Abstract
This study investigates the in vitro effects of oxidized low-density lipoproteins (ox-LDL), ‘physiological’ pro-oxidants, N-acetylcysteine (NAC), a free radical scavenger and glutathione precursor, and their combination on human peripheral blood mononuclear cell functions. We found that treatment with ox-LDL induced a significant down-regulation of proliferative response to mitogens, antigens and interleukin-2. Lipid extracts from ox-LDL were able to reproduce the same effect as the lipoprotein. On the other hand, NAC exposure induced a significant up-regulation of proliferative responses to all the stimuli used. Moreover, we showed that natural killer (NK) cell-mediated cytotoxic activity was significantly down-regulated by ox-LDL while treatment with NAC induced a significant up-regulation of NK-cell activity. Finally, we found that ox-LDL and NAC exerted opposite effects on the cytokine network, interfering both at the protein secretion level and the messenger RNA synthesis level. More importantly, when NAC was used in combination with ox-LDL the proliferative responses, NK-cell-mediated cytotoxic activity and cytokine production were restored to values comparable to controls. These data indicate that ox-LDL and NAC modulate immune functions, exerting opposite effects reflecting their pro-oxidant and antioxidant behaviours. Our results add new insights to the key role played by redox imbalance as a modulator of immune system homeostasis and suggest that an antioxidant drug such as NAC could be useful against pathologies associated with an increase in lipid peroxidation.
Introduction
It has been demonstrated that oxidative imbalance and the production of reactive oxygen species (ROS) cause, or are associated with, several human degenerative diseases. In fact, cardiovascular diseases,1 aging related diseases,2 infectious diseases3 [e.g. acquired immune-deficiency syndrome (AIDS)4–6], and non-infectious diseases7 (e.g. genetic pathologies8) share redox alterations and increase ROS production as a hallmark. However, at present, several studies are in progress in order to identify the putative key molecules involved in the redox dysregulation associated with such diseases. Among these, the low-density lipoproteins (LDL) could be of great physiological relevance. Several lines of evidence support the idea that oxidative modification of LDL plays a central role in the development of atherosclerosis.9 In fact, when LDL are oxidized (ox-LDL), they exert a pro-oxidant activity leading to the formation of intracellular ROS. This can lead, depending on the dose, to an alteration of cell activities and functions or, alternatively, given in vitro at highest concentrations, to apoptosis.10,11 For instance, in patients who are positive for human immunodeficiency virus, triglyceride metabolism disorders lead to an increase of some subclasses of LDL and to the appearance of ox-LDL. These are internalized via specific receptors in several cell types including the immune system.12,13 As a consequence, different forms of ox-LDL have been reported to elicit humoral immune responses in both experimental animals and in human patients14,15 and are able to modulate various cellular immune functions contributing to the pathogenesis of different immune diseases.14,16
On the other hand, antioxidant drugs can in turn act as modulators of immune response. In particular, N-acetylcysteine (NAC), interfering with or counteracting ROS-induced subcellular changes using different mechanisms, can modulate several cell functions. This drug can in fact work per se as a free radical scavanger, as a glutathione (GSH) precursor via a ‘thiol supplier’ activity, or as a structural analogue of GSH, enriching the GSH pool in the cellular environment.17,18
It was suggested that antioxidant drug therapy could be of relevance in a plethora of human diseases sharing oxidative imbalance as a common marker. For instance, regarding NAC, several studies have been performed in the field of infectious as well of non-infectious diseases.19–24
Because ox-LDL represent ‘physiological’ pro-oxidant agents relevant in human pathology and since receptors for both LDL and ox-LDL are expressed on immune cells such as lymphocytes, natural killer (NK) cells and macrophages, we analysed the immunomodulation induced both by ox-LDL and NAC on normal human peripheral blood mononuclear cells (PBMC). We found that ox-LDL can strongly down-regulate immune functions and that NAC can, at least partially, counteract this effect.
Materials and methods
LDL isolation and oxidation
LDL (1·019–1·063 g/ml) were prepared from pooled human plasma by density gradient ultracentrifugation.25 The LDL fraction was dialysed overnight against 0·15 m NaCl and then analysed for protein content by the Bradford method using bovine serum albumin as standard. Aliquots of LDL solution (2 mg/ml), sterilized by filtration through 0·2 µm Millipore membranes, were exposed to ultraviolet-B radiation (Philips TL 20 W/12) for 6 hr (42000 J/m2). In these conditions, the ultraviolet (UV) radiant flux density was verified by an Osram Centra UV-meter (Euroseps Instruments, Germany). In the present study LDL were exposed to UV radiation to obtain ox-LDL characterized by a relatively low content of lipid peroxidation derivatives.26–28
The same LDL preparations at the same concentration, without exposure to UV, were used as controls. Lipid extracts of native LDL and ox-LDL fractions, obtained according to Folch et al.29 were analysed for their lipid composition. The fatty acids composition of phosphatidylcholine (PC) and cholesteryl ester (CE) classes was determined by the thin layer chromatography/gas–liquid chromatography technique. Oxidation resulted in a lowering of polyunsaturated fatty acid content of about 15% in PC and 50% in CE. The extent of lipid peroxidation was estimated as malondialdehyde and 4-hydroxynonenal content by a commercial kit (LPO 586, Bioxytech S.A., Bonneuil Sur Marne, France) resulting in a mean value of 40·5 ± 8·2 nmol/mg of LDL protein.
Cells preparation
Human PBMC were isolated by Ficoll–Hypaque (Flow Laboratories, Irvine, UK) gradient separation of buffy coats obtained from healthy volunteer blood donors at the Transfusion Centre of the Croce Rossa Italiana (CRI), Rome, Italy. Promonocytic U937 cell line was maintained in RPMI-1640 medium supplemented with 10% heat-inactivated fetal calf serum, l-glutamine and penicillin-streptomycin.
