) standard curve (range 3–200 µm).
Detection of cytokines by enzyme-linked immunosorbent assay (ELISA)
Secretion of TNF-α protein in cell suspensions was assessed by an ELISA based on a previously described method.21 In summary, microtitre plates (Nunc Maxisorp; Nunc) were coated at 4° overnight with 5 µg/ml of mAb hamster anti-murine TNF-α (TN3.19.12). Culture supernatants were added to the plates in threefold serial dilutions prepared in R10 and incubated at 37° for 2 hr. The plates were then washed six times with ELISA washing buffer (PBS with 0·05% [vol/vol] Tween-20), and polyclonal rabbit anti-murine TNF-α antibodies (at a dilution of 1:1000) were added to the plates for 45 min at 37°. After another six washes, horseradish peroxidase-conjugated goat anti-rabbit immunoglobulin (Kirkgaard & Perry Laboratories, Gaithersburg, MD) was added at a dilution of 1:10 000, the plates incubated at 37° for a further 45 min, and 1,2 phenylenediamine (OPD) (Sigma) used to develop the reaction in the presence of 30% (vol/vol) hydrogen peroxide (Sigma). For detection of IL-10, undiluted culture supernatants were used in a protocol similar to that described above, but 1·5 µg/ml of the rat anti-murine IL-10 mAb JES5-SXC-1 (Cambridge Bioscience) was used as the capture reagent, followed by 1·5 µg/ml biotinylated rat anti-murine IL-10 mAb, JES5-2A5 (Cambridge Bioscience), for detection, and then streptavidin peroxidase conjugate (Tago Immunologicals of Bio Source, Camarillo, CA) at a dilution of 1:2000. The IL-10 ELISA was developed using OPD substrate (Sigma) in the presence of 30% (vol/vol) hydrogen peroxide (Sigma), according to the manufacturer's instructions. Recombinant murine TNF-α or IL-10, obtained from Cambridge Bioscience, was used to generate standard curves from which the respective cytokine concentrations were calculated. The absorbances for TNF-α and IL-10 ELISAs were read at 492 nm using a Multiscan Plus plate reader (Labsystems). All tests were performed in duplicate. The limits of detection of the assays were 4 ng/ml for TNF-α and 439 pg/ml for IL-10.
Statistical analysis
Results are expressed as the mean±standard error of the mean (SEM). Statistical analysis was performed using the VassarStat program by one-way analysis of variance followed by the Tukey Test where appropriate. A P-value of < 0·05 was considered significant.
Results
Adrenaline inhibits Mφ NO and TNF-α but enhances IL-10 responses to LPS
Initial experiments established the dose–response and kinetics of NO, TNF-α and IL-10 production from primary murine Mφs stimulated with LPS. LPS at 3, 10 and 30 µg/ml induced nitrite levels in culture supernatants of 48 ± 1, 71 ± 2 and 121 ± 2 µm, respectively, while cells cultured in medium alone produced only 5 ± 1 µm. In addition, these same LPS concentrations stimulated TNF-α (17 ± 3, 29 ± 1 and 41 ± 3 ng/ml, respectively, and cells in medium alone 6 ± 1 ng/ml) and IL-10 (765 ± 38, 1120 ± 24 and 1668 ± 52 pg/ml, respectively, and cells in medium alone 552 ± 11 pg/ml). The NO and TNF-α responses to LPS were detectable as early as 6 hr poststimulation, whereas IL-10 appeared later. All responses increased with time and were maximal at 48 hr. On the basis of these results, Mφs were stimulated in vitro with LPS (10 µg/ml) for 48 hr, and supernatants harvested for nitrite and cytokine determination.
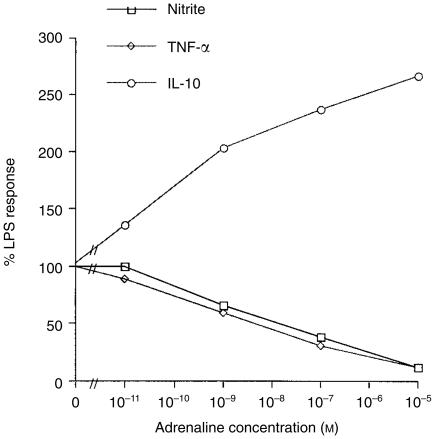
To assess the effects of adrenaline on these inflammatory mediators, Mφs were cultured in medium alone, or in medium containing LPS in the presence or absence of adrenaline. Additional control wells were cultured with adrenaline alone. LPS induced production of nitrite (medium alone: 4 ± 1 µm; LPS: 65 ± 1 µm), TNF-α (medium alone: 4 ± 1 ng/ml; LPS: 27 ± 1 ng/ml) and IL-10 (medium alone: 484 ± 64 pg/ml; LPS: 1543 ± 37 pg/ml). Adrenaline inhibited both the nitrite and TNF-α responses in a dose-dependent manner (see Fig. 1). At an adrenaline concentration of 10−5 m, both NO and TNF-α were inhibited to 12% and 11% of their control values, respectively. In contrast, adrenaline concomitantly augmented the IL-10 response above the values seen with LPS alone, such that at the maximal adrenaline concentration (10−5 m), the IL-10 response was 266% of that of the control value.