Treatments
LDL treatment
LDL and ox-LDL were added to the cultures according to the reported physiological concentrations in human plasma (0·2 mg/ml).30 Experiments were performed adding native LDL, ox-LDL, or their lipid extracts at the beginning of the culture period and left throughout. Experiments using trypan blue dye exclusion tests and Hoechst chromatin staining were always performed in the same conditions in order to exclude any aspecific toxicity of LDL and ox-LDL.
NAC treatment
The antioxidant compound NAC was purchased from the Zambon Group (Milano, Italy). The drug was dissolved in sterile distilled water as stock solution and diluted before experiments. The pH was adjusted to 7·4 by the addition of NaHCO3. A titration curve using 0·5, 5 and 50 mm NAC was performed. Any effect was obtained using 0·5 mm while significant effects were obtained using 5 mm and 50 mm (data not shown). Therefore, only experiments performed by using NAC at the concentration of 5 mm will be reported. Experiments by using trypan blue dye exclusion tests and Hoechst chromatin staining were always performed in order to exclude any aspecific toxicity of 0·5, 5 and 50 mm NAC.
The experiments employing ox-LDL and NAC in combination were performed adding them at the beginning of the cultures using 0·2 mg/ml ox-LDL and 5 mm NAC.
Measurement of intracellular glutathione content
Reduced GSH was measured by high-performance liquid chromatography (HPLC) according to the procedure of Reed et al.31 Briefly, PBMC (3 × 106 cells) were collected after separation and cellular pellets were deproteinized by rapid mixing with 250 ml 1% picric acid containing 0·5 mm desferal. After centrifugation at 500 g for 10 min, 100 ml acidic supernatant, containing soluble (non-protein) thiols, including GSH, cysteine and cystine, were derivatized for HPLC measurements. Samples (100 ml) were mixed with 50 ml of 100 mm iodoacetic acid solution in the presence of excess sodium bicarbonate. The reaction was allowed to proceed for 1 hr in the dark; then 100 ml of 0·75% of dinitrofluorobenzene in HPLC-grade absolute ethanol (Sanger reagent) was added and allowed to react overnight in the dark. Aliquots of 25 ml were subjected to HPLC analysis by using a 5-mm-spherisorb amino column (Phase Separation, 25·0 cm × 4·6 mm) in a Hewlett Packard 1090 Liquid Chromatograph with diode array detector. The column was eluted isocratically with solvent A (80% methanol, 20% water) for 10 min, followed by a 20-min gradient to 90% of solvent B (80% ammonium acetate buffer, 20% solvent A). GSH was calculated at 357 nm against known quantities of external standard (0·5–5·0 nmol).
Proliferative response
PBMC were resuspended at 1 × 106/ml in complete medium [RPMI-1640 supplemented with 10% heat-inactivated human serum (blood type AB), l-glutamine and penicillin–streptomycin] and stimulated with different mitogens, antigen and recombinant interleukin-2 (IL-2). The following doses were used: 10 µg/ml phytohaemmaglutinin (PHA), 1:50 dilution of pokeweed mitogen, 5 µg/ml concanavalin A, 20 µg/ml purified protein derivative from Mycobacterium tuberculosis, 50 µg/ml GMP (a mannoprotein-rich extract of Candida albicans cell wall that was a kind gift from Dr A. Cassone of this Institute), 20 U/ml IL-2. In some experiments PBMC were preincubated with 100 µg/ml fucoidan or 10 µg/ml anti-CD36 (Sigma Chemicals Co., St. Louis, MO) for 30 min at 37°. After 3 or 5 days, the cultures were pulsed for 18 hr with 0·5 µCi/well of [3H]TdR (specific activity 5 Ci/mm, Radiochemical Inc., Amersham, UK). Cells were then harvested onto glass fibre filters and [3H]TdR incorporation was measured by liquid scintillation spectroscopy. The results are expressed as mean counts per minute (c.p.m.) ± SD of the mean of triplicate cultures.
Cytotoxic assay
U937 target cells (1 × 106) were labelled with 51Cr by incubation with 100 µCi of 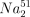 CrO4 (Amersham) for 1 hr at 37°.32 Effector cells (EC) were admixed with target cells (TC) (1 × 104 cells/well) at several ratios ranging from 6·25:1 up to 100:1. All experiments were performed in triplicate. Cell mixtures were incubated for 4 hr at 37° in flat-bottom, 96-well microtitre plates and centrifuged at 800 g for 5 min at the end of incubation period. One hundred microlitres of the supernatant was then collected from each well and counted in a gamma-counter. Spontaneous release, maximum release and percentage of specific 51Cr release were determined as described previously.33 NK cells were pretreated overnight with ox-LDL, NAC, or ox-LDL/NAC prior to the cytotoxic assay.
CrO4 (Amersham) for 1 hr at 37°.32 Effector cells (EC) were admixed with target cells (TC) (1 × 104 cells/well) at several ratios ranging from 6·25:1 up to 100:1. All experiments were performed in triplicate. Cell mixtures were incubated for 4 hr at 37° in flat-bottom, 96-well microtitre plates and centrifuged at 800 g for 5 min at the end of incubation period. One hundred microlitres of the supernatant was then collected from each well and counted in a gamma-counter. Spontaneous release, maximum release and percentage of specific 51Cr release were determined as described previously.33 NK cells were pretreated overnight with ox-LDL, NAC, or ox-LDL/NAC prior to the cytotoxic assay.
Cytokine assay
Detection in cell supernatants
To induce cytokine production, PBMC were cultured at 1 × 106 cells/ml in 24-well plates (Costar 3524) in complete medium, stimulated with 10 µg/ml PHA and treated with native LDL, ox-LDL and NAC. Supernatants were harvested 48 hr later and cytokine contents were measured using a sandwich ELISA (R & D System, Minneapolis, MN) according to the manufacturer's instructions.
Reverse transcription–polymerase chain reaction (RT-PCR) and densitometric analysis
For cytokine mRNA analysis, cells were cultured at 5 × 106/ml and stimulated with 10 µg/ml PHA with or without native LDL, ox-LDL and NAC. After 6 and 24 hr of culture, total cellular RNA was extracted using the RNeasy Kit (Quiagen, Hilden, Germany). RNA was reverse transcribed into cDNA and amplified by PCR using the Access RT-PCR System (Promega, Madison, WI) according to the manufacturer's instruction. The GAPDH set of primers was synthesized by MWG Biotech (Edersberg, Germany); the following couples of primers were used: GAPDH: sense 5′-GTC TTC ACC ATG GAG AAG GTC-3′; antisense 5′-CAT GCC AGT GAG CTT CCC GTT CA-3′; IL-1β, IL-2, IL-4, IL-6, IL-10, tumour necrosis factor-α (TNF-α) and interferon-γ (IFN-γ) sets of primers were purchased from Clontech; IL-15 set of primers was kindly provided by Dr S. Ferrini (Istituto Nazionale per la Ricerca sul Cancro, Genova, Italy). To avoid contamination all experiments included control RT-PCR without RNA. Densitometric analysis was performed as previously described.34,35
Statistical analysis
Statistical significance of results obtained from different experiments was calculated using a Student's t-test. A P value less than 0·05 was considered statistically significant.
Results
NAC and ox-LDL exert opposite effects on lymphocyte proliferative response
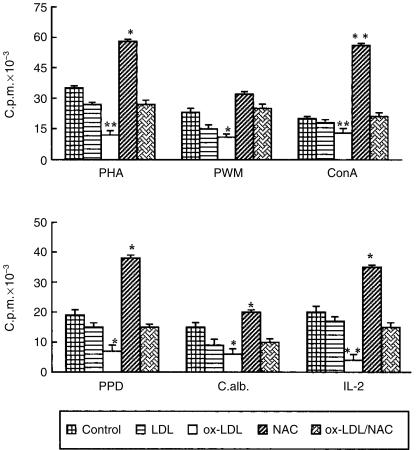
The effects of ox-LDL and NAC, added at the beginning of the culture and left throughout the culture period, on mitogen-induced, antigen-induced and IL-2-induced activation of normal human PBMC are illustrated in Fig. 1. The ox-LDL induced a significant down-regulation of proliferative response either with mitogens or with antigens and IL-2. By contrast, NAC induced a significant up-regulation of proliferative responses with all the stimuli used. More importantly, when NAC was added to the cultures in combination with ox-LDL, the proliferative responses were restored to values similar to control samples.
Figure 1.
NAC and ox-LDL exert opposite effects on lymphocyte proliferative response based on [3H]TdR uptake. Normal human PBMC were stimulated in vitro with mitogen (top; PHA, phytohaemmaglutinin; PWM, pokeweed mitogen; Con A, concanavalin A) or antigen (PPD, protein purified derivative from M. tuberculosis; C. alb., Candida albicans) and IL-2 (bottom) and untreated (control) or treated with native LDL, ox-LDL, NAC and ox-LDL/NAC. Results are expressed as mean c.p.m. ± SD of triplicate cultures. Data represent values from one representative experiment of at least five performed. (*P < 0·05; **P < 0·001 versus control cells).
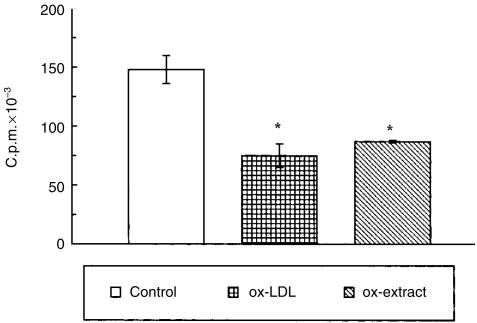
Different control experiments were also performed in order to assess the specificity of the above results. These experiments indicated, first, a similar viability in ox-LDL-exposed, NAC-exposed and control cultures. This was performed to exclude the possibility that inhibition of cell proliferation was due to a non-specific toxicity or induction of apoptosis. In fact, no signs of cytotoxicity were found after trypan blue dye exclusion assay as well as Hoechst chromatin staining (data not shown). Second, no inhibitory effects were observed on proliferative response after mitogen-induced, antigen-induced and IL-2-induced activation when native LDL were added to the cultures at the same concentrations as the ox-LDL (Fig. 1). Third, and notably, lipid extracts from ox-LDL were able to reproduce the same effect as the lipoprotein (Fig. 2).
Figure 2.
Lipid extract of ox-LDL down-regulated PHA-induced proliferative response of normal human PBMC based on [3H]TdR uptake. Results are expressed as mean c.p.m. ± SD of triplicate cultures. Data represent values from one representative experiment of at least three performed (*P < 0·05 versus control cells). The effect of native LDL was tested and the results obtained were comparable to control values.
As NAC acts as a precursor for GSH synthesis, we evaluated intracellular GSH level in NAC- or ox-LDL-treated PBMC. Our data indicate that the content of GSH in PBMC is: 1·117 nmol/106 cells in control cells; 1·340 nmol/106 cells in NAC-treated cells and 0·446 nmol/106 cells in ox-LDL-treated cells.