Figure 1.
Effects of adrenaline on macrophage (Mφ) nitrite, tumour necrosis factor-α (TNF-α) and interleukin-10 (IL-10) responses to lipopolysaccharide (LPS) stimulation. Murine peritoneal Mφs were cultured in medium alone, with LPS (10 µg/ml), or with LPS in the presence or absence of adrenaline. Additional control wells were cultured with adrenaline alone. After 48 hr, culture supernatants were harvested and nitrite levels determined by the Griess reaction, and TNF-α and IL-10 by specific enzyme-linked immunosorbent assay (ELISA). Results are expressed as mean±SEM and are representative of three similar experiments.
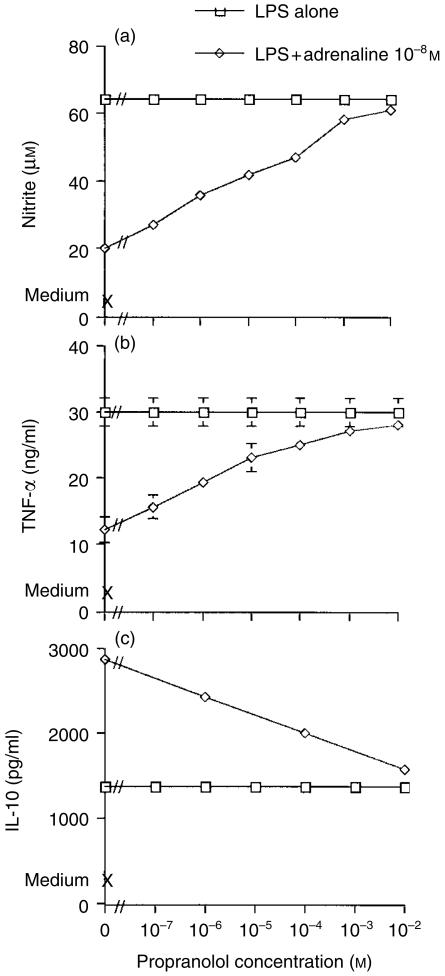
We have previously shown that adrenaline-induced inhibition of the Mφ LPS nitrite response was mediated via β1- and β2-adrenergic receptors.19 To examine the role of β-receptors in the cytokine changes effected by adrenaline, cells were cultured in medium alone, with LPS alone, or with LPS plus adrenaline (10−8 m) in the presence or absence of propranolol, a non-selective β-receptor antagonist. As observed previously, LPS induced nitrite (medium alone: < 4 ± 1 µm; LPS: 64 ± 1 µm), TNF-α (medium alone: 4 ng/ml; LPS: 30 ± 2 ng/ml) and IL-10 (medium alone: 550 ± 63 pg/ml; LPS: 1355 ± 55 pg/ml) from Mφ cultures. Nitrite and TNF-α were inhibited to 31% and 40% of the control values, respectively, by adrenaline at 10−8 m, but in contrast IL-10 was augmented to 212%. Propranolol alone had no effect on Mφ nitrite or cytokine responses to LPS. However, propranolol attenuated the adrenaline effects on nitrite, TNF-α and IL-10 (see Fig. 2). Similar results on the effects of propranolol were obtained even in the presence of adrenaline at 10−5 m (results not shown). In all experiments, the ameliorating effect of propranolol on nitrite and TNF-α was consistently more profound than its effect on the IL-10 response. These results therefore confirm our previous observations regarding the role of β-receptors in modulating NO,19 and also suggest a role for β-adrenergic regulation of Mφ-derived TNF-α and IL-10.
Figure 2.
Effects of propranolol on adrenaline-mediated modulation of macrophage (Mφ) nitrite, tumour necrosis factor-α (TNF-α) and interleukin-10 (IL-10) responses to lipopolysaccharide (LPS). Murine peritoneal Mφs were cultured in medium alone, with LPS (10 µg/ml), or with LPS plus adrenaline (10−8 m) in the presence or absence of the β-receptor antagonist propranolol. Additional control wells were cultured with either adrenaline or propranolol alone. After 48 hr, culture supernatants were harvested and nitrite levels determined by the Griess reaction, and TNF-α and IL-10 by specific enzyme-linked immunosorbent assay (ELISA). Results are expressed as mean±SEM and are representative of three similar experiments.
Macrophage NO response to LPS is regulated by TNF-α and IL-10
Macrophages concurrently produce the proinflammatory cytokine TNF-α and the immunosuppressive cytokine IL-10. Therefore, experiments were subsequently performed to examine how TNF-α and IL-10 affect Mφ NO production.