Further experiments were carried out in order to evaluate ox-LDL internalization. The down-regulating effect on PHA proliferative response induced by ox-LDL (80%) was not abrogated by either the preincubation with fucoidan (76%), a polysaccharide ligand that competes for the ligand-binding domain of class A scavenger receptors,36–38 or with anti-CD36 (86%), a monoclonal antibody that blocks ox-LDL binding to CD36 (a class B scavenger receptor).12,13,39–41
NAC and ox-LDL exert opposite effects on NK-cell-mediated cytotoxicity activity
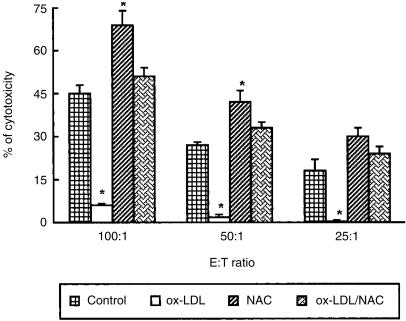
In order to evaluate the effect of NAC and ox-LDL on NK-cell-mediated cytotoxicity, effector cells were preincubated overnight with ox-LDL, NAC, or with their combination. As shown in Fig. 3, a significant decrease of NK-cell activity (P < 0·05) was observed when ox-LDL were added, while a significant increase was detected after the exposure to NAC at all the effector to target cell ratios considered. More importantly, when NAC was added to the cultures together with ox-LDL, the NK-cell activity was completely restored to values similar to that of control samples.
Figure 3.
NAC and ox-LDL exert opposite effects on NK-cell-mediated cytotoxicity based on 51Cr release. Effector cells (EC) were preincubated overnight with ox-LDL, NAC or their combination. Results are expressed as percentage of cytotoxicity (% of specific 51Cr release). Data are expressed as mean±SD from three independent experiments. *P < 0·05 vs. control. In all experiments the spontaneous release was less than 10%. The effect of native LDL was tested and the results obtained were comparable to control values.
NAC and ox-LDL exert opposite effects on cytokine production
Since cytokines drive both T-cell and NK-cell responses, we analysed the pattern of the production of these mediators by PBMC exposed to ox-LDL, NAC and their combination.
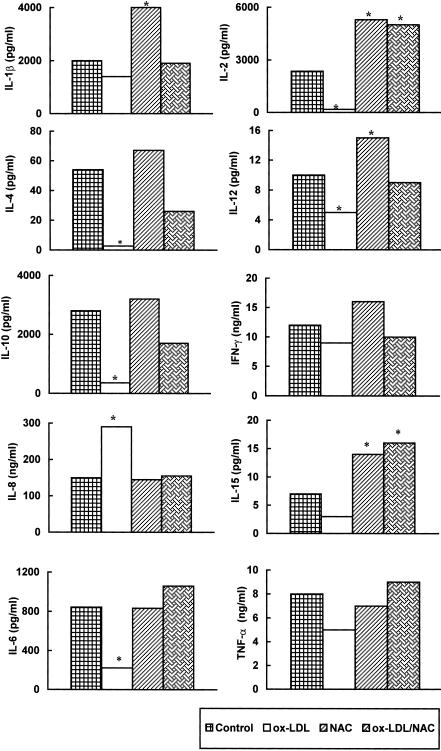
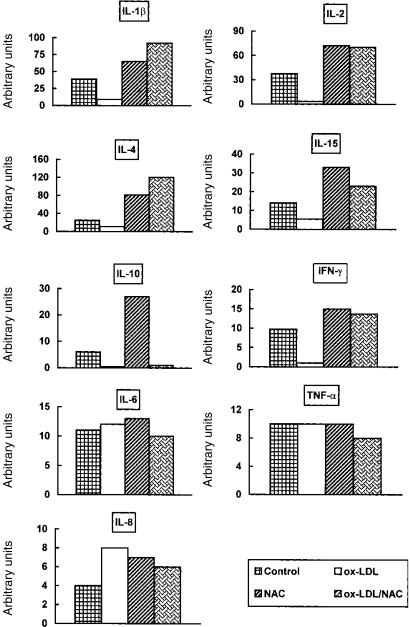
In vitro exposure of PHA-stimulated PBMC to ox-LDL caused a significant (P < 0·05) down-regulation of IL-2, IL-4, IL-12, IL-10 and IL-6 release in culture supernatants while no significant decrease of IL-1β, IFN-γ, IL-15 and TNF-α content was found in the same supernatants (Fig. 4). In contrast, IL-8 production was significantly up-regulated after ox-LDL treatment.
Figure 4.
NAC and ox-LDL modulate cytokine supernatant release. PHA-stimulated PBMC were treated with ox-LDL, NAC, or their combination for 48 hr. Results are expressed as pg/ml or ng/ml. Data represent values from one experiment representative of at least three performed, in which the same trend of values was observed. (*P < 0·05). The effect of native LDL was tested and the results obtained were comparable to control values.
On the other hand, 48-hr NAC treatment induced a significant (P < 0·05) up-regulation of IL-1β, IL-2, IL-12 and IL-15 production while production of IL-4, IL-10, IFN-γ, IL-8, IL-6 and TNF-α was unchanged. More importantly, when NAC was used in combination with ox-LDL cytokine secretion was restored to control values (Fig. 4). Notably, using the combination, IL-2 and IL-15 production was comparable to that induced by NAC.