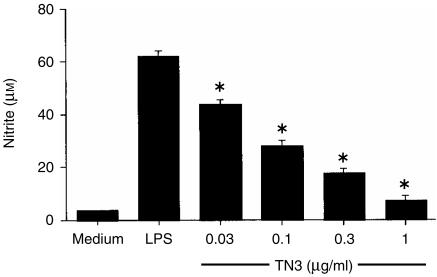
To examine the role of TNF-α in the LPS nitrite response, Mφs were cultured in medium alone, or with LPS (10 µg/ml) in the presence or absence of neutralizing anti-TNF-α antibodies. LPS induced a nitrite response (medium alone: 7 ± 1 µm, LPS: 62 ± 2 µm) which was inhibited in a dose-dependent manner by TN3.19.12, such that the highest concentration of mAb (1 µg/ml) suppressed the nitrite response to 12% of the LPS-alone value (Fig. 3). This result suggests that in this in vitro model, TNF-α is necessary for NO production.
Figure 3.
Effect of tumour necrosis factor-α (TNF-α) neutralization on the macrophage (Mφ) nitrite response to lipopolysaccharide (LPS). Murine peritoneal Mφs were cultured in medium alone, or with LPS (10 µg/ml) in the presence or absence of the neutralizing anti-TNF-α antibody TN3.19.12. After 48 hr, culture supernatants were harvested and nitrite levels determined by the Griess reaction. Results are expressed as mean±SEM and are representative of two similar experiments. *P < 0·01, TN3.19.12 treatment compared to the LPS-alone control.
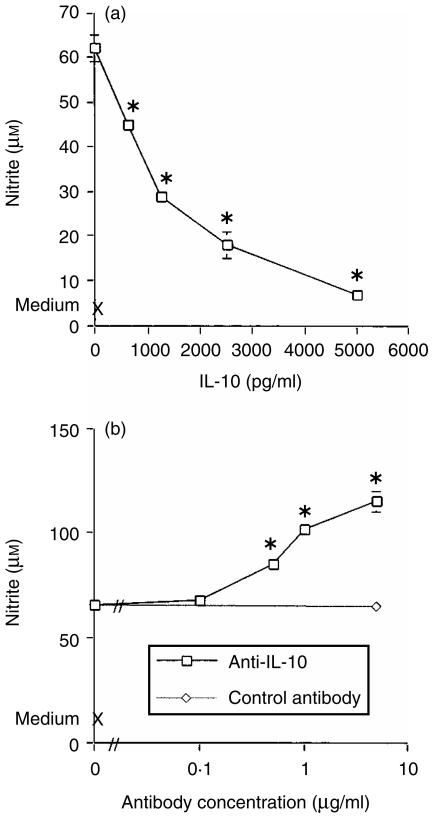
Further experiments were conducted to investigate the role of IL-10 in NO production. Here, Mφs were stimulated with LPS in the presence of exogenous IL-10. LPS induced nitrite (62 ± 1 µm), which was inhibited in a dose-dependent manner by IL-10, with suppression to 11% of the LPS-alone value at an IL-10 concentration of 5000 pg/ml (Fig. 4a). In contrast to these observations, the addition of specific anti-IL-10 antibodies to cultures potentiated the nitrite response to levels above those induced by LPS alone. However, the irrelevant isotype-matched control antibody, J51D, had no effect on the nitrite response (Fig. 4b). Anti-IL-10 mAb had no effect on nitrite production in the absence of LPS (data not shown). These results suggest that TNF-α and IL-10 have contrasting effects on Mφ NO production: TNF-α is required for NO production whereas IL-10 suppresses the response.
Figure 4.
Effects of interleukin-10 (IL-10) on the macrophage (Mφ) nitrite response to lipopolysaccharide (LPS). Murine peritoneal Mφs were cultured in medium alone, with LPS (10 µg/ml), or with LPS in the presence of either IL-10 (a), or the anti-IL-10 neutralizing antibody SXC-1 and its irrelevant isotype-matched control antibody, J51D (b). After 48 hr, culture supernatants were harvested and nitrite levels determined by the Griess reaction. Results are expressed as mean±SEM and are representative of two similar experiments. *P < 0·01 treatment groups compared to the LPS-alone control.
Adrenaline-mediated augmentation of IL-10 contributes to inhibition of nitrite
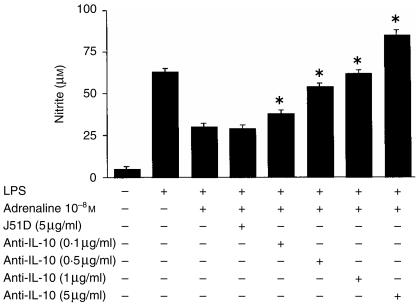
The above results showed that IL-10 inhibited Mφ production of NO. As the presence of adrenaline augmented the Mφ IL-10 response to LPS, we postulated that this increase may contribute to the suppression of NO by adrenaline. Therefore, to investigate this, Mφs were cultured in medium alone, with LPS alone (10 µg/ml), with LPS plus adrenaline at 10−8 m and with LPS plus adrenaline in the presence of neutralizing anti-IL-10 mAb or its irrelevant control antibody. As observed previously, the LPS-induced nitrite response was inhibited by the presence of adrenaline (medium alone: 5 ± 1 µm, LPS alone: 60 ± 2 µm, LPS plus adrenaline: 26 ± 2 µm). Specific neutralizing anti-IL-10 antibodies at 5 µg/ml completely abrogated the suppressive effect of adrenaline on the NO response, with an ameliorating effect observed with as little as 0·1 µg/ml of the antibody. In contrast, even the highest dose of the control antibody, J51D, had no effect on the adrenaline-mediated inhibition of nitrite (see Fig. 5).
Figure 5.
Interleukin-10 (IL-10) neutralization restores the nitrite response in the presence of adrenaline. Murine peritoneal macrophages (Mφs) (105) were cultured in medium alone, with lipopolysaccharide (LPS) alone (10 µg/ml), or with LPS plus adrenaline (10−8 m) in the presence or absence of either the anti-IL-10 neutralizing antibody, SXC-1, or its irrelevant isotype-matched control antibody, J51D. After 48 hr, culture supernatants were harvested and nitrite levels determined by the Griess reaction. Results are expressed as mean±SEM and are representative of two similar experiments. *P < 0·01, SXC-1 treatment compared to LPS plus adrenaline.
Exogenous TNF-α restores the nitrite response to LPS in the presence of adrenaline
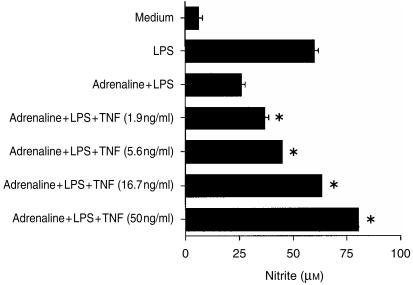
Additional experiments were performed to examine the effects of exogenous TNF-α on adrenaline's inhibition of nitrite. We have already established that TNF-α is required for nitrite production, and as IL-10 inhibits Mφ TNF-α,6 we postulated that IL-10 could suppress nitrite indirectly, via inhibition of TNF-α. Therefore, Mφs were cultured in medium alone, with LPS alone (10 µg/ml), with LPS plus adrenaline (at 10−8 m) and with LPS plus adrenaline in the presence of increasing concentrations of TNF-α, and nitrite levels were determined in supernatants after 48 hr in culture (see Fig. 6). Additional control cells were cultured with TNF-α alone. Cells cultured in medium alone produced little nitrite (5 + 1 µm). TNF-α alone at 1·9, 5·6, and 16·7 ng/ml in this experiment induced 19 ± 1, 27 ± 1 and 40 ± 1 µm nitrite, respectively, and therefore was less effective compared to high-dose LPS, which stimulated a nitrite response of 60 ± 2 µm. The LPS NO response was inhibited to 43% of the LPS-alone value by adrenaline (Fig. 6). Exogenous TNF-α attenuated adrenaline's suppression of NO in a dose-dependent manner, and TNF-α at 16·7 ng/ml completely abrogated adrenaline's effect on NO. Similar observations were made, even in the presence of a higher adrenaline concentration, where adrenaline at 10−5 m suppressed nitrite to 26% of the LPS-alone value and TNF-α at 50 ng/ml ameliorated the effect to 81% of the LPS response.
Figure 6.
Exogenous tumour necrosis factor-α (TNF-α) restores the nitrite response in the presence of adrenaline. Murine peritoneal macrophages (Mφs) (105) were cultured in medium alone, with lipopoysaccharide (LPS) alone (10 µg/ml), or with LPS plus adrenaline (10−8 m) in the presence or absence of recombinant murine TNF-α. After 48 hr, culture supernatants were harvested and nitrite levels determined by the Griess reaction. Results are expressed as mean+SEM and are representative of three similar experiments. *P < 0·01, TNF-α treatment compared to LPS plus adrenaline.