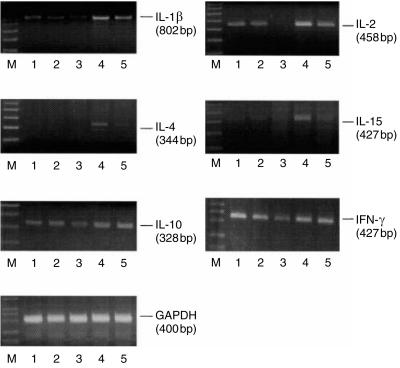
To verify whether ox-LDL and NAC affected cytokine gene expression we performed a molecular analysis of mRNA level. Figure 5 shows that ox-LDL inhibited IL-1β, IL-2, IL-15, IL-10 and IFN-γ gene expression and did not affect IL-4, IL-6 and TNF-α mRNA transcription. More importantly, NAC was able to abrogate the inhibitory effects induced by ox-LDL at transcriptional level on all the cytokines analysed except for IL-10. We investigated the early (6 hr) effect of ox-LDL, NAC and their combination on cytokine mRNA synthesis. Interestingly, mRNA inhibition induced by ox-LDL was already detectable after 6 hr of treatment for all the cytokines analysed except for IL-10 (Fig. 6).
Figure 5.
NAC and ox-LDL modulate cytokine mRNA levels. PHA-stimulated PBMC were treated with ox-LDL, NAC, or their combination for 24 hr. Results are expressed in arbitrary units. Data represent values from one experiment representative of three performed considering different donors in which the same trend of values was observed. The effect of native LDL was tested and the results obtained were comparable to control values.
Figure 6.
NAC and ox-LDL modulate cytokine mRNA levels. PHA-stimulated PBMC were treated with ox-LDL, NAC or their combination for 6 hr. Data represent values from one experiment representative of three performed considering different donors in which the same trend of values was observed. Lane M, 100 bp ladder; lane 1, control; lane 2, LDL; lane 3, ox-LDL; lane 4, NAC; lane 5, ox-LDL/NAC. GAPDH cDNA levels were equivalent in all cell samples analysed, as indicated.
Treatment of PHA-activated PBMC with native LDL did not exert any modulation of cytokine production in any of the experimental conditions considered (data not shown).
Discussion
In the present study we analysed the in vitro effects of ox-LDL and NAC, used either alone or in combination, on different immune functions of normal human PBMC. We found that ox-LDL and NAC exerted opposite effects down-regulating and up-regulating immune functions, respectively. Therefore, we set up experiments in order to verify whether the antioxidant NAC added in combination with ox-LDL was able to counteract the down-regulating effect of the ox-LDL. We found that NAC re-established the lymphocyte proliferative response, the NK-cell-mediated cytotoxic activity and the cytokine production to values comparable to controls. Native LDL used as control did not exert any inhibitory effects either on proliferative responses or on NK-cell activity and cytokine production.
We found that the lipid moiety of ox-LDL was able to reproduce the same effect as the lipoprotein, suggesting its role in the immunomodulation. In addition, we found that the inhibitory effect induced by ox-LDL on proliferative responses is not receptor-mediated. We blocked the two major receptors that bind and internalize ox-LDL: CD36 (a class B scavenger receptor) and the macrophage scavenger receptor (MSR, a class A scavenger receptor).39–41 In addition to modified lipoproteins, a different group of polyanionic ligands are recognized by the MSR, including sulphated polysaccharides such as fucoidan.36–38 The inhibitory effect on PHA proliferative response induced by ox-LDL was not abrogated either by the preincubation with anti-CD36 monoclonal antibody or with fucoidan. This might suggest that modulation induced by ox-LDL is not due to the entire lipoprotein but to the lipid moiety entry inside the cell. However, we cannot rule out the possibility that, without directly entering the cells, ox-LDL could trigger a signalling cascade inside the cells. This needs to be further analysed in future studies.
In the present study we observed that the NK-cell-mediated cytotoxic activity was significantly down-regulated by ox-LDL while treatment with NAC induced a significant enhancement in NK-cell cytotoxic activity. On the basis of our previous studies,42,43 we hypothesize that cytoskeleton integrity and function, prerequisites for NK-cell activity, could be oxidatively altered by ox-LDL leading to a decreased NK-cell function. On the other hand, the antioxidant activity exerted by NAC on cytoskeletal thiol groups can lead to an increased NK-cell-mediated cytotoxic binding and killing.44–47 Therefore, in this study we analysed the effect of NAC added in combination with ox-LDL on NK-cell-mediated cytotoxic activity. We found that NAC restored NK-cell activity to values comparable to controls.
It has been reported that the cytokine network is altered in ox-LDL-related diseases.48,49 Here we showed that ox-LDL down-regulated the production of IL-2, IL-4, IL-10, IL-12, IL-10 and IL-6 in PHA-stimulated PBMC. In contrast, ox-LDL induced a significant up-regulation of IL-8 production, confirming previous results by other authors.50,51 We found that IL-1β and IFN-γ mRNA levels were inhibited up to 80–90% by ox-LDL, while secretion of these proteins was not significantly down-regulated. Thus, the inhibitory effect of ox-LDL on IL-1β and IFN-γ production may be due to an early event at transcriptional level suggesting that the products of pre-existing mRNA were secreted and that ox-LDL inhibited mRNA neosynthesis.
Similarly to proliferative responses and NK-cell-mediated cytotoxic activity, NAC treatment exerted an up-regulating effect on the cytokine network increasing production of IL-1β, IL-2, IL-12, IL-15. Interestingly, cytokine production was restored when NAC was used in combination with ox-LDL. However, it should be noted that NAC was not able to counteract the inhibitory effect of ox-LDL on IL-10 mRNA transcription. Interestingly, mRNA inhibition induced by ox-LDL was already detectable after 6 hr of treatment for all the cytokines analysed except for IL-10, suggesting that NAC is not able to abrogate a late (24 hr) effect of ox-LDL.
Our results suggest that ox-LDL and NAC modulate immune functions exerting opposite effects reflecting their pro-oxidant and antioxidant behaviour and we hypothesized that they may act by an indirect mechanism involving the cytokine network. In particular, the significant decrease or the significant increase in IL-2 and IL-15 production, cytokines known for their activating effect on T cells, may account for the down-regulation or up-regulation exerted on proliferative responses by ox-LDL or NAC, respectively. Similarly the induction (increase) or inhibition (decrease) of IL-12, IL-15 and IFN-γ production by NAC and ox-LDL, respectively, might be responsible of the modulation of NK-cell activity.