IFN-γ antagonizes the inhibitory effect of adrenaline on the Mφ nitrite response to LPS
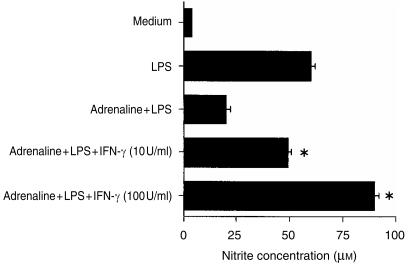
IFN-γ is a potent Mφ-activating cytokine. We have previously demonstrated that the presence of IFN-γ during Mφ activation enhances TNF-α production and concurrently suppresses IL-10.13 Thus, IFN-γ also differentially regulates TNF-α and IL-10, but in a contrasting manner to that of adrenaline. We therefore conducted additional experiments to determine whether IFN-γ could modulate adrenaline's suppression of nitrite. Mφs were cultured in medium alone, with IFN-γ alone (10 U/ml and 100 U/ml), with LPS alone (10 µg/ml), with LPS plus IFN-γ and with LPS plus adrenaline (10−7 m), in the presence or absence of recombinant IFN-γ. Cells cultured in medium alone or with IFN-γ alone produced little nitrite. LPS stimulated the production of 61 ± 2 µm nitrite, which was augmented to 123% and 148% of the control value by the presence of IFN-γ at 10 U/ml and 100 U/ml, respectively. Adrenaline suppressed the LPS NO response to 33% of the LPS-alone value, but IFN-γ at 10 U/ml ameliorated the adrenaline effect and completely abrogated it when added at 100 U/ml (see Fig. 7). Similar effects were observed with IFN-γ, even in the presence of adrenaline at 10−6 m, which inhibited NO to 13% of the LPS-alone control response.
Figure 7.
Interferon-γ (IFN-γ) abolishes the suppressive effect of adrenaline on nitrite. Murine peritoneal macrophages (Mφs) (105) were cultured in medium alone, with lipopolysaccharide (LPS) alone (10 µg/ml), with LPS plus IFN-γ (at 10 U/ml or 100 U/ml), or with LPS plus adrenaline (10−7 m) in the presence or absence of recombinant murine IFN-γ. After 48 hr, culture supernatants were harvested and nitrite levels determined by the Griess reaction. Results are expressed as mean+SEM and are representative of three similar experiments. *P < 0·01, IFN-γ treatment compared to LPS plus adrenaline.
Discussion
This study showed that the acute stress hormone, adrenaline, inhibited NO and TNF-α but concurrently up-regulated the IL-10 response to LPS, partly through a mechanism involving β-adrenergic receptors. We demonstrated that TNF-α was necessary for NO production in this in vitro model, but that IL-10 inhibited it. In addition, we examined the effects of the cytokine changes mediated by adrenaline in modulating the nitrite response of murine peritoneal Mφs to LPS. We showed first that augmentation of IL-10 by adrenaline contributed to suppression of NO and, second, that exogenous TNF-α restored the nitrite response in the presence of adrenaline. Finally, we demonstrated that the Mφ-activating cytokine, IFN-γ, antagonized the inhibitory effect of adrenaline on nitrite. Therefore, our results provide evidence that the anti-inflammatory effects of adrenaline during sepsis may be mediated, in part, by differential regulation of Mφ TNF-α versus IL-10, which in turn down-regulates NO.
Adrenaline exerts its effects by binding to and activating α and β cell-surface adrenergic receptors.22 We have previously shown that specific β1- and/or β2-adrenergic activation mediated the inhibition of NO by adrenaline; in contrast, α-receptors played no role.19 The present study confirms the role of β-receptors in NO inhibition by adrenaline, and also suggests that stimulation of β-receptors partly contributes to differential regulation of TNF-α and IL-10. These results do not exclude the possibility of a role for α-receptors in IL-10 up-regulation, as complete reversal of the adrenaline effect could not be achieved even at the highest concentration of propranolol used in this study. Indeed, other investigators have attributed a role for both α- and β-receptors in contributing to the potentiation of the IL-10 response in the presence of adrenaline.23 β-receptor stimulation activates cellular adenyl cyclase, which converts ATP to cAMP.24 Several studies have shown that agents which augment Mφ intracellular cAMP potentiate IL-10 release,15,25 and others have demonstrated the presence of cAMP response elements upstream of the IL-10 gene.26 Elevated intracellular cAMP also suppresses TNF-α and inducible nitric oxide synthase (iNOS) transcription.15,27
Our results confirm and extend previous studies which showed that adrenaline and/or β-adrenergic agonists differentially regulate TNF-α versus IL-10.23,28 Szabo et al. also showed that in addition to changes in TNF-α and IL-10, the selective β-agonist, isoproterenol, concurrently suppressedplasma nitrate and nitrite following in vivo challenge with LPS in mice.29 Our own in vitro findings, as reported here, suggest that augmentation of IL-10 in the presence of adrenaline has functional consequences which may partly explain the suppression of nitrite observed by Szabo et al. However, in contrast to our observations, stimulation of RAW 264.7 cells with LPS in the presence of a β-receptor agonist suppressed both TNF-α and NO, as well as IL-10.30 It is possible that cell lines behave differently from primary cells in culture and this may partly explain the differences in the results obtained.
In this study we showed that TNF-α contributed to NO production by LPS-stimulated Mφs, as suppression of this cytokine inhibited NO. Our observations are consistent with a previous report of in vivo studies, where endotoxin-induced TNF-α contributed to significant elevation of plasma nitrite and nitrate.31 In our study we also demonstrated an inhibitory effect of IL-10 on NO production, as reported previously.32 Therefore, in this model, TNF-α and IL-10 have contrasting effects on the Mφ NO response.
We noted that the β-adrenergic antagonist propranolol prevented down-regulation of nitrite by adrenaline and concurrently opposed the differential regulation of TNF-α and IL-10, as shown in Fig. 2. As IL-10 directly inhibits TNF-α,6 we postulated that potentiation of IL-10 by adrenaline may inhibit NO indirectly via IL-10 suppression of endogenous TNF-α. Here, we provide evidence for this mechanism by showing, first, that exogenous TNF-α (in a dose range comparable to that induced by LPS stimulation in this in vitro model) restored the nitrite response in the presence of adrenaline. Second, we demonstrated that IFN-γ, a cytokine that opposes the effects of adrenaline by augmenting TNF-α and concurrently inhibiting IL-10,13 abolished the suppressive effects of adrenaline on nitrite. IFN-γ has previously been shown to attenuate the inhibitory effects of glucocorticoids on monocytes,33 but, to the best of our knowledge, this is the first report to show that IFN-γ may also antagonize the effects of adrenaline on these cells. These observations may have important clinical implications as the presence of IFN-γ during sepsis may diminish any beneficial effects of adrenaline therapy.
The results of our study therefore provide evidence that IL-10 may be partly responsible for the inhibitory action of adrenaline on nitrite, through suppression of TNF-α. Additional evidence for the presence of such a mechanism is provided by reports in the literature that up-regulation of IL-10 by β-agonists does indeed contribute to TNF-α inhibition28 and, furthermore, that adrenaline's suppression of human whole-blood IL-1β is the result of both enhancement of IL-10 and inhibition of TNF-α.34 Together, these findings suggest the presence of complex interactions between hormonal and cytokine networks during sepsis.
Adrenaline and β-adrenergic agonists have to date been implicated in the regulation of several Mφ-derived cytokines, including TNF-α, IL-1β, IL-6, IL-8, IL-10 and IL-12.23,28,29,34–36 Modulation of these cytokines may have downstream consequences on other Mφ activities, for example inhibition of the Mφ microbicidal agent NO, as we have shown here. Similarly, noradrenaline, another catecholamine hormone, modulates peripheral blood mononuclear cell cytokine production through β-adrenergic receptors, resulting in accelerated replication of the human immunodeficiency virus (HIV).37 Collectively, these studies illustrate the importance of catecholamines in regulating immunity and thus contributing to the pathophysiology of inflammatory and/or immune disorders. Finally, the regulatory effects of β-adrenergic agonists on Mφ inflammatory mediators may have therapeutic benefits in clinical situations where chronic and/or excessive inflammation exist.38 The mechanisms described in our report may partly contribute to understanding the anti-inflammatory actions of these agents.
Acknowledgments
This study was funded by the Research Board of the University of Zimbabwe.
References
- 1.Wei X, Charles IG, Smith A, et al. Altered immune response in mice lacking inducible nitric oxide synthetase. Nature. 1995;375:408–11. doi: 10.1038/375408a0. [DOI] [PubMed] [Google Scholar]
- 2.Beutler B, Milsark IW, Cerami AC. Passive immunisation against cachectin/tumor necrosis factor protects mice from lethal effects of endotoxin. Science. 1985;229:869–71. doi: 10.1126/science.3895437. [DOI] [PubMed] [Google Scholar]
- 3.Casey LC, Balk RA, Bone RC. Plasma cytokine and endotoxin levels correlate with survival in patients with the sepsis syndrome. Ann Intern Med. 1993;119:771–8. doi: 10.7326/0003-4819-119-8-199310150-00001. [DOI] [PubMed] [Google Scholar]