The results of the present study could add new insights to the key role played by redox imbalances as modulators of immune homeostasis, contributing to the clarification of the pathogenic mechanisms of different human degenerative diseases including ageing related diseases and AIDS. Moreover, an antioxidant drug such as NAC could be useful against pathologies associated with an increase in lipid peroxidation.
In conclusion, since it has been demonstrated that in vivo an increase of ox-LDL modulates cellular and humoral immune responses, our in vitro model employing experimentally oxidized LDL could be a useful tool to test the efficacy of novel antioxidant drugs before their clinical use.
Acknowledgments
This work was partially supported by National Research Project on AIDS to M.V. and W.M.
Abbreviations
- ox-LDL
oxidized low-density lipoprotein
- NAC
N-acetylcysteine
References
- 1.Halliwell B. Free radicals, antioxidants and human disease. Curiosity, cause or consequence. Lancet. 1994;344:431–438. doi: 10.1016/s0140-6736(94)92211-x. [DOI] [PubMed] [Google Scholar]
- 2.Scarfiotti C, Fabris F, Cestaro B, Giuliani A. Free radicals, atherosclerosis, ageing, and related dysmetabolic pathologies: pathological and clinical aspects. Eur J Cancer Prev. 1997;1:31–6. doi: 10.1097/00008469-199703001-00007. [DOI] [PubMed] [Google Scholar]
- 3.Schwarz KB. Oxidative stress during viral infection: a review. Free Rad Biol Med. 1996;21:641–9. doi: 10.1016/0891-5849(96)00131-1. [DOI] [PubMed] [Google Scholar]
- 4.Muller F. Reactive oxygen intermediates and human immunodeficiency virus (HIV) infection. Free Rad Biol Med. 1992;13:651–7. doi: 10.1016/0891-5849(92)90039-j. [DOI] [PubMed] [Google Scholar]
- 5.Pace GW, Leaf CD. The role of oxidative stress in HIV disease. Free Rad Biol Med. 1995;19:523–8. doi: 10.1016/0891-5849(95)00047-2. [DOI] [PubMed] [Google Scholar]
- 6.Legrand-Poels S, Vaira D, Pincemail J, Van de Vorst A, Piette J. Activation of human immunodeficiency virus type 1 by oxidative stress. AIDS Res Hum Retroviruses. 1990;6:1389–97. doi: 10.1089/aid.1990.6.1389. [DOI] [PubMed] [Google Scholar]
- 7.Gatè PJ, Nguyen Ba Gtew KD, Tapiero H. Oxidative stress induced in pathologies: the role of antioxidants. Biomed Pharmacother. 1999;53:169–80. doi: 10.1016/S0753-3322(99)80086-9. [DOI] [PubMed] [Google Scholar]
- 8.Pagano G, Korkina LG, Brunk UT, et al. Congenital disorders sharing oxidative stress and cancer proneness as phenotypic hallmarks: prospects for joint research in pharmacology. Med Hypotheses. 1998;51:253–66. doi: 10.1016/s0306-9877(98)90084-6. [DOI] [PubMed] [Google Scholar]
- 9.Steinberg D. Oxidative modification of LDL and atherogenesis. Circulation. 1997;95:1062–71. doi: 10.1161/01.cir.95.4.1062. [DOI] [PubMed] [Google Scholar]
- 10.Escargueil-Blanc I, Salvayre R, Nègre-Salvayre A. Necrosis and apoptosis induced by oxidized low density lipoproteins occur through two calcium-dependent pathways in lymphoblastoid cells. FASEB J. 1994;8:1075–80. doi: 10.1096/fasebj.8.13.7926374. [DOI] [PubMed] [Google Scholar]
- 11.Alcouffe J, Caspar-Bauguil S, Garcia V, Salvayre R, Thomsen M, Benoist H. Oxidized low density lipoproteins induce apoptosis in PHA-activated peripheral blood mononuclear cells and in the Jurkat T-cell line. J Lipid Res. 1999;40:1200–10. [PubMed] [Google Scholar]
- 12.Steinbrecher UP. Receptors for oxidized low density lipoprotein. Biochim Biophys Acta. 1999;1436:279–98. doi: 10.1016/s0005-2760(98)00127-1. [DOI] [PubMed] [Google Scholar]
- 13.Dhaliwal BS, Steinbrecher UP. Scavenger receptors and oxidized low density lipoproteins. Clin Chim Acta. 1999;286:191–205. doi: 10.1016/s0009-8981(99)00101-1. [DOI] [PubMed] [Google Scholar]
- 14.Lopes-Virella MF, Virella G. Atherosclerosis and autoimmunity. Clin Immunol Immuno-Pathol. 1994;73:155–67. doi: 10.1006/clin.1994.1184. [DOI] [PubMed] [Google Scholar]
- 15.Hodis H-N, Kramsch DM, Avogaro P, Bittolo-Bon G, Cazzolato G, Hwang J, Peterson H, Sevanian A. Biochemical and cytotoxic characteristics of an in vivo circulating oxidized low density lipoprotein (LDL-) J Lipid Res. 1994;35:669–77. [PubMed] [Google Scholar]
- 16.Schuh J, Novogrodsky A, Haschemeyer RH. Inhibition of lymphocyte mitogenesis by autoxidized low-density lipoprotein. Biochem Biophys Res Comm. 1978;84:763–8. doi: 10.1016/0006-291x(78)90770-2. [DOI] [PubMed] [Google Scholar]
- 17.Burgunder JM, Varriale A, Lauterburg BH. Effect of N-acetylcysteine on plasma cysteine and glutathione following paracetamol administration. Eur J Clin Pharmacol. 1989;36:127–31. doi: 10.1007/BF00609183. [DOI] [PubMed] [Google Scholar]