- 4.Damas P, Ledoux D, Nys M, Vrindts Y, De Groote P, Franchimont P, Lamy M. Cytokine serum levels during severe sepsis in humans: IL-6 as a marker of severity. Ann Surg. 1992;215:356–62. doi: 10.1097/00000658-199204000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wysocka M, Kubin M, Vieira LQ, Ozmen L, Garotta G, Scott P, Trinchieri G. Interleukin-12 is required for interferon-γ production and lethality in lipopolysaccharide-induced shock in mice. Eur J Immunol. 1995;25:672–6. doi: 10.1002/eji.1830250307. [DOI] [PubMed] [Google Scholar]
- 6.Fiorentino DF, Zlotnik A, Mosmann TR, Howard M, O'Garra A. IL-10 inhibits cytokine production by activated macrophages. J Immunol. 1991;147:3815–22. [PubMed] [Google Scholar]
- 7.Tsunawaki S, Sporn M, Ding A, Nathan C. Deactivation of macrophages by transforming growth factor-beta. Nature. 1998;334:260–02. doi: 10.1038/334260a0. [DOI] [PubMed] [Google Scholar]
- 8.Pfeffer K, Matsuyama T, Kundig TM, et al. Mice deficient for the 55 kD tumor necrosis factor receptor are resistant to endotoxic shock, yet succumb to L. monocytogenes infection. Cell. 1993;73:457–67. doi: 10.1016/0092-8674(93)90134-c. [DOI] [PubMed] [Google Scholar]
- 9.Marchant A, Bruyns C, Vandenabeele P, et al. Interleukin 10 controls interferon-γ and tumor necrosis factor production during experimental endotoxaemia. Eur J Immunol. 1994;24:1167–71. doi: 10.1002/eji.1830240524. [DOI] [PubMed] [Google Scholar]
- 10.Gerard C, Bruyns C, Marchant A, et al. Interleukin 10 reduces the release of tumor necrosis factor and prevents lethality in experimental endotoxaemia. J Exp Med. 1993;177:547–50. doi: 10.1084/jem.177.2.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Walley KR, Lukacs NW, Standiford TJ, Strieter RM, Kunkel SL. Balance of inflammatory cytokines related to severity and mortality of murine sepsis. Infect Immun. 1996;64:4733–8. doi: 10.1128/iai.64.11.4733-4738.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bermudez LE, Champsi J. Infection with Mycobacterium avium induces production of interleukin-10 (IL-10), and administration of anti-IL-10 antibody is associated with enhanced resistance to infection in mice. Infect Immun. 1993;61:3093–7. doi: 10.1128/iai.61.7.3093-3097.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kelly JP, Bancroft GJ. Administration of interleukin 10 abolishes innate resistance to Listeria monocytogenes. Eur J Immunol. 1996;26:356–64. doi: 10.1002/eji.1830260214. [DOI] [PubMed] [Google Scholar]
- 14.Strassmann G, Patil KV, Finkelman F, Fong M, Kambayashi T. Evidence for the involvement of interleukin 10 in the differential deactivation of murine peritoneal macrophages by prostaglandin E2. J Exp Med. 1994;180:2365–70. doi: 10.1084/jem.180.6.2365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eigler A, Siegmund B, Emmerich U, Baumann KH, Hartmann G, Endres S. Anti-inflammatory activities of cAMP-elevating agents: enhancement of IL-10 synthesis and concurrent suppression of TNF production. J Leukoc Biol. 1998;63:101–7. doi: 10.1002/jlb.63.1.101. [DOI] [PubMed] [Google Scholar]
- 16.Tripp CS, Beckerman KP, Unanue ER. Immune complexes inhibit antimicrobial responses through interleukin 10 production. Effects in severe combined immunodeficient mice during Listeria infection. J Clin Invest. 1995;95:1628–34. doi: 10.1172/JCI117837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Besedovsky HO, delRey AD. Immune–neuro–endocrine interactions: facts and hypotheses. Endocr Rev. 1996;17:64–102. doi: 10.1210/edrv-17-1-64. [DOI] [PubMed] [Google Scholar]
- 18.Wilder RL. Neuroendocrine–immune system interactions and autoimmunity. Annu Rev Immunol. 1995;13:307–38. doi: 10.1146/annurev.iy.13.040195.001515. [DOI] [PubMed] [Google Scholar]
- 19.Sigola LB, Zinyama RB. Adrenaline inhibits nitric oxide production through β1 and β2 adrenergic receptors. Immunology. 2000;100:359–63. doi: 10.1046/j.1365-2567.2000.00029.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Green LC, Wagner DA, Glogowski J, Skipper PL, Wishnok JS, Tannenbaum SR. Analysis of nitrate, nitrite and [15]nitrate in biological fluids. Anal Biochem. 1982;126:131–8. doi: 10.1016/0003-2697(82)90118-x. [DOI] [PubMed] [Google Scholar]
- 21.Cross CE, Bancroft GJ. Ingestion of acapsular Cryptococcus neoformans occurs via mannose and beta-glucan receptors, resulting in cytokine production and increased phagocytosis of the encapsulated form. Infect Immun. 1995;63:2603–11. doi: 10.1128/iai.63.7.2604-2611.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Van Zwiten PA. Adrenergic and cholinergic receptors. In: Bannister R, Mathias CJ, editors. Autonomic FailureA Textbook of Clinical Disorders of the Autonomic Nervous System. Oxford: Oxford University Press; 1992. pp. 94–106. [Google Scholar]
- 23.van der Poll T, Coyle SM, Barbosa K, Braxton C, Lowry SF. Epinephrine inhibits tumor necrosis factor-α and potentiates interleukin-10 production during human endotoxaemia. J Clin Invest. 1996;97:713–9. doi: 10.1172/JCI118469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kobilka B. Adrenergic receptors as models for G protein-coupled receptors. Annu Rev Neurosci. 1992;15:87–114. doi: 10.1146/annurev.ne.15.030192.000511. [DOI] [PubMed] [Google Scholar]
- 25.Kambayashi T, Jacob CO, Zhou D, Mazurek N, Fong M, Strassmann G. Cyclic nucleotide phosphodiesterase type IV participates in the regulation of IL-10 and in the subsequent inhibition of TNF-alpha and IL-6 release by endotoxin-stimulated macrophages. J Immunol. 1995;155:4909–16. [PubMed] [Google Scholar]
- 26.Platzer C, Meisel C, Vogt K, Platzer M, Volk HD. Up-regulation of monocytic IL-10 by tumor necrosis factor-alpha and cAMP elevating drugs. Int Immunol. 1995;7:517–23. doi: 10.1093/intimm/7.4.517. [DOI] [PubMed] [Google Scholar]
- 27.Bulut V, Severn A, Liew FY. Nitric oxide production by murine macrophages is inhibited by prolonged elevation of cyclic AMP. Biochem Biophys Res Commun. 1993;195:1134–8. doi: 10.1006/bbrc.1993.2162. [DOI] [PubMed] [Google Scholar]
- 28.Suberville S, Bellocq A, Fouqueray B, Phillipe C, Lantz O, Perez J, Baud L. Regulation of interleukin-10 production by beta-adrenergic agonists. Eur J Immunol. 1996;26:2601–5. doi: 10.1002/eji.1830261110. [DOI] [PubMed] [Google Scholar]
- 29.Szabo C, Hasko G, Zingarelli B, Nemeth ZW, Salzman AL, Kvetan V, Pastores SM, Vizi ES. Isoproterenol regulates tumour necrosis factor, interleukin-10 and nitric oxide production and protects against the development of vascular hyporeactivity in endotoxaemia. Immunology. 1997;90:95–100. doi: 10.1046/j.1365-2567.1997.00137.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hasko G, Nemeth ZH, Szabo C, Zsilla G, Salzman AL, Vizi ES. Isoproterenol inhibits IL-10, TNF alpha and nitric oxide production in RAW 264.7 macrophages. Brain Res Bull. 1998;45:183–7. doi: 10.1016/s0361-9230(97)00337-7. [DOI] [PubMed] [Google Scholar]
- 31.Cunha FQ, Assreuy J, Moss DW, et al. Differential induction of nitric oxide synthase in various organs of the mouse during endotoxaemia: role of TNF-alpha and IL-1-beta. Immunology. 1994;81:211–5. [PMC free article] [PubMed] [Google Scholar]
- 32.Cunha FQ, Moncada S, Liew FY. Interleukin-10 (IL-10) inhibits the induction of nitric oxide synthase by interferon-gamma in murine macrophages. Biochem Biophys Res Commun. 1992;182:1155–9. doi: 10.1016/0006-291x(92)91852-h. [DOI] [PubMed] [Google Scholar]
- 33.Schaffner A, Rellstab P. Gamma-interferon restores listericidal activity and concurrently enhances release of reactive oxygen metabolites in dexamethasone-treated human monocytes. J Clin Invest. 1988;82:913–9. doi: 10.1172/JCI113698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Van der Poll T, Lowry SF. Epinephrine inhibits endotoxin-induced IL-1 beta production: roles of tumor necrosis factor alpha and IL-10. Am J Physiol. 1997;273:R1885–90. doi: 10.1152/ajpregu.1997.273.6.R1885. [DOI] [PubMed] [Google Scholar]
- 35.van der Poll T, Lowry SF. Lipopolysaccharide-induced interleukin-8 production by human whole blood is enhanced by epinephrine and inhibited by hydrocortisone. Infect Immun. 1997;65:2378–81. doi: 10.1128/iai.65.6.2378-2381.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Panina-Bordignon P, Mazzeo D, Di Lucia P, D'Ambrosio D, Lang R, Fabbri L, Self C, Sinigaglia F. β2-agonists prevent Th1 develpoment by selective inhibition of interleukin 12. J Clin Invest. 1997;100:1513–9. doi: 10.1172/JCI119674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cole SW, Korin YD, Fahey JL, Zack JA. Norepinephrine accelerates HIV replication via protein kinase A-dependent effects on cytokine production. J Immunol. 1998;161:610–6. [PubMed] [Google Scholar]
- 38.Malfait A, Malik AS, Marinova-Mutafchieva L, Butler DM, Mainin RV, Feldmann M. The β2-adrenergic agonist salbutamol is a potent suppressor of established collagen-induced arthritis: mechanisms of action. J Immunol. 1999;162:6278–83. [PubMed] [Google Scholar]