- 18.De Flora S, Rossi GA, De Flora AD. Metabolic desmutagenic and anticarcinogenic effect of N-acetylcysteine. Respiration. 1986;50:43–9. doi: 10.1159/000195087. [DOI] [PubMed] [Google Scholar]
- 19.Morini M, Cai T, Abrigi MG, et al. The role of the thiol N-acetylcysteine in the prevention of tumor invasion and angiogenesis. Int J Biol Markers. 1999;14:268–71. doi: 10.1177/172460089901400413. [DOI] [PubMed] [Google Scholar]
- 20.Sen CK. Redox signaling and the emerging therapeutic potential of thiol antioxidants. Biochem Pharmacol. 1998;55:1747–58. doi: 10.1016/s0006-2952(97)00672-2. [DOI] [PubMed] [Google Scholar]
- 21.Look MP, Rockstroh JK, Rao GS, et al. Sodium selenite and N-acetylcysteine in antiretroviral-naive HIV-1-infected patients: a randomized, controlled pilot study. Eur J Clin Invest. 1998;28:389–97. doi: 10.1046/j.1365-2362.1998.00301.x. [DOI] [PubMed] [Google Scholar]
- 22.Malorni W, Rivabene R, Santini MT, Donelli G. N-acetylcysteine inhibits apoptosis and decreases viral particles in HIV-chronically infected U937 cells. FEBS Lett. 1993;1:75–8. doi: 10.1016/0014-5793(93)81043-y. [DOI] [PubMed] [Google Scholar]
- 23.Malorni W, Rivabene R, Lucia BM, Ferrara R, Mazzone AM, Cauda R, Paganelli R. The role of oxidative imbalance in progression to AIDS. Effect of the thiol supplier N-acetylcysteine. AIDS Res Hum Retroviruses. 1998;14:1589–96. doi: 10.1089/aid.1998.14.1589. [DOI] [PubMed] [Google Scholar]
- 24.Eylar E, Rivera-Quinones C, Molina C, Baez I, Molina F, Mercado CM. N-acetylcysteine enhances T cell functions and T cell growth in culture. Int Immunol. 1993;5:97–101. doi: 10.1093/intimm/5.1.97. [DOI] [PubMed] [Google Scholar]
- 25.Aver RJ, Eder HA, Bragdon JH. The distribution and chemical composition of ultracentrifugally separated lipoproteins in human serum. J Clin Invest. 1995;34:1345–52. doi: 10.1172/JCI103182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Steinberg D, Parthasarathy S, Carew TE, Khoo JC, Witztum JL. Beyond cholesterol. Modifications of low-density lipoprotein that increase its atherogenicity. N Engl J Med. 1989;320:915–24. doi: 10.1056/NEJM198904063201407. [DOI] [PubMed] [Google Scholar]
- 27.Esterbauer H, Dieber-Rotheneder M, Waeg G, Striegl G, Jurgens G. Biochemical, structural, and functional properties of oxidized low-density lipoprotein. Chem Res Toxicol. 1990;3:77–92. doi: 10.1021/tx00014a001. [DOI] [PubMed] [Google Scholar]
- 28.Nègre-Salvayre A, Alomar Y, Troly M, Salvayre R. UV-treated lipoproteins as a model system for the study of the biological effect of lipid peroxides on cultured cells. III. The protective effect of antioxidants (procubol, catechin, vitamin E) against the cytotoxicity of oxidized LDL occurs in two different ways. Biochim Biophys Acta. 1991;1096:291–300. doi: 10.1016/0925-4439(91)90065-h. [DOI] [PubMed] [Google Scholar]
- 29.Folch J, Lees M, Sloane-Stanley GH. A simple method for isolation and purification of total lipids from animal tissues. J Biol Chem. 1957;226:497–509. [PubMed] [Google Scholar]
- 30.Nègre-Salvayre A, Lopez M, Levade T, Pieraggi MT, Dousset N, Douste-Blazy L, Salvayre R. Ultraviolet-treated lipoproteins as a model system for the study of the biological effects of lipid peroxides on cultured cells. II. Uptake and cytotoxicity of ultraviolet-treated LDL on lymphoid cell lines. Biochim Biophys Acta. 1990;1045:224–32. doi: 10.1016/0005-2760(90)90124-g. [DOI] [PubMed] [Google Scholar]
- 31.Reed DJ, Babson RJ, Beatty PW, Brodie AE, Ellis WW, Potter DW. High-performance liquid chromatography analysis of nanomole levels of glutathione, glutathione disulfide, and related thiols and disulfides. Ann Biochem. 1980;106:55–62. doi: 10.1016/0003-2697(80)90118-9. [DOI] [PubMed] [Google Scholar]
- 32.Zarcone D, Tilden AB, Grossi VG. Radiation sensitivity of resting and activated nonspecific cytotoxic cells of T lineage and NK lineage. Blood. 1989;73:615–21. [PubMed] [Google Scholar]
- 33.Tilden AB, Zarcone D, Prasthofer EF, Lane VG. X-linked codominant genes control all types of non-major histocompatibility complex-restricted cytotoxicity. Hum Immunol. 1991;30:208–14. doi: 10.1016/0198-8859(91)90036-9. [DOI] [PubMed] [Google Scholar]
- 34.Viora M, Camponeschi B. Down-regulation of interleukin-2 receptor gene activation and protein expression by dideoxynucleoside analogs. Cell Immunol. 1995;163:289–95. doi: 10.1006/cimm.1995.1128. [DOI] [PubMed] [Google Scholar]
- 35.Haraguchi S, Good RA, James-Yarish M, Cianciolo GJ, Day NK. Differential modulation of Th-1 and Th-2 related cytokine mRNA expression by a syntetic peptide homologous to a conserved domain within retroviral envelope protein. Proc Natl Acad Sci USA. 1995;92:3611–15. doi: 10.1073/pnas.92.8.3611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Krieger M, Herz J. Structures and functions of multiligand lipoprotein receptors: macrophage scavenger receptors and LDL receptor-related protein (LRP) Annu Rev Biochem. 1994;63:601–37. doi: 10.1146/annurev.bi.63.070194.003125. [DOI] [PubMed] [Google Scholar]
- 37.Falcone DJ, Ferenc MJ. Acetyl-LDL stimulates macrophage-dependent plasminogen activation and degradation of extracellular matrix. J Cell Physiol. 1988;135:387–96. doi: 10.1002/jcp.1041350305. [DOI] [PubMed] [Google Scholar]
- 38.Falcone DJ, McCaffrey TA, Vergilio JA. Stimulation of macrophage urokinase expression by polyanions is protein kinase C-dependent and requires protein and RNA synthesis. J Biol Chem. 1991;226:22726–32. [PubMed] [Google Scholar]
- 39.Calvo D, Gòmez-Coronado D, Lasunciòn MA, Vega MA. CLA-1 is an 85-kD plasma membrane glycoptrotein that acts as a high affinity receptor for both native (HDL, LDL and VLDL) and modified (ox-LDL and acLDL) lipoprotein. Arterioscler Thromb Vasc Biol. 1997;7:2341–9. doi: 10.1161/01.atv.17.11.2341. [DOI] [PubMed] [Google Scholar]
- 40.Calvo D, Gòmez-Coronado D, Suàrez Y, Lasunciòn MA. Human CD36 is a high affinity receptor for the native lipoproteins HDL, HDL, and VLDL. J Lipid Res. 1998;39:777–88. [PubMed] [Google Scholar]
- 41.El Khoury J, Hickman Thomas CA, Cao L, Silverstein SC, Loike JD. Scavenger receptor-mediated adhesion of microglia to β-amyloid fibrils. Nature. 1996;382:716–19. doi: 10.1038/382716a0. [DOI] [PubMed] [Google Scholar]
- 42.Malorni W, Straface E, Di Genova G, Fattorossi A, Rivabene R, Camponeschi B, Masella R, Viora M. Oxidized low-density lipoproteins affect natural killer cell activity by impairing cytoskeleton function and altering the cytokine network. Exp Cell Res. 1997;236:436–45. doi: 10.1006/excr.1997.3736. 10.1006/excr.1997.3736. [DOI] [PubMed] [Google Scholar]
- 43.Malorni W, D'Ambrosio A, Rainaldi G, Rivabene R, Viora M. Thiol supplier N-acetylcysteine enhances conjugate formation between natural killer cells and K562 or U937 targets but increases the lytic function only against the latter. Immunol Lett. 1994;43:209–14. doi: 10.1016/0165-2478(94)90225-9. [DOI] [PubMed] [Google Scholar]
- 44.Giovannini C, Straface E, Modesti D, Coni E, Cantafora A, De Vincenzi M, Malorni W, Masella R. Tyrosol, the major olive oil biophenol, protects against oxidized-LDL-induced injury in Caco-2 cells. J Nutr. 1999;129:1269–77. doi: 10.1093/jn/129.7.1269. [DOI] [PubMed] [Google Scholar]
- 45.Masella R, Straface E, Giovannini C, Di Benedetto R, Scazzocchio B, Viora M, Cantafora A, Malorni W. Subcellular alterations induced by UV-oxidized low density lipoproteins in ephitelial cells can be counteracted by alpha-tocopherol. Photochem Photobiol. 2000;71:97–102. doi: 10.1562/0031-8655(2000)071<0097:saibuo>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 46.Sirianni MC, Soddu S, Malorni W, Arancia G, Aiuti F, Soddus S. Mechanism of defective natural killer cell activity in patients with AIDS is associated with defective distribution of tubulin. J Immunol. 1988;140:2565–8. [PubMed] [Google Scholar]
- 47.Bellomo G, Mirabelli F, Vairetti M, Iosi F, Malorni W. Cytoskeleton as a target in menadione-induced oxidative stress in cultured mammalian cells. I. Biochemical and immunocytochemical features. J Cell Physiol. 1990;143:118–28. doi: 10.1002/jcp.1041430116. [DOI] [PubMed] [Google Scholar]
- 48.Lopes-Virella MF, Virella G. Cytokines, modified lipoproteins, and arteriosclerosis in diabetes. Diabetes. 1996;3:40–4. doi: 10.2337/diab.45.3.s40. [DOI] [PubMed] [Google Scholar]
- 49.Uyemura K, Demer LL, Castle SC, et al. Cross-regulatory roles of interleukin (IL) -12 and IL-10 in atherosclerosis. J Clin Invest. 1996;97:2130–8. doi: 10.1172/JCI118650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang N, Tabas I, Winchester R, Ravalli S, Rabbani LE, Tall A. Interleukin 8 is induced by cholesterol loading of macrophages and expressed by macrophage foam cells in human atheroma. J Biol Chem. 1996;271:8837–42. doi: 10.1074/jbc.271.15.8837. [DOI] [PubMed] [Google Scholar]
- 51.Liu Y, Hulten LM, Wiklund O. Macrophages isolated from human atherosclerotic plaques produce IL-8, and oxysterol may have a regulatory function for IL-8 production. Arterioscler Thromb Vasc Biol. 1997;17:317–23. doi: 10.1161/01.atv.17.2.317. [DOI] [PubMed] [Google Scholar]